Abstract
Many species of songbirds exhibit dramatic seasonal variation in song output. Recent evidence suggests that seasonal changes in auditory processing are coincident with seasonal variation in vocal output. Here, we show, for the first time, that frequency selectivity and temporal resolution of the songbird auditory periphery change seasonally and in a sex-specific manner. Male and female house sparrows (Passer domesticus) did not differ in their frequency sensitivity during the non-breeding season, nor did they differ in their temporal resolution. By contrast, female house sparrows showed enhanced frequency selectivity during the breeding season, which was matched by a concomitant reduction of temporal resolution. However, males failed to show seasonal plasticity in either of these auditory properties. We discuss potential mechanisms generating these seasonal patterns and the implications of sex-specific seasonal changes in auditory processing for vocal communication.
Keywords: house sparrow, auditory-evoked potentials, seasonality, communication, plasticity
1. Introduction
The behavioural salience of mate attraction and courtship signals changes seasonally in many songbird species [1]. Recent evidence suggests that plasticity in neural sensory representation may play an important role in generating this variation in the behavioural salience of song [2–5]. For instance, female white-throated sparrows (Zonotrichia albicollis) in breeding condition show differential neural responses, as measured with immediate early gene activity, to conspecific song versus tones [5]. These same females will also produce a behavioural response (copulation solicitation display) in response to playback of male song [5]. However, females that are not in breeding condition do not show differential neural responses to conspecific songs and tones, and playback of the male song does not induce the behavioural response of a copulation solicitation display [5].
The neural representation of sound begins at the auditory periphery. Yet, we know substantially less about seasonal changes in peripheral auditory processing in songbirds, compared with central auditory processing. However, recent evidence suggests that plasticity in sensory processing correlated with reproduction may begin at the periphery [6–9]. Because peripheral auditory filters gate information travelling to the central auditory system, they are expected to play an important role in determining the salience of spectral and temporal vocal features.
The peripheral auditory filters are especially important in the resolution of an important trade-off between frequency resolution and temporal resolution [10,11]. This trade-off is known as the ‘uncertainty principle’ (see [12] for an application to the analysis of birdsong). This trade-off arises because auditory filters that integrate a signal over a long period of time provide enhanced frequency resolution [10]. However, patterns in the temporal domain can be lost when the integration time of an auditory filter is long [11]. The anatomical resolution of the trade-off between timing and frequency information is determined in part by the bandwidth of the auditory filter [10,11]. Auditory filters with narrow bandwidths tend to have longer integration times and generally enhance frequency resolution, while broad bandwidth auditory filters have short integration times and enhance temporal resolution. Thus, if the bandwidth of the auditory filters changes seasonally, the information being passed to the central auditory system will probably alter the representation of vocal signals in the central auditory system.
Here, we investigated sex and seasonal variation in house sparrow auditory filters and temporal resolution. Females show a seasonal change in their response to male vocalizations [13,14]. Therefore, we predicted that female house sparrows would show seasonal plasticity in the bandwidth of their auditory filters and, concomitantly, temporal resolution. Currently, little is known about the vocal features that elicit female sexual response in house sparrows; therefore, we could not predict the direction of change (i.e. whether auditory filters would increase or decrease in bandwidth, or whether temporal resolution would be diminished or improved). Additionally, we predicted that males and females would differ in their auditory processing during the breeding season. During the non-breeding season, males and females show behavioural responses similar to conspecific vocalizations [13,14]. Therefore, we predicted that during the non-breeding season, males and females would not differ in their auditory processing.
2. Methods
(a). Capture, housing and experimental design
All breeding season individuals (four females, 14 males) were collected between 15 February and 15 April 2011. All non-breeding season individuals (eight females, 10 males) were collected between 22 October and 1 November 2011. We trapped house sparrows with baited-treadle traps at a private residence in Lafayette, Indiana, and transported the individuals to Purdue University for housing. Birds were housed individually or in pairs in 1 m3 steel cages and provided ad libitum with seed, grit, eggshell and vitamin-treated water. All breeding season individuals showed evidence of breeding condition (e.g. darkened bills, cloacal protuberance, brood patch). Non-breeding-season individuals had completed moult, and males had light bills typical of non-breeding birds [13,14]. Auditory-evoked potentials were recorded from individuals between 24 and 36 h after collection of a blood sample and not more than 48 h after capture.
(b). Hormone assay
We punctured the alar wing vein with a sterile 16-gauge needle and collected a blood sample with a heparinized collection tube (RAM Scientific Safe-T-Fill). All blood samples were collected between 11.00 and 13.00 h. Plasma was separated by centrifugation and stored at −20°C. Hormone levels were determined with Assay Designs ELISA kits for testosterone (ADI-910-065; males) and oestradiol (ADI-900-174; females). These kits have been previously validated for other passerines [9,15]. Kit instructions were followed for both testosterone and oestradiol samples. Briefly, for each bird, we added steroid displacement buffer (1% of plasma volume) to 10 µl (males) or 50 µl (females) of plasma. The total sample volume was then brought to 200 µl with assay buffer. This resulted in a final dilution of 1 : 20 for males and 1 : 4 for females. Each sample was run in duplicate (100 µl each) on a plate that also contained either five testosterone standards (0.008–2.000 ng ml−1) or 10 oestrogen standards (0.0156–30.00 ng ml−1). We add 50 µl of antibody and incubated the plates for 1 h. We then added 50 µl of alkaline phosphatase-conjugated steroid and incubated for 1 h. The plates were then emptied, and all sample wells were washed in triplicate with 400 µl of wash buffer. We then added 200 µl of substrate to each well. After a 1 h incubation, we added 50 µl of stop solution to each well and read the plates at 405 nm on a SpectraMax M5 microplate reader (Molecular Devices, Chicago, IL, USA). The average coefficient of variation for the duplicate samples was 3.1 per cent for males and 4.7 per cent for females. We excluded individuals from hormone analysis if their coefficient of variation was greater than 10 per cent. One autumn female was excluded from the hormone analysis based on this criterion. This female was still included in the analysis of auditory processing.
(c). Auditory-evoked potentials
The auditory-evoked potential recording procedure and stimuli used in this study have been described in detail previously [16–18]. Briefly, auditory-evoked potentials were recorded from anaesthetized individuals (midazolam: 5 mg kg−1; ketamine: 50 mg kg−1; xylazine 3 mg ml−1) using a TDT System II (Tucker Davis Technologies, Gainesville, FL, USA). There was no difference between the sexes or seasons in the amount of drug required for sedation or the amount of time individuals remained sedated. Recordings were conducted in a chamber (1.2 × 1.2 × 1.4 m) lined with acoustic foam (Acoustic Solutions, Richmond, VA, USA). Stimuli were created in SigGen32 on a computer with an AP2 sound processing card, and then passed through a digital to analogue converter (TDT DA1), 31 band equalizer (Behringer Ultragraph model FBQ6200), Crown D75 amplifier and shielded speaker (RCA Model 40–5000; 140–20 000 Hz frequency response). Responses were passed from needle electrodes to a head stage (TDT HS4), biological amplifier (TDT DB4), analogue to digital converter (TDT AD2) and recorded on a computer. Filtering, amplification and artefact-rejection levels were identical to those published previously [16–18].
(d). Frequency selectivity and auditory filters
Stimuli for the frequency selectivity protocol were 8-ms tonebursts with 2-ms cos2 gating presented at 2, 3 and 4 kHz and ranging in intensity from 16 to 72 dB SPL in 8 dB steps. We choose 2, 3 and 4 kHz because this is the range of best sensitivity in house sparrows, and these frequencies are well represented in their vocalizations [8]. Tonebursts were presented in spectrally notched white-noise (spectrum level = 15.3±2 dB re: 20 µPa2 outside of spectral notch) generated by two signal generators (TDT WG1) and two filters (TDT PF1, roll-off 156 dB octave−1). The spectral notches were centred on the toneburst frequency, and the notches were 0–80% of centre frequency. For each frequency–notchwidth combination, we determined the threshold using a cross-correlation method in which the auditory brainstem response (ABR) evoked to the 72 dB stimulus was used as a template that was cross-correlated with each subsequent response. We then regressed the cross-correlation value on stimulus intensity and determined the intercept of that function with the noise floor (see the electronic supplementary material).
We determined auditory filter shape using a notched noise masking technique [10,19,20]. The masked threshold (Ps) can be expressed as
where K represents the SNR required to evoke a response, W(f) is a weighting function and N(f) is the long-term average power spectrum of the masking noise. In turn, the weighting function W(f) was modelled as a two parameter rounded exponential model [roex(p,r)]. In this model, p defines the slope of the filter near the centre frequency, whereas r constrains the dynamic range of the filter. The weight of the filter then becomes
where g is the normalized width of the silent spectral notch in the masking noise. If we use this equation for the weight of the filter, the equation for the ABR masked threshold becomes
where nw is the width of the silent spectral notch and K′ is the efficiency constant expressed in decibels (10 × log10K). PSDnw(i) is the power spectral density of the noise divided into 25 Hz bins, each of which is multiplied by the weight of the filter:
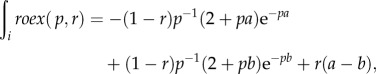 |
where a and b are the upper and lower frequency limits of each bin, respectively. This function is then summed over the number of bins (N) in the largest notchwidth. The Ps(nw)′ and PSDnw(i) were measured directly from our ABRs and stimuli, respectively. We solved for K′, p and r using an iterative Gauss–Newton polynomial fitting procedure in SAS (Proc NLIN; SAS Institute Inc., v. 9.2).
We then determined the equivalent rectangular bandwidth (ERB) using the formula ERB = 4/p × centre frequency. The ERB is a standard measure of auditory filter width that describes a rectangle with the same height and area as an auditory filter. Larger ERB values indicate broader tuning and suggest better temporal resolution. Smaller ERB values indicate sharper tuning and suggest poor temporal resolution. We also determined the quality of the filter (QERB = centre frequency/ERB). QERB allows the sharpness of filters with different centre frequencies to be directly compared, by expressing filter tuning as a function of both filter bandwidth and filter centre frequency. Filters with larger QERB values have sharper tuning and provide better frequency discrimination than filters with smaller QERB values.
(e). Temporal resolution
Temporal resolution stimuli were 0.67 ms tonebursts with a 0.25 ms Blackman gating, centred at 3 kHz, and presented at 60 dB SPL. Tonebursts were presented either singly or in pairs separated by 0.7–25 ms. For each inter-stimulus interval, the single toneburst (700 repetitions) was alternated with the paired tonebursts (700 repetitions), and the responses were recorded in separate averaging buffers. We determined the peak-to-peak amplitude of the ABR to the single toneburst. We then used a point-to-point subtraction to isolate the ABR to the second toneburst and determined the peak-to-peak amplitude. ABR recovery for each inter-stimulus interval was then calculated as the amplitude of the ABR to the second toneburst divided by the amplitude of the ABR to a single toneburst. Additional methodological details have previously been reported [17,18].
(f). Statistical analysis
We analysed the hormone data with t-tests. All auditory-evoked potential data were analysed with repeated measures ANOVAs in PROC MIXED in SAS v. 9.2, with individual as a random factor. Auditory filter and temporal resolution data were analysed separately. For the auditory filters, we modelled both the ERB and the QERB as dependent variables. The independent variables were frequency, sex, season and their interactions. In the temporal resolution model, the dependent variable was relative amplitude of the ABR to the second click, and the independent variables were inter-click interval, sex, season and their interactions. Non-significant interaction effects were dropped from the models. For each model, we used an autoregressive covariance structure and calculated denominator degrees of freedom using the Kenward–Rogers algorithm. Significant effects were explored post hoc with the diff option in the LSMEANS statement. These data have been deposited in the Dryad digital repository (doi:10.5061/dryad.31nd3).
3. Results
Non-breeding males had significantly lower testosterone levels (mean±s.e. = 1.75±0.2 ng ml−1) than breeding season males (mean±s.e. = 4.1±0.79 ng ml−1; t22 = 2.4, p = 0.025). Similarly, non-breeding females had lower oestradiol levels (mean±s.e. = 0.19±0.05 ng ml−1) than the breeding females (mean±s.e. = 1.1±0.4 ng ml−1; t9 = 3.2, p = 0.01). Testosterone levels agreed well with previous estimates in wild-caught house sparrows [13]. Oestradiol estimates for non-breeding individuals also agreed well with previous estimates in wild-caught birds; however, our estimates of oestradiol levels in breeding condition birds were approximately double those of previous estimates [13].
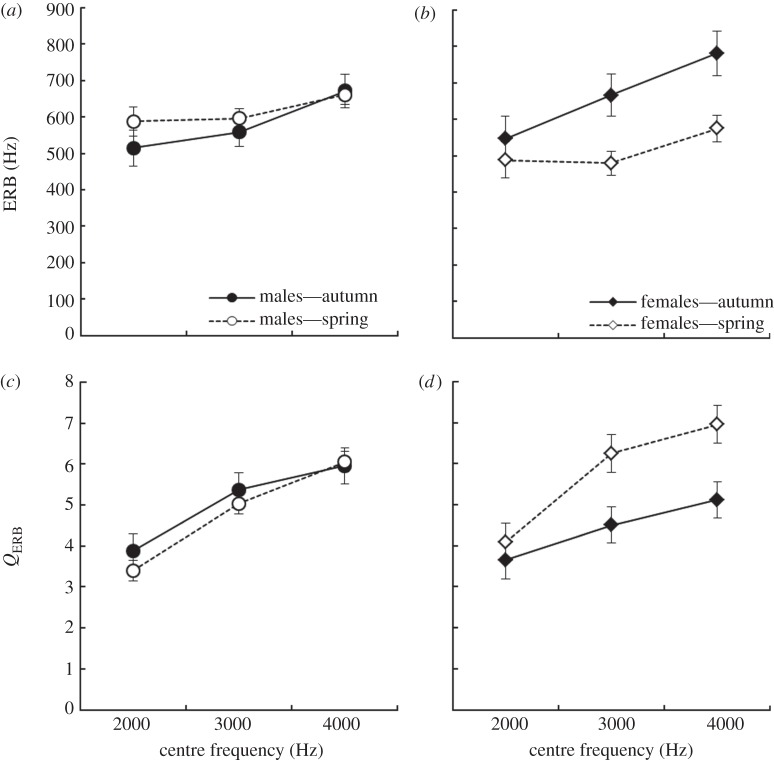
Auditory filter bandwidth, auditory filter quality and temporal resolution all varied seasonally in similar sex-specific ways. Generally, females exhibited seasonal plasticity in their auditory processing, whereas males did not. ERB varied with the season main effect (F1,49 = 4.05, p = 0.049), but not with the main effect of sex (F1,49 = 0.12, p = 0.73). However, as predicted, there was a significant sex × season interaction on ERB (F1,49 = 8.02, p = 0.006; figure 1). Breeding season females had narrower filters than non-breeding females (t48 = 3.03, p = 0.004). Females also had narrower filters than males during the breeding season (t42 = 2.4, p = 0.02), but did not differ from males during the non-breeding season (t57 = 1.66, p = 0.10). Finally, males did not differ seasonally (t53 = 0.68, p = 0.49). Filter ERB was significantly affected by frequency (F2102 = 10.8, p < 0.001; figure 2) with bandwidth increasing with increasing centre frequency.
Figure 1.
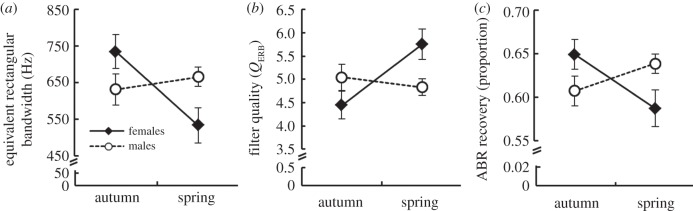
(a) Auditory filter equivalent rectangular bandwidth (ERB), (b) auditory filter quality (QERB) and (c) auditory brainstem response (ABR) recovery as a function of sex and season in the house sparrow. ABR recovery was measured as the amplitude of the ABR to a second click in a paired-click paradigm relative to the amplitude of the ABR to a single click. An increase in filter quality led to a concomitant decrease in temporal resolution ability. LSMEANS±s.e. were generated with the lsmeans statement in PROC MIXED in SAS v. 9.2 for the significant interaction term sex × season. Note that these values are averaged across frequencies (a,b) and inter-click intervals (c).
Figure 2.
(a,b) Auditory filter equivalent rectangular bandwidth (ERB) and (c,d) auditory filter quality (QERB) as a function of sex, season and frequency in house sparrows. Smaller ERB values and larger QERB values indicate greater frequency selectivity. LSMEANS±s.e. were generated with the lsmeans statement in PROC MIXED in SAS v. 9.2.
Neither sex (F1,48 = 0.34, p = 0.56) nor season (F1,48 = 3.95, p = 0.05) had a significant main effect on QERB. However, as predicted, there was a significant effect of the sex × season interaction term on QERB (F1,48 = 7.69, p = 0.008; figure 1). In the breeding season, females had significantly sharper filters than non-breeding females (t46 = 2.97, p = 0.005), whereas males did not differ between the seasons (t52 = 0.66, p = 0.5). Additionally, females had sharper filters than males during the breeding season (t41 = 2.52, p = 0.016), but were not different than males during the non-breeding season (t56 = 1.47, p = 0.15). QERB also varied with centre frequency (F2104 = 39.5, p < 0.001), with sharpness increasing with increasing centre frequency (figure 2).
There were no significant main effects of sex (F1,66 = 0.08, p = 0.77) or season (F1,66 = 0.8, p = 0.36) on ABR recovery. There was, however, a significant effect of the sex × season interaction on ABR recovery (F1,66 = 7.66, p = 0.007; figure 1). During the non-breeding season, there was no difference between males and females (t64 = 0.75, p = 0.46). Similarly, there was no difference in ABR recovery between the seasons for males (t69 = 1.54, p = 0.13). However, in the breeding season, females had poorer ABR recovery than males (t59 = 2.17, p = 0.03). Additionally, ABR recovery was greater in non-breeding females than in breeding season females (t64 = 2.3, p = 0.02), as would be expected based on the auditory filter results. Not surprisingly, there was a significant effect of inter-stimulus interval on the recovery of the ABR (F9484 = 500, p < 0.001; figure 3), with greatest recovery at the longest inter-click intervals.
Figure 3.
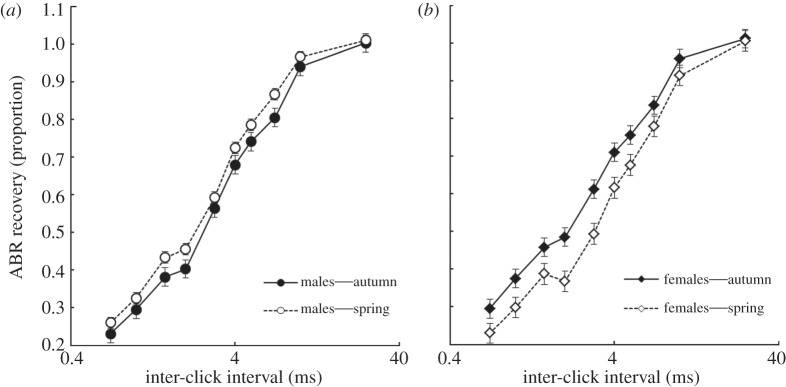
(a,b) Auditory brainstem response (ABR) recovery as a function of sex, season and inter-stimulus interval in house sparrows. ABR recovery is a measure of temporal resolution from a paired-click paradigm and is equal to the amplitude of the ABR to the second click in a pair of clicks relative to the amplitude of an ABR to a single click. Higher ABR recovery values indicate greater temporal resolution. LSMEANS±s.e. was generated with the lsmeans statement in PROC MIXED in SAS v. 9.2.
4. Discussion
Overall, we found that frequency selectivity and temporal resolution varied seasonally, in sex-specific ways. Females showed strong upregulation of frequency selectivity, as measured by auditory filter bandwidth and quality, during the breeding season. This upregulation of frequency selectivity came at the expense of temporal resolution. Males, however, did not exhibit a strong shift in either frequency selectivity or temporal resolution in the breeding season. These results suggest that males and females have convergent processing of acoustic stimuli during the non-breeding seasons, but divergent processing of acoustic stimuli during the breeding season. Seasonal changes in the neural representation of song [3–5] are therefore likely to begin at the auditory periphery and may be important for the seasonal change in the behavioural salience of vocal signals.
(a). Seasonality in auditory processing
Our understanding of seasonal changes in auditory processing, particularly at the auditory periphery and in the midbrain, has advanced tremendously in the last few decades. There is now substantial evidence to suggest that there are seasonal- or reproductively related changes in the auditory periphery and midbrain of fish [21,22], frogs [23–25], birds [6–9] and mammals [26]. Most of these studies have focused on sensitivity to tones, noisebursts and synthetic mating calls. However, different properties of the auditory system (e.g. responses to simple versus complex stimuli) are not necessarily linearly related [27,28]. Therefore, a change (or the lack thereof) in one aspect of auditory processing does not necessarily preclude, nor does it suggest, that other properties of the auditory system will show similar patterns. To the best of our knowledge, this is the first work to show that there are seasonal changes in the frequency selectivity of the songbird auditory periphery.
Auditory processing is almost universally upregulated in reproductively active animals when compared with animals that are not reproductively active, although the exact nature of these patterns varies across species. Female midshipman fish in reproductive condition show improved phase-locking to the frequencies of male courtship vocalizations compared with females that are not in breeding condition, and this upregulation can be mimicked by implanting non-breeding individuals with steroid hormones [21,29]. Similarly, in male Northern leopard frogs (Rana p. pipiens), midbrain neurons of reproductively active individuals show enhanced sensitivity to low frequencies and enhanced phase-locking to amplitude modulation in synthetic mating calls when compared with non-breeding individuals [24]. Injections of oestradiol result in enhanced sensitivity of Northern leopard frog auditory midbrain neurons to toneburst [30].
Similarly, in green treefrogs (Hyla cinerea), neurons in the auditory midbrain of females are more responsive to low frequencies in reproductively active females that have not mated compared with females that have already mated [25]. Green treefrogs also show sex-specific variation in auditory processing, with females having greater sensitivity to natural vocalizations than males and males having greater sensitivity to low-frequency pure tones than females [31]. Testosterone does not affect the auditory processing of males, but diminishes sensitivity in some parts of the audiograms for females [31]. This may be related to reproductive behaviour because testosterone levels are lower in reproductively active individuals and relatively high in individuals that have not yet become reproductively active.
Natural seasonal variation in the auditory processing of wild-caught songbirds suggests that peripheral processing is upregulated during the breeding season [6–8] or does not exhibit seasonal change [8]. For instance, house sparrows show enhanced suprathreshold ABRs to tones in the frequency range of their vocalizations during the breeding season, compared with the non-breeding season, but there is no seasonal change in the threshold to these frequencies [8]. Henry & Lucas [8] also found a significant interaction between sex and the intensity of the stimulus on the amplitude of the ABR, with males having steeper ABR amplitude by intensity functions than females. Carolina chickadees, tufted titmice, downy woodpeckers and white-breasted nuthatches all exhibit seasonal changes in their auditory-evoked responses to clicks and tones [6,7]. Recent work has also revealed sex differences in the frequency sensitivity and selectivity of Carolina chickadees [32] and brown-headed cowbirds [16,33], although seasonal patterns have yet to be described.
There has been only one study investigating the effects of steroid hormones on peripheral auditory processing in songbirds [9]. The authors found that non-breeding white-crowned sparrows of both sexes that were implanted with steroid hormone showed diminished ABR amplitudes, increased latency and elevated thresholds to tones compared with a placebo group. However, naturally occurring seasonal variation in white-crowned sparrow peripheral auditory processing has yet to be documented. Frequency selectivity and temporal resolution, as measured using distortion product otoacoustic emissions, did not differ between the treatment groups [9]. Unfortunately, frequency selectivity and temporal resolution were measured only in males; so it remains to be seen whether female white-crowned sparrows exhibit seasonal variation in frequency selectivity and temporal resolution. Nonetheless, work in the auditory forebrain of songbirds now strongly suggests that oestrogen can strengthen neural responses to conspecific song [2–5,34].
Taken together, these results suggest that seasonal variation in auditory processing is a widespread phenomenon across vertebrate taxa. In most species, some aspect of auditory processing is upregulated during times of reproductive behaviour. In some taxa, seasonal variation appears to be similar for both sexes, while in others, seasonal variation in sex-specific. The exact nature of this variation may depend strongly on the ecology and reproductive behaviours of the species of interest. Understanding seasonal variation from a comparative perspective will provide greater perspective into the aspects of ecology and animal behaviour that may result in season- and sex-specific variation in auditory processing.
(b). Physiological mechanisms
There are several mechanisms that could potentially result in seasonal changes in peripheral auditory processing, including the top-down effects (e.g. ‘efferent effects’), the addition or replacement of hair cells, changes in the electrical tuning properties of the hair cells or some combination of these mechanisms. There is currently very little information to suggest which of these mechanisms is operating in songbirds. However, in fish, recent evidence suggests that the addition of hair cells to the saccule during the breeding season may be responsible for improved peripheral coding of courtship songs [22]. Songbirds are capable of regenerating hair cells [35–37]; however, it is unclear whether this phenomenon operates seasonally.
Circumstantial evidence also suggests that changes in the tuning properties of hair cells could be responsible for seasonal changes in peripheral auditory processing. In fish, frogs and birds, the tuning of the auditory periphery is largely a result of electrical tuning of the hair cells [38–42]. The electrical tuning of the hair cells results from differential expression of voltage-gated calcium and calcium-sensitive big potassium (BK) ion channels in the hair cells. Frequency-specific resonance is the result of differential kinetics of the BK channels [43]. These differential kinetics are due in part to the large number of splice-variants of slo1, the pore-forming α-subunit of the BK channel, which are differentially expressed across the cochlea [44,45]. This leads to differential tuning of hair cells in different parts of the tonotopic map. In mammals, oestrogen responsive elements are involved in the regulation of Slo transcription, suggesting that differences in circulating hormone levels may lead to differential expression of BK channels [46–48].
Several lines of evidence suggest that hormonal regulation of ion channels is a plausible mechanism for seasonal auditory regulation. Seasonal changes in auditory processing in songbirds [6–8] are coincident with the timing of seasonal changes in hormone levels. Furthermore, oestrogen receptors and aromatase, an enzyme involved in the conversion of testosterone to oestrogen, have been found in auditory hair cells of both sexes of zebra finches when in breeding condition [49]. Taken together, these results suggest that seasonal changes in auditory processing could be mediated by genomic effects of circulating hormone levels. Local variation in the sensitivity of splice-variants to oestrogen could produce different sex- or species-specific upregulation of auditory processing in the breeding season. This promises to be a fruitful avenue for future investigation.
5. Conclusions
In conclusion, we demonstrate that seasonal plasticity in auditory processing can result in seasonal changes in both frequency selectivity and temporal resolution. However, seasonal changes in frequency selectivity and temporal resolution were found only in females, suggesting that seasonal changes in auditory processing may represent adaptive plasticity for mate selection during the breeding season. Therefore, females with different hormone titres may exhibit differential sensitivity to male vocalizations as a result of differences in auditory processing.
Acknowledgements
All methods were approved by the Purdue University Animal Care and Use Committee under protocol no. 08-132.
This work was supported by NSF (IOS-1121728), an NSF doctoral dissertation improvement grant (IOS-1109677) and an Animal Behavior Society graduate student research award.
References
- 1.Baptista LF, Trail PW, DeWolfe BB, Morton ML. 1993. Singing and its functions in female white-crowned sparrows. Anim. Behav. 46, 511–524 10.1006/anbe.1993.1219 (doi:10.1006/anbe.1993.1219) [DOI] [Google Scholar]
- 2.Remage-Healey L, Coleman ME, Oyama RK, Schlinger BA. 2010. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc. Natl Acad. Sci. USA 107, 3852–3857 10.1073/pnas.0906572107 (doi:10.1073/pnas.0906572107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. 2008. Estradiol modulates neural responses to song in a seasonal songbird. J. Comp. Neurol. 511, 173–186 10.1002/cne.21830 (doi:10.1002/cne.21830) [DOI] [PubMed] [Google Scholar]
- 4.Maney DL, Pinaud R. 2011. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front. Neuroendocrinol. 32, 287–303 10.1016/j.yfrne.2010.12.002 (doi:10.1016/j.yfrne.2010.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoder KM, Vicario DS. 2011. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav. Neurosci. 126, 17–28 10.1037/a0026673 (doi:10.1037/a0026673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maney DL, Cho E, Goode CT. 2006. Estrogen-dependent selectivity of genomic responses to birdsong. Eur. J. Neurosci. 23, 1523–1529 10.1111/j.1460-9568.2006.04673.x (doi:10.1111/j.1460-9568.2006.04673.x) [DOI] [PubMed] [Google Scholar]
- 7.Lucas JR, Freeberg TM, Krishnan A, Long GR. 2002. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J. Comp. Physiol. A 188, 981–992 10.1007/s00359-002-0359-x (doi:10.1007/s00359-002-0359-x) [DOI] [PubMed] [Google Scholar]
- 8.Lucas JR, Freeberg TM, Long GR, Krishnan A. 2007. Seasonal variation in avian auditory evoked responses to tones, a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J. Comp. Physiol. A 192, 201–215 10.1007/s00359-006-0180-z (doi:10.1007/s00359-006-0180-z) [DOI] [PubMed] [Google Scholar]
- 9.Henry KS, Lucas JR. 2009. Vocally-correlated seasonal auditory variation in the house sparrow (Passer domesticus). J. Exp. Biol. 212, 3817–3822 10.1242/jeb.033035 (doi:10.1242/jeb.033035) [DOI] [PubMed] [Google Scholar]
- 10.Caras ML, Brenowitz E, Rubel EW. 2010. Peripheral auditory processing changes seasonally in Gambel's white-crowned sparrow. J. Comp. Physiol. A 196, 581–599 10.1007/s00359-010-0545-1 (doi:10.1007/s00359-010-0545-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore BCJ. 1993. Frequency analysis and pitch perception. In Human psychophysics (eds Yost WA, Popper AN, Fay RR.), pp. 56–115 New York, NY: Springer [Google Scholar]
- 12.Viemeister NF, Plack CJ. 1993. Time analysis. In Human psychophysics (eds Yost WA, Popper AN, Fay RR.), pp. 116–154 New York, NY: Springer [Google Scholar]
- 13.Beecher MD. 1988. Spectrographic analysis of animal vocalizations: implications of the ‘uncertainty principle’. Bioacoustics 1, 187–208 10.1080/09524622.1988.9753091 (doi:10.1080/09524622.1988.9753091) [DOI] [Google Scholar]
- 14.Anderson TB. 2006. Biology of ubiquitous house sparrow: from genes to populations. New York, NY: Oxford University Press [Google Scholar]
- 15.Lowther PE, Cink CL. 2006. House sparrow (Passer domesticus), The birds of North America online. Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna/species/012.
- 16.Swett MB, Breuner CW. 2008. Interaction of testosterone, corticosterone and corticosterone binding globulin in the white-throated sparrow (Zonotrichia albicollis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 226–231 10.1016/j.cbpa.2008.06.031 (doi:10.1016/j.cbpa.2008.06.031) [DOI] [PubMed] [Google Scholar]
- 17.Gall MD, Lucas JR. 2010. Sex differences in auditory filters of brown-headed cowbirds (Molothrus ater). J. Comp. Physiol. A 196, 559–567 10.1007/s00359-010-0543-3 (doi:10.1007/s00359-010-0543-3) [DOI] [PubMed] [Google Scholar]
- 18.Henry KS, Gall MD, Lucas JR. 2011. Songbirds trade off auditory frequency resolution and temporal resolution. J. Comp. Physiol. A 197, 351–359 10.1007/s00359-010-0619-0 (doi:10.1007/s00359-010-0619-0) [DOI] [PubMed] [Google Scholar]
- 19.Gall MD, Henry KS, Lucas JR. 2012. Two measures of temporal resolution in brown-headed cowbirds (Molothrus ater). J. Comp. Physiol. A 198, 61–68 10.1007/s00359-011-0687-9 (doi:10.1007/s00359-011-0687-9) [DOI] [PubMed] [Google Scholar]
- 20.Patterson RD. 1976. Auditory filter shape derived with noise stimuli. J. Acoust. Soc. Am. 59, 640–654 10.1121/1.380914 (doi:10.1121/1.380914) [DOI] [PubMed] [Google Scholar]
- 21.Patterson RD, Nimmo-Smith I, Weber DL, Milroy R. 1982. The deterioration of hearing with age: frequency selectivity, the critical ratio, the audiogram, and speech threshold. J. Acoust. Soc. Am. 72, 1788–1803 10.1121/1.388652 (doi:10.1121/1.388652) [DOI] [PubMed] [Google Scholar]
- 22.Sisneros JA, Forlano PM, Deitcher DL, Bass AH. 2004. Steroid-dependent auditory plasticity lead to adaptive coupling of sender and receiver. Science 305, 404–407 10.1126/science.1097218 (doi:10.1126/science.1097218) [DOI] [PubMed] [Google Scholar]
- 23.Coffin AB, Morh RA, Sisneros JA. 2012. Saccular-specific hair cell addition correlates with reproductive state-dependent changes in the auditory saccular sensitivity of a vocal fish. J. Neurosci. 32, 1366–1376 10.1523/jneurosci.4928-11.2012 (doi:10.1523/jneurosci.4928-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillery CA. 1984. Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia 1984, 844–852 10.2307/1445327 (doi:10.2307/1445327) [DOI] [Google Scholar]
- 25.Goense JBM, Feng AS. 2005. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. J. Neurobiol. 65, 22–36 10.1002/neu.20172 (doi:10.1002/neu.20172) [DOI] [PubMed] [Google Scholar]
- 26.Miranda JA, Wilczynski W. 2009. Female reproductive state influences the auditory midbrain response. J. Comp. Physiol. A 195, 341–349 10.1007/s00359-008-0410-7 (doi:10.1007/s00359-008-0410-7) [DOI] [PubMed] [Google Scholar]
- 27.Elkind-Hirsch KE, Stoner WR, Stach BA, Jerger JF. 1992. Estrogen influence auditory brainstem responses during the normal menstrual cycle. Hear. Res. 60, 143–148 10.1016/0378-5955(92)90016-G (doi:10.1016/0378-5955(92)90016-G) [DOI] [PubMed] [Google Scholar]
- 28.Theunissen FR, Sen K, Doupe AJ. 2000. Spectral-temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. J. Neurosci. 20, 2315–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolley SM, Gill PR, Theunissen FR. 2006. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. J. Neurosci. 26, 2499–2512 10.1523/jneurosci.3731-05.2006 (doi:10.1523/jneurosci.3731-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sisneros JA. 2009. Steroid-dependent auditory plasticity for the enhancement of acoustic communication: recent insights from a vocal teleost fish. Hear. Res. 252, 9–14 10.1016/j.heares.2008.12.007 (doi:10.1016/j.heares.2008.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yovanof S, Feng AS. 1983. Effects of estradiol on auditory evoked responses from the frog's auditory midbrain. Neurosci. Lett. 36, 291–297 10.1016/0304-3940(83)90015-0 (doi:10.1016/0304-3940(83)90015-0) [DOI] [PubMed] [Google Scholar]
- 32.Miranda JA, Wilczynski W. 2009. Sex differences and androgen influences on midbrain auditory thresholds in the green treefrog, Hyla cinerea. Hear. Res. 252, 79–88 10.1016/j.heares.2009.04.004 (doi:10.1016/j.heares.2009.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry KS, Lucas JR. 2010. Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis. Anim. Behav. 80, 497–507 10.1016/j.anbehav.2010.06.012 (doi:10.1016/j.anbehav.2010.06.012) [DOI] [Google Scholar]
- 34.Gall MD, Brierley LE, Lucas JR. 2011. Species and sex effects on auditory processing in brown-headed cowbirds and red-winged blackbirds. Anim. Behav. 81, 973–982 10.1016/j.anbehav.2011.01.032 (doi:10.1016/j.anbehav.2011.01.032) [DOI] [Google Scholar]
- 35.Remage-Healy L, Coleman ME, Oyama RK, Schilinger BA. 2010. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc. Natl Acad. Sci. USA 107, 3852–3857 10.1073/pnas.0906572107 (doi:10.1073/pnas.0906572107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marean GC, Burt JM, Beecher MD, Rubel EW. 1998. Auditory perception following hair cell regeneration in the European starling (Sturnus vulgaris): frequency and temporal resolution. J. Acoust. Soc. Am. 103, 3567–3580 10.1121/1.423085 (doi:10.1121/1.423085) [DOI] [PubMed] [Google Scholar]
- 37.Woolley SMN, Rubel EW. 2002. Vocal memory and learning in adult Bengalese finches with regenerated hair cells. J. Neurosci. 22, 7774–7787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dooling RJ, Ryals BM, Manabe K. 1997. Recovery of hearing and vocal behavior after hair-cell regeneration. Proc. Natl Acad. Sci. USA 94, 14 206–14 210 10.1073/pnas.94.25.14206 (doi:10.1073/pnas.94.25.14206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashmore JF. 1983. Frequency tuning in a frog vestibular organ. Nature 304, 536–538 10.1038/304536a0 (doi:10.1038/304536a0) [DOI] [PubMed] [Google Scholar]
- 40.Fuchs PA, Nagai T, Evans MG. 1988. Electrical tuning in hair cells isolated from the chick cochlea. J. Neurosci. 8, 2460–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugihara I, Furukawa T. 1989. Morphological and functional aspects of two different types of hair cells in the goldfish sacculus. J. Neurophysiol. 62, 1330–1343 [DOI] [PubMed] [Google Scholar]
- 42.Steinacker A, Romero A. 1992. Voltage-gated potassium current and resonance in toadfish saccular hair cells. Brain Res. 574, 229–236 10.1016/0006-8993(92)90821-P (doi:10.1016/0006-8993(92)90821-P) [DOI] [PubMed] [Google Scholar]
- 43.Rohmann KN, Deitcher DL, Bass AH. 2009. Calcium-activated potassium (BK) channels are encoded by duplicate slo1 genes in teleostfishes. Mol. Biol. Evol. 26, 1509–1521 10.1093/molbev/msp060 (doi:10.1093/molbev/msp060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fettiplace R, Fuchs PA. 1999. Mechanisms of hair cell tuning. Annu. Rev. Physiol. 61, 809–834 10.1146/annurev.physiol.61.1.809 (doi:10.1146/annurev.physiol.61.1.809) [DOI] [PubMed] [Google Scholar]
- 45.Jones EM, Gray-Keller M, Fettiplace R. 1999. The role of Ca2+-activated K+ channel spliced variants in the tonotopic organization of the turtle cochlea. J. Physiol. 518, 653–665 10.1111/j.1469-7793.1999.0653p.x (doi:10.1111/j.1469-7793.1999.0653p.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramanathan K, Michael TH, Jiang GJ, Hiel H, Fuchs PA. 1999. A molecular mechanism for electrical tuning of cochlear hair cells. Science 283, 215–217 10.1126/science.283.5399.215 (doi:10.1126/science.283.5399.215) [DOI] [PubMed] [Google Scholar]
- 47.Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. 2005. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 579, 4856–4860 10.1016/j.febslet.2005.07.069 (doi:10.1016/j.febslet.2005.07.069) [DOI] [PubMed] [Google Scholar]
- 48.Kundu P, Alioua A, Stefani E, Toro L. 2007. Regulation of mouse Slo gene expression: multiple promoters, transcription start sites, and genomic action of estrogen. J. Biol. Chem. 282, 27 478–27 492 10.1074/jbc.M704777200 (doi:10.1074/jbc.M704777200) [DOI] [PubMed] [Google Scholar]
- 49.Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balhazart J, Ball GF. 2009. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear. Res. 252, 49–55 10.1016/j.heares.2009.04.012 (doi:10.1016/j.heares.2009.04.012) [DOI] [PubMed] [Google Scholar]