Abstract
Most algae regularly experience periods of darkness ranging from a few hours to a few days. During this time, they are unable to photosynthesize, and so must consume stored energy products. However, some organisms such as polar algae and some microalgal cysts and spores are exposed to darkness for months to years, and these must use alternative strategies to survive. Some taxa, such as dinoflagellates, form cysts and become dormant. Others use physiological methods or adopt mixotrophy. The longest documented survival of more than a century was for dinoflagellates buried in sediments in a Norwegian fjord. Seasonal changes in daylight hours are naturally unaffected by climate change. This means that polar microalgae will in the future need to survive the same period of seasonal darkness but at higher temperatures, and this will require a greater drawdown of stored energy. Recent experimental work has shown that both Arctic and Antarctic phytoplankton are able to survive increases of up to 6°C in the dark.
Keywords: dark survival, Antarctic, winter, microalgae, phytoplankton
1. Introduction
Most phototrophic organisms regularly experience periods of darkness. For many, this is a daily occurrence experienced during night-time but for others, such as phytoplankton cells in deeply mixed oceans, it can occur over periods of hours to days. Organisms in some terrestrial habitats can, as a result of inundation or burial, experience episodic periods of more prolonged darkness that can last for days to weeks or even longer. In polar areas also, many phototrophic organisms routinely survive periods of several months in the dark during the long polar winter. Perhaps the longest periods of darkness that have been endured by phototrophs are those experienced by some algal cysts or spores, examples of which have germinated after more than a century in darkness (figure 1) [1].
Figure 1.
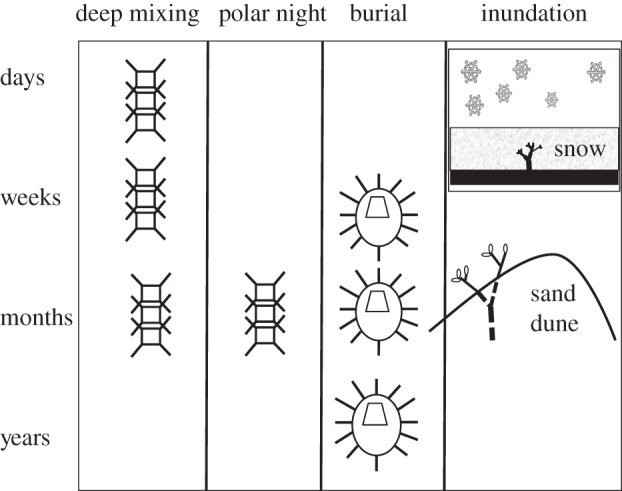
Duration of dark survival by phytoplankton, dinoflagellate/diatom cysts/spores and terrestrial plants buried by sand or snow.
To remain viable in the dark, cells must be able to retain membrane, organelle and DNA integrity. Damage is constantly occurring naturally through exposure to environmental stresses such as heat and oxidation [2]. Active cells are able to repair this incremental damage as it happens, but dormant cells are not. While there is some evidence that some dormant bacterial spores can repair DNA damage, there is as yet no evidence that eukaryotic cells have this capacity [3]. Cells that become dormant, though, can reduce this damage by production of a resistant wall covering such as in some dinoflagellates and chrysophytes. Alternatively, cells can settle in environments where external stresses are minimized. This includes very cold, dark and anaerobic environments. In these environments, the accumulation of damage can be reduced to very low levels, and some cells are able to survive for long periods.
Plants have different physiological responses to low light than they have to darkness. Therefore, it is important to define the difference between very low light and darkness in natural environments. Midday photosynthetically active radiation (PAR; 400–700 nm wavelength) at the Earth's surface is approximately 2000 µmol photons m–2 s–1. However, some light can still be detected at the Earth's surface on the darkest of nights, but then values are less than 1 µmol photons m–2 s–1. The compensation irradiance—that is, the light intensity at which oxygen consumption (respiration) is equal to oxygen production by photosynthesis—determines cell viability at low irradiances, and is thus an appropriate threshold to consider for dark survival. For phytoplankton, this value is typically 1–4 µmol photons m–2 s–1, although for some light-adapted cells it can exceed 30 µmol photons m–2 s–1, and for some extreme shade-adapted cells it is less than 1 µmol photons m–2 s–1 [4].
Here, we examine the different strategies used by phototrophs, predominantly marine algae, for surviving long periods in the dark. We comment on the environmental implications of this capacity and speculate on what are the likely effects of surviving in the dark as global climate change causes winter temperatures to rise.
2. Mechanisms of surviving the dark
Organisms use different strategies to survive long periods of darkness. Some produce cysts or resting stages, others are facultatively heterotrophic (i.e. mixotrophic) and others are able to adjust their metabolic rates or rely on energy storage products [5,6]. Temperature also plays an important role in dark survival [5,7], with increasing temperatures causing a reduction in long-term cell viability.
(a). Cyst/spore production
Taxa from many algal divisions are known to produce resting spores or cysts. For some species, this is only a short, transient stage as part of their normal reproductive cycle. For other taxa, however, resting cysts are produced in response to adverse environmental conditions and can remain dormant in sediments for many years. Some organisms use this strategy to survive the winter in the sediments and then rely on re-suspension in spring to resume their life cycle [8]. These cysts, although retaining a photosynthetic capacity, remain inactive during dormancy. This capacity to survive has enabled some nuisance species to form blooms from cyst beds deposited many years previously [9]. Many species of diatoms, dinoflagellates and chrysophytes produce cysts at the end of summer or in response to external environmental stimuli, such as nutrient limitation, and remain dormant until the following spring. Some dinoflagellates and chrysophytes produce cysts with resistant cell walls that allow them to remain buried in sediment, but viable for many years. The resistant cell walls of some of these taxa can survive degradation for thousands to millions of years, and these have been used by geologists for palaeo-environmental interpretations (figure 2). The longest documented survival period for marine diatom spores or dinoflagellate cysts is approximately a century for buried dinoflagellates [1]. Other reported extended survival periods include 3 years for diatom spores [10] and 16.5 years for a freshwater dinoflagellate [11]. Kaefer et al. [12] estimated the half-life for buried dinoflagellate cysts to be between 2 and 10 years.
Figure 2.
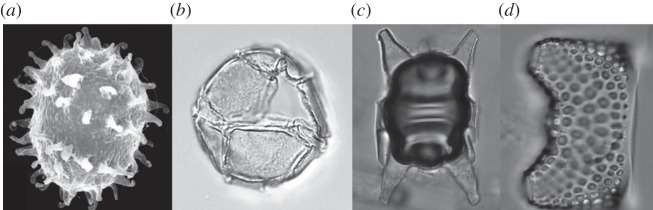
Cysts and spores. (a) Polarella glacialis, Antarctic dinoflagellate cyst; (b) Impagidinium sphericum, temperate marine dinoflagellate cyst; (c) Odontella sp., Antarctic diatom spore; (d) Eucampia antarctica, Antarctic diatom spore.
Resting cysts and spores are mostly non-photosynthetic [13], and are known to contain elevated levels of storage lipids and glucose [14]. Spore and cyst formation is most commonly induced by nutrient limitation, but also in response to changes in salinity and temperature [15], and they are known to germinate in response to light [12] or nutrient addition [16]. Others have an endogenous cycle and germinate after specific periods of time or in response to photoperiod. In these organisms, melatonin (the hormone that regulates sleep, sexual behaviour and circadian rhythms in animals) has been found to play a key role [17]. Dark survival of most spores and cysts is achieved by minimizing energy expenditure by becoming dormant. Cell viability and survival is enhanced if the cyst resides in dark, cold or anaerobic conditions. Each of these environmental conditions reduces the oxidative damage that would otherwise cause membrane and DNA degradation [18].
(b). Nutritional versatility
Mixotrophy is the use of both phototrophy (i.e. photosynthesis) and heterotrophy (the use of organic carbon for growth) by the same cell to obtain its necessary energy requirements. In well-lit environments, phytoplankton primarily use light energy for cell maintenance and growth. However, in environments where light levels or nutrients are low, many microalgal taxa are additionally able to use a wide variety of organic substrates, including amino acids and saturated fatty acids, to sequester their required carbon [19,20]. While these active uptake mechanisms also operate in the light, they have been shown to significantly upregulate at low irradiances or in darkness [20]. For example, Palmisano & Sullivan [21] and Rivkin & Putt [22] demonstrated that there was a light-dependent uptake of amino acids and glucose in extreme shade-adapted microalgae from Antarctic sea ice. Other Antarctic phytoplankton cells have also been observed to take up large organic molecules and particles, including bacteria, to supplement their energy requirements in winter [23]. While mixotrophy often provides a significant competitive advantage in low-light or dark environments, it is unclear whether it is ever sufficient to compensate for a complete lack of light and allow long-term survival.
(c). Physiological changes
Two different physiological responses occur in phytoplankton taxa in response to exposure to darkness: reduction of their metabolic rate [24], and use of energy storage products (e.g. lipids, starch etc). Increased levels of starch and other storage products have often been detected in polar phytoplankton taxa in late autumn and winter [25,26]. Photosynthetic carbon allocation in phytoplankton is strongly affected by both irradiance and nutrient levels [27]. At low temperatures and at low light levels (less than 20 μmol photons m–2 s–1) protein is the main product of photosynthesis, and the proportion of polysaccharide and lipid increases with radiation fluxes. High carbon fixation into low-molecular-weight metabolites is also associated with light-limited growth [28]. During darkness, protein synthesis occurs at the expense of consumption of low-molecular-weight compounds and carbohydrates [29–31], and this may result in a more rapid drawdown of energy reserves, which will consequently shorten the dark survival period of many taxa.
Another long-term response to darkness is a decrease in photosynthetic pigments and photosynthetic performance [32]. Luder et al. [33], working with Antarctic seaweeds, reported a decrease in the photosynthetic parameters ETRmax and Fv/Fm after three months of darkness, degradation of light harvesting antennae after four months, and degradation of light harvesting complex 1 and/or reaction centres of PSII and/or PSI after five months. Pigment content and photosynthetic performance were at a minimum at the end of six months. However, they were able to resume photosynthesis within 24 hrs of the light returning. Most algae have some ability to acclimate to changes in light and temperature, but relatively little is known about the extent of, or mechanisms involved in, the downregulation of respiration [34].
Metabolic function is strongly temperature-dependent, with all rates increasing to an optimum at higher temperatures before declining if temperatures exceed this threshold. Higher metabolic rates in the dark could lead to a more rapid drawdown of stored energy products, and consequently shorter dark survival times. Increased temperature can also raise the compensation point irradiance [35], which might lead to increased mortality and a decrease in biomass. Experiments with polar algae, however, indicate that dark survival rates are not affected at small to moderate temperature increases [36].
3. Dark survival scenarios
(a). Diurnal cycle (approximately 12 hrs)
All metabolic processes in algal cells require energy. During the day, this energy is constantly made available using solar energy to synthesize glucose during photosynthesis. In the dark, however, cells continue to respire and so require access to a continued source of energy. Normally, enough glucose is produced and stored during the day for them to easily survive the lack of light during the night. However, most cells only have a limited storage capacity for glucose, and consequently must convert excess energy into storage products such as starch and lipids. Recently, the production and use of these storage products for large-scale algal biofuel production has become the focus of intense interest, as it is hoped that in the future they might provide a carbon-neutral, sustainable alternative to burning fossil fuels [37].
Circadian (daily) rhythms, which are ubiquitous in algae, are known to regulate many cell functions, including cell division, photosynthetic capacity, gene expression and phototaxis [38]. These rhythms are controlled by internal clocks and are closely tied to the photoperiod, causing different physiological responses in day to night. It is thought that circadian rhythms provide an anticipation of environmental change (e.g. in light), which provides an adaptive advantage [39]. Strong circadian rhythms in oxygen production in continuous white light, expression of light harvesting complex genes and nuclear division have all been demonstrated. Dark respiration has not been found to vary with circadian rhythm, but effective quantum yield (ΔF/Fm′) and non-photochemical quenching have [39]. The regulation of cellular processes such as photosynthesis by circadian rhythms is thus an important short-term adaptive response to daily darkness.
(b). Microphytobenthos (hours to days)
The microphytobenthos (MPB) is the community of microalgal cells, predominantly diatoms, living at the sediment surface of shallow intertidal or subtidal environments. These communities are constantly exposed to extremes of light and temperature. At low tide, for instance, temperatures can vary from below freezing point in winter to above 40°C in summer. Likewise, if low tide coincides with midday, cells can be exposed to irradiances above 2000 µmol photons m–2 s–1. Many of the cells in these communities are motile and are able to migrate into the sediments to avoid excessively high solar radiation. The light environment within sediments is interesting. In sandy sediments, intense scattering at the sediment surface can cause an increase in light intensity by up to 280 per cent [40]. However, light is rapidly attenuated with depth and in most sediment types less than 1 per cent of the surface irradiance is present at a depth of only 3 mm [41]. Consequently, most MPB cells spend much of their time in the dark, but regulate their energy absorption and usage by vertical migration to and from the surface. The sediment–water interface in intertidal and subtidal environments is highly dynamic, with active resuspension. Furthermore, just a few millimetres beneath the surface the sediment usually becomes strongly anaerobic and thus hostile to normal cellular metabolism. Both physical processes (such as sedimentation) and biological processes (such as bioturbation by benthic infauna) can act to bury MPB below the euphotic zone, often for extended periods. Cell survival has been observed to exceed 12 months, although the proportion of surviving cells is very low [42]. Survival is facilitated by spore production, although there is also some evidence for heterotrophy [42,43]. In some of these organisms, there also seems to be a strong correlation between their dark survival capacity and their ability to metabolize stored intracellular nitrate [44].
(c). Ocean mixing (hours to days)
Deep mixing can expose cells to darkness on time scales of hours to days. Convection cells in exposed water bodies, caused by densification of surface waters through either cooling or increased salinity, can mix surface waters to depths greater than 500 m [45]. By comparison, the euphotic depth—the depth to which there is sufficient light for photosynthesis to exceed respiration (usually defined as 1% of surface irradiance)—is rarely greater than 100 m. When this vertical mixing is sustained for long periods, such as over winter, entrained phytoplankton cells receive insufficient light to survive, and this contributes to the typically low biomass levels in open oceans during winter. Cells isolated from a deep chlorophyll maximum (200 m) off the coast of California were found to survive for up to eight weeks in the dark and then rapidly resumed photosynthesis on re-exposure to light [46].
(d). Polar winter (months)
Polar marine plants need to survive long periods (up to several months annually) of darkness during the polar winter. These low levels of surface light are further exacerbated by the presence of a sea ice cover, which, together with snow, can cut out 99.9 per cent of the remaining surface irradiance. Measured midday light levels beneath the sea ice in summer are frequently less than 5 μM photons m–2 s–1 (compared with approx. 2000 μM photons m–2 s–1 at the surface) and are usually below detection levels in winter [47]. Several studies have reported on the ability of Antarctic phytoplankton and sea ice taxa to survive long periods of darkness [48–50]. Some Antarctic phytoplankton taxa have been able to resume photosynthesis after periods of up to 10 months in the dark, and one seaweed species was able to resume photosynthesis within 24 hrs after a six-month period of total darkness [33]. Bunt & Lee [50] reported cells remaining active after 2 years in a refrigerator, although these were subjected to infrequent, brief exposures light when the refrigerator was opened.
Polar phototrophic cells use different strategies to survive long periods of darkness. Some produce cysts or resting stages, others are facultatively heterotrophic and others are able to adjust their metabolic rates or rely on energy storage products [5,6]. Spore production is relatively uncommon in Antarctic phytoplankton taxa, and seems to be limited to a few Chaetoceros, Eucampia, Odontella and Thalassiosira diatom species [51], and a small number of dinoflagellates, such as Polarella (figure 2) [52]. Facultative heterotrophy, on the other hand, is widespread, particularly among the dinoflagellates and many of the smaller taxa [23,53].
Phytoplankton in some areas of Antarctica can survive and even photosynthesize throughout winter. Laybourn-Parry et al. [23] recorded mid-winter surface PAR of approximately 9 µmol photons m–2 s–1 at lakes near Davis, Antarctica (68°34′35″ S, 77°58′08″ E). They noted small differences in morphologies in some species, which may have been resting stages, but generally concluded that the phytoplankton functioned throughout the year in lakes mostly by using nutritional versatility (heterotrophy). As phytoplankton compensation irradiances in these lakes are less than 1 µmol photons m–2 s–1 [54], these responses, although from an Antarctic winter, might better be considered a response to low light rather than darkness.
Effective understanding and modelling of polar ecosystems requires knowledge of phytoplankton biomass and primary production throughout all of the year. However, very little winter biomass and productivity data exists for either the Arctic or the Southern Ocean. Most of what little Antarctic biomass data exists comes from the Weddell Sea and adjacent Antarctic Peninsula. Here, winter biomass levels were found to be between 10 and 60 mg C m–2 [55,56], 20 mg chla m–2 [57] and less than 0.1 mg chla m–3 [58]. These values are approximately two to four orders of magnitude less than summer values. Apart from a few studies at coastal sites [59–61], no data exist for the remaining 90 per cent of the area. There are even fewer winter primary productivity estimates and these are predominantly derived from near-shore locations [60,62]. While satellites have made a contribution to filling this gap (e.g. SeaWIFS), they are unable to produce chlorophyll or productivity estimates south of 50° S during the austral winter because of the high solar angles and insufficient light [60,63].
Micronutrients such as iron are now generally considered to limit primary productivity in much of the Southern Ocean in summer [64], but it is still thought that productivity is mostly light-limited in autumn and winter [65]. Significantly higher temperatures are likely to raise the requirement for light energy, mostly through higher respiration rates, and this will exacerbate the effect of light limitation. Respiration rates of phytoplankton are poorly known in any environment [32], and in the low-chlorophyll Southern Ocean waters community respiration rates (as measured by O2 uptake) can be high, and have been seen to periodically exceed photosynthesis (O2 production), making these waters (temporarily at least) a net source of CO2 rather than a sink [66]. This is presumably achieved through a high incidence of heterotrophy.
The shortage of winter data from the Arctic is equally dire. While there are more biomass measurements from spring and summer [67,68], winter data are almost non-existent. This has made it very difficult to model ecosystem response to climate forcing [65]. Unlike the Antarctic, which is surrounded by deep and narrow continental shelves, more than 60 per cent of Arctic seas are above relatively shallow continental shelves. As in the Antarctic, many areas north of the Polar Circle still receive diffuse surface light even though the sun does not rise above the horizon. Water depth is an important factor in maintaining phytoplankton stocks. Over shallow seas, convective cells are able to constantly transport sinking and settled cells back to the euphotic zone [8]. Even over deep water these convective processes in winter are thought to be responsible for maintaining a critical ‘inoculum’ of phytoplankton biomass and low levels of primary production within the water column [8].
It is clear from the low-chlorophyll biomass levels documented that the quantity of phytoplankton able to survive the Antarctic winter is extremely low. Very little, however, is known about the physiological state of this surviving inoculum, and winter survival of Southern Ocean phytoplankton is therefore likely to affect the capacity of this ecosystem to initiate both spring and summer blooms.
(e). Effect of increased temperatures on dark survival
There is likely to be an increase in global sea surface temperatures of at least 2°C by the end of the century [69], and this is likely to lead to a reduced spatial and temporal distribution of sea ice in polar areas [70]. For most of the year, seawater temperatures around Antarctica remain close to the freezing point of seawater, which is approximately −1.9°C. Because seawater temperatures cannot normally go lower than this threshold, most marine organisms will never be exposed to temperatures lower than this. However, with a loss of sea ice, Antarctic organisms are increasingly likely to be exposed to increased seawater temperatures. Currently, during winter, seawater temperatures around Antarctica remain close to freezing point, but as the sea ice disappears in the future they are likely to increase.
While the biomass of phytoplankton and sea ice algal communities at the end of winter are extremely low, they are able to grow strongly with the return of the sun [61]. When Antarctic sea ice and phytoplankton species were incubated at elevated temperatures in the dark, it was found that there was no significant difference in survival between ambient temperature and 6°C above ambient, but a significantly poorer outcome at 12° above ambient [36,71]. In these experiments there were no significant differences in the drawdown in energy storage products or recovery rates at any temperature. When Arctic phytoplankton collected from Tromsø Sound in northern Norway (70° N) in winter were incubated in the dark, the ambient and 6°C above ambient treatments recovered well, whereas the 14°C above ambient treatment failed to recover [71]. These experiments suggest that both Arctic and Antarctic phytoplankton are resilient when grown in the dark at temperatures up to 6°C greater than natural and are only seriously impaired when exposed to temperatures greater than this.
4. Conclusions
Microalgae are able to survive extended periods of darkness by using a diverse range of strategies. These include the production of resting spores and cysts, obtaining nutrition from alternative sources, using stored energy reserves and reducing their metabolic rates. Cells naturally experience darkness of varying length as part of the diurnal cycle (i.e. night), through burial in sediments, deep ocean mixing and as part of seasonal cycles, such as the polar night. While most polar microalgae are able to survive periods of darkness, exposure to elevated temperatures in the dark reduces their viability. This reduction only occurs at temperatures that are unlikely to be experienced in a few centuries as a result of climate change.
References
- 1.Lundholm N, Ribeiro S, Andersen TJ, Koch T, Godhe A, Ekelund F, Ellegaard M. 2011. Buried alive: germination of up to a century-old marine protist resting stages. Phycologia 50, 629–640 10.2216/11-16.1 (doi:10.2216/11-16.1) [DOI] [Google Scholar]
- 2.Britt AB. 1996. DNA damage and repair in plants. Ann. Rev. Physiol. Plant Mol. Biol. 47, 75–100 10.1146/annurev.arplant.47.1.75 (doi:10.1146/annurev.arplant.47.1.75) [DOI] [PubMed] [Google Scholar]
- 3.Durban E, Grecz N, Farkas J. 1974. Direct enzymatic repair of deoxyribonucleic acid single-strand breaks in dormant spores. J. Bacteriol. 118, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk JTO. 2011. Light and photosynthesis in aquatic ecosystems, 3rd edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Smayda TJ, Mitchell-Innes B. 1974. Dark survival of autotrophic planktonic marine diatoms. Mar. Biol. 25, 195–202 10.1007/BF00394965 (doi:10.1007/BF00394965) [DOI] [Google Scholar]
- 6.Zhang Q, Gradinger R, Zhou Q. 2003. Competition within the marine microalgae over the polar dark period in the Greenland Sea of high Arctic. Acta Oceanol. Sin. 22, 233–242 [Google Scholar]
- 7.Popels LC, Hutchins DA. 2002. Factors affecting dark survival of the brown tide alga Aureococcus anophagefferens (Pelagophyceae). J. Phycol. 38, 738–744 10.1046/j.1529-8817.2002.01115.x (doi:10.1046/j.1529-8817.2002.01115.x) [DOI] [Google Scholar]
- 8.Backhaus JO, Hegseth EN, Wehde H, Irigoien X, Hatten K, Logemann K. 2003. Convection and primary production in winter. Mar. Ecol. Prog. Ser. 251, 1–14 10.3354/meps251001 (doi:10.3354/meps251001) [DOI] [Google Scholar]
- 9.McGillicuddy DJ, Anderson DM, Lynch DR, Townsend DW. 2005. Mechanisms regulating large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: results from a physical–biological model. Deep Sea Res. II 52, 2698–2714 10.1016/j.dsr2.2005.06.021 (doi:10.1016/j.dsr2.2005.06.021) [DOI] [Google Scholar]
- 10.Lewis J, Harris ASD, Jones KJ, Edmonds RL. 1999. Long term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. J. Plankton Res. 21, 343–354 10.1093/plankt/21.2.343 (doi:10.1093/plankt/21.2.343) [DOI] [Google Scholar]
- 11.Huber G, Nipkow F. 1923. Experimentelle utersuchungen uber die entwicklung und formbildung von Ceratium hirundinella O.F.M. Flora New Ser. 16, 114–215 [Google Scholar]
- 12.Kaefer BA, Buesseler KO, Anderson DM. 1992. Burial of living dinoflagellate cysts in estuarine and nearshore sediments. Mar. Micropaleontol. 20, 147–161 10.1016/0377-8398(92)90004-4 (doi:10.1016/0377-8398(92)90004-4) [DOI] [Google Scholar]
- 13.Binder BI, Anderson DM. 1987. Physiological and environmental-control of germination in Scrippsiella trochoidea (Dinophyceae) resting cysts. J. Phycol. 23, 99–107 10.1111/j.1529-8817.1987.tb04431.x (doi:10.1111/j.1529-8817.1987.tb04431.x) [DOI] [Google Scholar]
- 14.Doucette GI, Fryxell GA. 1983. Thalassiosira antarctica: vegetative and resting stage chemical composition of an ice-related marine diatom. Mar. Biol. 78, 1–6 10.1007/BF00392964 (doi:10.1007/BF00392964) [DOI] [Google Scholar]
- 15.Figueroa RI, Vazquez JA, Massanet A, Murado MA, Bravo I. 2011. Interactive effects of salinity and temperature on planozygote and cyst formation of Alexandrium minutum (Dinophyceae) in culture. J. Phycol. 47, 13–24 10.1111/j.1529-8817.2010.00937.x (doi:10.1111/j.1529-8817.2010.00937.x) [DOI] [PubMed] [Google Scholar]
- 16.Oku O, Kamatani A. 1999. Resting spore formation and biochemical composition of the marine planktonic diatom Chaetoceros pseudocurvisetus in culture: ecological significance of decreased nucleotide content and activation of the xanthophyll cycle by resting spore formation. Mar. Biol. 135, 425–436 10.1007/s002270050643 (doi:10.1007/s002270050643) [DOI] [Google Scholar]
- 17.Balzar I. 1996. Encystment of Gonyaulax polyedra: dependence on light. Biol. Rhythm Res. 27, 386–389 10.1076/brhm.27.3.386.12961 (doi:10.1076/brhm.27.3.386.12961) [DOI] [Google Scholar]
- 18.Rintala J-M, Spilling K, Blomster J. 2007. Temporary cyst enables long-term dark survival of Scrippsiella hangoei (Dinophyceae). Mar. Biol. 152, 57–62 10.1007/s00227-007-0652-x (doi:10.1007/s00227-007-0652-x) [DOI] [Google Scholar]
- 19.Neilson AH, Lewin RA. 1974. The uptake and utilization of organic carbon by algae; an essay in comparative biochemistry. Phycologia 13, 227–264 10.2216/i0031-8884-13-3-227.1 (doi:10.2216/i0031-8884-13-3-227.1) [DOI] [Google Scholar]
- 20.Tuchman NC, Schollett MA, Rier ST, Geddes P. 2006. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 561, 167–177 10.1007/s10750-005-1612-4 (doi:10.1007/s10750-005-1612-4) [DOI] [Google Scholar]
- 21.Palmisano AC, Sullivan CW. 1985. Pathways of photosynthetic carbon assimilation in sea-ice microalgae from McMurdo Sound, Antarctica. Limnol. Oceanogr. 30, 674–678 10.4319/lo.1985.30.3.0674 (doi:10.4319/lo.1985.30.3.0674) [DOI] [Google Scholar]
- 22.Rivkin RB, Putt M. 1987. Heterotrophy and photoheterotrophy by Antarctic microalgae: light-dependent incorporation of amino acids and glucose. J. Phycol. 23, 442–452 10.1111/j.1529-8817.1987.tb02530.x (doi:10.1111/j.1529-8817.1987.tb02530.x) [DOI] [Google Scholar]
- 23.Laybourn-Parry J, Marshall WA, Marchant HJ. 2005. Flagellate nutritional versatility as a key to survival in two contrasting Antarctic saline lakes. Freshwater Biol. 50, 830–838 10.1111/j.1365-2427.2005.01369.x (doi:10.1111/j.1365-2427.2005.01369.x) [DOI] [Google Scholar]
- 24.Jochem FJ. 1999. Dark survival strategies in marine phytoplankton assessed by cytometric measurement of metabolic activity with fluorescein diacetate. Mar. Biol. 135, 721–728 10.1007/s002270050673 (doi:10.1007/s002270050673) [DOI] [Google Scholar]
- 25.Fryxell GA. 1989. Marine phytoplankton at the Weddell Sea ice edge: seasonal changes at the specific level. Polar Biol. 10, 1–18 10.1007/BF00238285 (doi:10.1007/BF00238285) [DOI] [Google Scholar]
- 26.McKnight DM, Howes BL, Taylor CD, Goehringer DD. 2000. Phytoplankton dynamics in a stably stratified Antarctic lake during winter darkness. J. Phycol. 36, 852–861 10.1046/j.1529-8817.2000.00031.x (doi:10.1046/j.1529-8817.2000.00031.x) [DOI] [Google Scholar]
- 27.Mock T, Gradinger R. 2000. Changes in photosynthetic carbon allocation in algal assemblages of Arctic sea ice with decreasing nutrient concentrations and irradiance. Mar. Ecol. Prog. Ser. 202, 1–11 10.3354/meps202001 (doi:10.3354/meps202001) [DOI] [Google Scholar]
- 28.Madariaga I. 2002. Short-term variations in the physiological state of phytoplankton in a shallow temperate estuary. Hydrobiologia 475, 345–358 10.1023/A:1020391425989 (doi:10.1023/A:1020391425989) [DOI] [Google Scholar]
- 29.Smith REH, Clement P, Head EJ. 1990. Night metabolism of recent photosynthate by sea ice algae in the high Arctic. Mar. Biol. 107, 255–261 10.1007/BF01319824 (doi:10.1007/BF01319824) [DOI] [Google Scholar]
- 30.Lindqvist K, Lignell R. 1997. Intracellular partitioning of 14CO2 in phytoplankton during a growth season in the northern Baltic. Mar. Ecol. Prog. Ser. 152, 41–50 10.3354/meps152041 (doi:10.3354/meps152041) [DOI] [Google Scholar]
- 31.Fernandez E, De Madariaga I, Serret P. 1991. Photosynthate partitioning by natural phytoplankton populations in a shallow coastal front. Sci. Mar. 55, 599–604 [Google Scholar]
- 32.Falkowski PG, Raven JA. 1998. Aquatic photosynthesis. London, UK: Blackwell Scientific [Google Scholar]
- 33.Luder UH, Wiencke C, Knoetzel J. 2002. Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipens (Rhodophyta): a study to simulate Antarctic winter sea ice cover. J. Phycol. 38, 904–913 10.1046/j.1529-8817.2002.t01-1-01071.x (doi:10.1046/j.1529-8817.2002.t01-1-01071.x) [DOI] [Google Scholar]
- 34.Starr G, Oberbauer SF. 2003. Photosynthesis of Arctic evergreens under snow: implications for tundra ecosystem carbon balance. Ecology 84, 1415–1420 10.1890/02-3154 (doi:10.1890/02-3154) [DOI] [Google Scholar]
- 35.Tilzer MM, Dobinsky Z. 1987. Effects of temperature and day length on the mass balance of Antarctic phytoplankton. Polar Biol. 7, 35–42 10.1007/BF00286822 (doi:10.1007/BF00286822) [DOI] [Google Scholar]
- 36.Reeves S, McMinn A, Martin A. 2011. The effect of prolonged darkness on the growth, recovery and survival of Antarctic sea ice diatoms. Polar Biol. 34, 1019–1032 10.1007/s00300-011-0961-x (doi:10.1007/s00300-011-0961-x) [DOI] [Google Scholar]
- 37.Singh A, Nigam PS, Murphy JD. 2011. Mechanism and challenges in commercialisation of algal biofuels. Bioresour. Technol. 102, 26–34 10.1016/j.biortech.2010.06.057 (doi:10.1016/j.biortech.2010.06.057) [DOI] [PubMed] [Google Scholar]
- 38.Suzuki L, Johnson CH. 2001. Algae know the time of day: circadium and photoperiodic programs. J. Phycol. 37, 933–942 10.1046/j.1529-8817.2001.01094.x (doi:10.1046/j.1529-8817.2001.01094.x) [DOI] [Google Scholar]
- 39.Schubert H, Gerbersdorf S, Titlyanov E, Titlyanova T, Granbom M, Pape C, Luning K. 2004. Circadian rhythm of photosynthesis in Kappaphycus alvarezii (Rhodophyta): independence of the cell cycle and possible photosynthetic clock targets. Eur. J. Phycol. 39, 423–430 10.1080/09670260400009924 (doi:10.1080/09670260400009924) [DOI] [Google Scholar]
- 40.Kuhl M, Lassen C, Jorgensen BB. 1994. Light penetration and light intensity in sandy marine-sediments measured with irradiance and scalar irradiance fiberoptic microprobes. Mar. Ecol. Prog. Ser. 105, 139–148 10.3354/meps105139 (doi:10.3354/meps105139) [DOI] [Google Scholar]
- 41.Ichimi K, Tada K, Montani S. 2008. Simple estimation of penetration rate of light in inter-tidal sediments. J. Oceanogr. 64, 399–404 10.1007/s10872-008-0033-1 (doi:10.1007/s10872-008-0033-1) [DOI] [Google Scholar]
- 42.Veuger B, van Oevelen D. 2011. Long-term pigment dynamics and diatom survival in dark sediment. Limnol. Oceanogr. 56, 1065–1074 10.4319/lo.2011.56.3.1065 (doi:10.4319/lo.2011.56.3.1065) [DOI] [Google Scholar]
- 43.Cullen JJ. 1982. The deep chlorophyll maximum: comparing vertical profiles of chlorophyll a. Can. J. Fish. Aquat. Sci. 39, 791–803 10.1139/f82-108 (doi:10.1139/f82-108) [DOI] [Google Scholar]
- 44.Kamp A, deBeer D, Nitsch JL, Lavik G, Stief P. 2011. Diatoms respire nitrate to survive dark and anoxic conditions. Proc. Natl Acad. Sci. USA 108, 5649–5654 10.1073/pnas.1015744108 (doi:10.1073/pnas.1015744108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paluszkiewicz T, Garwood RW, Denbo DW. 1994. Deep convection plumes in the ocean. Oceanography 7, 37–44 10.5670/oceanog.1994.01 (doi:10.5670/oceanog.1994.01) [DOI] [Google Scholar]
- 46.Murphy AM, Cowles TJ. 1997. Effects of darkness on multi-excitation in vivo fluorescence and survival in a marine diatom. Limnol. Oceanogr. 42, 1444–1453 10.4319/lo.1997.42.6.1444 (doi:10.4319/lo.1997.42.6.1444) [DOI] [Google Scholar]
- 47.McMinn A, Ashworth C, Ryan K. 1999. Growth and productivity of Antarctic sea ice algae under PAR and UV irradiances. Botanica Marina 42, 401–407 10.1515/BOT.1999.046 (doi:10.1515/BOT.1999.046) [DOI] [Google Scholar]
- 48.Peters E, Thomas DN. 1996. Prolonged darkness and diatom mortality. I. Marine Antarctic species. J. Exp. Mar. Biol. Ecol. 207, 25–41 10.1016/S0022-0981(96)02520-8 (doi:10.1016/S0022-0981(96)02520-8) [DOI] [Google Scholar]
- 49.Palmisano AC, Sullivan CW. 1983. Physiology of sea ice diatoms. 2. Dark survival of three polar diatoms. Can. J. Microbiol. 29, 157–158 10.1139/m83-026 (doi:10.1139/m83-026) [DOI] [Google Scholar]
- 50.Bunt JS, Lee CC. 1972. Date on the composition and dark survival of four sea ice microalgae. Limnol. Oceanogr. 17, 458–461 10.4319/lo.1972.17.3.0458 (doi:10.4319/lo.1972.17.3.0458) [DOI] [Google Scholar]
- 51.Hoban MA, Fryxell GA, Buck KR. 2008. Biddulphioid diatoms: resting spores in Antarctic Eucampia and Odontella. J. Phycol. 16, 591–602 10.1111/j.1529-8817.1980.tb03078.x (doi:10.1111/j.1529-8817.1980.tb03078.x) [DOI] [Google Scholar]
- 52.Thomson P, McMinn A, Kiessling I, Watson M, Goldsworthy P. 2006. Composition and succession of dinoflagellates and chrysophytes in the upper fast ice of Davis Station, East Antarctica. Polar Biol. 29, 337–345 10.1007/s00300-005-0060-y (doi:10.1007/s00300-005-0060-y) [DOI] [Google Scholar]
- 53.Palmisano AC, Sullivan CW. 1982. Physiology of sea ice diatoms. I. Response of three polar diatoms to a simulated summer–winter transition. J. Phycol. 18, 489–498 10.1111/j.1529-8817.1982.tb03215.x (doi:10.1111/j.1529-8817.1982.tb03215.x) [DOI] [Google Scholar]
- 54.Priddle J, Heywood RB. 1980. Evolution of Antarctic lake ecosystems. Biol. J. Linn. Soc. 14, 51–66 (doi:10.1111/j.1095-8312.1980.tb00097.x) [Google Scholar]
- 55.Garrison DL, Buck KR, Gowing MM. 1991. Plankton assemblages in the ice edge zone of the Weddell Sea during the austral winter. J. Mar. Syst. 2, 123–130 10.1016/0924-7963(91)90018-P (doi:10.1016/0924-7963(91)90018-P) [DOI] [Google Scholar]
- 56.Garrison DL, Close AR. 1993. Winter ecology of the sea ice biota in Weddell Sea pack ice. Mar. Ecol. Prog. Ser. 96, 17–31 10.3354/meps096017 (doi:10.3354/meps096017) [DOI] [Google Scholar]
- 57.Satoh H, Watanabe K. 1988. Primary productivity in the fast ice area near Syowa Station, Antarctica, during spring and summer 1983/84. J. Oceanogr. 44, 287–292 10.1007/BF02302571 (doi:10.1007/BF02302571) [DOI] [Google Scholar]
- 58.Scharek R, Smetacek V, Fahrbach E, Gordon LI, Rohardt G, Moore S. 1994. The transition from winter to early spring in the eastern Weddell Sea, Antarctica: plankton biomass and composition in relation to hydrography and nutrients. Deep Sea Res. I 41, 1231–1250 10.1016/0967-0637(94)90042-6 (doi:10.1016/0967-0637(94)90042-6) [DOI] [Google Scholar]
- 59.Dayton PK, Watson D, Palmisano A, Barry JP, Oliver JS, Rivera D. 1986. Distribution patterns of benthic microalgal standing stock at McMurdo Sound, Antarctica. Polar Biol. 6, 207–213 10.1007/BF00443397 (doi:10.1007/BF00443397) [DOI] [Google Scholar]
- 60.Smith RC, Baker KS, Byers ML, Stammerjohn SE. 1998. Primary productivity of the Palmer long term ecological research area and the Southern Ocean. J. Mar. Syst. 17, 245–259 10.1016/S0924-7963(98)00041-4 (doi:10.1016/S0924-7963(98)00041-4) [DOI] [Google Scholar]
- 61.McMinn A, Martin A, Ryan KG. 2010. Phytoplankton and sea ice biomass and physiology during the transition between winter and spring (McMurdo Sound, Antarctica). Polar Biol. 33, 1547–1566 10.1007/s00300-010-0844-6 (doi:10.1007/s00300-010-0844-6) [DOI] [Google Scholar]
- 62.Palmisano AC, Soohoo JB, Soohoo SL, Kottmeier ST, Craft LL, Sullivan CW. 1986. Photoadaptation in Phaeocystis pouchetii advected beneath annual sea ice in McMurdo Sound, Antarctica. J. Plankton Res. 8, 891–906 10.1093/plankt/8.5.891 (doi:10.1093/plankt/8.5.891) [DOI] [Google Scholar]
- 63.Moore JK, Abbott MR. 2000. Phytoplankton chlorophyll distributions and primary production in the Southern Ocean. J. Geophys. Res. 105, 28 709–28 722 10.1029/1999JC000043 (doi:10.1029/1999JC000043) [DOI] [Google Scholar]
- 64.Timmermans KR, Davey MS, van der Wagt B, Snoek J, Geider RJ, Veldhuis MJW, Gerringa LJA, de Baar HJW. 2001. Co-limitation by iron and light of Chaetoceros brevis, C. dichaeta and C. calcitrans (Bacillariophyceae). Mar. Ecol. Prog. Ser. 217, 287–297 10.3354/meps217287 (doi:10.3354/meps217287) [DOI] [Google Scholar]
- 65.van Oijen T, van Leeuwe MA, Granum E, Weissing FJ, Bellerby RGJ, Gieskes WWC, de Baar HJW. 2004. Light rather than iron controls photosynthate production and allocation in Southern Ocean phytoplankton populations during austral autumn. J. Plankton Res. 26, 885–900 10.1093/plankt/fbh088 (doi:10.1093/plankt/fbh088) [DOI] [Google Scholar]
- 66.Agusti S, Satta MP, Mura MP. 2004. Summer community respiration and pelagic metabolism in upper surface Antarctic waters. Aquat. Microb. Ecol. 35, 197–205 10.3354/ame035197 (doi:10.3354/ame035197) [DOI] [Google Scholar]
- 67.Hill V, Cota G. 2005. Spatial patterns of primary production on the shelf, slope and basin of the western Arctic in 2002. Deep Sea Res. II 52, 2244–3354 10.1016/j.dsr2.2005.10.001 (doi:10.1016/j.dsr2.2005.10.001) [DOI] [Google Scholar]
- 68.Hegseth EN. 1998. Primary production in the northern Barents Sea. Polar Res. 17, 113–123 10.1111/j.1751-8369.1998.tb00266.x (doi:10.1111/j.1751-8369.1998.tb00266.x) [DOI] [Google Scholar]
- 69.IPCC 2007. Climate change 2007: synthesis report. In Contribution of Working Groups I, II and III to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri RK, Reisinger A.), p. 104 Geneva, Switzerland: IPCC [Google Scholar]
- 70.Turner J, Colwell SR, Marshall GJ, Lachlan-Cope TA, Carleton AM, Jones PD, Lagun V, Reid PA, Iagovkina S. 2005. Antarctic climate change during the last 50 years. J. Climatol. 25, 279–294 10.1002/joc.1130 (doi:10.1002/joc.1130) [DOI] [Google Scholar]
- 71.Martin A, McMinn A, Heath M, Hegseth EN, Ryan KG. 2012. The physiological response to increased temperature in over-wintering sea-ice algae and phytoplankton in McMurdo Sound, Antarctica and Tromsø, Norway. J. Exp. Mar. Biol. Ecol. 428, 57–66 10.1016/j.jembe.2012.06.006 (doi:10.1016/j.jembe.2012.06.006) [DOI] [Google Scholar]


