Abstract
The ability of many animals to recognize kin has allowed them to evolve diverse cooperative behaviours; such ability is less well studied for plants. Many plants, including Artemisia tridentata, have been found to respond to volatile cues emitted by experimentally wounded neighbours to increase levels of resistance to herbivory. We report that this communication was more effective among A. tridentata plants that were more closely related based on microsatellite markers. Plants in the field that received cues from experimentally clipped close relatives experienced less leaf herbivory over the growing season than those that received cues from clipped neighbours that were more distantly related. These results indicate that plants can respond differently to cues from kin, making it less likely that emitters will aid strangers and making it more likely that receivers will respond to cues from relatives. More effective defence adds to a growing list of favourable consequences of kin recognition for plants.
Keywords: communication, eavesdropping, kin recognition, volatiles, Artemisia tridentata, herbivory
1. Introduction
Many animals are able to distinguish close relatives from strangers and to act differently towards their kin [1–3]. Individuals are expected to behave preferentially towards kin to increase their inclusive fitness [4]. For example, recognition of kin allows individuals to direct altruism towards kin, avoiding the costs of behaving altruistically towards strangers [5]. Kin recognition and kin bias have long been assumed to be beyond the abilities of plants, although various pollen self-incompatibility mechanisms have been well accepted [6,7]. Recent evidence also indicates that allocation patterns in some plants differ if their roots encounter relatives compared with strangers [8–10]. In general, plants grew roots or stems more aggressively when strangers were encountered compared with kin.
A growing number of plants have been found to respond to volatile cues released when neighbours are damaged by herbivores to prime or increase their defences to future risk of attack [11]. Sagebrush (Artemisia tridentata) responded most effectively when volatile cues were emitted by genetically identical clones compared with strangers, suggesting the ability to distinguish self from non-self and to respond more strongly to ‘self’ signals [12]. The ability to discriminate between volatile cues released by close relatives (rather than clones) versus strangers and to respond differentially has not been demonstrated for this or any other plant species. Here, we report that sagebrush responds more effectively to volatile cues emitted by closely related individuals to reduce levels of leaf damage experienced under natural conditions.
2. Material and methods
We conducted four field experiments over 3 years that compared the proportion of leaves which were damaged by herbivores over the growing season when plants were provided with volatile cues from a clipped close relative versus cues from a distant relative. The first two experiments were the most natural but had relatively few replicates. Cues were emitted at the start of the season by experimentally clipping plants that varied in their relatedness to rooted receiver plants, as determined using microsatellites.
Our fieldwork was conducted at Taylor Meadow, UC Sagehen Creek Field Station, North of Truckee, California. We produced potted clones of sagebrush by taking stem cuttings during winter, trimming them to a single vegetative bud plus 2–3 cm of stem, and dipping the base of the stems in talc that contained 0.8 per cent indole-3-butyric acid to stimulate root initiation [13]. Stem bases were placed in vermiculite, and the cuttings were maintained in a misting chamber until they rooted. Potted clones were returned to the field and used as volatile donors. In the spring, immediately following snow melt when naturally rooted plants were actively growing, two potted clones were placed within 10 cm of two assay receiver branches of a large, naturally rooted plant (figure 1a). The distal half of 25 per cent of the leaves was clipped with scissors on one branch of each potted volatile donor. Volatiles were allowed to pass naturally between clipped donor branches and receiver assay branches in the open air for 24 h, after which time potted plants were removed. The two donors were at least 1 m apart to minimize contamination of volatile cues. Sagebrush branches do not have highly functional vascular connections and are not well-integrated [14]; volatile cues were found to be active in this system up to distances of 60 cm [15]. At the end of the growing season, we counted the number of leaves with chewing damage and the total number of leaves on the assay branches of plants that had received volatiles from closely or distantly related donors.
Figure 1.
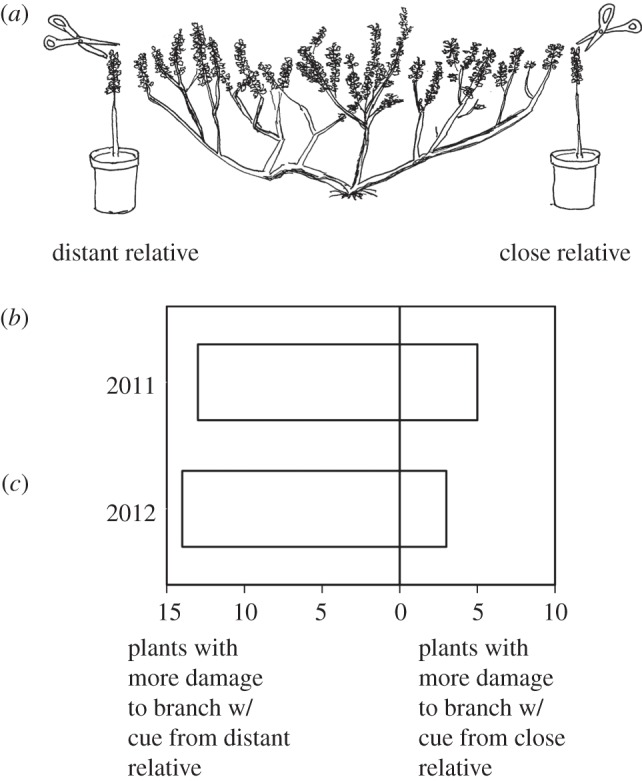
(a) Experimental set-up showing a rooted receiver plant with two potted volatile donor plants. Potted donors were placed within 10 cm of two branches of the receiver. The two potted donor plants were selected such that one was closely related to the receiver and one was distantly related. Leaves of the potted donors were clipped with scissors at the start of the growing season (June 2011 or May 2012). At the end of the season, natural levels of damage caused by herbivores were measured for the two branches of each receiver plant. (b,c) The number of receiver plants with more herbivore damage on the branch near a donor that was either a distant or a close relative. In 2011, 13 out of 18 receiver plants experienced more damage on the branch near the clipped distant relative. In 2012, 14 out of 17 receiver plants experienced more leaf damage on the branch near the clipped distant relative.
We determined relatedness using seven microsatellites that varied among individual sagebrush clones as described in Ishizaki et al. [16]. Relatedness (r) was estimated using the method described in Queller & Goodnight [17] with values ranging from −1 to 1. Two potted plants were selected for each receiver to maximize the difference in r between the closely and distantly related pairs. For each receiver (assay) plant, we compared the proportion of leaves damaged on the branch near the clipped closely related donor and distantly related donor. We evaluated the effect of relatedness using a binomial test with the null expectation that an equal number of branches would be more damaged near closely and distantly related clipped donors.
To increase our sample size and isolate the role of volatile cues, we conducted a third experiment in 2011 in which we moved volatiles from the headspace of clipped plants to the headspace of receivers. We determined levels of relatedness among 99 plants in Taylor meadow using techniques described above [16,17]. We designated 65 plants to be assays that received volatiles from one of 33 clipped donor plants (figure 2a). Plants were haphazardly selected such that our sample of pairs of clipped donors and receiver assay plants spanned the range from distantly to closely related. All plants were separated by greater than 1 m. We clipped the distal half of 25 per cent of the leaves on one branch of each donor and immediately enclosed the clipped branch in a clear plastic bag, attached at the stem with a wire twist–tie. Volatiles emitted by the clipped branch collected in the plastic bag for 24 h. After 24 h, one branch on each of the receiver assay plants was enclosed in a new plastic bag with a twist–tie around its stem. One litre of air from the headspace of the donor plant was transferred to the plastic bag on the receiver plant using a 1 l syringe (model S-1000, Hamilton Co., Reno, NV, USA). The branch on the receiver plant was incubated with air from the clipped plant for 24 h, after which the plastic bag was removed. All receiver assay plants were covered for 24 h; only the source of the volatiles varied. We modelled the proportion of damaged leaves on the receiver plants using binomial generalized linear mixed models with a random intercept for plant identity and included spatial block. The rates of herbivore damage were higher for plants on the west side of the meadow than the east side and rates of herbivory exhibited significant spatial autocorrelation (Moran's I, p < 0.01). To account for this strong effect of space on herbivory, we split the study into east and west blocks and found no spatial autocorrelation within blocks (Moran's I, p > 0.05). We fit models with all combinations of blocks and relatedness between donor and receiver as fixed effects and compared models using Akaike's information criteria (AIC) and deviance information criteria (DIC; [18]). All of the models had the same random effects structure. DIC values were similar to AIC and are not presented. The results were qualitatively similar when we modelled the effect of space by using the east–west spatial coordinates as a continuous explanatory variable, suggesting that our conclusion did not depend on how we represented space.
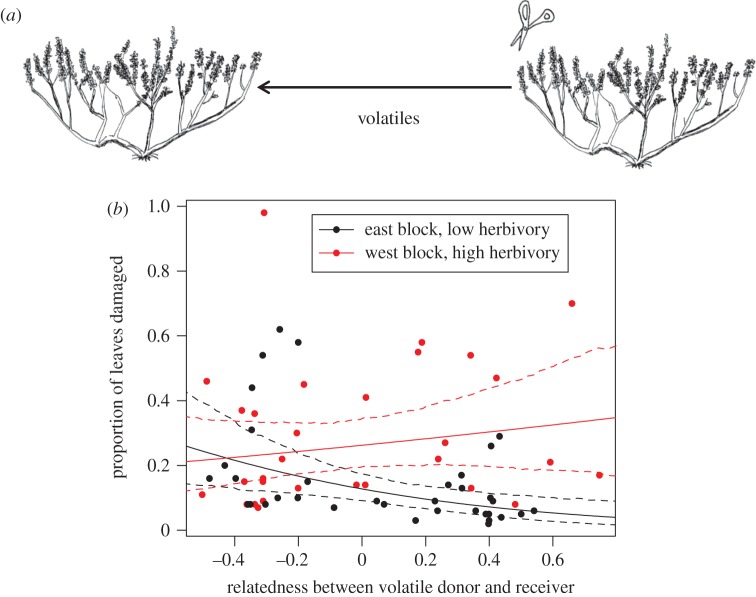
Figure 2.
(a) Experimental set-up showing the transfer of headspace volatiles collected for 24 h from an experimentally clipped branch and delivered to a receiver branch on another plant. The receiver branch was incubated for 24 h in a clear plastic bag containing the volatiles from the headspace of the clipped donor plant. (b) The relationship between relatedness of the donor and receiver plants, and the proportion of leaves damaged by herbivores on the receiver branch. For plants in the east block, rates of herbivore damage decreased as the relatedness between the volatile donor and receiver plants increased. For plants in the west block, rates of herbivore damage were high and showed no relationship to the level of relatedness between volatile donor and receiver.
In 2012, we used a paired design and incubated two branches of each receiver assay plant with volatiles from clipped closely and distantly related plants. This allowed a relatively large number of replicates and the paired design controlled for spatial patchiness in herbivory (figure 3a). We marked 25 receiver plants and determined two donor plants that were closely and distantly related to each receiver plant. One branch of each donor plant was clipped and volatiles were collected in plastic bags for 24 h as described above. Headspace volatiles were transferred using a 1 l syringe to bags surrounding two branches on each receiver assay plant in spring and branches incubated with volatiles for 24 h. The proportion of leaves damaged by herbivores was determined at the end of the season for pairs of assay branches that had received volatiles from clipped closely and distantly related donors. We compared the proportion of leaves damaged by herbivores using a paired t-test.
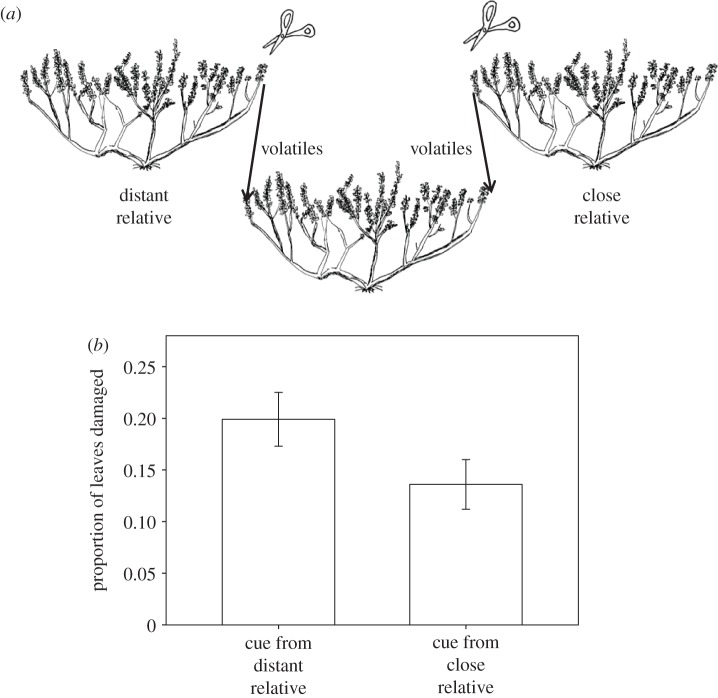
Figure 3.
(a) Experimental set-up showing the transfer of headspace volatiles from one branch of a clipped distant relative and from one branch of a clipped close relative. The two branches of the receiver plant were incubated with these headspace volatiles for 24 h early in the season. At the end of the season, we recorded the proportion of leaves damaged by herbivores on these two assay branches of receiver plants. (b) Branches on receiver plants incubated with headspace volatile cues from clipped distant relatives had a greater proportion of leaves damaged by herbivores than branches incubated with cues from clipped close relatives.
3. Results
The first two experiments compared herbivore damage on assay branches that received volatile cues from clipped potted plants which differed in their level of relatedness to the receiver. In the first experiment in 2011, we placed two potted plants near two assay branches of 18 large rooted sagebrush receiver plants (figure 1a). One of the potted plants was closely related to the rooted receiver plant (Queller & Goodnight's [17], r = 0.234±0.031) and a second potted plant was distantly related to the rooted receiver (r = −0.372±0.014). Leaves of both potted plants were experimentally clipped to provide volatile cues to the assay branches for 24 h. At the end of the season, branches near the closely related clipped plant had received less damage by herbivores than branches near the distantly related clipped plant in significantly more cases (figure 1b, mean proportional reduction±1 s.e. = 0.41±0.08, binomial test p = 0.05).
This experiment was repeated in 2012 using 17 large sagebrush plants with different combinations of closely and distantly related neighbours. Branches with closely related (r = 0.194±0.047) clipped neighbours received less leaf damage than branches with distantly related (r =−0.286±0.026) clipped neighbours (figure 1c; mean proportional reduction±1, s.e. = 0.42±0.07, binomial test p = 0.01).
Sample sizes in these experiments were limited by difficulties in producing cloned sagebrush. In the next two experiments, we took advantage of previous findings that moving air collected from the headspace of experimentally clipped donor plants was effective at inducing resistance in receiver branches [19]. When we recorded herbivore damage at the end of the 2011 season, we observed that damage was much greater on one side of the meadow than the other and these were designated as blocks. We used model building techniques and AIC to assess the roles of spatial block, relatedness between clipped donor and receiver assay plants, and their interaction on the proportion of leaves damaged by herbivores. The preferred model by 4.5 AIC units included a decrease in herbivory with increasing relatedness between donor and receiver in the low herbivory block and no relationship between relatedness and herbivory in the high-herbivory block (figure 2b and table 1). Rates of herbivory, particularly by grasshoppers (Cratypedes neglectus and Camnula pellucida), were unusually high in 2011 (y-axis in figure 2b compared with other years); in areas with very high herbivory, effects of communication and relatedness were undetectable but in areas with lower herbivory, effects of communication particularly between more related individuals demonstrably reduced leaf damage.
Table 1.
Model comparison results ordered by AIC. (‘r’ is relatedness between volatile donor and receiver and ‘block’ is spatial block within the field.)
| parameter estimates ± 95% CIs |
||||||
|---|---|---|---|---|---|---|
| model | intercept | r | block | r × block | ΔAIC | AIC weight |
| r × block | −1.92 ± 0.37 | −1.58 ± 1.08 | 0.89 ± 0.53 | 2.09 ± 1.51 | 0 | 0.84 |
| block | −2.01 ± 0.39 | — | 0.97 ± 0.57 | — | 4.5 | 0.09 |
| r + block | −1.98 ± 0.39 | −0.52 ± 0.80 | 0.93 ± 0.56 | — | 4.9 | 0.07 |
| r | −1.55 ± 0.30 | −0.67 ± 0.85 | — | — | 12.7 | 0 |
| intercept | −1.56 ± 0.30 | — | — | — | 13.0 | 0 |
A fourth field experiment was conducted in 2012 that moved volatiles rather than plants but used a paired design to compare effects of volatiles from closely and distantly related clipped donor plants transferred to two different branches on 25 receiver assay plants (figure 3a). Rates of herbivory were lower in 2012 than in 2011 and the paired design controlled for spatial patchiness in damage. Branches that received volatiles from closely related (r = 0.462±0.027) clipped plants experienced less damage than branches on the same assay plants that received volatiles from distantly related (r =−0.362±0.019) clipped plants (figure 3b; mean difference±s.e. = 0.063±0.027, t24 ratio = 2.31, p = 0.03).
4. Discussion
Plants responded more effectively to volatile cues from close relatives than from distant relatives in all four experiments and communication reduced levels of leaf damage experienced over the three growing seasons. This result was unlikely to be caused by volatiles repelling or poisoning insect herbivores [20]. Because volatiles were not directly repellent, absorption and re-emission of deterrents were not likely to have caused the result. Volatile signals are the only known means of coordinating systemic induced resistance among branches of attacked sagebrush individuals [15]. Whether these phenomena are termed communication depends upon how this term is defined and there is currently no consensus in the literature [21,22]. Most definitions converge to describe situations in which emissions or displays of cues are plastic and the responses of receivers are conditional on receiving the cue. In this case, emission is plastic because cues are not released unless plants are attacked or experimentally clipped and receivers respond only after exposure to cues they recognize.
Some definitions of communication require that both the sender and receiver benefit by engaging in the behaviour [21,23]. Sagebrush is a long-lived perennial, making estimates of the costs and benefits of communication difficult although plants that responded to volatile cues from damaged neighbours experienced greater survival at the seedling stage and greater production of new branches and inflorescences over 12 years [24]. Other workers have found that damage by leaf chewing herbivores can have large negative effects on sagebrush fitness [25]. Effective communication between related individuals that reduces herbivory has the potential to benefit both the responder [24] and the emitter if levels of relatedness are high between the individuals communicating [4]. Populations of sagebrush are genetically structured so that an individual is likely to be surrounded by other individuals that are close relatives ([14], E. D. McArthur 2002, personal communication). The volatiles that are emitted by experimentally clipped sagebrush are highly variable among individuals ([12], appendix S1). We found a significant correlation between genetic relatedness (estimated as Queller & Goodnight's [17] ‘r’ and volatile similarity (Mantel test p = 0.0028). Plants that were more closely related had more similar volatile profiles although it is not known which volatiles act as cues that affect defence. Plants may respond to volatiles that are similar to their own, since they share volatile blends with close relatives. High variability in volatiles may make it less likely that strangers can eavesdrop on cues.
Viscous populations with limited dispersal and resulting genetic structure can more easily evolve cooperative behaviours, such as communication, than populations lacking these traits [23,26]. The ability to recognize kin makes such evolution more likely [5,23,26]. Here, we have demonstrated that plants communicate more effectively with kin and that this communication increases plant resistance to herbivores. Plants responded more effectively to the cues emitted by kin rather than strangers. This did not involve kin recognition by the emitters because the phenomenon occurred when volatiles were transferred artificially with no opportunity for the sender to sense whether the receiver was close kin or stranger. There was no evidence that emission of volatiles depended on the relatedness of potential receivers. The greater effectiveness of cues from kin indicates kin recognition by the receiver or eavesdropping plant. One possible mechanism for this recognition is similarity in volatile profiles. Volatile cues from close kin may be more easily perceived by kin or may provide more reliable information about probable risk. The ability to differentially communicate based on relatedness makes possible a wide variety of social behaviours for plants that have previously been thought to be solely within the repertoire of animals [1–3].
Acknowledgements
We thank Ian Pearse, Masashi Ohara, Rika Ozawa, Gen-ichiro Arimura and Kathy Hughes for help with these experiments and Jeff Brown and Faerthen Felix for facilitating our work at UC Sagehen Creek Reserve in Tahoe National Forest. Comments by Susan Dudley and Jos Schall improved the manuscript. This work was supported by grants from the JSPS.
References
- 1.Wilson EO. 1975. Sociobiology. Cambridge, UK: Harvard University Press [Google Scholar]
- 2.Waldman B. 1988. The ecology of kin recognition. Annu. Rev. Ecol. Syst. 19, 543–571 10.1146/annurev.es.19.110188.002551 (doi:10.1146/annurev.es.19.110188.002551) [DOI] [Google Scholar]
- 3.Penn DJ, Fromman JG. 2010. Kin recognition: an overview of conceptual issues, mechanisms and evolutionary theory. In Animal behaviour: evolution and mechanisms (ed. Kappeler P.), pp. 55–85 Heidelberg, Germany: Springer [Google Scholar]
- 4.Hamiliton WD. 1964. The genetical evolution of social behavior, I & II. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 5.Gardner A, West SA. 2010. Greenbeards. Evolution 64, 25–38 10.1111/j.1558-5646.2009.00842.x (doi:10.1111/j.1558-5646.2009.00842.x) [DOI] [PubMed] [Google Scholar]
- 6.Nasrallah JB. 2002. Recognition and rejection of self in plant reproduction. Science 296, 305–308 10.1126/science.296.5566.305 (doi:10.1126/science.296.5566.305) [DOI] [PubMed] [Google Scholar]
- 7.Charlesworth D, Vekemans X, Castric V, Glemin S. 2005. Plant self-incompatibility systems: a molecular evolutionary perspective. New Phytol. 168, 61–69 10.1111/j.1469-8137.2005.01443.x (doi:10.1111/j.1469-8137.2005.01443.x) [DOI] [PubMed] [Google Scholar]
- 8.Dudley SA, File AL. 2007. Kin recognition in an annual plant. Biol. Lett. 3, 435–438 10.1098/rsbl.2007.0232 (doi:10.1098/rsbl.2007.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy GP, Dudley SA. 2009. Kin recognition: competition and cooperation in Impatiens (Balsaminaceae). Am. J. Bot. 96, 1990–1996 10.3732/ajb.0900006 (doi:10.3732/ajb.0900006) [DOI] [PubMed] [Google Scholar]
- 10.Biedrzycki ML, Jilany TA, Dudley SA, Bais HP. 2010. Root exudates mediate kin recognition in plants. Commun. Integr. Biol. 3, 28–35 10.4161/cib.3.1.10118 (doi:10.4161/cib.3.1.10118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heil M, Karban R. 2010. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144 10.1016/j.tree.2009.09.010 (doi:10.1016/j.tree.2009.09.010) [DOI] [PubMed] [Google Scholar]
- 12.Karban R, Shiojiri K. 2009. Self-recognition affects plant communication and defense. Ecol. Lett. 12, 502–506 10.1111/j.1461-0248.2009.01313.x (doi:10.1111/j.1461-0248.2009.01313.x) [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Cordero E, McKell CM. 1979. Stem cutting propagation of big sagebrush (Artemisia tridentata Nutt.). J. Range Manag. 32, 141–143 10.2307/3897559 (doi:10.2307/3897559) [DOI] [Google Scholar]
- 14.Cook CW, Stoddart LA. 1960. Physiological responses of big sagebrush to different types of herbivore removal. J. Range Manag. 13, 14–16 10.2307/3894891 (doi:10.2307/3894891) [DOI] [Google Scholar]
- 15.Karban R, Shiojiri K, Huntzinger M, McCall AC. 2006. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87, 922–930 10.1890/0012-9658(2006)87[922:DRISVA]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[922:DRISVA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 16.Ishizaki S, Kubota S, Shiojiri K, Karban R, Ohara M. 2010. Development of eight microsatellite markers in big sagebrush (Artemisia tridentata Nutt.). Mol. Ecol. Res. 10, 232–236 10.1111/j.1755-0998.2009.02796.x (doi:10.1111/j.1755-0998.2009.02796.x) [DOI] [Google Scholar]
- 17.Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–273 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 18.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information theoretic approach. New York, NY: Springer [Google Scholar]
- 19.Karban R, Shiojiri K, Ishizaki S. 2010. An air transfer experiment confirms the role of volatile cues in communication between plants. Am. Nat. 176, 381–384 10.1086/655222 (doi:10.1086/655222) [DOI] [PubMed] [Google Scholar]
- 20.Karban R, Baxter KJ. 2001. Induced resistance in wild tobacco with clipped sagebrush neighbors: the role of herbivore behavior. J. Insect Behav. 14, 147–156 10.1023/A:1007893626166 (doi:10.1023/A:1007893626166) [DOI] [Google Scholar]
- 21.Scott-Phillips TG. 2008. Defining biological communication. J. Evol. Biol. 21, 387–395 10.1111/j.1420-9101.2007.01497.x (doi:10.1111/j.1420-9101.2007.01497.x) [DOI] [PubMed] [Google Scholar]
- 22.Schenk HJ, Seabloom EW. 2010. Evolutionary ecology of plant signals and toxins: a conceptual framework. In Plant communication from an ecological perspective (eds Baluska F, Ninkovic V.), pp. 1–19 Berlin, Germany: Springer [Google Scholar]
- 23.Maynard Smith J, Harper DGC. 2003. Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- 24.Karban R, Ishizaki S, Shiojiri K. 2012. Long-term demographic consequences of eavesdropping for sagebrush. J. Ecol. 100, 932–938 10.1111/j.1365-2745.2012.01974.x (doi:10.1111/j.1365-2745.2012.01974.x) [DOI] [Google Scholar]
- 25.Takahashi M, Huntley N. 2010. Herbivorous insects reduce growth and reproduction of big sagebrush (Artemisia tridentata). Arthropod Plant Interact. 4, 257–266 10.1007/s11829-010-9108-1 (doi:10.1007/s11829-010-9108-1) [DOI] [Google Scholar]
- 26.Platt TG, Bever JD. 2009. Kin competition and the evolution of cooperation. Trends Ecol. Evol. 24, 370–377 10.1016/j.tree.2009.02.009 (doi:10.1016/j.tree.2009.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]