Abstract
Melanin production is often considered costly, yet beneficial for thermoregulation. Studies of variation in melanization and the opposing selective forces that underlie its variability contribute greatly to understanding natural selection. We investigated whether melanization benefits are traded off with predation risk to promote observed local and geographical variation in the warning signal of adult male wood tiger moths (Parasemia plantaginis). Warning signal variation is predicted to reduce survival in aposematic species. However, in P. plantaginis, male hindwings are either yellow or white in Europe, and show continuous variation in melanized markings that cover 20 to 90 per cent of the hindwing. We found that the amount of melanization increased from 40 to 59 per cent between Estonia (58° N) and north Finland (67° N), suggesting melanization carries thermoregulatory benefits. Our thermal measurements showed that more melanic individuals warmed up more quickly on average than less melanic individuals, which probably benefits flight in cold temperatures. With extensive field experiments in central Finland and the Alpine region, we found that more melanic individuals suffered increased predation. Together, our data suggest that warning signal efficiency is constrained by thermoregulatory benefits. Differences in relative costs and benefits of melanin probably help to maintain the geographical warning signal differences.
Keywords: aposematism, signal size, colour polymorphism, predation, opposing selection, Parasemia plantaginis
1. Introduction
Studies of coloration form much of the groundwork for studying natural selection [1–3], especially those pertaining to lepidopterans [4,5]. Camouflage is one coloration-based predator defence strategy and benefits from variable coloration [6], which prevents predators from developing a search image for the most abundant phenotype [6,7]. At the opposite end of the predator aversion strategy spectrum is aposematism, where prey use conspicuous warning signals to advertise secondary defences [7]. Warning signals of aposematic organisms are not expected to vary much because predators should learn to avoid a uniform signal more efficiently than a variable signal [8,9], leading to greater survival.
Contrary to the predictions for warning signal homogeneity, many organisms possess variable warning signals [10–13]. Changes in the warning signal appearance, though, can be expected over the geographical range of an aposematic species, and are commonly thought to represent local adaptations to predators [11,13,14] (but see [12]). Local (intra-population) warning signal variation, though, still presents challenges to explain because it should cause increased mortality owing to less efficient association by predators between the signal and unpalatability [9]. To better describe warning signal variation, we use the term ‘warning signal diversity’, because warning signal variation can manifest itself in multiple ways in the same signal or geographical area (i.e. through polymorphisms, continuous variation or a combination of these). The warning signal diversity in many aposematic organisms on different spatial scales suggests that selective forces other than predation influence the signal [12,15,16]. Trade-offs between protection from predators and other fitness-related traits can cause increased local warning signal diversity [16,17].
Melanin is one aspect of animal coloration and many warning signals, and is thought to trade off with other fitness-related traits often because it is costly [17,18]. More melanic individuals may be developmentally hindered because the melanin pigment requires nitrogen [18,19], which is typically a limited resource for herbivorous lepidopterans [20]. However, melanin can also contribute to a more robust immune system [21] and can increase the ability to absorb solar radiation [2,22], which can benefit fitness [23]. At least two documented instances in lepidopterans show increases in melanin coverage with elevation [24,25] and latitude [25,26], suggesting a general link with thermoregulation (see [22] for more examples). Increased melanization can result in faster warm-up times in lepidopterans [27], which can be important for predator aversion via escape ability [28]. Thermoregulation in flight is also important, where the primary source of heat gain is solar radiation [29]. Additional fitness-related benefits of more melanin in lepidopteran wings include increased flight duration [30,31] and greater ability to move across cooler habitat patches [31,32], which can be important for courting, egg laying and feeding [30]. Melanization can also decrease egg maturation time in Colias [33]. Hindwing melanization itself is thought to be particularly important for thermoregulation during flight in at least one species, Paranassius phoebus [32]. In aposematic species, melanization may limit the amount of other pigments important in the warning signal, thereby setting the stage for a relatively unexplored trade-off between defensive signalling and thermoregulation [17,34].
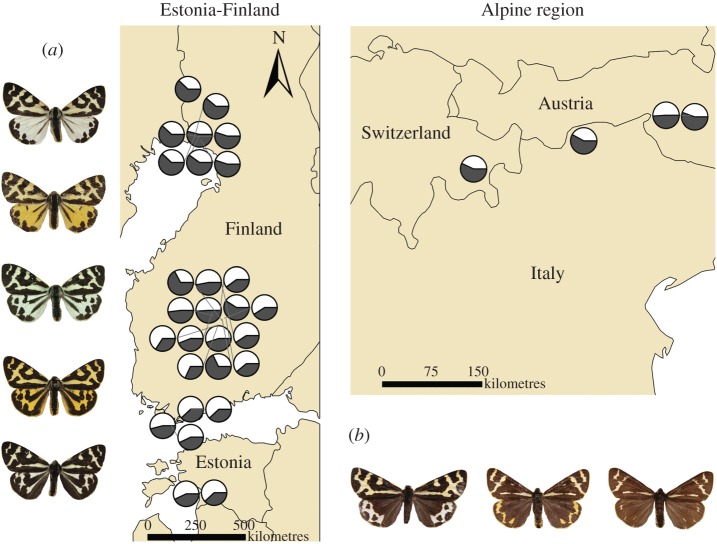
The wood tiger moth (Parasemia plantaginis) is an aposematic diurnal arctiid moth with a holarctic distribution. On both a local and broad geographical scale, P. plantaginis exhibits substantial warning signal diversity. European male P. plantaginis have either yellow or white hindwings with variable black patterning that serve as a warning signal [16] (figure 1). Herein, we refer to the yellow or white base hindwing colour of males as ‘colour’, and refer to the black pattern as ‘melanization’. The black melanin-based pattern typically covers 20 to 70 per cent of the hindwing area, but can be over 90 per cent in the Alps (figure 1). Throughout Estonia and Finland, only 4.6 per cent of males have hindwing melanization above 70 per cent, compared with nearly 27 per cent of sampled individuals from the Alpine region (R. H. Hegna, O. Nokelainen, J. Mappes, unpublished data). Although the extent of forewing melanization also varies, the range is typically less (50–80% throughout Europe). The yellow or white pigmentation in males and melanization are heritable traits [35], which makes both traits possible targets for natural selection. Female coloration varies gradually between red and yellow, and functions as a warning signal as well [36]. Hindwing melanization in females is typically at the higher end of the male melanization range (70–90%). Examining geographical patterns in female phenotype is more difficult because they do not fly as frequently as males, which makes them harder to collect in sufficient numbers. In this paper, we focus on the adaptive significance of variation in male hindwing melanization.
Figure 1.
A map showing the sampling sites between Estonia and northern Finland, and the Alpine region. Each site is represented by a pie chart with varying amounts of black representing the mean percentage of hindwing melanization of individuals captured at that site. Moths featured in (a) display the range of melanization observed in Estonia, Finland and the Alpine region, and (b) represent other levels of melanization found only in the Alpine Region. (Online version in colour.)
We studied whether variation in male hindwing melanization is linked to thermoregulation and/or involved in a trade-off with protective benefits of colour. We first examined whether melanization increases with latitude, which would indicate a thermoregulatory function for melanin in P. plantaginis. We then determined whether increased amounts of melanin result in an improved ability to warm up. Lastly, we conducted a field-based predation experiment in Finland and the Alpine region to see if more melanization (and less colour) leads to an increased likelihood of attack by avian predators. We hypothesized that greater amounts of hindwing melanization in adult male moths would increase the ability of individuals to warm up, but would also make them more vulnerable to predation. We also compared the variation in melanization between the two regions where the predation experiment was conducted because higher levels of warning signal diversity are sometimes associated with lower predation pressure [12,13,37]. We expected that the greater range of hindwing melanization in the Alpine region might be explained, in part, by lower predation pressure or reduced selection against less effective warning signals.
2. Material and methods
(a). Geographic trends in melanization
A total of 166 males were collected in 2009 from 26 sites across Finland (67° N) and another 25 males from two sites in Estonia (58° N; figure 1). Fifty-four males were also collected from four sites across the Alpine region in 2009. Moth specimens were collected using nets and pheromone traps containing live females, killed, and photographed to determine the amount of melanization present. We used the program paint.net to obtain the percentage of hindwing melanization from the photographs. Specifically, we counted the number of pixels comprising the melanized portion of the wing using the contrast threshold selection tool (i.e. the magic wand tool, set to 40%) and compared that with the total number of pixels comprising the hindwing.
We investigated the possibility that a melanization cline exists between Estonia and north Finland using a linear mixed model analysis in PASW v. 18. The analysis included the hindwing melanization (%) as the dependent variable, with latitude as the explanatory variable. Sampling sites were included in the model as a random factor nested under latitudes to control for multiple individuals collected at each sampling site.
Alpine specimens were not included in the cline analysis because elevation differed significantly from the Estonian and Finnish sites (1800–2500 m compared with 5–200 m in elevation, respectively). Instead, we used the Alpine specimens to compare the average melanization level and overall variation in the melanization between central Finland (n = 62) and the Alpine region (n = 54). We investigated differences in variability using Levene's test of unequal variances and mean melanization with a t-test. We also visually determined and recorded the hindwing colour of 69 individuals in central Finland and 55 individuals in the Alpine region. We were able to determine hindwing colour successfully from eight more individuals than melanization because damaged wings give unreliable estimates of melanization, but still show whether the hindwing was yellow or white.
To relate any melanization variation to natural conditions, we obtained records of monthly mean temperatures from April through August (between 1981 and 2010) from meteorological agencies in Finland, Switzerland and Austria. The mean temperatures in Switzerland and Austria were taken from two elevations representing a range of where P. plantaginis occurred in each location. Data on rainfall, average temperature highs and lows, and the number of days above 15°C (minimum observed flight temperature) were also obtained (see the electronic supplementary material, S1).
(b). Thermal property measurements
To determine whether more melanized individuals warm up faster, we measured the temperature gained under a heat source over a fixed time across males with varying amounts of melanin and of different colours (yellow or white). Fifty-five dead individuals were used and originated from Finland (n = 31), the Alpine region of Europe (n = 13) and non-Alpine central Europe (n = 11). All individuals were undamaged and had their wings spread completely open. Because small-bodied lepidopterans typically lose heat during flight, this position should be relevant [29,38,39], even though P. plantaginis and other moths do not routinely rest for long periods with their wings in the same position. However, individuals can be observed with their wings partly extended shortly before flight and when threatened, which does expose the dorsal surface of the hindwings to solar radiation. The use of dead moths allowed us to control for any differences in thermoregulation-related behaviour, such as movement, repositioning of wings or differences in metabolic activity that might exist among differently melanized individuals. Previous studies have also used either dry [32] or fresh [23] dead individuals for similar reasons when exploring links between thermoregulation and coloration.
Measurements were taken with a thermal imaging camera (InfraCAM, FLIR Systems) in a controlled environment (mean = 15.6°C, s.d. = 1.27), representing the lowest temperature at which we observed flying individuals in the field (R.H.H. & J.M. 2009, personal observation). The environment was created using a plastic container filled with approximately 3 cm of ice. We fixed a piece of dry foam (1 × 1 × 4 cm) in the centre to provide a platform on which the specimen was mounted using an insect pin. To ensure that all measurements started in similar conditions, we checked the initial temperature of the moth with the camera, and the temperature of the environment with both the camera and a sensor mounted at the same level as the moth. The initial temperature was included as a covariate in the analysis as well. The heat source for the temperature gain measurements was a 60 W incandescent heat lamp, mounted 52 cm above the specimen. We took each temperature reading once after 45 s. Measurements were taken from the thorax of each specimen between the attachment points of both forewings, being careful to not take readings of the pin. We systematically alternated among colours and relative amounts of melanin to prevent any sample order effects confounding the results.
In analysing our data, we adhered to the lumped system model of heat transfer, which assumes small variations in temperature throughout the object [40]. The lumped system method is expected to apply to the wood tiger moth, given its small size and thin shape. Therefore, we examined and analysed the raw temperature differences for each individual directly to determine whether melanization predicted heat gain. Including surface area as a covariate to control for specimen size did not change the results. The percentage of melanization in the hindwing was calculated from photographs using the threshold discrimination tool in paint.net.
We analysed the temperature gain data with a one-way analysis of covariance (ANCOVA). We used the amount of temperature gained as the response variable with colour as a fixed factor and percentage of the hindwing covered by melanization as the covariate, along with the initial temperature. The interaction term between colour and hindwing melanization was also included in the model.
(c). Predation experiment
We used artificial paper moths with plasticine clay bodies to test whether colour or melanization of P. plantaginis influenced predation risk under natural conditions (figure 2). We carried out the experiment in Finland from 21 June to 6 July 2010, and in the Alpine region of Europe (Switzerland and Austria) from 6 to 31 July 2010. Experiment timing was chosen to coincide with the same level of flight activity in both locations, as alpine populations emerge later than Finnish populations (unpublished data).
Figure 2.
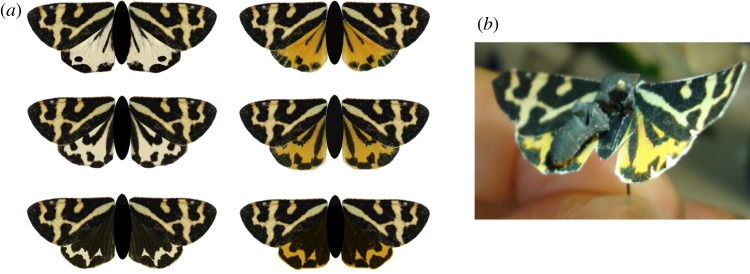
(a) The artificial moths of one forewing colour type (n = 6/12 total model types) used in the predation experiment. (b) Each day models were checked to see if avian attacks had occurred. (Online version in colour.)
Artificial models were created with GIMP (v. 2.6.3) using one picture of the real moth for each type, and printed on Rite in the Rain paper (J L Darling Corporation, Tacoma, WA; www.riteintherain.com). We chose pictures of undamaged real moths best representing the ranges of the actual phenotypes, based on our image analysis data. Artificial moths possessed either white or yellow ‘base’ hindwing coloration and one of three degrees of melanization (37, 58 or 87%). The forewing pattern used in the models represents the most common forewing type found in Europe (R. H. Hegna et al. 2009, unpublished data). The hue of the forewing ‘white’ pattern is correlated to some degree with hindwing colour [41]. Individuals with yellow hindwings tend to have more yellowish forewings compared with white individuals. We used both forewing colours with both yellow and white hindwings to isolate the effect of hindwing coloration. Thus, we had 12 model types in total, with one model type for each combination of hindwing colour (yellow/white), forewing colour (yellow/white) and melanization level (37, 58 or 87%). The position of the wings in the models (figure 2) was designed to look similar to males when they are threatened or moving through the grass in pre-flight posture (similar to when they are also close to locating females).
We fastened models with an insect pin to a green background made from a circular piece of corkboard painted green with Tikkurila Helmi M384 paint (figure 2). The circular platform was then mounted on a 0.35 m wooden stake. We used avian vision modelling to ensure that the each moth model colour (i.e. yellow, white, black) was easily detectable to avian predators against the green background (table 1; for detailed methods see electronic supplementary material, S2). We were also able to ensure that the coloration of the artificial moths corresponded with the real moth colours against the background (table 1). The contrasts are reported as just noticeable difference values (JNDs) [42]. The model predicts that values of less than 1 are indistinguishable, values between 1 and 3 are hard to distinguish unless under optimal conditions, and values greater than 5 are easy to tell apart under most conditions (D. Osorio 2012, personal communication; see also [42]).
Table 1.
Chromatic (‘colour’) and achromatic (‘luminance’) contrasts comparing models and real moths against the standard green background used in the predation experiment (reported as JND vaues).
| object | colour | luminance |
|---|---|---|
| real yellow moth | 10.6 | 9.2 |
| yellow model | 13.0 | 10.4 |
| real white moth | 8.6 | 20.0 |
| white model | 10.5 | 20.3 |
| real melanization | 11.6 | 11.2 |
| model melanization | 11.5 | 12.0 |
Artificial moth models were placed 15 m apart along a linear transect. A total of 840 models were placed along transects in central Finland and 864 along transects in the Alpine region (see the electronic supplementary material, S3). In central Finland, we placed 60 models along nine transects and 50 models along six transects (because of logistical constraints), and 24 models were along each transect in the Alpine region (36 transects). Alpine region transects were shorter because our ability to place long continuous transects was limited by the presence of large geophysical obstacles. Our sampling in the Alpine region indicated the moths mostly occupy areas of higher elevation (1800–2500 m), but we could not rule out that they also occupy lower-elevation habitats at lower densities. Transects in the Alpine region were placed at varying levels of elevation (1200–2500 m) to account for its indirect effects on predation (such as changes in vegetation and community). We randomized the order of model types with approximately equal dispersion of all colour morphs. All transects were placed at least 500 m apart to ensure good dispersion of sites, and to decrease the probability that the same birds visited several transects. In the Alps we made models available to predators for 72 h, and in central Finland we left models available for 120 h, but we only used predation events within the first 72 h when both datasets were combined in an overall analysis. The models were checked once a day and recorded as ‘attacked’ if beak marks were apparent in the plasticine body (figure 2). Analyses included only those individuals we could positively identify as attacked by birds.
To document the visual environment in which each model was placed, we recorded the habitat type in each model's location. Our broad categories included meadow, forest, alpine meadow and power line. Alpine meadows were distinguished from other meadows by the existence of short alpine vegetation and were typically at higher elevations. We used the power line ‘habitat’ category in central Finland because vegetation in these areas is of a particular height and density (usually 1–2 m tall and can be very dense) not fitting into other categories.
To analyse the data, we first combined all data from the Alps and central Finland to look for overall general trends in predation and compare the two regions. We then analysed each region (central Finland and the Alps) separately to examine local factors that may influence predation (i.e. elevation and habitat). In the second analysis, attacks from the additional 2 days of observation time were included for central Finland.
We used logistic regression to analyse the artificial moths' vulnerability to predation with the status of each model (attacked or not attacked) as the dependent variable, and hindwing melanization, colour and forewing colour as explanatory variables. Habitat was an explanatory variable in the separate regional analyses for both regions. Elevation, which differed for each transect, was an explanatory variable in the Alpine dataset analysis. Transects in central Finland were all at approximately the same elevation, but we included ‘transect’ as an explanatory variable for the Finnish dataset to account for variation in attacks at each location. All statistical models and data met the assumptions of the logistic regression analysis, including the goodness of model fit assumption determined by the Hosmer–Lemeshow test (p > 0.40 for all models used). In the final models, we removed all non-significant interactions (all interaction p-values were >0.10).
3. Results
(a). Geographic melanization and climate patterns
The amount of melanization increased with increasing latitude in P. plantaginis males from Estonia to north Finland (F1,12.1 = 11.9, p = 0.005; table 2). In addition, individuals in the Alps had a significantly greater amount of melanin covering their hindwings than those in central Finland (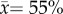 versus 43%, respectively; p < 0.001, t = −4.6, d.f. = 91.6). Melanization also varied more among individuals in the Alpine region compared with central Finland (s² = 0.022 versus 0.012, respectively; Levene's test, F1,114 = 9.1, p = 0.003).
versus 43%, respectively; p < 0.001, t = −4.6, d.f. = 91.6). Melanization also varied more among individuals in the Alpine region compared with central Finland (s² = 0.022 versus 0.012, respectively; Levene's test, F1,114 = 9.1, p = 0.003).
Table 2.
Parameter estimates from the mixed model analysis showing that melanization increased with latitude between Estonia and Finland.
| parameter | estimate | s.e. | d.f. | T | p |
|---|---|---|---|---|---|
| intercept | −1.127285 | 0.83466 | 12.053 | −1.351 | 0.202 |
| latitude | 0.046208 | 0.013343 | 12.086 | 3.463 | 0.005 |
Climate in central Finland tends to be warmer than in the Alpine region (see the electronic supplementary material, S1). There are fewer days above the minimum observed flight temperature of 15°C and the average temperature is also lower.
(b). Thermal measurements
The ANCOVA analysis indicated that both colour and melanin explained the average rate at which P. plantaginis males warmed up (F1,4 = 28.4, p < 0.001; table 3). The statistical model (including colour, melanization and their interaction) accounted for 69 per cent of the variance in the amount of temperature gained. There was an interaction between colour and melanization because white males benefitted more from increased amounts of melanin compared with yellow males (F1,1 = 12.2, p = 0.001; figure 3). Colour alone accounted for 36 per cent of the observed variance in temperature gain, with yellow males warming up more quickly on average than white males (F2,1 = 28.1, p < 0.001). For white males, melanization explained 65 per cent of variation in temperature gain, whereas melanization explained 45 per cent of variation for yellow males (figure 3). Greater melanization also increased the average rate for warming up, and accounted for 59 per cent of the variance when controlling for colour (F1,1 = 72.1, p < 0.001). Initial temperature of the specimens had no effect (F1,1 = 0.4, p = 0.53).
Table 3.
ANCOVA table results showing that melanization, colour, and an interaction between melanization and colour influenced the temperature gained over 45 s.
| source | sum of squares | d.f. | ms | F | p | partial eta squared |
|---|---|---|---|---|---|---|
| corrected model | 15.218 | 4 | 3.804 | 28.393 | <0.001 | 0.694 |
| intercept | 0.146 | 1 | 0.146 | 1.087 | 0.302 | 0.021 |
| colour | 3.765 | 1 | 3.765 | 28.102 | <0.001 | 0.36 |
| hindwing melanin (%) | 9.667 | 1 | 9.667 | 72.145 | <0.001 | 0.591 |
| initial temperature (°C) | 0.053 | 1 | 0.053 | 0.398 | 0.531 | 0.008 |
| colour×hindwing melanin | 1.631 | 1 | 1.631 | 12.173 | 0.001 | 0.196 |
| error | 6.699 | 50 | 0.134 | |||
| total | 276.800 | 55 | ||||
| corrected total | 21.917 | 54 |
Figure 3.
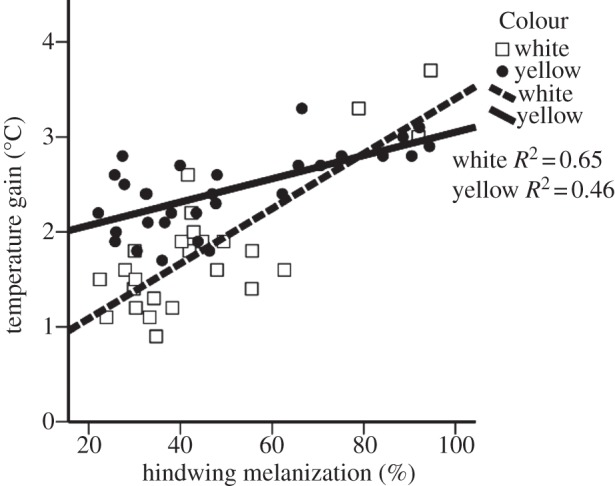
The relationship between hindwing melanization and the temperature gain over a fixed amount of time.
(c). Predation experiment
We found that melanization predicted being attacked when all the data from central Finland and the Alpine region were analysed together (figure 4). Every 10 per cent increase in the amount of melanin resulted in a 16 per cent increase in the odds of being attacked (p < 0.001, odds ratio = 1.015 per unit of melanin increase, CI95 = 1.007–1.023, Wald = 13.4). Hindwing colour was not a predictor of predation (p = 0.77). Forewing colour was also not a significant predictor of predation (p = 0.34, Wald = 0.90). Models in central Finland were 1.85 times more likely to be attacked than in the Alps (p < 0.001, odds ratio = 1.85, CI95 = 1.34–2.55, Wald = 14.2). No interaction was statistically significant, but the interaction between region and coloration was weak (p = 0.12). The weak interaction suggests predators attacked the hindwing colours differently in the two regions.
Figure 4.
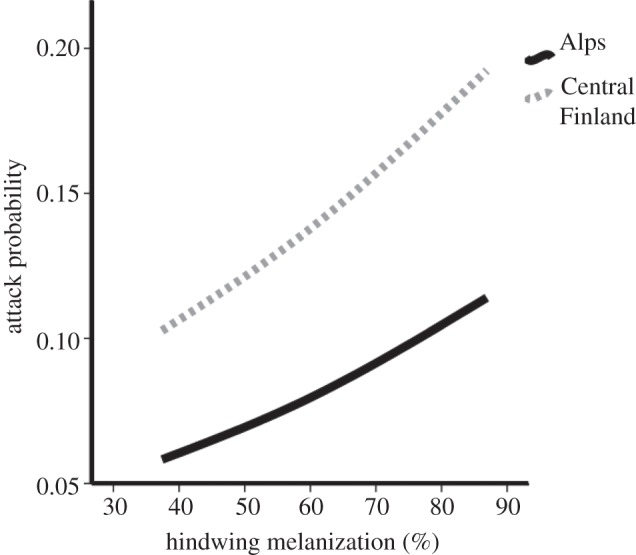
The predicted probability of being attacked as melanization increases in both the Alps and central Finland.
When the Alpine region's data were analysed separately, melanization and habitat type were independently significant predictors of being attacked and were not involved in any interaction effects. Our results indicated that every 10 per cent increase in the amount of melanin covering the hindwing resulted in a 22 per cent increase in the odds of being attacked (p = 0.002, odds ratio = 1.020 per unit of melanin increase, CI95 = 1.007–1.032, Wald = 9.7). Hindwing colour did not predict being attacked (p = 0.33, Wald = 0.9) and neither did forewing colour (p = 0.30, Wald = 1.6). Habitat type was a significant predictor of predation within the Alpine region. Artificial models placed in forest (regardless of elevation) or in meadows were more likely to be attacked than models in alpine meadows (p = 0.015, Wald = 8.4). The only significant interaction in our analysis was between elevation and location as predictors of predation (p = 0.02, Wald = 5.4). In a separate logistic regression analysis looking at elevation as a predictor of being attacked for each separate location (Switzerland and Austria), we found that every 500 m increase in elevation decreased the odds of attack by 39 per cent in Austria (p = 0.006, odds ratio = 0.999 m−1 gained in elevation, CI95 = 0.998–1.000, Wald = 7.5). However, elevation was not a significant predictor of being attacked in Switzerland (p > 0.5, Wald = 0.01).
In central Finland our results differed from the combined model, as hindwing colour (p = 0.004) was a significant predictor of predation, whereas melanin was only a weak predictor (p = 0.086, Wald = 2.9). We found that white males were nearly twice as likely to be attacked as yellow males (p = 0.004, odds ratio = 1.96, Wald = 8.4), although colour was involved in a significant interaction with the transect site (p = 0.006, Wald = 30.6). The interaction was found to emanate from two transects where yellow models were more likely to be attacked than white models (out of the 15 transects established). By itself, transect was not a significant predictor of predation (p = 0.20, Wald = 16.9). Neither forewing colour (p = 0.76, Wald = 0.1) nor habitat type were predictors of predation (p = 0.20, Wald = 0.2).
4. Discussion
Taken together, our results show the likely existence of a trade-off between thermoregulation and predation risk with respect to the amount of melanin present on the hindwing in P. plantaginis. Specifically, we found that greater amounts of melanin in both white and yellow males resulted not only in an increased ability to absorb radiation, but also increased the likelihood of attack by avian predators. One mechanistic explanation for the increased predation risk of more melanized individuals is that the amount of colour (yellow or white) decreases as the proportion of the hindwing covered by melanin increases. Nokelainen et al. [16] showed that both yellow and white pigments in the hindwings of male P. plantaginis serve as warning signals, even though yellow males were avoided more by avian predators than white males. Larger warning signals increase detection by predators [43,44], which should facilitate recognition and avoidance [7,45]. Therefore, diminished signal size can reduce the effectiveness of the signal [44,46], as we observed in our results. Alternatively, increased amounts of black pigment might have the effect of enhancing internal contrast within the hindwing. Internal contrasting patterns have often been thought to improve warning signal efficacy [47], but evidence of it improving warning signal efficacy in natural systems is mixed [48,49]. In the current study, we found no evidence for an ‘optimal level of internal contrasts’ because increased melanization increased the probability of being attacked almost linearly. Despite decreasing the efficacy of the warning signal, higher levels of hindwing melanization impart thermoregulatory benefits.
Although adult P. plantaginis tend to rest with only their forewings exposed, hindwing melanization would still be beneficial for thermoregulation because they are exposed to solar radiation (and predators) just before flight and continuously during flight. Indeed, gaining heat is not only important for pre-flight thermoregulation [27,50], particularly for smaller lepidopterans that are more prone to losing heat [29,39]. Hindwing melanization can benefit the maintenance of the appropriate temperature necessary for flight when moving across different habitat types and in changing weather patterns [27,31,32,50]. Therefore, our result demonstrating that P. plantagins males warm up more quickly when they have greater amounts of melanin in their hindwings represents a relevant benefit. Our finding that melanization increases from Estonia (58° N) to northern Finland (67° N) further supports the importance of hindwing melanin to thermoregulation.
Energetic trade-offs between production costs and benefits of melanization have received much attention [18,19,51,52], whereas intra- or interspecific signalling trade-offs represent a less explored aspect. However, recent focus has shifted to recognize the importance of melanin to signalling. Ellers & Boggs [24] found that more melanized adult female Colias philodice butterflies attracted fewer males than less melanized females, despite the thermoregulatory benefits of melanin at high elevations. Thermoregulation was also shown to constrain warning signal size in P. plantaginis larvae [17], representing one of the few examples of an interspecific signalling trade-off involving melanin. Our results, along with previous studies examining both inter- and intraspecific signalling, demonstrate that increased melanin for thermoregulation purposes can reduce signalling efficacy and create trade-offs.
In addition to showing support for a trade-off, our results pose an explanation for the geographical difference in the hindwing warning signal between central Finland and the Alpine region. Temperatures at elevations where P. plantaginis occur in the Alps are generally cooler, with fewer days reaching above our observed flight threshold of 15°C (see the electronic supplementary material, S1). The net effect of the cooler climate should make melanization an important fitness-related trait because it should benefit mate-finding opportunities in the Alps more compared with central Finland. Similarly, Punzalan et al. [23] found that the thermoregulatory benefits of melanin increased fitness in the ambush bug (Phymata americana), but only when temperatures were at the lower limit of tolerance for the species. Although we did not directly link warm-up ability to fitness in our study, previous work on lepidopterans has shown fitness benefits of increased melanization [30–32,53]. Therefore, cool temperatures at high elevations in the Alps are likely to make the benefits of additional melanin outweigh its predation costs and tip the balance of selection in favour of highly melanized phenotypes. We should also note that melanization could benefit escape ability from predators [28] and other behavioural mechanisms our experiment did not test.
The lower attack rate we observed in the Alpine region may also make the increased selection against melanized forms by predators more easily overcome by melanization's potential benefits. Evidence for this possibility exists because the lower attack rate also coincided with greater variation in the amount of melanization in the hindwing (and therefore in warning signal size). Studies of predation in poison frogs have found evidence that areas of higher warning signal diversity appear to experience less predation [12,13]. Our results appear to corroborate the findings in poison frogs, at least from the perspective of warning signal size. Explaining the co-occurrence of both white and yellow males in central Finland and the Alpine region is still a challenge.
Differences in predation trends with respect to hindwing colour that existed between central Finland and the Alpine region also applied in the ratio of males with white or yellow hindwings. Predators in central Finland paid greater attention to hindwing colour than to melanization, with white males being attacked nearly twice as much as yellow individuals. At the same time, the population in central Finland is unexpectedly comprised of mostly white individuals (54/69 males). Our results mirror those of Nokelainen et al. [16], who found that yellow individuals possessed a more effective warning signal than white individuals in the Åland Archipelago of Finland. Nokelainen et al. [16] also found that white males have greater mating success, which may explain how they can still comprise a large portion of the population where they are attacked more. In contrast to central Finland, the Alpine region is predominantly yellow (36/55 males) and only melanization was shown to impact predation in the regional analysis. The ability of yellow males to warm up more quickly at lower melanization levels might help explain why the Alpine population is mostly yellow. Darker colours were also recently found to be beneficial for thermoregulation in the monarch butterfly [54]. Increased mating success of white males in the Alpine region may help to explain the persistence of white males there as well. In general, our results suggest that positive frequency-dependent selection by predation does not occur with P. plantaginis in central Finland or the Alpine region, because the most common morphs were not attacked less by predators. Although predation-driven frequency-dependent selection appears not to be a primary driving force in the maintenance of the male colour polymorphism, predators may still help explain the geographical differences.
Different responses to the importance of hindwing colour between central Finland and the Alpine region may reflect differences in behaviour of local predators, rather than predator learning environment (i.e. the frequency of differently coloured male hindwings). There is both theoretical [10] and empirical evidence [36,55] showing that different predators can vary in their responses to aposematic prey, which in turn can influence warning signal efficiency. For example, omnivorous birds having less experience with aposematic insects than insectivorous birds have been found to attack aposematic prey more eagerly [55]. Thus, direction and strength of selection on aposematic prey can vary significantly with predator communities [36]. It seems that predation alone does not explain either large or small-scale geographical variation in warning signal diversity in P. plantaginis. Although we suspect differences in, and interactions among, predation, temperature and sexual selection to be the largest drivers of geographical differences in warning signal appearance of P. plantaginis, it remains a challenge to show the exact extent to which these factors are responsible. Furthermore, higher levels of gene flow may contribute to some of the observed local warning signal diversity and male colour polymorphism [41].
When multiple selection pressures affect a trait, it is often challenging to identify how they interact to produce a particular phenotype. Hindwing melanization appears to be important for thermoregulatory purposes in male P. plantaginis, whereas hindwing colour (white or yellow) is the primary warning signal. Hindwing colour is also a warning signal in female P. plantaginis [36], but whether melanin produces similar thermoregulatory benefits and signalling costs in females requires further study. Our results provide evidence that the differences in costs and benefits of melanin in the two locations can drive phenotypic differences in the warning signal of males on broad and local geographical scales. Mating trade-offs [16] or other geographically related factors, such as predator or alternate prey community, could contribute to differences in dominant colours locally between central Finland and the Alpine region as well. Our study shows that predation is not the only factor influencing warning signal diversity and demonstrates a need for additional studies to examine geographical variation in warning signals in an integrated manner.
Acknowledgements
We thank Markus Lackner and Janne Valkonen for field-work help, Carita Lindstedt for avian vision model help and comments, and Eira Ihalainen, Juan Galarza, Mika Mökkönen and two anonymous reviewers for helpful comments. Data are deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.82c67). This work was conducted with permission of the Swiss National Park (permit no. CH-4168), and both the Salzburg (permit no. 21303-96/8/11-2010) and Karnten (permit no. SP3-NS-1545/2010) sections of Hohe Tauern National Park in Austria. We thank the Finnish Centre of Excellence in Biological Interactions (project number 252411; J.M., O.N., R.H.H.), the Biological Interactions Graduate School (R.H.H.), the Academy of Finland (J.M.) and the Palm Beach Atlantic University Quality Initiative Research Grant Program (J.R.H.) for the financial support.
References
- 1.Gray SM, McKinnon JS. 2007. Linking color polymorphism maintenance and speciation. Trends Ecol. Evol. 22, 71–79 10.1016/j.tree.2006.10.005 (doi:10.1016/j.tree.2006.10.005) [DOI] [PubMed] [Google Scholar]
- 2.True JR. 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18, 640–647 10.1016/j.tree.2003.09.006 (doi:10.1016/j.tree.2003.09.006). [DOI] [Google Scholar]
- 3.Endler JA. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91 10.2307/2408316 (doi:10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 4.Majerus MEN. 1998. Melanism: evolution in action. New York, NY: Oxford University Press [Google Scholar]
- 5.Cook LM, Grant BS, Saccheri IJ, Mallet J. 2012. Selective bird predation on the peppered moth: the last experiment of Michael Majerus. Biol. Lett. (doi:10.1098/rsbl.2011.1136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond AB, Kamil AC. 2002. Visual predators select for crypticity and polymorphism in virtual prey. Nature 415, 609–613 10.1038/415609a (doi:10.1038/415609a) [DOI] [PubMed] [Google Scholar]
- 7.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- 8.Beatty CD, Beirinckx K. 2004. The evolution of müllerian mimicry in multispecies communities. Nature 431, 63–66 10.1038/nature02818 (doi:10.1038/nature02818) [DOI] [PubMed] [Google Scholar]
- 9.Joron M, Mallet JLB. 1998. Diversity in mimicry: paradox or paradigm? Trends Ecol. Evol. 13, 461–466 10.1016/S0169-5347(98)01483-9 (doi:10.1016/S0169-5347(98)01483-9) [DOI] [PubMed] [Google Scholar]
- 10.Endler JA, Mappes J. 2004. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547 10.1086/382662 (doi:10.1086/382662) [DOI] [PubMed] [Google Scholar]
- 11.Noonan BP, Comeault AA. 2009. The role of predator selection on polymorphic aposematic poison frogs. Biol. Lett. 5, 51–54 10.1098/rsbl.2008.0586 (doi:10.1098/rsbl.2008.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegna RH, Saporito RA, Donnelly MA. 2013. Not all colors are equal: predation and color polytypism in the aposematic poison frog Oophaga pumilio. Evol. Ecol. 1–15 10.1007/s10682-012-9605-z (doi:10.1007/s10682-012-9605-z) [DOI] [Google Scholar]
- 13.Chouteau M, Angers B. 2012. Wright's shifting balance theory and the diversification of aposematic signals. PLoS ONE 7, e34028. 10.1371/journal.pone.0034028 (doi:10.1371/journal.pone.0034028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallet J, Barton NH. 1989. Strong natural selection in a warning-color hybrid zone. Evolution 43, 421–431 10.2307/2409217 (doi:10.2307/2409217) [DOI] [PubMed] [Google Scholar]
- 15.Estrada C, Jiggins CD. 2008. Interspecific sexual attraction because of convergence in warning colouration: is there a conflict between natural and sexual selection in mimetic species? J. Evol. Biol. 21, 749–760 10.1111/j.1420-9101.2008.01517.x (doi:10.1111/j.1420-9101.2008.01517.x) [DOI] [PubMed] [Google Scholar]
- 16.Nokelainen O, Hegna RH, Reudler JH, Lindstedt C, Mappes J. 2012. Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc. R. Soc. B 279, 257–265 10.1098/rspb.2011.0880 (doi:10.1098/rspb.2011.0880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindstedt C, Lindström L, Mappes J. 2009. Thermoregulation constrains effective warning signal expression. Evolution 63, 469–478 10.1111/j.1558-5646.2008.00561.x (doi:10.1111/j.1558-5646.2008.00561.x) [DOI] [PubMed] [Google Scholar]
- 18.Talloen W, Dyck HV, Lens L. 2004. The cost of melanization: butterfly wing coloration under environmental stress. Evolution 58, 360–366 10.1111/j.0014-3820.2004.tb01651.x (doi:10.1111/j.0014-3820.2004.tb01651.x) [DOI] [PubMed] [Google Scholar]
- 19.Ojala K, Lindström L, Mappes J. 2007. Life-history constraints and warning signal expression in an arctiid moth. Funct. Ecol. 21, 1162–1167 10.1111/j.1365-2435.2007.01322.x (doi:10.1111/j.1365-2435.2007.01322.x) [DOI] [Google Scholar]
- 20.Mattson WJ., Jr 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11, 119–161 10.1146/annurev.es.11.110180.001003 (doi:10.1146/annurev.es.11.110180.001003) [DOI] [Google Scholar]
- 21.Siva-Jothy MT. 2000. A mechanistic link between parasite resistance and expression of a sexually selected trait in a damselfly. Proc. R. Soc. Lond. B 267, 2523–2527 10.1098/rspb.2000.1315 (doi:10.1098/rspb.2000.1315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trullas SC, Wyk JHV, Spotila JR. 2007. Thermal melanism in ectotherms. J. Therm. Biol. 32, 235–245 10.1016/j.jtherbio.2007.01.013 (doi:10.1016/j.jtherbio.2007.01.013) [DOI] [Google Scholar]
- 23.Punzalan D, Rodd FH, Rowe L. 2008. Sexual selection mediated by the thermoregulatory effects of male colour pattern in the ambush bug Phymata americana. Proc. R. Soc. B 275, 483–492 10.1098/rspb.2007.1585 (doi:10.1098/rspb.2007.1585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellers J, Boggs CL. 2003. The evolution of wing color: male mate choice opposes adaptive wing color divergence in Colias butterflies. Evolution 57, 1100–1106 10.1111/j.0014-3820.2003.tb00319.x (doi:10.1111/j.0014-3820.2003.tb00319.x) [DOI] [PubMed] [Google Scholar]
- 25.Guppy CS. 1986. Geographic variation in wing melanism of the butterfly Parnassius phoebus F. (Lepidoptera: Papilionidae). Can. J. Zool. 64, 956–962 10.1139/z86-145 (doi:10.1139/z86-145) [DOI] [PubMed] [Google Scholar]
- 26.Tuomaala M, Kaitala A, Rutowski RL. 2012. Females show greater changes in wing colour with latitude than males in the green-veined white butterfly, Pieris napi (Lepidoptera: Pieridae). Biol. J. Linn. Soc. 107, 899–909 10.1111/j.1095-8312.2012.01996.x (doi:10.1111/j.1095-8312.2012.01996.x) [DOI] [Google Scholar]
- 27.Watt WB. 1968. Adaptive significance of pigment polymorphisms in Colias butterflies. I. variation of melanin pigment in relation to thermoregulation. Evolution 22, 437–458 10.2307/2406873 (doi:10.2307/2406873) [DOI] [PubMed] [Google Scholar]
- 28.Kingsolver JG. 1987. Predation, thermoregulation, and wing color in pierid butterflies. Oecologia 73, 301–306 10.1007/bf00377522 (doi:10.1007/bf00377522) [DOI] [PubMed] [Google Scholar]
- 29.Tsuji JS, Kingsolver JG, Watt WB. 1986. Thermal physiological ecology of Colias butterflies in flight. Oecologia 69, 161–170 10.1007/bf00377616 (doi:10.1007/bf00377616) [DOI] [PubMed] [Google Scholar]
- 30.Roland J. 1982. Melanism and diel activity of alpine Colias (Lepidoptera: Pieridae). Oecologia 53, 214–221 10.1007/bf00545666 (doi:10.1007/bf00545666) [DOI] [PubMed] [Google Scholar]
- 31.Van Dyck H, Matthysen E. 1998. Thermoregulatory differences between phenotypes in the speckled wood butterfly: hot perchers and cold patrollers? Oecologia 114, 326–334 10.1007/s004420050454 (doi:10.1007/s004420050454) [DOI] [PubMed] [Google Scholar]
- 32.Guppy CS. 1986. The adaptive significance of alpine melanism in the butterfly Parnassius phoebus F. (Lepidoptera: Papilionidae). Oecologia 70, 205–213 10.1007/bf00379241 (doi:10.1007/bf00379241) [DOI] [PubMed] [Google Scholar]
- 33.Ellers J, Boggs CL. 2004. Functional ecological implications of intraspecific differences in wing melanization in Colias butterflies. Biol. J. Linn. Soc. 82, 79–87 10.1111/j.1095-8312.2004.00319.x (doi:10.1111/j.1095-8312.2004.00319.x) [DOI] [Google Scholar]
- 34.Speed MP, Ruxton GD. 2007. How bright and how nasty: explaining diversity in warning signal strength. Evolution 61, 623–635 10.1111/j.1558-5646.2007.00054.x (doi:10.1111/j.1558-5646.2007.00054.x) [DOI] [PubMed] [Google Scholar]
- 35.Nokelainen O, Lindstedt C, Mappes J. In press Environment mediated morph-linked immune and life-history responses in the aposematic wood tiger moth. J. Anim. Ecol. 10.1111/1365-2656.12037 (doi:10.1111/1365-2656.12037) [DOI] [PubMed] [Google Scholar]
- 36.Lindstedt C, Eager H, Ihalainen E, Kahilainen A, Stevens M, Mappes J. 2011. Direction and strength of selection by predators for the color of the aposematic wood tiger moth. Behav. Ecol. 22, 580–587 10.1093/beheco/arr017 (doi:10.1093/beheco/arr017) [DOI] [Google Scholar]
- 37.Puurtinen M, Kaitala V. 2006. Conditions for the spread of conspicuous warning signals: a numerical model with novel insights. Evolution 60, 2246–2256 10.1111/j.0014-3820.2006.tb01862.x (doi:10.1111/j.0014-3820.2006.tb01862.x) [DOI] [PubMed] [Google Scholar]
- 38.Heinrich B. 1993. The hot-blooded insects: strategies and mechanisms of thermoregulation. Cambridge, MA: Harvard University Press [Google Scholar]
- 39.Heinrich B. 1986. Comparative thermoregulation of four montane butterflies of different mass. Physiol. Zool. 59, 616–626 [Google Scholar]
- 40.Mills AF. 1999. Heat transfer, 2nd edn Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- 41.Galarza JA, Nokelainen O, Hegna RH, Ashrafi R, Mappes J. In preparation. Spatio-temporal variation in the warning signal and populations of European wood tiger moths.
- 42.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633 10.1007/s003590050286 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 43.Bohlin T, Tullberg BS, Merilaita S. 2008. The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo). Anim. Behav. 76, 577–584 10.1016/j.anbehav.2008.02.012 (doi:10.1016/j.anbehav.2008.02.012) [DOI] [Google Scholar]
- 44.Lindstedt C, Lindström L, Mappes J. 2008. Hairiness and warning colours as components of antipredator defence: additive or interactive benefits? Anim. Behav. 75, 1703–1713 10.1016/j.anbehav.2007.10.024 (doi:10.1016/j.anbehav.2007.10.024) [DOI] [Google Scholar]
- 45.Lindström L, Alatalo RV, Mappes J. 1999. Reactions of hand-reared and wild-caught predators toward warningly colored, gregarious, and conspicuous prey. Behav. Ecol. 10, 317–322 10.1093/beheco/10.3.317 (doi:10.1093/beheco/10.3.317) [DOI] [Google Scholar]
- 46.Gamberale G, Tullberg BS. 1996. Evidence for a peak-shift in predator generalization among aposematic prey. Proc. R. Soc. Lond. B 263, 1329–1334 10.1098/rspb.1996.0195 (doi:10.1098/rspb.1996.0195) [DOI] [PubMed] [Google Scholar]
- 47.Guilford T. 1990. The evolution of aposematism. In Insect defenses: adaptive mechanisms and strategies of prey and predators (eds Evans DL, Schmidt JO.), pp. 23–62 Albany, NY: State University of New York Press [Google Scholar]
- 48.Hegna RH, Saporito RA, Gerow KG, Donnelly MA. 2011. Contrasting colors of an aposematic poison frog do not affect predation. Ann. Zool. Fenn. 48, 29–38 10.5735/086.048.0103 (doi:10.5735/086.048.0103) [DOI] [Google Scholar]
- 49.Dolenská M, Nedved O, Veselý P, Tesarová M, Fuchs R. 2009. What constitutes optical warning signals of ladybirds (Coleoptera: Coccinellidae) towards bird predators: colour, pattern or general look? Biol. J. Linn. Soc. 98, 234–242 10.1111/j.1095-8312.2009.01277.x (doi:10.1111/j.1095-8312.2009.01277.x) [DOI] [Google Scholar]
- 50.Kingsolver JG. 1983. Thermoregulation and flight in Colias butterflies: elevational patterns and mechanistic limitations. Ecology 64, 534–545 10.2307/1939973 (doi:10.2307/1939973) [DOI] [Google Scholar]
- 51.Nijhout HF. 1991. The development and evolution of butterfly wing patterns. Smithsonian Series in Comparative Evolutionary Biology (USA). Washington, DC: Smithsonian Institution Scholarly Press [Google Scholar]
- 52.Graham SM, Watt WB, Gall LF. 1980. Metabolic resource allocation versusmating attractiveness: adaptive pressures on the ‘alba’ polymorphism of Colias butterflies. Proc. Natl Acad. Sci. USA 77, 3615. 10.1073/pnas.77.6.3615 (doi:10.1073/pnas.77.6.3615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rawlins JE. 1980. Thermoregulation by the black swallowtail butterfly, Papilio polyxenes (Lepidoptera: Papilionidae). Ecology 61, 345–357 10.2307/1935193 (doi:10.2307/1935193) [DOI] [Google Scholar]
- 54.Davis AK, Chi J, Bradley C, Altizer S. 2012. The redder the better: wing color predicts flight performance in monarch butterflies. PLoS ONE 7, e41323. 10.1371/journal.pone.0041323 (doi:10.1371/journal.pone.0041323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Exnerová A, Landova E, Stys P, Fuchs R, Prokopova M, Cehlarikova P. 2003. Reactions of passerine birds to aposematic and non-aposematic firebugs (Pyrrhocoris apterus; Heteroptera). Biol. J. Linn. Soc. 78, 517–525 10.1046/j.0024-4066.2002.00161.x (doi:10.1046/j.0024-4066.2002.00161.x) [DOI] [Google Scholar]