Abstract
Animals that maintain cooperative relationships show gains in longevity and offspring survival. However, little is known about the cognitive or hormonal mechanisms involved in cooperation. Indeed, there is little support for a main hypothesis that non-human animals have the cognitive capacities required for bookkeeping of cooperative exchanges. We tested an alternative hypothesis that cooperative relationships are facilitated by an endocrinological mechanism involving oxytocin, a hormone required for bonding in parental and sexual relationships across mammals. We measured urinary oxytocin after single bouts of grooming in wild chimpanzees. Oxytocin levels were higher after grooming with bond partners compared with non-bond partners or after no grooming, regardless of genetic relatedness or sexual interest. We ruled out other possible confounds, such as grooming duration, grooming direction or sampling regime issues, indicating that changes in oxytocin levels were mediated by social bond strength. Oxytocin, which is thought to act directly on neural reward and social memory systems, is likely to play a key role in keeping track of social interactions with multiple individuals over time. The evolutionary linkage of an ancestral hormonal system with complex social cognition may be the primary mechanism through which long-term cooperative relationships develop between both kin and non-kin in mammals.
Keywords: oxytocin, social bonds, cooperation, emotional bookkeeping, grooming, chimpanzee
1. Introduction
In non-human primates and other social animals, strong, enduring social bonds are typically seen between genetically related individuals [1–5]. Enduring relationships between non-kin, same-sex individuals also occur [5–15], but their evolution is more difficult to explain [5,16,17]. In both cases, these relationships are usually defined in terms of high rates of cooperative behaviours, including grooming [1,2,5–8,11]. Although the maintenance of enduring social bonds is associated with fitness benefits, whether these are between kin [1,2] or non-kin [6,11], the underlying mechanism of how such relationships are maintained over time is unclear. Contingent reciprocity, where one remembers a service given by another and then offers a service in return at a later date, offers a possible explanation, although this mechanism is rare in animals [16,17] and has been found among individuals that interact at low rates [18]. By contrast, individuals that interact at high rates and have strong, stable social bonds typically show short-term imbalances in services that are more equitable when calculated over months [19–21]. Whether or not some animal species have the cognitive capacity to remember social exchanges over time is currently unclear [17,21,22]. A cognitively less demanding mechanism underlying exchange of cooperative acts may be based on an uncalculated mediation of reciprocity [21,23] whereby services given from animal A to animal B promote a positive emotion, which increases the likelihood that B will interact again with A. The underlying physiological mechanism of such a process could act on neural reward and social memory circuits [24–31], promoting social choices that are considerably influenced by underlying emotions [21,24].
The neuropeptide hormone oxytocin plays a central role in facilitating bonding between kin and mating partners in humans and other social mammals [24–27]. Exogenously administered oxytocin increases rates of several cooperative behaviours in genetically related meerkats [31] and promotes reciprocity between genetically unrelated humans [32,33]. Furthermore, centrally administered oxytocin increases huddling behaviour between unrelated female meadow voles, but only with their preferred same-sex social partner [34]. This suggests that the physiological mechanisms promoting parental and reproductive relationships in social mammals, such as in kin and pair bonds, may be similar to those governing non-kin cooperative relationships in non-reproductive contexts in humans.
We determined whether oxytocin is involved in mediating the enduring, cooperative relationships that can be observed among both related and unrelated chimpanzees [7–10]. We predicted that oxytocin levels should be higher after subjects have experienced a grooming interaction with a strongly bonded social partner compared with another individual or after no such social interaction. This should be the case, whether or not strongly bonded social partners are kin.
Chimpanzees are a good model species for investigating the physiological underpinnings of social bonds. Although laboratory chimpanzees largely fail tests of contingent reciprocity [22,35,36], in the wild they maintain strong, enduring social bonds with both non-kin and kin beyond sexual interests [7–10]. In both kin and non-kin dyads, high rates of grooming, coalitionary support and food sharing can be observed, especially within male–male dyads [7,8], and to a lesser degree also in female–female and male–female dyads [9,10,37].
Oxytocin is produced in the magnocellular neurosecretory cells of the supraoptic and paraventricular nuclei, and stored in the posterior pituitary, from where it is released into the periphery [38]. Although still debated [39], a growing body of evidence suggests a coordinated release of central and peripheral oxytocin [40,41]. Also, peripheral measures of oxytocin from plasma or urine correlate positively with its biobehavioural functions, such as aversion reduction in male mice [42], social contact compared with isolation in marmosets [43], lactation compared with non-lactation in rhesus macaques [44] and rates of affiliative behaviours in pair-bonded tamarins [45]. To investigate the potential role of oxytocin in cooperation in wild chimpanzees, we used a non-invasive sampling method in a field setting to detect changes to urinary oxytocin levels following single bouts of a specific social behaviour: mutual grooming.
We collected urine from chimpanzees 15–60 min after they were observed: (i) grooming with a close maternal relative (i.e. mother, offspring or maternal sibling) with whom they maintained a strong social bond (kin bond partner condition); (ii) grooming with an individual with whom they maintained a strong social bond but who was not a close maternal relative (non-kin bond partner condition); (iii) grooming with an individual with whom the subject did not have a strong social bond (non-bond partner condition); or (iv) feeding or resting, with no grooming or other social interactions for 1 h (no grooming control condition).
2. Material and methods
(a). Subjects and observational data
Chimpanzees were from the habituated Sonso community, Budongo Forest, Uganda, which is a 428 km2 moist, semi-deciduous tropical forest at an altitude of 1100 m [46]. Data collection took place between February 2008 and July 2010. During this time, the Sonso community consisted of 62–77 individuals (adults: six to eight males aged more than 15 years, 19–21 females aged more than 14 years; sub-adults: seven to nine males aged 10–15 years, four to five females aged 10–14 years). Given that we wanted to sample subjects in four different behavioural conditions, our criteria for inclusion of individuals as subjects was that they were frequently observed and were more than 10 years old. We thus collected urine from 33 chimpanzees subjects (females: 15 adults and four sub-adults; males: seven adults and seven sub-adults) with number of urine samples per chimpanzee being (mean±s.d.) 4.15±2.14 (n=148 samples). A minority of individuals who ranged mainly in the peripheral parts of the territory were not seen every month and were therefore not sampled (n = 6 females with offspring, n = 3 nulliparous females and n = 1 old adult male). There is no reason to suspect that the urinary oxytocin responses of these 10 non-sampled individuals would differ from those of the more central chimpanzees that were the subjects of this study. Furthermore, subjects were not sampled if they had an infant less than 2 years of age, as frequent lactation is known to increase basal oxytocin levels [44].
Observational data of key behaviours were collected in two ways, either by focal animal sampling [47] or on an ‘all occurrence’ basis [47]. ‘All occurrence’ in fission–fusion chimpanzees meant recording all observed occurrences of a particular behaviour within the current subgroup—or party—of immediately visible chimpanzees by R.M.W., C.C. and four field assistants (M.D., J.A., O.J., S.A.) where ‘party’ is defined as all chimpanzees within visual range (less than 30 m) of the focal chimpanzee (see electronic supplementary material S2). Social interactions were defined as any affiliative or aggressive body contact, including copulation, but excluding vocal exchanges without body contact, and non-grooming interactions with ‘dependent’ offspring (i.e. infants less than 5 years that always travel with their mother). We also recorded the oestrous status of all females (see electronic supplementary material S1), as well as 15 min scan samples on party composition (i.e. the identities of chimpanzees in visual range—less than 30 m—of the subject). All human observers showed high inter-rater reliability test scores (Pearson's r > 0.9, n = 60 sample points).
(b). Assessing dyadic social bond strength
We assessed the quality of relationships by calculating behavioural rates of the following behaviours over the current and preceding annual quarters from either ‘all occurrence’ or focal data: coalitionary support, food sharing, grooming, resting in (less than 1 m) proximity and aggression [48,49] (see electronic supplementary material S2 for definitions). Each occurrence of behaviour was recorded as a single event. As duration was not recorded for events (e.g. food sharing, support and aggression), it was not included in the calculation. However, long-term behavioural data from Budongo chimpanzees have shown that grooming duration correlates strongly with number of grooming events (J. Lehmann 2011, personal communication).
From the resulting rates of each type of behavioural event, we calculated the composite relationship index (CRI) [49], which is indicative of social bond strength. The CRI is derived from the composite sociality index [1], but includes socio-negative, as well as socio-positive, behaviours [48,49]. The CRI gives socio-positive (given or received food transfer, coalitionary support, social grooming and resting in less than 1 m proximity) and socio-negative (aggression given or received) behaviours equal and opposite weights, such that the CRI = (SP1ij/SP1ave + SP2ij/SP2ave)/2−SNij/SNave, where SP1 = rate of grooming events plus rate of resting in 1 m proximity; SP2 = rate of food sharing plus rate of coalitionary support; SN = rate of aggression, i = individual and j = dyad partner. The index is positive when an individual within a dyad is the actor in, on average, more socio-positive than socio-negative interactions with its partner. The CRI was calculated for each individual within the dyad, whereas the dyadic CRI, which represents the relationship value across a dyad, is the sum of the two individual CRIs. ‘Bond partners’ were defined as dyads having (i) a mutual socio-positive relationship, where the CRI > 0 for each dyad member during the annual quarter of the urine sample collection and the preceding quarter; or (ii) a large mutual socio-positive relationship, where the CRI > 0 for each dyad member and the dyadic CRI > 10 during one of the quarters, with a mutual socio-neutral or positive relationship (dyadic CRI ≥ 0) during the other quarter (see electronic supplementary material S2). According to these criteria, on average, 1.9 per cent of kin dyads (n = 21) and 1.6 per cent of non-kin dyads (n = 18) reached bond partner status, from an average total of 1122 dyads, for each six-month block.
(c). Urine sample collection and extraction
Samples were collected after an allogrooming event lasting at least 10 min, or after 1 h of no social interaction. We assumed a time window of urinary clearance of oxytocin of 30–60 min, as previously established for primates [50]. Urine samples were not collected if the first urination did not occur within a time window of 15–60 min after the start of grooming (we extended the window of sample collection time to increase sample size and owing to the finding that some urinary oxytocin levels were elevated less than 30 min after the start of grooming; see the electronic supplementary material, figure S4). In addition, we did not collect samples if the first urination following a target event could not be collected or if the subjects engaged in another social interaction, including copulation, in the hour prior to urination.
Urine samples were collected on plastic sheets or leaves and then transferred with a disposable plastic pipette into a 5 ml vial. Following urine collection, 1.1 ml of urine was transferred with an Eppendorf pipette into a cryo vial containing 100 µl of 0.5 N phosphoric acid. Cryo vials were stored in a Thermos can containing ice cubes until arrival at camp, 15 min to 7 h later, where urine samples were centrifuged with a hand centrifuge for 3 min. Sep-Pak Light C18 cartridges (55–150 µm 50/box; WAT023501) were conditioned with 1 ml MeOH, then 1 ml distilled water. Urine (1 ml) was transferred to a syringe and loaded onto the cartridge by slowly pushing the syringe so that the flow rate was approximately 1 ml per min. The cartridge was then washed with 1 ml of 10 per cent acetonitrile (ACN) containing 0.1 per cent TFA. Samples were then eluted with 1 ml ACN/H20 80 : 20 and stored in liquid nitrogen or at −20°C in a freezer until they were shipped frozen on dry ice to the Assay Services Unit at the Wisconsin National Primate Research Center (Madison, WI).
In the laboratory, 1 ml of the extracted samples was brought to room temperature and then dried down in a water bath with air stream and reconstituted in assay buffer supplied in the 96-well enzyme immunoassay kit used (Assay Designs; catalogue no. 901–153). To compensate for the variation in the volume and concentration of the voided urine, we measured creatinine concentrations in each urine sample [43] and expressed all oxytocin values as pg mg−1 creatinine. Oxytocin validations of parallelism and accuracy were conducted satisfactorily (see electronic supplementary material S3). We sampled 13 subjects twice on a single day. Otherwise, the average time interval between samples per individual was (mean±s.d.) 2.27±1.69 months (see electronic supplementary material S3). Although it was not possible to completely rule out measurement errors, it was highly unlikely that any such errors occurred in a direction that supported the examined hypothesis.
(d). Genetic analysis
Dyads were classified as kin or non-kin according to a combination of (i) parentage analyses based on autosomal microsatellites and (ii) mitochondrial DNA and Y-chromosome microsatellite haplotype sharing information. We were able to demonstrate that all kin bond partners were either mother offspring (n = 15) or maternal sibling (n = 3) pairs, and that none of the non-kin bond partners and non-bond partners were such close maternal relatives. In most cases, we were able to exclude a close paternal relationship (i.e. father–offspring, paternal siblings) for the non-kin bond partners and non-bond partners (see electronic supplementary material S4 and table S2). We considered relatedness through the father to be of low importance given that chimpanzees do not show a preference for paternal kin as cooperation partners [7].
(e). Statistics and variable distributions
We examined the source of variation in a continuous response variable, oxytocin (pg mg−1 creatinine), using a general linear mixed model (GLMM) [51]. We estimated coefficients using the maximum likelihood (rather than using restricted maximum likelihood) feature in IBM SPSS 20. Because the dataset contained incomplete values (not all subjects could be tested in all conditions) and because different individuals appeared a different number of times as subjects or grooming partners, we included identity of subject as a random factor in all models. In addition, identity of grooming partner and identity of dyad were included as random factors in models that examined factors on a dyadic level [19,51]. Across the three grooming conditions, there were 36 grooming partners, with each partner occurring with a frequency of 2.05±1.36 (mean±s.d.) in the dataset, and there were 57 different dyads each occurring with a frequency of 1.3±0.79 (mean±s.d.) in the dataset.
Descriptions and distributions of the predictor variables are as follows. Age: n = 22 adults, n = 11 sub-adult; sex: n = 19 females, n = 14 males; diurnal: variation in urine sampling time, whether before (n = 66 samples) or after (n = 71 samples) 12.00 h; condition: three grooming conditions depending on subject's relationship to grooming partner: kin bond partner (n = 19 subjects, 23 samples), non-kin bond partner (n = 13 subjects, 21 samples), non-bond partner (n = 20 subjects, 34 samples) and no grooming control (n = 29 subjects, 59 samples); grooming durationlog10: time (min) from start of grooming within a dyad to the end of grooming, with no pause in grooming more than 1 min; grooming direction: groomer, groomee (more than 90% of grooming duration), bi-directional grooming (remaining cases); grooming durationlog10 (mean±s.d.: 1.3±0.26 min), latency startlog10 (1.67±0.22 min), latency end (25.2±23.5 min): latency from start and end of grooming to urination, respectively.
As some continuous predictor variables (latency start and grooming duration) and the response variable (oxytocin pg mg−1 crea) were not normally distributed, they were transformed using a log function, resulting in more symmetrical distributions. As a few values were extremely high (see electronic supplementary material, figure S1), we excluded outliers greater than 2 s.d. from the mean for each behavioural condition to prevent extreme values from disproportionately affecting the results [52,53]. Nonetheless, GLMMs run with the 11 outliers included, produced similar main results (see electronic supplementary material S5 and table S1). Variables did not exhibit problems of collinearity [51,54] (Pearson's and Kendall's tau r < 0.7; variance inflation factor less than three in all cases), suggesting that each predictor variable accounted for a portion of the variance. As a check of the overall significance of all predictor variables, we ran likelihood ratio tests comparing the full model with the respective null model (comprising only the random effects). We considered only the effect of the individual predictors if the full model reached significance. Likelihood ratio tests comparing full and null models: GLMM 1: χ2 = 18.8, d.f. = 4, n = 137, p < 0.005 (table 1a); GLMM 2: χ2 = 21.5, d.f. = 6, n = 78, p < 0.005 (table 1b) [18,49].
Table 1.
Factors influencing urinary oxytocin (pg mg−1 creatinine). Bold: p < 0.05. Parameter estimates: conditions with 0 are compared with remaining conditions; estimates of variables in italics were taken from a re-run of the same model. (a) General linear mixed model (GLMM) 1: influence of general predictors on oxytocin concentrations, includes all four behavioural conditions—137 samples across 33 subjects. (b) GLMM 2: influence of grooming-specific variables on oxytocin concentrations, includes the three grooming conditions only (all grooming samples listed in (a) within n = 31 subjects, n = 78), and includes dyadic predictor variables.
| predictor variable | F | d.f. | P | condition | estimate | s.e. | t | p |
|---|---|---|---|---|---|---|---|---|
| (a) | ||||||||
| sex (f/m) | 0.02 | 1 | 0.88 | female | −0.04 | 0.09 | −0.47 | 0.88 |
| age (adult/sub-adult) | 0.98 | 1 | 0.33 | adult | 0.13 | 0.09 | 1.38 | 0.33 |
| diurnal (<12 h >) | 0.03 | 1 | 0.86 | <12 h | 0.04 | 0.07 | 0.54 | 0.86 |
| condition | 6.14 | 3 | 0.001 | kin bond partner | 0.35 | 0.12 | 3.30 | 0.001 |
| non-kin bond partner | 0.24 | 0.12 | 2.22 | 0.038 | ||||
| non-bond partner | 0.02 | 0.08 | −0.77 | 0.44 | ||||
| no grooming control | 0 | 0 | 0 | 0 | ||||
| (b) | ||||||||
| sex of dyad | 2.03 | 2 | 0.14 | male–female | 0.24 | 0.13 | 1.78 | 0.08 |
| male–male | 0.05 | 0.14 | 0.35 | 0.73 | ||||
| female–female | 0 | 0 | 0 | 0 | ||||
| groom direction | 0.48 | 2 | 0.62 | bi-directional | −0.07 | 0.13 | −0.51 | 0.62 |
| subject receives | 0.60 | 0.16 | 0.39 | 0.69 | ||||
| subject gives | 0 | 0 | 0 | 0 | ||||
| groom duration (log10) | 0.12 | 1 | 0.73 | 0.09 | 0.26 | 0.35 | 0.73 | |
| latency start (log10) | 1.22 | 1 | 0.28 | −0.42 | 0.38 | −1.10 | 0.27 | |
| latency end | 0.22 | 1 | 0.64 | −0.00 | 0.02 | −0.47 | 0.62 | |
| condition | 5.81 | 3 | 0.005 | kin bond partner | 0.43 | 0.13 | 3.3 | 0.002 |
| non-kin bond partner | 0.28 | 0.12 | 2.35 | 0.022 | ||||
| non-bond partner | 0 | 0 | 0 | 0 | ||||
| non-kin bond partner | −0.15 | 0.13 | −1.20 | 0.25 | ||||
| kin bond partner | 0 | 0 | 0 | 0 | ||||
3. Results
To determine whether there was an influence of grooming or social partner on subjects' urinary oxytocin levels, we ran two GLMMs. In the first GLMM, we assessed the predictive power of the four behavioural conditions (kin bond grooming, non-kin bond grooming, non-bond grooming and no grooming control; table 1a) relative to that of general properties of subjects (age, sex) and urination time (morning/afternoon) on urinary oxytocin levels. In the second model (table 1b), we investigated the influence of properties specific to grooming interactions (sex combination of groomers, duration of grooming, direction of grooming and the urination time in relation to the grooming interaction—latency from onset of grooming and from end of grooming) on the subjects' urinary oxytocin levels. In order to determine the influence of dyadic factors associated with the three grooming conditions on urinary oxytocin levels, we excluded the no grooming control samples from this model.
In both models, condition was the only significant predictor (model 1: F3,137 = 6.14; p = 0.001; model 2: F2,78 = 5.84; p = 0.002). The GLMM parameter estimates are shown in table 1. Urinary oxytocin levels (log10 mean±s.e.) were significantly higher in kin bond partner (1.20±0.1 pg mg−1 crea, n = 19, 23 samples) and non-kin bond partner (1.06±0.1 pg mg−1 crea, n = 13, 21 samples) grooming conditions compared with both non-bond partner grooming (0.8±0.07 pg mg−1 crea, n = 20, 34 samples) and no grooming control (0.84±0.05 pg mg−1 crea, n = 29, 59 samples) samples. Urinary oxytocin levels in the non-bond partner condition were not different from no grooming control samples. Also, urinary oxytocin levels in the kin bond partner condition were not significantly different from the non-kin bond partner condition (figure 1). Possible confounds—namely the subject's sex or age class, diurnal variation in sampling time, grooming duration, sex combination of the grooming dyad, whether the subject was giving or receiving grooming, and latency between grooming and urination—did not have a significant influence on urinary oxytocin levels (see figures 2 and 3; electronic supplementary material S4 and S5, but see electronic supplementary material, table S1 and figure S3 in relation to the predictor variable sex combination of the grooming dyad, which showed a significant effect in the multivariate analysis when outliers were included).
Figure 1.
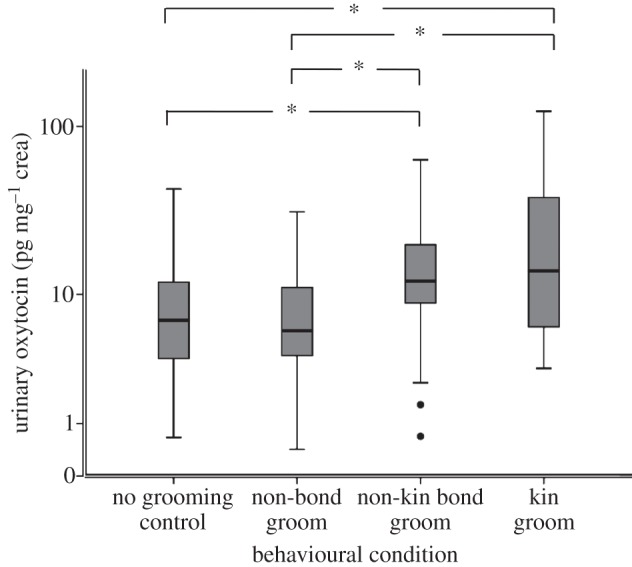
The influence of relationship quality and recent grooming on urinary oxytocin levels (n = 33 subjects, n = 137 samples). Urinary oxytocin levels following a single bout of grooming (more than 10 min) with a genetically related bond partner, an unrelated bond partner, a non-bond partner or following resting or feeding (control). Box plots show median and quartiles, whiskers show the 95% CI, and circles indicate values >95% CI. Differences across behavioural conditions: *p < 0.05 (table 1b).
Figure 2.
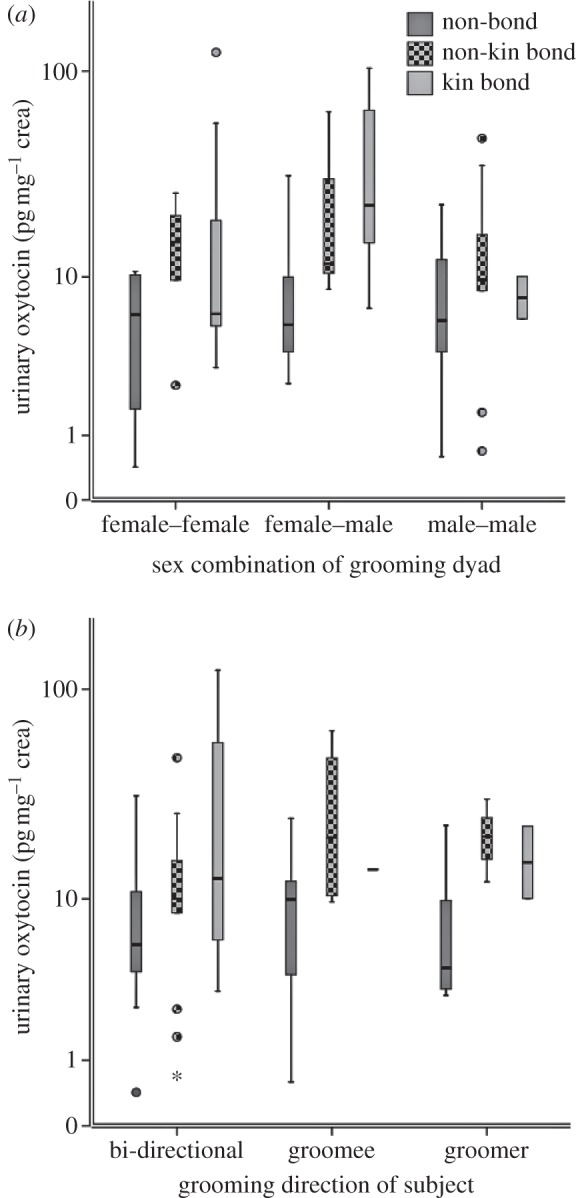
The distribution of grooming-related predictor variables with respect to grooming condition and urinary oxytocin levels (n = 31 subjects, n = 78 samples). Predictor variables: (a) sex combination of dyad (number of urine samples per condition: female–female dyads: n = 22 [non-bond = 4, non-kin bond = 6, kin = 12]; male–female dyads: n = 26 [non-bond = 11, non-kin bond = 6, kin = 9]; male–male dyads: n = 30 [non-bond = 19, non-kin bond = 9, kin = 2]; (ii) direction of grooming (bidirectional: subject gives and receives grooming: n = 50 [non-bond = 16, non-kin bond = 14, kin = 20]; groomee (subject receives grooming): n = 13 [non-bond = 8, non-kin bond = 4, kin = 1]; groomer (subject gives grooming): n = 15 [non-bond = 10, non-kin bond = 3, kin = 2]. Box plots contain data from all subjects, show median and quartiles, and whiskers show 95% CI. Circles and asterisk show values > 95% CI (see table 1b).
Figure 3.
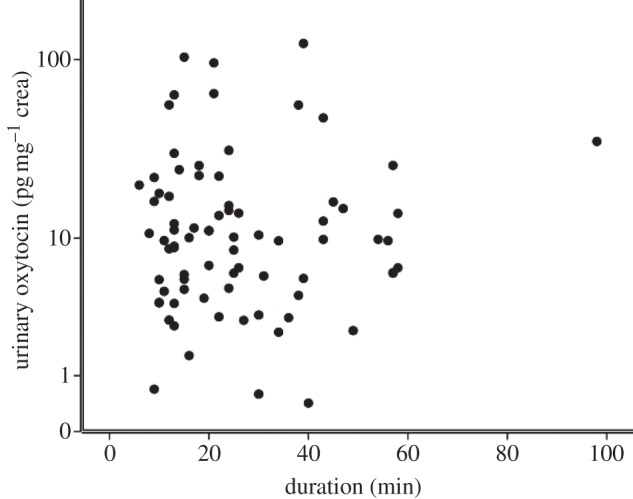
Grooming duration relative to urinary oxytocin levels across three grooming conditions. Urine samples from kin bond partner grooming, non-kin bond partner grooming and non-bond partner grooming conditions included.
We ran a third GLMM to assess whether high oxytocin levels were due to the act of grooming with a bond partner, as opposed to mere presence of the bond partner (being less than 30 m from the subject). This is relevant because, in humans, mothers' comforting words are sufficient to raise children's oxytocin levels in the absence of physical contact [55]. In the fission–fusion societies of chimpanzees, subjects can be separated from their bond partners for hours or days at a time. We tested whether the presence of at least one kin or non-kin bond partner within the subjects' range of visibility, 15–60 min prior to urination, affected oxytocin levels. We included the samples from the two behavioural conditions where the variable presence of bond partner can vary (i.e. non-bond partner grooming and no grooming control). Identity of subject was included as a random factor. We found no effect of the mere presence of a bond partner on urinary oxytocin levels (GLMM: F1,73 = 0.58, p = 0.81), suggesting that, in chimpanzees, the mere physical presence of a bond partner is not sufficient to significantly raise oxytocin levels.
4. Discussion
Our results demonstrate that a rise in oxytocin was dependent upon the combined effects of social grooming with an existing bond partner. Neither the occurrence of grooming nor the presence of a social bond partner alone was sufficient to increase oxytocin levels. Crucially, oxytocin levels were similarly high after grooming with non-kin and kin bond partners. This suggests that in chimpanzees oxytocin plays a key role in maintaining social relations beyond immediate genetic ties. It also suggests, against current arguments [24], that affiliative touch alone was not sufficient to raise oxytocin levels. Oxytocin levels were not increased even after 10 min of grooming, unless this was between bond partners. These results are in line with studies with exogenously administered oxytocin in humans [32] and other animals [30,34,42,56], suggesting that psychological as well as physical factors are associated with oxytocin secretion.
We acknowledge that our sample size, although sizable for a field study, was relatively small in general. Thus, it may be prudent to interpret some of the non-significant results conservatively. For instance, it is possible that grooming causes some increase in oxytocin, regardless of partner identity. However, the fact remains that the effects were substantially higher if the interaction involved a bond partner.
Although our study does not explicitly address the causality of the observed phenomenon, it seems likely that the grooming interaction between bond partners triggered the oxytocin release rather than the reverse, for the following reasons. If high oxytocin precipitated the grooming interaction, we would expect oxytocin to be similarly increased after all grooming bouts, whether grooming was with bond partners or with non-bond partners. In addition, given that actors are the ones motivated to groom, we would expect oxytocin to be higher in the actor than the receiver. However, neither of these predictions was supported. Both multivariate and univariate GLMMs showed significant differences in oxytocin levels across grooming bouts depending on the relationship of the grooming partners, and no significant difference in oxytocin levels between actor and receiver.
In addition, our results show that measuring peripheral oxytocin relates well to the target behaviour, in that a single social event was non-randomly associated with the subsequent urinary oxytocin level. Whether peripheral oxytocin levels relate to central oxytocin levels, and in particular, whether or not peripheral and central levels of oxytocin release are coordinated, remains unclear (for review, see [57]). One line of evidence in favour of a coordinated release is that neurons responsible for central oxytocin release also protrude into the pituitary, which is responsible for peripheral oxytocin release [38]. A second line of evidence is that peripherally and centrally administered oxytocin can trigger similar behavioural responses in a range of animals [31,56,58–61]. Finally, genetically caused, abnormally low central oxytocin secretion is also associated with low plasma oxytocin levels in both mice [62] and humans [53].
Independent of whether or not central and peripheral oxytocin release is coordinated, there are good reasons to examine the relation between social behaviour and peripheral oxytocin. First, the oxytocinergic system that supports bond formation in mammals also functions as a feedback loop, in which socio-positive behaviours, such as physical contact between mothers and their offspring, increase the expression of oxytocin [61]. Similarly, peripheral administration of oxytocin reliably leads to maternal behaviour [63,64], suggesting that bond formation operates through behaviourally induced oxytocin secretion that feeds back to the brain through either central or peripheral pathways. Such a feedback loop might explain how a psychological element, namely a positive attitude [21,23] towards specific social partners, becomes reinforced, perhaps increasingly so with each subsequent encounter [65].
Overall, the data suggest a correlation between social interactions indicative of central excretion of oxytocin and peripherally measured oxytocin levels. Whether this is because peripherally secreted oxytocin is fed back to the brain from afferent peripheral tissues [57] or because of a coordinated release of central and peripheral oxytocin levels is currently unresolved. Based on the available evidence, we conclude that the question of whether peripheral oxytocin directly feeds back to the brain is of only secondary importance.
Our results are consistent with neurological models that posit that an effect of oxytocin on social memory and positive feedback in neural reward circuits facilitates repeated interactions with social partners with whom positive interactions have already occurred [25–27,29]. However, other neurological pathways might also be relevant, such as observed effects of oxytocin in the amygdala in relation to fear mediation and aggression reduction [34].
Our main result shows a relationship between oxytocin and grooming with social bond partners in chimpanzees. This result was independent of genetic ties or sexual interests and as such may represent an important mechanism through which close relationships have evolved in non-kin. The ability to form strong social bonds with non-kin provides animals with more flexible options to increase their reproductive success [6,7,11]. The mechanisms underlying non-kin bond formation in non-reproductive contexts have remained elusive, particularly whether oxytocin plays a part in bond formation between unrelated individuals [24,27]. In this study, we defined close social bonds in terms of high rates of exchange of cooperative behaviours. As such, our results provide support for the hypothesis that enduring, cooperative relationships among non-kin are mediated by hormonal (and not purely cognitive) mechanisms [5,21,27,30]. How such endocrinological mechanisms interplay with cognitive processes remains to be investigated. We suggest that such an endocrine feedback loop, in conjunction with cognitive processes, provides a mechanism that enables individuals to engage in reciprocal social exchanges by building trust [32] between individuals, even when they are not genetically related.
In using a non-invasive sampling technique that measures changes in urinary oxytocin levels in relation to changes in specific social events, we have developed a tool with which cross-species comparisons can eventually be made of social mammals in their natural environment in terms of how and when social bonding takes place and the relevance of social bonds during cooperation, whether occurring between kin or non-kin.
Acknowledgements
We thank F. Babweteera and our field assistants M. Gideon, J. Okuti, S. Adue and J. Aoli for all their help with logistical support and data collection, A. Weltring for organizing sample shipments, L. Vigilant, V. Reynolds and Z. Zommers for providing additional faecal samples, C. Rowney for laboratory analyses of the genetic samples, and three anonymous reviewers for their helpful comments. The Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and the President's office of Uganda gave permission to conduct this study. We acknowledge the Royal Zoological Society of Scotland for providing core funding to the Budongo Conservation Field Station. Funding was provided by the British Academy, the Wenner-Gren Foundation for Anthropological Research, the Leverhulme Trust (Research Leadership Award), the Max Planck Institute for Evolutionary Anthropology, and the Wisconsin National Primate Research Center (NIH NCRR000167 support of laboratory). The data for this study are available on request.
References
- 1.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 10.1126/science.1088580 (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 2.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice L, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361 10.1016/j.cub.2010.05.067 (doi:10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 3.Krützen M, Sherwin WB, Connor RC, Barré LM, Van de Casteele T, Mann J, Brooks R. 2003. Contrasting relatedness patterns in bottlenose dolphins (Tursiops spp.) with different alliance strategies. Proc. R. Soc. Lond. B 270, 497–502 10.1098/rspb.2002.2229 (doi:10.1098/rspb.2002.2229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss CJ, Croze H, Lee PC. (eds) 2001. The Amboseli elephants: a long-term perspective on a long-lived mammal. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Seyfarth RM, Cheney DL. 2012. The evolutionary origins of friendship. Annu. Rev. Psychol. 63, 4.1–4.25 10.1146/annurev-psych-120710-100337 (doi:10.1146/annurev-psych-120710-100337) [DOI] [PubMed] [Google Scholar]
- 6.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853 10.1073/pnas.0900639106 (doi:10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langergraber KE, Mitani JC, Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790 10.1073/pnas.0611449104 (doi:10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitani JC. 2009. Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640 10.1016/j.anbehav.2008.11.021 (doi:10.1016/j.anbehav.2008.11.021) [DOI] [Google Scholar]
- 9.Langergraber KE, Mitani JC, Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840–851 10.1002/ajp.20711 (doi:10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]
- 10.Lehmann J, Boesch B. 2009. Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees, Pan troglodytes. Anim. Behav. 77, 377–387 10.1016/j.anbehav.2008.09.038 (doi:10.1016/j.anbehav.2008.09.038) [DOI] [Google Scholar]
- 11.Schulke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210 10.1016/j.cub.2010.10.058 (doi:10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 12.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PloS Med. 7, 1–20 10.1371/journal.pmed.1000316 (doi:10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McShea WJ. 1990. Social tolerance and proximate mechanisms of dispersal among winter groups of meadowvoles, Microtus pennsylvanicus. Anim. Behav. 39, 346–351 10.1016/S0003-3472(05)80880-2 (doi:10.1016/S0003-3472(05)80880-2) [DOI] [Google Scholar]
- 14.Parker KJ, Lee TM. 2003. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J. Comp. Psychol. 117, 283–289 10.1037/0735-7036.117.3.283 (doi:10.1037/0735-7036.117.3.283) [DOI] [PubMed] [Google Scholar]
- 15.Weidt A, Hofmann SE, Konig B. 2008. Not only mate choice matters: fitness consequences of social partner choice in female house mice. Anim. Behav. 75, 801–808 10.1016/j.anbehav.2007.06.017 (doi:10.1016/j.anbehav.2007.06.017) [DOI] [Google Scholar]
- 16.Clutton-Brock T. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57 10.1038/nature08366 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 17.Cheney DL. 2011. Extent and limits of cooperation in animals. Proc. Natl Acad. Sci. USA 108, 10 902–10 909 10.1073/pnas.1100291108 (doi:10.1073/pnas.1100291108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheney DL, Moscovice LR, Heesenc M, Mundry R, Seyfarth RM. 2010. Contingent cooperation between wild female baboons. Proc. Natl Acad. Sci. USA 107, 9562–9566 10.1073/pnas.1001862107 (doi:10.1073/pnas.1001862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes CM, Mundry R, Boesch C. 2009. Long-term reciprocation of grooming in wild West African chimpanzees. Proc. R. Soc. B 276, 699–706 10.1098/rspb.2008.1324 (doi:10.1098/rspb.2008.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silk JB, et al. 2010. Female chacma baboons form strong, equitable and enduring social bonds. Behav. Ecol. Sociobiol. 64, 1733–1747 10.1007/s00265-010-0986-0 (doi:10.1007/s00265-010-0986-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schino G, Aureli F. 2009. Reciprocal altruism in primates: partner choice, cognition, and emotions. Adv. Stud. Behav. 39, 45–69 10.1016/S0065-3454(09)39002-6 (doi:10.1016/S0065-3454(09)39002-6) [DOI] [Google Scholar]
- 22.Melis AP, Semmann D. 2010. How is human cooperation different? Phil. Trans. R. Soc. B 365, 2663–2674 10.1098/rstb.2010.0157 (doi:10.1098/rstb.2010.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Waal FBM. 2000. Attitudinal reciprocity in food sharing among brown capuchin monkeys. Anim. Behav. 60, 253–261 10.1006/anbe.2000.1471 (doi:10.1006/anbe.2000.1471) [DOI] [PubMed] [Google Scholar]
- 24.Curley JB, Keverne EB. 2005. Genes, brains and mammalian social bonds. Trends. Ecol. Evol. 20, 561–567 10.1016/j.tree.2005.05.018 (doi:10.1016/j.tree.2005.05.018) [DOI] [PubMed] [Google Scholar]
- 25.Goodson JL, Thompson RR. 2010. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 20, 784–794 10.1016/j.conb.2010.08.020 (doi:10.1016/j.conb.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 26.Insel TR. 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–779 10.1016/j.neuron.2010.03.005 (doi:10.1016/j.neuron.2010.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soares MC, Bshary R, Fusani L, Goymann W, Hau M, Hirschenhauser K, Oliveira RF. 2010. Hormonal mechanisms of cooperative behaviour. Phil. Trans. R. Soc. B 365, 2737–2750 10.1098/rstb.2010.0151 (doi:10.1098/rstb.2010.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. 2005. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc. Natl Acad. Sci. USA 102, 17 237–17 240 10.1073/pnas.0504767102 (doi:10.1073/pnas.0504767102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. 2000. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288 10.1038/77040 (doi:10.1038/77040) [DOI] [PubMed] [Google Scholar]
- 30.Dantzer R, Bluthe RM, Koob GF, Moal M. 1987. Modulation of social memory in male rats by neurohypophyseal peptides. Psychoparmacology 91, 363–368 10.1007/BF00518192 (doi:10.1007/BF00518192) [DOI] [PubMed] [Google Scholar]
- 31.Madden JR, Clutton-Brock TH. 2011. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc. R. Soc. B 278, 1189–1194 10.1098/rspb.2010.1675 (doi:10.1098/rspb.2010.1675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature 435, 673–676 10.1038/nature03701 (doi:10.1038/nature03701) [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. 2009. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650 10.1016/j.neuron.2008.04.009 (doi:10.1016/j.neuron.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 34.Beery AK, Zucker I. 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169, 665–673 10.1016/j.neuroscience.2010.05.023 (doi:10.1016/j.neuroscience.2010.05.023) [DOI] [PubMed] [Google Scholar]
- 35.Brosnan SF, Silk JB, Henrich J, Mareno MC, Lambeth SP, Schapiro SJ. 2009. Chimpanzees (Pan troglodytes) do not develop contingent reciprocity in an experimental task. Anim. Cogn. 12, 587–597 10.1007/s10071-009-0218-z (doi:10.1007/s10071-009-0218-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melis AP, Hare B, Tomasello M. 2008. Do chimpanzees reciprocate favours? Anim. Behav. 76, 951–962 10.1016/j.anbehav.2008.05.014 (doi:10.1016/j.anbehav.2008.05.014) [DOI] [Google Scholar]
- 37.Wittig RM, Boesch C. 2010. Receiving post-conflict affiliation from the enemy's friend reconciles former opponents. PLoS ONE 5, e13995. 10.1371/journal.pone.0013995 (doi:10.1371/journal.pone.0013995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. 2009. Characterization of the oxytocin system regulating affiliative behaviour in female prairie voles. Neuroscience 162, 892–903 10.1016/j.neuroscience.2009.05.055 (doi:10.1016/j.neuroscience.2009.05.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12, 524–538 10.1038/nrn3044 (doi:10.1038/nrn3044) [DOI] [PubMed] [Google Scholar]
- 40.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. 2007. Oxytocin, vasopressin and sociality. Prog. Brain Res. 170, 131–136 [DOI] [PubMed] [Google Scholar]
- 41.Ross HE, Young LJ. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 30, 534–547 10.1016/j.yfrne.2009.05.004 (doi:10.1016/j.yfrne.2009.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ring RH, et al. 2006. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 185, 218–225 10.1007/s00213-005-0293-z (doi:10.1007/s00213-005-0293-z) [DOI] [PubMed] [Google Scholar]
- 43.Seltzer LJ, Ziegler TE. 2007. Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Horm. Behav. 51, 436–442 10.1016/j.yhbeh.2006.12.012 (doi:10.1016/j.yhbeh.2006.12.012) [DOI] [PubMed] [Google Scholar]
- 44.Maestripieri D, Hoffman CL, Anderson GM, Carter S, Higley JD. 2009. Mother–infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol. Behav. 96, 613–619 10.1016/j.physbeh.2008.12.016 (doi:10.1016/j.physbeh.2008.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. 2010. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm. Behav. 58, 614–618 10.1016/j.yhbeh.2010.06.014 (doi:10.1016/j.yhbeh.2010.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds V. 2005. Chimpanzees of the Budongo forest. Oxford, UK: Oxford University Press [Google Scholar]
- 47.Altmann J. 1974. Observational study of behavior: sampling methods. Behavior 49, 227–267 10.1163/156853974X00534 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 48.Fraser ON, Schino G, Aureli F. 2008. Components of relationship quality in chimpanzees. Ethology 104, 834–843 10.1111/j.1439-0310.2008.01527.x (doi:10.1111/j.1439-0310.2008.01527.x) [DOI] [Google Scholar]
- 49.Crockford C, Wittig RM, Mundry R, Zuberbühler K. 2012. Wild chimpanzees inform ignorant group members of danger. Curr. Biol. 22, 142–146 10.1016/j.cub.2011.11.053 (doi:10.1016/j.cub.2011.11.053) [DOI] [PubMed] [Google Scholar]
- 50.Amico JA, Ulbrecht JS, Robinson AG. 1987. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J. Clin. Endocrinol. Metab. 64, 340–345 10.1210/jcem-64-2-340 (doi:10.1210/jcem-64-2-340) [DOI] [PubMed] [Google Scholar]
- 51.Baayen RH. 2008. Analyzing linguistic data. Cambridge, UK: Cambridge University Press [Google Scholar]
- 52.Emory-Thompson M, Muller MN, Kahlenberg SM, Wrangham RW. 2010. Dynamics of social and energetic stress in wild female chimpanzees. Horm. Behav. 58, 440–449 10.1016/j.yhbeh.2010.05.009 (doi:10.1016/j.yhbeh.2010.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. 2012. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry 72, 175–181 10.1016/j.biopsych.2011.12.025 (doi:10.1016/j.biopsych.2011.12.025) [DOI] [PubMed] [Google Scholar]
- 54.Tabachnick BG, Fidell LS. 2007. Using multivariate statistics, 5th edn Boston, MA: Allyn and Bacon [Google Scholar]
- 55.Seltzer LJ, Ziegler TE, Pollak SD. 2010. Social vocalizations can release oxytocin in humans. Proc. R. Soc. B 277, 2661–2666 10.1098/rspb.2010.0567 (doi:10.1098/rspb.2010.0567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liberzon MD, Trujillo KA, Akil H, Young EA. 1997. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology 17, 353–359 10.1016/S0893-133X(97)00070-5 (doi:10.1016/S0893-133X(97)00070-5) [DOI] [PubMed] [Google Scholar]
- 57.Churchland PS, Winkielman P. 2012. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm. Behav. 61, 392–399 10.1016/j.yhbeh.2011.12.003 (doi:10.1016/j.yhbeh.2011.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arletti R, Benelli A, Bertolini A. 1992. Oxytocin involvement in male and female sexual behavior. Annu. N.Y. Acad. Sci. 652, 180–193 10.1111/j.1749-6632.1992.tb34354.x (doi:10.1111/j.1749-6632.1992.tb34354.x) [DOI] [PubMed] [Google Scholar]
- 59.Caldwell JD, Walker CH, O'Rourke ST, Faggin BM, Morris M, Mason GA. 1996. Analogies between oxytocin systems of the uterus and brain. Horm. Metabol. Res. 28, 65–74 10.1055/s-2007-979131 (doi:10.1055/s-2007-979131) [DOI] [PubMed] [Google Scholar]
- 60.McCarthy MM, Kow LM, Pfaff DW. 1992. Speculations concerning the physiological significance of central oxytocin in maternal behavior. Annu. N.Y. Acad. Sci. 652, 70–82 10.1111/j.1749-6632.1992.tb34347.x (doi:10.1111/j.1749-6632.1992.tb34347.x) [DOI] [PubMed] [Google Scholar]
- 61.Petersson M, Alster P, Lunderberg T, Uvnäs-Moberg K. 1996. Oxytocin increases nociceptive thresholds in a long-term perspective in female and male rats. Physiol. Behav. 60, 1311–1315 10.1016/0304-3940(96)12773-7 (doi:10.1016/0304-3940(96)12773-7) [DOI] [PubMed] [Google Scholar]
- 62.Jin D, et al. 2007. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45 10.1038/nature05526 (doi:10.1038/nature05526) [DOI] [PubMed] [Google Scholar]
- 63.Francis DD, Young LJ, Meaney MJ, Insel TR. 2002. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J. Neuroendocrinol. 14, 349–353 10.1046/j.0007-1331.2002.00776.x (doi:10.1046/j.0007-1331.2002.00776.x) [DOI] [PubMed] [Google Scholar]
- 64.Pedersen CA, Ascher JA, Monroe YL, Prange AJ. 1982. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650 10.1126/science.7071605 (doi:10.1126/science.7071605) [DOI] [PubMed] [Google Scholar]
- 65.Cushing BS, Carter CS. 2000. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm. Behav. 37, 49–56 10.1006/hbeh.1999.1558 (doi:10.1006/hbeh.1999.1558) [DOI] [PubMed] [Google Scholar]


