Abstract
Because most species in an ecological assemblage are rare, much of the species richness we value is due to taxa with few individuals or a restricted distribution. It has been apparent since the time of ecological pioneers such as Bates and Darwin that tropical systems have disproportionately large numbers of rare species, yet the distribution and abundance patterns of these species remain largely unknown. Here, we examine the diversity of freshwater fish in a series of lakes in the Amazonian várzea, and relate relative abundance, both as numbers of individuals and as biomass, to the occurrence of species in space and time. We find a bimodal relationship of occurrence that distinguishes temporally and spatially persistent species from those that are infrequent in both space and time. Logistic regression reveals that information on occurrence helps distinguish those species that are rare in this locality but abundant elsewhere, from those that are rare throughout the region. These results form a link between different approaches used to evaluate commonness and rarity. In doing so, they provide a tool for identifying species of high conservation priority in poorly documented but species rich localities.
Keywords: species abundance distribution, occurrence, várzea, freshwater fish, rare biosphere, Amazon
1. Introduction
In 1863, Bates [1] noted that while the density of butterflies in the Amazon was similar to that in UK, the number of species represented by those individuals was vastly greater. Growing concern about the state of the world's biological diversity, much of which is found in tropical regions, underlines the need to learn more about the structure of these diverse assemblages, the majority of which are poorly documented [2,3]. However, we do know that much of this unknown diversity will be comprised of rare taxa. The universal pattern in species abundance distributions is that both rare and common species are found in every community [4], with the fraction of rare species increasing in rich habitats. Indeed, surveys of tropical arthropod assemblages routinely find that many species are present in a sample only as singletons or represented by a few individuals [5,6]. This preponderance of rare species raises particular problems from a conservation standpoint. Because rarity is thought to increase the risk of extinction, either through demographic stochasticity or because species that occupy a restricted habitat are vulnerable if it is modified, being rare is a factor than can be taken into account in decisions to list threatened species [7] or in developing local management plans. On the other hand, if most species are rare it is difficult to know which ones to prioritize. Moreover, while average local abundance tends to be higher for species that occur in many sites [8–10], species that are rare in one locality are not necessarily scarce everywhere because their abundance may be depressed as a result of local conditions (e.g. unsuitable habitat) [11] or through random chance. An important challenge, therefore, for researchers and conservationists working in species rich but poorly documented localities, is to distinguish species that are rare throughout their range from those that just happen to be rare in a particular site.
To address this issue, we need to learn more about the structure of tropical assemblages—particularly those that have been relatively little studied. While a comprehensive evaluation of tropical biodiversity would be an immense and impracticable task [12,13], we do know that different types of diversity pattern(s) are linked [14–17]. McGill [16], for instance, points out that extent and abundance tend to covary, meaning that there will typically be some species that are common in terms of range size, occupancy and abundance, and others that are rare by the same metrics. He dubs this the ‘common is common’ pattern. By the same token, information on different aspects of rarity could help distinguish species that have low abundance in the region as a whole—the ‘rare throughout’ species—from those that happen to be rare in a particular locality or habitat—the ‘rare locally’ species.
Here, we examine the diversity of fish in lakes within the Amazonian flooded forest (várzea) to ask how the pattern of species occurrence, in space and in time, is linked to the relative abundance of species. Occurrence is a measure of the number of sites or samples that a species is present in [17]. A bimodal or U-shaped relationship of species occurrence is often seen if the quadrats used to sample an assemblage are divided into bins, representing species that are progressively more widespread [16,18]. Interestingly, a similar bimodal pattern of species occurrence appears if data are accumulated at the same place through time [19]. Given the extent to which autocorrelation influences spatial patterns of biodiversity [16], it seems likely that species that occur frequently in space are the same as those that are persistent through time. We therefore predict that if we track species simultaneously over space and time, we will detect a mode of species infrequent in both space and time, and a second mode of species that are prevalent in both space and time. This pattern will be strongest at local scales, where behaviour and ecology promote clumping of individuals. Species abundance distributions on the other hand tend to be unimodal (but see [20])—often resembling a lognormal. However, Preston [21] asserted that species abundance and species occurrence distributions are different expressions of the same underlying pattern. We therefore argue that species abundance distributions can be deconstructed to reveal ordered groupings of species that are increasingly persistent in space or time. Finally, we propose that species that occur in the ‘persistent’ mode, yet have low numerical abundance, are more likely to be in the ‘rare locally’ than in the ‘rare throughout’ category. We suggest that these ‘signatures’ of rarity can be a useful aid to researchers characterizing the diversity of rich assemblages and to managers charged with conserving these systems.
We test these ideas using data collected during an intensive survey of fish communities in five small lakes (figure 1) sampled monthly for 18 months. This highly seasonal habitat is inundated for around 6 months per year [22]. Very few of the fish species found here have had their conservation status formally evaluated (see the electronic supplementary material, S1).
Figure 1.
Study area (with sampled lakes numbered 1–5).
2. Material and methods
(a). Site description
This study was carried out at the Mamirauá Sustainable Development Reserve (MSDR), in the Brazilian Central Amazon floodplain (figure 1), a wetland site of international importance recognized under the UN Ramsar Convention [23]. Mamirauá Reserve is the largest protected area of flooded forests—known as várzea—in Brazil. The reserve's 1 124 000 ha are completely flooded for 3 to 6 months every year during the flooding pulse. During this time, the water level varies by more than 10 m [22]. Four seasons can be identified, based on water level monitoring since 1990 [17]. These are (i) the cheia, or high water season, from May until mid-July; (ii) the vazante or falling water season from mid-July to September; (iii) the seca or low water season in the months of September, October and November; and (iv) and the enchente or rising water season from December to April.
The mosaic of forests [24] and water bodies, lakes and channels, at Mamirauá is typical of the várzea environment (figure 1). These water bodies support a large fish fauna [25], with diverse communities in each of the major aquatic habitats present [26].
Floating meadows form an important aquatic habitat at Mamirauá Reserve [26,27] and vary in extent as the water level rises and falls [28]. Although these meadows can be highly diverse, they are usually dominated by a small number of plant species. The more abundant grass and macrophyte species found at Mamirauá are the Poaceae Paspalum repens (among other Paspalum species), Echinochloa polystachia, the Potenderiaceae Eichornia crassipes, the Araceae Pistia stratiotes, the Azollaceae Azolla spp. and the Salviniaceae Salvinia minima [26].
(b). Sampling
Five lakes in Mamirauá Reserve (lakes Araçazinho, Juruá Grande, Juruazinho, Pagão and Tracajá; figure 1) were sampled monthly in the period from September 2003 to March 2005. The lakes are inundated during the high water season, but during the dry season, it is possible to calculate lake size; this varied from 11.1 to 102.3 ha. Each sample was made up of five sample units. These units consisted of 16 m2 of vegetation which was separated from the larger blocks of floating meadows, surrounded by a seine net (multifilament; 2 mm mesh size, 30 m long and 6 m wide), and then lifted into a boat. All plants were removed from the net and returned to the sample site. All fish were then collected and transported to the field laboratory in buckets where they were identified, measured and weighted. Whenever possible, fish were returned alive to the local water bodies, but when identification was not possible in the field, they were humanely killed and taken to the laboratory at Tefé, the nearest town in Amazonas State. In such cases, fish specimens were preserved in a 10 per cent formalin solution for transportation, and transferred to a 70 per cent alcohol solution in the laboratory. Total effort, which was consistent throughout, was 400 m2 of floating meadow per month across all five lakes. Fish were identified using the literature including [29–32] and the fish collection at Mamirauá Institute.
(c). Data analysis
In line with [33], we defined species as rare if they had less than 1 per cent of the total number of individuals overall. This gave 20 common species and 145 rare species and coincided with an inflection point in the rank abundance plot of the assemblage (figure 2). Rare species were then assigned to the categories ‘rare locally’ (i.e. those rare in these samples and thus in the floating meadow habitat in lakes, but frequently encountered elsewhere in the reserve) and ‘rare throughout’ (i.e. rare in the other aquatic habitats present in the reserve). To do this, we assembled data that had been collected during extensive routine surveys in six other habitat types within the reserve. These habitats were: open water; banks and margins; flooded forest; floating meadow within channels; forest temporary pools; and submerged dead trees. We were able to extract information on both the presence and relative abundance of 142 out of our 145 rare species in each of the other habitats (three species were excluded from the analysis owing to incomplete information). We used these independent data on the distribution and abundance of the species elsewhere in the reserve to distinguish between the two types of rare fish in our lake floating meadow fish. To explore how our conclusions are influenced by the way the distinction between ‘rare locally’ and ‘rare throughout’ is reached, we repeated this categorization four times using different criteria. The definitions of ‘rare locally’ adopted were (i) present in at least two other habitat types (n = 63 ‘rare locally’ species); (ii) present in at least three other habitat types (n = 41 ‘rare locally’ species); (iii) present in at least two other habitat types OR ≥ 0.5 per cent of total abundance when present anywhere (n = 74 ‘rare locally’ species); (iv) present in at least two other habitat types OR ≥ 1 per cent of total abundance when present anywhere (n = 69 ‘rare locally’ species). For more details, see electronic supplementary material, S2.
Figure 2.

Rank abundance plot (relative abundance of species in relation to species rank) of the várzea lake fish assemblage. Species with more than 1% of total abundance (0.01—indicated by the dash on the y-axis) are designated common species.
Logistic regression was used to ask whether information on persistence can be used to deduce a fish's rarity status, in other words to help distinguish between species that are ‘rare locally’ and those that are ‘rare throughout’. Here, the response variable was status (‘rare locally’ or ‘rare throughout’) while the predictor variables were body size (log10 mean wet weight in grams), and persistence (either the number of lakes (1–5) or the number of seasons (1–4) a species was detected in). Because researchers will typically have either spatial or temporal data rather than both, lake number and season number were analysed separately. Lake number and season were treated as factors. We repeated the analysis for each of our four definitions of ‘rare locally’ species. Analyses were conducted in R [34].
3. Results
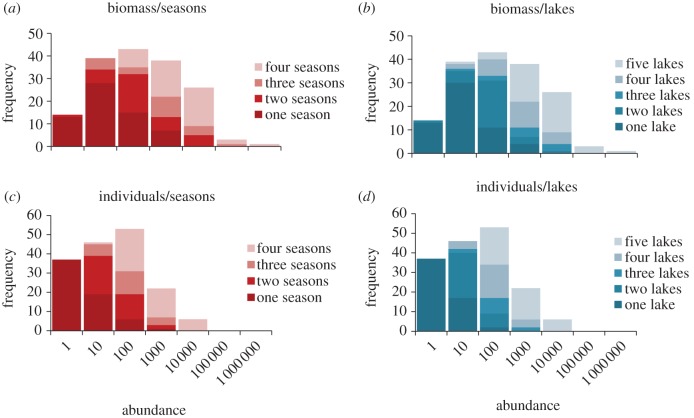
Sampling yielded greater than 20 000 individual fish belonging to 165 species in 99 genera and in 29 families. The distribution of biomass (figure 3) follows a typical lognormal distribution. Dominant species (with total biomass greater than 10 000 g) include the cichlids Cichla monochulus (peacock cichlid), Cichlasoma amazonarum (Amazon cichlid), Mesonauta insignis (flag cichlid) and Pterophyllum scalare (angelfish). The distribution of numerical abundance, on the other hand (figure 3), resembles a truncated lognormal with an excess of species in the low abundance classes. Thirty-seven species are represented only as singletons, whereas numerically abundant species (n > 1000) include both cichlids (Mesonauta insignis, Cichlasoma amazonarum) and characids (e.g. Pygocentrus nattereri (red-bellied piranha), Mylossoma duriventre (silver dollar), Moenkhausia intermedia (scissortail tetra) and Ctenobrycon spilurus (silver tetra)). For a list of species, see electronic supplementary material, S1.
Figure 3.
Species abundance distributions showing the frequency of species in log10 abundance classes for biomass (a,b) and numerical abundance (c,d). The legends indicate species that are present in one, two, three or four seasons, or one, two, three, four or five lakes.
Figure 4 reveals two distinct modes of occurrence, one of species found in a single season and lake, the other of species present in most lakes and most seasons. When these occurrence patterns are superimposed on the overall species abundance distributions (figure 3), it is clear that for both abundance currencies there is an ordered sequence of occurrence with increasingly persistent species progressively dominating the larger abundance classes.
Figure 4.

Frequency of species in relation to the number of lakes, and the number of seasons, in which they occur.
The logistic regression analysis indicates that the less frequently a rare species is detected in either space or time, the more likely it is to be ‘rare throughout’ the region. The analysis treated space (number of lakes) and time (number of seasons) as categorical values; their overall effect is shown in table 1. Table 1 also indicates that the different methods used to distinguish the ‘rare locally’ and ‘rare throughout’ species lead to similar conclusions indicating that local information on the occurrence of a species in either space or time is a robust indicator of its status. The details of the individual logistic regressions are provided in the electronic supplementary material, S3, which also graphs the predicted probabilities. From these graphs, it is clear that both the lake effect and season effect are stronger for species with larger body sizes. The distribution of body size is shown in the electronic supplementary material, S4. There is no interaction between body size and numbers of seasons or lakes. Overall, it appears that fish that are present in only one or two seasons, or fewer than four lakes, are the most likely to fall into the regionally rare group (see the electronic supplementary material, S3).
Table 1.
Wald test of the overall effect of space and time when predicting the probability that a species is ‘rare throughout’ rather than ‘rare locally’. The predictor values of interest here are the number of lakes or the number of seasons a species was present in during the investigation of lake floating meadow habitat; lake number and season number are treated as factors (see text for more details). The analysis, which is repeated for each of the definitions of ‘rare locally’ (see electronic supplementary material, S2), uses the aod package in R [35]. Full details of the logistic regression analyses are provided in electronic supplementary material, S3.
| definition of ‘rare locally’ | space (lakes) | time (seasons) |
|---|---|---|
| two habitats; n = 63 ‘rare locally’ species | χ2 = 12.3, d.f. = 4, p = 0.015 | χ2 = 13.7, d.f. = 3, p = 0.003 |
| three habitats; n = 41 ‘rare locally’ species | χ2 = 8.8, d.f. = 4, p = 0.067 | χ2 = 10.0, d.f. = 3, p = 0.018 |
| two habitats or ≥0.5%; n = 74 ‘rare locally’ species | χ2 = 14.3, d.f. = 4, p = 0.007 | χ2 = 11.9, d.f. = 3, p = 0.008 |
| two habitats or ≥1%; n = 69 ‘rare locally’ species | χ2 = 14.8, d.f. = 4, p = 0.005 | χ2 = 14.1, d.f. = 3, p = 0.003 |
4. Discussion
These results reveal, as expected, that many species of fish in this rich Amazonian system are rare, in terms of both numerical abundance and biomass. However, while the relative abundances of these species take the classic graded form from rare to common, resembling a typical ‘lognormal’ pattern (which is truncated in the case of numerical abundance), species occurrences fall into two clusters: a group of species that are infrequent in both space and time, and a group that is persistent in space and time. We can draw on this information to help distinguish between those species, which, while rare in terms of abundance in this habitat type and lake system, are common elsewhere, and those that are rare throughout. Because temporal and spatial occurrence are linked, information on either can be used to deduce species status.
This ability to pinpoint species that may be of particular conservation concern [7], and to discriminate between low abundance taxa is important, particularly in areas rich in biological diversity. Comprehensive surveys of diverse but little-studied systems require unrealistic inputs of time and resources [2]. Indeed, H.W. Bates's inventory of insects in the environs of Mamirauá, over 150 years ago, while far from complete, remains the best there is [12]. As we have shown, it is possible to make inferences about the status of species, and the likelihood that they are regionally rare, from small-scale surveys and using the types of data that field researchers typically gather. While this information could aid managers assessing species, particularly in the absence of more detailed data on range and local population size [36], the approach we have outlined here is potentially useful in other contexts too. For example, microbial communities are as yet very incompletely surveyed, and one of the biggest challenges facing researchers working in this area is characterizing the ‘rare biosphere’ [37]. Information on both occurrence (in space and/or time) and abundance could be used to distinguish transiently rare microbial taxa from those that are globally rare. Microbial systems, in the same way as the aquatic communities that inhabit the várzea, experience high levels of spatial and temporal turnover. When turnover is reduced, we would expect to see a lower ‘rare’ mode and a larger ‘common’ mode of occurrence, but that the overall patterns would be conserved [19]. Turnover will also influence the degree of spatial and temporal autocorrelation in a system [16].
Our findings have other implications. For example, the occurrence data reveal how a species abundance distribution is constructed by overlays of species that are increasingly persistent in time, and in space. Species abundance distributions are one of the oldest methods of examining diversity data, and provide a clear means of describing and comparing communities [4,38]. However, there is still considerable debate about why species abundance distributions take the form they do, and how they are shaped by the currency used to measure abundance [39]. Deconstructing species abundance distributions provides a means of examining the mechanisms that underpin them [4]. Our results show that species are ordered by occurrence with infrequent species predominant at the rare end of the distribution. Interestingly, we found that this pattern is the same for both numerical abundance, and biomass, and whether the distributions are assembled using temporal or spatial data. This knowledge will aid attempts to predict species abundances from occurrence data [40].
Although infrequent and persistent species are ordered in the same way in species abundance distributions that use the alternative currencies of numerical abundance and biomass, the form of these distributions differs. When biomass is plotted, the distribution is unimodal and close to a textbook representation of a lognormal distribution. By contrast, when abundance is measured as numbers of individuals, the distribution is asymmetric with an excess of species in the low abundance classes. This pattern is similar to the one that Connolly et al. [41] detected when assessing the diversity of marine fish and corals. Connolly and co-workers argued that it is only at very large scales (ca 10 000 km) that lognormal distributions of numerical abundance are fully unveiled. At the scale of the local community, differences in the shape of the species abundance distributions of biomass and numerical abundance can be explained by body size and the utilization of the spatial niche [42]. Resource allocations will primarily involve the persistent taxa—species that are usually present and generally more abundant. However, we suggest that the structural complexity of the floating meadow habitat [43,44] enables rare species to persist and this, combined with the cyclical inundation regime, contributes to high turnover that underpins the diversity of this system.
The observed separation of infrequent and persistent species resonates with Hanski's [45] core-satellite hypothesis. However, the core-satellite hypothesis predicts relatively rapid shifts in abundance so that a rare species may become a core one, and vice versa [46]. Our data imply that temporal and spatial rarity are linked, though the relatively short-term nature of the sampling does not provide a definitive test of this. Nonetheless, other investigations, where species have been tracked through decades, suggest that core species maintain their abundance and rank through time [47–49].
To date, most emphasis has been on spatial patterns of biological diversity. Growing concern about the fate of the world's biota underlines the need to understand and measure how ecological communities change through time [50]. Although temporal and spatial data can have different characteristics (such as the extent to which datasets are bounded) ‘space for time’ substitution is a tool used to interpret biodiversity patterns [51]. Our results illustrate how this substitution can work and underline the links between different patterns of diversity [16].
Acknowledgements
We thank Jonas Oliveira, Rose Chaves, Marilia Santos and Mauricio Zorro for their support in fish collection in the field and identification in the laboratory. Patrícia Fracarolli Canholi also helped in the processing of the samples. The Mamirauá Institute provided logistics, infrastructure and part of the funding. We are grateful to the reviewers and editors for their helpful advice. A.E.M. acknowledges support from the European Research Council (project BioTIME 250189), and thanks Maria Dornelas for stimulating discussions on temporal diversity patterns.
References
- 1.Bates H. 1863. The naturalist on the river Amazons. London, UK: John Murray [Google Scholar]
- 2.Lawton JH, et al. 1998. Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature 391, 72–76 10.1038/34166 (doi:10.1038/34166) [DOI] [Google Scholar]
- 3.Comita LS, Muller-Landau HC, Aguilar S, Hubbell SP. 2010. Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329, 330–332 10.1126/science.1190772 (doi:10.1126/science.1190772) [DOI] [PubMed] [Google Scholar]
- 4.McGill BJ, et al. 2007. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015 10.1111/j.1461-0248.2007.01094.x (doi:10.1111/j.1461-0248.2007.01094.x) [DOI] [PubMed] [Google Scholar]
- 5.Coddington JA, Agnarsson I, Miller JA, Kuntner M, Hormiga G. 2009. Undersampling bias: the null hypothesis for singleton species in tropical arthropod surveys. J. Anim. Ecol. 78, 573–584 10.1111/j.1365-2656.2009.01525.x (doi:10.1111/j.1365-2656.2009.01525.x) [DOI] [PubMed] [Google Scholar]
- 6.Novotny V, Basset Y. 2000. Rare species in communities of tropical insect herbivores: pondering the mystery of singletons. Oikos 89, 564–572 10.1034/j.1600-0706.2000.890316.x (doi:10.1034/j.1600-0706.2000.890316.x) [DOI] [Google Scholar]
- 7.Mace G, Collar N, Gaston K, Hilton-Taylor C, Akcakaya H, Leader-Willimas N, Milner-Gulland E, Stuart S. 2008. Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv. Biol. 22, 1424–1442 10.1111/j.1523-1739.2008.01044.x (doi:10.1111/j.1523-1739.2008.01044.x) [DOI] [PubMed] [Google Scholar]
- 8.Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- 9.Brown J. 1984. On the relationship between abundance and distribution of species. Am. Nat. 124, 255–279 10.1086/284267 (doi:10.1086/284267) [DOI] [Google Scholar]
- 10.Gaston K, Blackburn T, Greenwood J, Gregory R, Quinn R, Lawton J. 2000. Abundance–occupancy relationships. J. Appl. Ecol. 37(Suppl. 1), 39–59 10.1046/j.1365-2664.2000.00485.x (doi:10.1046/j.1365-2664.2000.00485.x) [DOI] [Google Scholar]
- 11.Rabinowitz D. 1981. Seven forms of rarity. In Biological aspects of rare plant conservation (ed. Synge H.), pp. 205–217 Chichester, UK: John Wiley [Google Scholar]
- 12.Magurran AE, Queiroz H. 2010. Evaluating tropical biodiversity: do we need a more refined approach? Biotropica 42, 537–539 10.1111/j.1744-7429.2010.00670.x (doi:10.1111/j.1744-7429.2010.00670.x) [DOI] [Google Scholar]
- 13.May RM. 2002. The future of biological diversity in a crowded world. Curr. Sci. 82, 1325–1331 [Google Scholar]
- 14.Blackburn TM, Gaston KJ. 2001. Linking patterns in macroecology. J. Anim. Ecol. 70, 338–352 10.1046/j.1365-2656.2001.00484.x (doi:10.1046/j.1365-2656.2001.00484.x) [DOI] [Google Scholar]
- 15.McGill BJ. 2010. Towards a unification of unified theories of biodiversity. Ecol. Lett. 13, 627–642 10.1111/j.1461-0248.2010.01449.x (doi:10.1111/j.1461-0248.2010.01449.x) [DOI] [PubMed] [Google Scholar]
- 16.McGill BJ. 2011. Linking biodiversity patterns by autocorrelated random sampling. Am. J. Bot. 98, 481–502 10.3732/ajb.1000509 (doi:10.3732/ajb.1000509) [DOI] [PubMed] [Google Scholar]
- 17.Gaston KJ, He F. 2011. Species occurrence and occupancy. In Biological diversity: frontiers in measurement and assessment (eds Magurran AE, McGill BJ.), pp. 141–151 Oxford, UK: Oxford University Press [Google Scholar]
- 18.Raunkaier C. 1934. Life forms and statistical plant geography. Oxford, UK: Oxford University Press [Google Scholar]
- 19.Williams CB. 1964. Patterns in the balance of nature and related problems in quantitative ecology. London, UK: Academic Press [Google Scholar]
- 20.Dornelas M, Connolly SR. 2008. Multiple modes in a coral species abundance distribution. Ecol. Lett. 11, 1008–1016 10.1111/j.1461-0248.2008.01208.x (doi:10.1111/j.1461-0248.2008.01208.x) [DOI] [PubMed] [Google Scholar]
- 21.Preston FW. 1948. The commonness, and rarity, of species. Ecology 29, 254–283 10.2307/1930989 (doi:10.2307/1930989) [DOI] [Google Scholar]
- 22.Ramalho EE, Macedo J, Vieira TM, Valsecchi J, Calvimontes J, Marmontel M, Queiroz HL. 2009. Ciclo hidrológico nos ambientes de várzea da Reserva de Desenvolvimento Sustentável Mamirauá—Médio Rio Solimões, Período de 1990 a 2008. Uakari 5, 61–87 [Google Scholar]
- 23.Magurran A. 2009. Threats to freshwater fish. Science 325, 1215. 10.1126/science.1177215 (doi:10.1126/science.1177215) [DOI] [PubMed] [Google Scholar]
- 24.Wittmann F, Schôngart J, Montero J, Motzer T, Junk W, Piedade M, Queiroz H, Worbes M. 2006. Tree species composition and diversity gradients in white water forests across the Amazon Basin. J. Biogeogr. 33, 1334–1347 10.1111/j.1365-2699.2006.01495.x (doi:10.1111/j.1365-2699.2006.01495.x) [DOI] [Google Scholar]
- 25.Henderson P, Crampton W. 1997. A comparison of fish diversity and abundance between nutrient-rich and nutrient-poor lakes in the Upper Amazon. J. Trop. Ecol. 13, 175–198 10.1017/S0266467400010403 (doi:10.1017/S0266467400010403) [DOI] [Google Scholar]
- 26.Henderson P, Hamilton H. 1995. Standing crop and distribution of fish in drifting and attached floating meadow within an Upper Amazonian varzea lake. J. Fish Biol. 47, 266–276 10.1111/j.1095-8649.1995.tb01894.x (doi:10.1111/j.1095-8649.1995.tb01894.x) [DOI] [Google Scholar]
- 27.Junk W. 1970. Investigations on the ecology and production-biology of the floating meadows (Paspalo-Echinochloetum) on the Middle Amazon. Amazoniana 2, 449–495 [Google Scholar]
- 28.Mitsch W, Gosselink J, Zhang L, Anderson C. 2009. Wetland ecosystems. London, UK: John Wiley & Sons Inc [Google Scholar]
- 29.Gery J. 1977. Characoids of the world. Mail Neptune, NJ: TFH Publication Inc [Google Scholar]
- 30.Burgess W. 1989. An atlas of freshwater and marine catfishes: a preliminary survey of the Siluriformes. Neptune City, NJ: T.F.H. Publications [Google Scholar]
- 31.Keith P, Le Bail P, Planquette P. 2000. Atlas des Poissons d'Eau Douce de Guyane: Tome 2-Fascicule I, Batrachoidiformes, Mugiliformes, Beloniformes, Cyprinodontiformes, Synbranchiformes, Perciformes, Pleuronectiformes, Tetraodontiformes. Collection Patrimoines Naturels, 43(1). Museum National d'Histoire Naturelle, Institut d'Écologie et de Gestion de la Biodiversité - Service du Patrimoine Naturel Paris, France: MNHN/SPN [Google Scholar]
- 32.Le Bail P, Keith P, Planquette P. 2000. Atlas de Poissons díeau douce de Guyane. Tome 2, fascicule II. Siluriformes. Paris: Patrimoines naturels (MNHN/SPN) [Google Scholar]
- 33.Matias MG, Chapman MG, Underwood AJ, O'Connor NE. 2012. Increasing density of rare species of intertidal gastropods: tests of competitive ability compared with common species. Mar. Ecol. Prog. Ser. 453, 107–116 10.3354/meps09639 (doi:10.3354/meps09639). [DOI] [Google Scholar]
- 34.RCoreTeam 2012. R: a language and environment for statistical computing.Vienna, Austria: See http://www.R-project.org [Google Scholar]
- 35.Lesnoff M, Lancelot R. 2010. Analysis of overdispersed data. R package version 12. Vienna, Austria: See http://cran.r-project.org/package=aod. [Google Scholar]
- 36.Pritt JJ, Frimpong EA. 2010. Quantitative determination of rarity of freshwater fishes and implications for imperiled-species designations. Conserv. Biol. 24, 1249–1258 10.1111/j.1523-1739.2010.01488.x (doi:10.1111/j.1523-1739.2010.01488.x) [DOI] [PubMed] [Google Scholar]
- 37.Sogin ML, Morrison HG, Huber JA, Welch DM, Huse SM, Neal PR, Arrieta JM, Herndl GJ. 2006. Microbial diversity in the deep sea and the underexplored ‘rare biosphere’. Proc. Natl Acad. Sci. USA 103, 12 115–12 120 10.1073/pnas.0605127103 (doi:10.1073/pnas.0605127103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May RM. 1975. Patterns of species abundance and diversity. In Ecology and evolution of communities (eds Cody ML, Diamond JM.), pp. 81–120 Cambridge, MA: Harvard University Press [Google Scholar]
- 39.Morlon H, et al. 2009. Taking species abundance distributions beyond individuals. Ecol. Lett. 12, 488–501 10.1111/j.1461-0248.2009.01318.x (doi:10.1111/j.1461-0248.2009.01318.x) [DOI] [PubMed] [Google Scholar]
- 40.Conslik E, Conslik J, Enquist BJ, Thompson J, Harte J. 2009. Improved abundance prediction from presence–absence data. Glob. Ecol. Biogeogr. 18, 1–10 10.1111/j.1466-8238.2008.00427.x (doi:10.1111/j.1466-8238.2008.00427.x) [DOI] [Google Scholar]
- 41.Connolly SR, Hughes TP, Bellwood DR, Karlson RH. 2005. Community structure of corals and reef fishes at multiple scales. Science 309, 1363–1364 10.1126/science.1113281 (doi:10.1126/science.1113281) [DOI] [PubMed] [Google Scholar]
- 42.Henderson PA, Magurran AE. 2010. Linking species abundance distributions in numerical abundance and biomass through simple assumptions about community structure. Proc. R. Soc. B 277, 1561–1570 10.1098/rspb.2009.2189 (doi:10.1098/rspb.2009.2189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grenouillet G, Pont D, Seip K. 2002. Abundance and species richness as a function of food resources and vegetation structure: juvenile fish assemblages in rivers. Ecography 25, 641–650 10.1034/j.1600-0587.2002.250601.x (doi:10.1034/j.1600-0587.2002.250601.x) [DOI] [Google Scholar]
- 44.Vidotto A, Carvalho E. 2007. Composition and structure of fish community in a stretch of the Santa Barbara River influenced by Nova Avanhandava Reservoir (low Tiete River, Sao Paulo State, Brazil). Acta Limnol. Brasiliensia 19, 233–245 [Google Scholar]
- 45.Hanski I. 1982. Dynamics of regional distribution: the core and satellite species hypothesis. Oikos 38, 210–221 10.2307/3544021 (doi:10.2307/3544021) [DOI] [Google Scholar]
- 46.Gotelli N, Simberloff D. 1987. The distribution and abundance of tallgrass prairie plants: a test of the core-satellite hypothesis. Am. Nat. 130, 18–35 10.1086/284695 (doi:10.1086/284695) [DOI] [Google Scholar]
- 47.Magurran AE, Henderson PA. 2003. Explaining the excess of rare species in natural species abundance distributions. Nature 422, 714–716 10.1038/nature01547 (doi:10.1038/nature01547) [DOI] [PubMed] [Google Scholar]
- 48.Magurran A, Henderson P. 2010. Temporal turnover and the maintenance of diversity in ecological assemblages. Phil. Trans. R. Soc. B 365, 3611–3620 10.1098/rstb.2010.0285 (doi:10.1098/rstb.2010.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackburn T, Gaston K, Greenwood J, Gregory R. 1998. The anatomy of the interspecific abundance–range size relationship for the British avifauna. II. Temporal dynamics. Ecol. Lett. 1, 47–55 10.1046/j.1461-0248.1998.00005.x (doi:10.1046/j.1461-0248.1998.00005.x) [DOI] [Google Scholar]
- 50.Magurran AE, Dornelas M. 2010. Biological diversity in a changing world. Phil. Trans. R. Soc. B 365, 3593–3597 10.1098/rstb.2010.0296 (doi:10.1098/rstb.2010.0296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dornelas M, et al. 2012. Quantifying temporal change in biodiversity: challenges and opportunities. Proc. R. Soc. B. 10.1098/rspb.2012.1931 (doi:10.1098/rspb.2012.1931) [DOI] [PMC free article] [PubMed] [Google Scholar]