Abstract
An emerging problem in conservation is whether listed morpho-species with broad distributions, yet specialized lifestyles, consist of more than one cryptic species or functionally distinct forms that have different ecological requirements. We describe extreme regional divergence within an iconic endangered butterfly, whose socially parasitic young stages use non-visual, non-tactile cues to infiltrate and supplant the brood in ant societies. Although indistinguishable morphologically or when using current mitochondrial and nuclear sequence-, or microsatellite data, Maculinea rebeli from Spain and southeast Poland exploit different Myrmica ant species and experience 100 per cent mortality with each other's hosts. This reflects major differences in the hydrocarbons synthesized from each region by the larvae, which so closely mimic the recognition profiles of their respective hosts that nurse ants afford each parasite a social status above that of their own kin larvae. The two host ants occupy separate niches within grassland; thus, conservation management must differ in each region. Similar cryptic differentiation may be common, yet equally hard to detect, among the approximately 10 000 unstudied morpho-species of social parasite that are estimated to exist, many of which are Red Data Book listed.
Keywords: chemical mimicry, host specificity, butterfly, conservation, Maculinea, Phengaris
1. Introduction
To set meaningful priorities in conservation and for practical remedies to succeed, it is vital to ascertain whether the threatened morpho-species named in Red Data lists are likely to consist of more than one cryptic species [1,2] or functionally distinct genotypes. In theory, regional (co-)adaptations may be amplified in closely coupled biological systems [3], such as obligate mutualisms [4] and host–parasite arms races [5]. In the case of insect–insect interactions, both parties may be susceptible to strong selection within small spatial scales, even between neighbouring landscapes [6,7]. Thus, unexpected subsets of host specificity, rapid evolution and cryptic speciation are an emerging feature of insect-parasitoid studies [8], and apparently exist, possibly in extreme forms, among the estimated 100 000 morpho-species of poorly studied insects that interact with ants (myrmecophiles) [9].
DNA analysis transformed biologists' ability to detect cryptic species within the described morpho-species [10,11], and with a burgeoning array of reference sequences available (e.g. Consortium for the Barcode of Life), an increasing number of species complexes are being identified [2]. Nevertheless, commonly used, affordable techniques may be insensitive to detecting differentiation that has arisen recently or from selection on one or a few genes that affect the phenotype in major ways [12–14]. Maculinea (large blue) butterflies exemplify this problem: all six recognized morpho-species are iconic flagship insects, long identified as global conservation priorities [15–17], that possess attributes associated with cryptic speciation [2], including socially parasitic young stages that use non-visual, non-tactile cues, including chemical and acoustical mimicry, to infiltrate and exploit ant societies [15–19]. Recent analyses of mitochondrial- (COI, COII) and nuclear sequence data (EF1α, wingless), as well as microsatellite data, suggest that each morpho-species of Maculinea that has a predatory lifestyle contains cryptic lineages, but recognized cuckoo species (species that are fed directly on regurgitations by ants; see the electronic supplementary material, figure S1e) could not be distinguished [15,20–22], despite their closer integration and local coevolution with ant societies and, typically, more extreme host specificity [9,23,24].
We studied the regional divergence that nevertheless appeared to exist within the endangered cuckoo butterfly Maculinea rebeli (Hirschke), which was itself indistinguishable from a close relative, Maculinea alcon (Denis & Schiffermüller), in recent molecular studies [20,21]. Prior to 1991, each of these congeners was classed as globally vulnerable [25], but uncertainty about their taxonomic status led to exclusion from subsequent lists. It is not disputed that Ma. rebeli and Ma. alcon are distinct ecotypes (or putative ecospecies) which inhabit different ecosystems, xerophytic and moist grassland, respectively, and exploit different plant and Myrmica species [9,22]. Both are extreme specialists whose respective larvae feed initially on the flowerheads of Gentiana cruciata and Gentiana pneumonanthe on typical sites, a distinction widely used, including here, to classify the two types. Larvae then infiltrate Myrmica ant colonies in their final instar, where they live for 11–23 months and acquire more than 98 per cent of their ultimate biomass (see the electronic supplementary material, figure S1) [9]. They achieve this transition by abandoning their host plant and secreting simple cocktails of hydrocarbons that resemble the chemical signatures of Myrmica grubs sufficiently well to trick foraging workers of any Myrmica species to ‘rescue’ the mimic and carry it into the underground brood chambers [9,18]. However, although each caterpillar is adopted indiscriminately by the first forager to encounter it [9], each Myrmica species whose nest it enters represents not only a different food but also a different enemy, chemical template to mimic [26] and living environment for 92–96% of the intruder's life: unsurprisingly, caterpillars typically survive with the single, or occasionally sibling, model ant species that they mimic best [9]. Thus, within colonies of the model host species, the intruding larvae successfully compete with the ant brood for worker attention and are soon fed (and rescued) preferentially by the nurse ants (see the electronic supplementary material, figure S1e), a subterfuge that is achieved by synthesizing additional hydrocarbons shortly after adoption that more precisely mimic their host Myrmica species (but other Myrmica species less) [27]. By contrast, caterpillars carried into nests of other Myrmica species suppress their secretions and rely on the passive acquisition of their host's gestalt odour for social integration [27]. Acquired camouflage alone, however, is insufficient to survive periods of stress or deprivation, when nurse ants become discriminatory and xenophobic [28].
We noticed that populations of Ma. rebeli in southwest Europe and Poland appear to exploit very different species of Myrmica, Myrmica schencki and Myrmica sabuleti, respectively [9,29]: ants whose chemical recognition profiles differ more than any other known pairs of Myrmica species [26] and which occupy different niches within grassland. We therefore studied the exclusivity of host specificity that has evolved in each region by measuring survival both in natural populations and in the laboratory. We then devised behavioural experiments to assess the social status achieved by Spanish and Polish larvae after infiltrating the two host ant societies, and also identified the mechanism responsible for host specificity by analysing the mimetic chemicals secreted by pre- and post-adoption larvae from each region. Finally, we described the key attribute of the niche occupied by each ecotype (or cryptic species) of this endangered butterfly, which provides the essential knowledge for their future conservation [16].
2. Material and methods
(a). Measuring host specificity in natural populations
Host specificity was measured by comparing the proportions of caterpillars that were adopted into different Myrmica nests with the proportions that survived to adulthood or pupation. Data were obtained from three populations for 5 consecutive years near Panticosa in the Spanish Pyrenees [9,30] and in one population for 4 years near Przemyśl, southeast Poland [22]. The proportion of larvae adopted by different ants was estimated by baiting beneath stratified random samples of gentians and by counting the number of eggs on each plant [30,31]. Previous work had shown that there was no difference in egg or larval survival on gentians growing in different ant territories, nor in the ratio of larvae retrieved from beneath plants by different ant species: the first Myrmica worker to encounter a larva retrieved it, and where two species overlapped, there was no bias in retrieval towards one species [30,32]. The distribution of the egg population on gentians is, therefore, an accurate surrogate for the distribution of the final-instar population entering nests of each Myrmica species [30,32]. Adult estimates of Ma. rebeli were obtained by recording eclosing individuals along stratified transects across sites, and identifying the nest after confirming that it contained an empty pupal case [30,31]. Additional data were obtained by excavating all Myrmica nests near gentians along stratified transects that had supported known densities of eggs the previous year and by counting the pupae they contained. No mortality has been recorded in pupae in Myrmica cells before eclosion [30–32].
A map of regional host specificity (figure 1) was compiled from our published results [9,29–32] supplemented by additional field data. The distributions are considered to be near complete for Poland [22,29], the French and Spanish Pyrenees and southern Alps [30–32], but the northern Alps and Massif Central were less comprehensively sampled and may contain greater complexity in host use. Host use in Italy, Hungary and central Switzerland was not mapped.
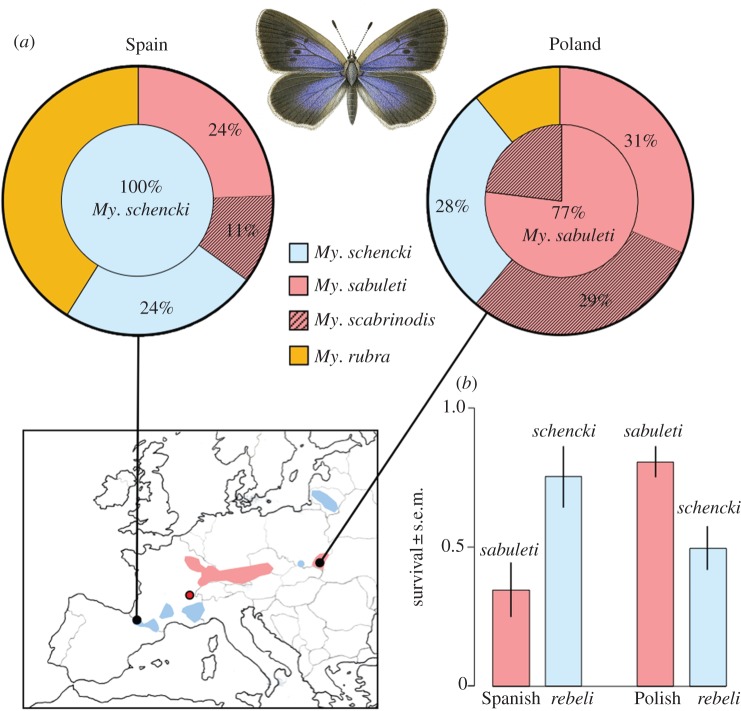
Figure 1.
Host specificity of Maculinea rebeli in Spain and southeast Poland. (a) Field survival: outer circle, egg distribution (=larval adoption, see §2a) in different Myrmica ant territories (n = 2859 Spain, 102 Poland); inner circle, ant species where Ma. rebeli survived to pupae or adults (n = 148 Spain, 548 Poland). Map: blue, My. schencki recorded as sole host; pink, My. sabuleti primary host; red circle, source of laboratory test ants in Alps. (b) Larval survival after 17 days in paired laboratory ant colonies set from the same naive French source nests.
(b). Laboratory experiments of host specificity
Host specificity from the two regions was measured using naiïve laboratory My. schencki and My. sabuleti colonies, collected from the Jura, east France, midway between the Pyrenees and Przemyśl in a landscape lacking the butterfly (figure 1). Six nests of each species were excavated and divided into subcolonies, maintained on a standard diet in Brian nests [33]. After 6 to 8 weeks acclimatization, more than 100 G. cruciata flower spikes were randomly collected from the Polish and Spanish sites, and the resultant final-instar larvae were used within 12 h of leaving their foodplant to establish experiments (§2b–d). First, larval survival was measured in 22 laboratory cultures, each containing 50 workers and five ant larvae, established from the stock nests (six pairs of My. schencki, five pairs of My. sabuleti). A total of 123 Ma. rebeli larvae from Poland and 97 from Spain were introduced in groups of seven so that each set of cultures contained matching pairs of colonies, each derived from the same stock nest. Larvae that died in the first 7 days were replaced, and total survival in each nest was recorded after 17 days. Statistical analysis was conducted by paired two-tailed t-test.
(c). Social status achieved in natural and unnatural host colonies
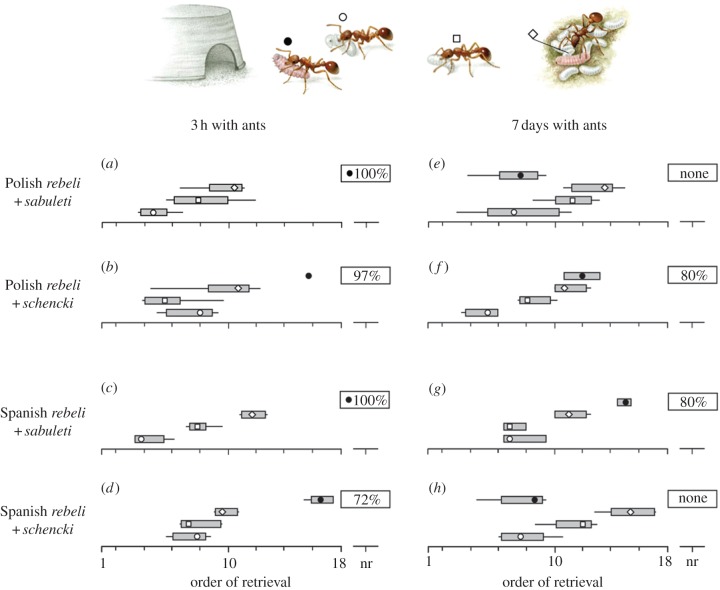
We assessed the social status achieved by each form of Ma. rebeli within colonies of each Myrmica species in a standard bioassay [23] that involved perturbing laboratory ant colonies and recording the order in which the ants' own brood or the mimetic caterpillars were rescued. Groups of five butterfly larvae from each region were adopted into matching colonies of naïive French My. schencki and My. sabuleti, using separate replicate nests to those used in §2b. Every test colony also contained five brood items each of kin ant pupae, large and small larvae, making a total of 20 immature individuals and 20 workers per replicate. Cultures were established in 413 cm2 boxes containing a small moist sponge pad beneath an inverted 6 cm diameter saucer with a notched entrance, under which the ants gathered their brood and Ma. rebeli (figure 2). Three hours after the Ma. rebeli caterpillars had been introduced, we perturbed the experimental colonies by uncovering the brood chamber and relocating it over another pad nearby; we then recorded the order in which the nurse ants rescued their 15 brood items or the five Ma. rebeli and carried them into the new nest (figure 2). The same experiment was repeated 7 days later, which represents a sufficient period for Ma. rebeli caterpillars to attain their maximum potential integration with a host society, yet remaining a similar size to when first adopted, i.e. the same size or smaller than the Myrmica pupae and large larvae [23,32]. The number of replicates for each ant–butterfly combination tested varied owing to a paucity of ant pupae and butterfly deaths (especially with unnatural hosts): n = 8 (figure 2a,b), n = 6 (figure 2c,e), n = 5 (figure 2d,h), n = 4 (figure 2f) and n = 3 (figure 2g). Fisher's exact tests were used to ascertain differences in the probability of a class of item being retrieved or abandoned by worker ants after perturbations. We also made three types of non-parametric analysis of the rank order in which chosen items were retrieved, within or between treatments: Kruskal–Wallis to establish whether ants rescued items randomly or selectively; Wilcoxon to test for changes in the order of selected items after Ma. rebeli had lived for 7 days with the ants compared with the initial 3 h; Mann–Whitney to test for differences in the order in which ant brood or butterfly caterpillars were selected within each of the eight combinations of ants and butterflies shown in figure 2. In addition to Mann–Whitney analysis, we used a randomization procedure whereby ranks were assigned at random for each trial twice. We recorded the difference in median between the two draws and repeated the procedure 10 000 times providing a frequency distribution for differences in medians to arise without selection. We then compared the observed differences in median between the item classes and assessed their likelihood to occur at random (see the electronic supplementary material, table S1).
Figure 2.
Status achieved by Maculinea rebeli within natural and unnatural Myrmica host societies. (a–h) The order that disturbed workers rescued ant brood or butterfly larvae 3 h and 7 days after adoption. Each replicate involved a choice between kin ant pupae (open circle), large kin ant larvae (open square), small kin ant larvae (open diamond), Ma. rebeli larvae (filled circle). Boxplots show means of median orders of rescue (symbol), 25–75% quartiles (box), and first and last individuals (tails); ‘nr’ = per cent Ma. rebeli larvae not retrieved by ants after 30 min. All treatments showed significant differences in the order in which items were retrieved (Kruskal–Wallis, H = 10.38–25.84, d.f. = 3, p = 0.016 to < 0.001), with fewer Ma. rebeli rescued than ant brood in (a–d,f and g) (z = −12.83 to −46.66, p < 0.001). After 7 days with their natural hosts (e,h), Ma. rebeli were rescued first equal with kin pupae (Mann–Whitney W = 38.5, p = 1.000; W = 24.0, p = 0.5309, respectively), significantly ahead of kin larvae (p = 0.024, 0.027). See the electronic supplementary material, table S1 for full statistical tests.
(d). Analysis of surface semio-chemicals on Maculinea rebeli larvae
To test whether observed regional differences in Ma. rebeli's host specificity could be explained by variation in mimetic semio-chemicals, we tracked the changing chemical profiles of caterpillars from each region when reared with each ant, from uncontaminated pre-adoption final instars (see the electronic supplementary material, figure S1b), to individuals 6 weeks after adoption (see the electronic supplementary material, figure S1e), and finally to the latter after isolation from ants for 5 days, which allows acquired semio-chemicals to dissipate and prompts the hungry caterpillars to release their own secretions [27]. Hexane extracts of surface chemicals were obtained [18,26,27] from: (i) unparasitized My. schencki and My. sabuleti on our study sites in the Pyrenees, Poland and the naive ants in France that had not experienced Ma. rebeli (figure 1; n = 5 workers from five nests of each ant species per locality); (ii) eight batches per region of pre-adoption final-instar Ma. rebeli larvae sampled after leaving G. cruciata before contact with ants (see the electronic supplementary material, figure S1b; n = 5 larvae per batch, ∑40 individuals for each type of Ma. rebeli); (iii) Ma. rebeli larvae after living 6 weeks with naiïve French ants (see the electronic supplementary material, figure S1e), n = 5 (Spanish + schencki and Polish + sabuleti), n = 3 (Spanish + sabuleti and Polish + schencki); and (iv) Ma. rebeli larvae reared as in (iii), then isolated from ants and kept unfed and singly in sterile conditions for 5 days; n = 5 (Spanish + schencki), n = 4 (Polish + schencki), n = 3 (Spanish + sabuleti and Polish + sabuleti).
The chemical and statistical analyses of extracts followed an established protocol [26,27]. Maculinea extracts were concentrated to 20 μl, ant workers to 50 μl and 2 μl of every sample were analysed by gas chromatography with mass spectrometric detection using a HP 5890II gas chromatograph and HP 5971A mass selective detector, and ultra-high purity helium as the carrier gas with 10 psi column head pressure. Mass spectral data were acquired in full scan mode over 40–600 m/z. Mass chromatograms were initially screened for hydrocarbons by examining the selected ion chromatogram of m/z = 57. The chromatogram was integrated at a threshold value of 12 (HP integrator) to obtain the areas under the peaks measuring the total ion count. With each sequence of samples, we also analysed alkane standards (n-C20–n-C36), and the position of each peak within that range in a sample was calculated as an equivalent chain length (ECL) [26]. Mass chromatograms were inspected to ensure that they were free of gross interferences and that peaks of interest, such as branched and straight alkanes and alkenes, were chromatographically distinct and symmetrical. We excluded peaks that were column bleed, siloxanes or phthalate plasticizers as indicated by a characteristic abundant ion at m/z 149. Peaks of interest were tentatively identified by a combination of ECL number and inspections of their full scan mass spectra and matching with the NIST-97/08 mass spectral database.
For statistical analysis, the area under each peak was expressed as the proportion of the sum of all peaks in the chromatogram [26]. Samples were compared using multivariate and non-parametric multi-dimensional scaling on the ranks of the Bray–Curtis similarities [34]. The extent of a final lack of fit was assessed by a STRESS statistic [26] before pairwise differences between species and treatments were assessed using an analysis of similarities [35] in Primer-e v6. We used the average pairwise distance between groups, and assessed two averages with a two-sample t-test, to compare differences in the shift of similarities between groups.
(e). Myrmica niches on Maculinea rebeli sites
Baits were placed under 223 flowering G. cruciata plants in Spain to record the species of Mymica foraging around them [31]. Vegetation structure (height) was measured using Stewart's direct method [36] at four diagonal points 5 cm from each plant. Species' niches were compared using two-tailed t-tests having confirmed normality of the data.
3. Results
(a). Host specificity
In three Ma. rebeli populations over 5 years in the Spanish Pyrenees, we found that eggs were laid indiscriminately [37] on G. cruciata growing in the territories of four species of Myrmica, yet 100 per cent of adults emerged from My. schencki nests the following summers, despite only 24 per cent of the larval population being adopted by that ant (figure 1a; z =−96.81, p = <0.001). By contrast, for 4 years near Przemyśl, Poland, 28 per cent of Ma. rebeli larvae were adopted by My. schencki, but no adult emerged from their nests (z =−9.66, p = <0.001). Instead, 77 per cent of adults emerged from My. sabuleti nests and the remainder from Myrmica scabrinodis, a chemically similar [26] close sibling to My. sabuleti [38] (survival with My. sabuleti > My. scabrinodis; z =−3.85, p = <0.001). In Poland, we observed My. schencki workers eject mutilated Ma. rebeli larvae from the nests into which foragers had retrieved larvae a few hours earlier, with each corpse showing clear signs of attack by their putative hosts.
Well-fed captive ants are more tolerant of intruders than in the wild [28]. Nevertheless, we obtained a similar pattern of differential survival by Ma. rebeli within 17 days of introduction to standardized laboratory My. sabuleti or My. schencki cultures established from naive colonies from France (figure 1b; Spanish butterfly ≠ Polish butterfly survival with My. schencki, t5 = 2.51, p = 0.054; Spanish butterfly ≠ Polish butterfly survival with My. sabuleti, t4 = 4.10, p = 0.015).
(b). Social status of caterpillars in natural and unnatural host species' colonies
Maculinea rebeli larvae did not integrate with their host society in the first hours after their adoption, being the last items to be chosen by workers on the rare occasions they were rescued after colony perturbation by exposure to light (see figure 2a–d and the electronic supplementary material, table S1). As expected [23], workers generally selected their pupae ahead of their large larvae and retrieved both in preference to small ant larvae. However, after a week with their natural hosts, Ma. rebeli from Spain and Poland were chosen equal first with the kin pupae of My. schencki and My. sabuleti, respectively, and significantly ahead of the smaller ant larvae (see figure 2e,h and the electronic supplementary material, table S1). But when each was reared with its unnatural ant host, just 20 per cent of week-old butterfly larvae were rescued, and these were afforded lowly status, ranking well below small ant larvae (see figure 2f,g and the electronic supplementary material, table S1). Even this may overestimate integration, because many Ma. rebeli larvae were killed in unnatural host Myrmica nests, and we perhaps tested the least maladapted individuals in the most socially accepting colonies.
(c). Analysis of model and mimetic chemical profiles
Given the multi-functionality of hydrocarbons [39], perfect matches by Ma. rebeli secretions to the dissimilar recognition profiles of My. schencki or My. sabuleti [26] were not expected [27]. Nevertheless, Ma. rebeli from each region secreted a distinctive cocktail that mimicked its natural host's signature with increasing likeness (figure 3). After living 6 weeks with laboratory ants, both types of social parasite resembled their natural and artificial hosts significantly more closely than did pre-adoption larvae (t2–3 =−4.28 to −18.41, p = <0.001, see the electronic supplementary material, table S2 for full statistics). However, after 5 days of isolation, the profiles of Spanish rebeli reared unnaturally with My. sabuleti, and Polish rebeli with My. schencki, shifted to resemble their natural model more closely (t43 = 7.38, p = < 0.001 and t27 = 3.26, p = 0.003, respectively). In particular, the former lost one compound, tentatively identified as 1-methyl-tricosane (see the electronic supplementary material, table S3), which it had evidently acquired from My. sabuleti and which was absent from My. schencki, and instead started synthesizing heptacosane and 3-methyl-tricosane, diagnostic hydrocarbons of My. schencki which were absent or just detectable on My. sabuleti. Similarly, isolated Polish rebeli lost dotriacontane and octacosane acquired from My. schencki (but undetectable on My. sabuleti) and gained docosane, an n-alkane characteristic of My. sabuleti, but not of My. schencki. It is noteworthy that two of these three emerging mimetic hydrocarbons (docosane, 3-methyl-tricosane) synthesized by isolated 7-week-old Ma. rebeli larvae were absent from the simpler profiles secreted by pre-adoption larvae. By contrast, individuals reared with their natural host did not change significantly (Spanish rebeli with My. schencki, t35 = 0.99, p = 0.327) or became less like it (Polish rebeli with My. sabuleti, t19 = 5.03, p = < 0.001) after isolation.
Figure 3.
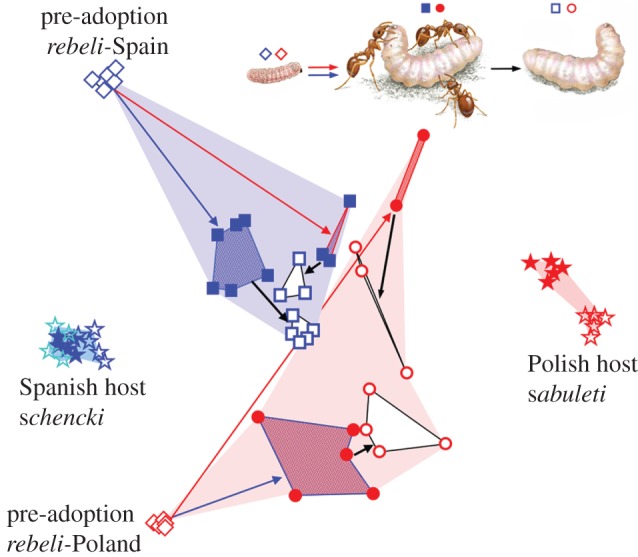
Changes in cuticular hydrocarbon profiles when Ma. rebeli larvae are reared with or without different Myrmica host species. The non-parametric multi-dimensional scaling plot shows profiles of final-instar butterfly larvae from Spain (blue symbols) and Poland (red) at pre-adoption before encountering ants (diamonds), after 6 weeks (solid squares/circles) with My. schencki (blue arrows, boundaries and stippling) and My. sabuleti (red arrows, boundaries, stippling), then removal from ants for 5 days (open squares, circles, black arrows and lines), when acquired ant chemicals dissipate and those synthesized by the butterfly accumulate. Stars My. schencki (blue), My. sabuleti (red); naive test colonies (solid), Spanish (dark), Polish (pale) sites.
(d). Niches of host ants in grassland
We found that My. schencki inhabits shorter turf than My. sabuleti in the xerotypic grasslands that support Ma. rebeli in the Pyrenees (table 1: My. schencki ≠ My. sabuleti t98 = 3.05, p = 0.003; My. sabuleti ≠ My. scabrinodis t29 = 6.37, p = 0.001; My. scabrinodis ≠ Myrmica rubra ns). Similarly, as befits the more thermophilous ant, we observed My. schencki predominantly in well-grazed swards on skeletal soils in Poland.
Table 1.
The niches within heterogeneous successional grassland occupied by host and non-host Myrmica species that adopted Maculinea rebeli larvae in the Pyrenees, Spain, represented by vegetation height (cm, n = 314).
| My. schencki turf (cm) | My. sabuleti turf (cm) | My. scabrinodis turf (cm) | Myrmica rubra turf (cm) | |
|---|---|---|---|---|
| sward height ± s.e.m. | 6.16 ± 0.26 | 7.60 ± 0.39 | 17.63 ± 1.50 | 18.23 ± 0.84 |
4. Discussion
Our results reveal a major difference in the physiology of populations of Ma. rebeli in Spain and southeast Poland, enabling each social parasite to infiltrate and exploit a very different Myrmica host society—a degree of specialization that makes each incompatible with the other's host species. By contrast, some taxonomists consider Ma. rebeli itself to be a mere ecotype of Ma. alcon rather than a true species. On current knowledge, the known hosts of these two cuckoo Maculinea belong to three distinct groups of Myrmica [38]: rubra (includes ruginodis), scabrinodis (includes sabuleti) and lobicornis (includes schencki), of which Ma. alcon exploits representatives from the first two groups [9] and Ma. rebeli from the second two. Field [40] and pre-adoption chemical [24] evidence suggest that similar exclusive differentiation may have evolved between the main European form of Ma. alcon that exploits My. scabrinodis and that of Scandinavia and the Pays-Bas that is adapted to My. rubra/ruginodis. Current molecular techniques compound the confusion, for no wide-scale differentiation was detected between or within Ma. rebeli and Ma. alcon [20,21], perhaps because current forms of these extreme specialists evolved rapidly in recent millennia [20] and/or very few genes are involved. Unfortunately, lycaenid butterflies in general, and Maculinea species in particular, are notoriously difficult to pair in captivity, making large-scale cross-breeding experiments on hybrids exceedingly difficult. Thus, although some morphologists and recent genetic analyses currently recognize one cuckoo species of Maculinea (Ma. alcon), ecological studies suggest two cryptic species (Ma. rebeli and Ma. alcon) [9], and our current functional/physiological studies point towards three (possibly four) recent siblings, drawn from the above, exploiting rubra-, scabrinodis- and lobicornis-taxa of Myrmica.
Whatever the taxonomic status of each form, all are ill-served by traditional conservation paradigms based on species listing. Like ecospecies [41], each type exploits a resource that occupies a different niche or biotope, and all are threatened by habitat degradation or destruction. In the case of Ma. rebeli, My. schencki requires more frequently grazed grassland than My. sabuleti and considerably more than My. scabrinodis. The successful restoration to the UK of Ma. arion resulted from creating optimum habitat for its host My. sabuleti [16]: similar management would promote Ma. rebeli in southeast Poland yet cause population extinctions elsewhere in Poland (figure 1a) and in Spain.
Regional host shifts are not unknown in social parasites, especially among cuckoo species [24,40,42]. However, the more different the ecology, physiology, defence and social organization of hosts, the less we consider it probable that the extreme adaptations required to exploit them will be expressed by phenotypes of a single species [43]. Indeed, all six morpho-species of insect social parasite whose ecology, mimicry, host use or genetics have been studied show evidence of cryptic speciation (Microdon hoverflies [43], predatory Maculinea [15,20,21]) or extreme differentiation (cuckoo Maculinea), making it likely that the phenomenon is common among the approximately 10 000 unstudied morpho-species [9] of insect social parasites, many of which are Red Data Book listed [15,25]. Other parasitic systems may be similar, particularly where species' interactions are governed by non-morphological cues such as chemical signalling or resistance [8,10,43]. Thus, while molecular techniques have strengthened the species paradigm by identifying cryptic species among certain types of listed morpho-species [8,10,11], conservationists cannot yet rely on them [12–14] to recognize functionally distinct forms or siblings of extreme specialists which perhaps differ by a single gene or which evolve and disappear over millennia rather than epochs, and yet are among the most interesting and threatened species on the Earth.
Acknowledgements
We thank P. W. H. Holland for illuminating discussions on speciation, R. Hails and S. Freeman for statistical advice, J. C. Wardlaw, S. Everett and A. Worgan for help with experimental insects, A. Górnicki for fieldwork, Richard Lewington for artwork and EU Framework programmes Macman and CLIMIT for funding.
References
- 1.Mace GM. 2004. The role of taxonomy in species conservation. Phil. Trans. R. Soc. Lond. B 359, 711–719 10.1098/rstb.2003.1454 (doi:10.1098/rstb.2003.1454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winke K, Ingram KK, Das I. 2006. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 22, 148–155 10.1016/j.tree.2006.11.004 (doi:10.1016/j.tree.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 3.Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press [Google Scholar]
- 4.Molbo D, Machado CA, Sevenster JG, Keller L, Herre EA. 2003. Cryptic species of fig-pollinating wasps: implications for the evolution of the fig–wasp mutualism, sex allocation, and precision of adaptation. Proc. Natl Acad. Sci. USA 100, 5867–5872 10.1073/pnas.0930903100 (doi:10.1073/pnas.0930903100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greischar MA, Koskella B. 2007. A synthesis of experimental work on parasite local adaptation. Ecol. Lett. 10, 418–434 10.1111/j.1461-0248.2007.01028.x (doi:10.1111/j.1461-0248.2007.01028.x) [DOI] [PubMed] [Google Scholar]
- 6.Finlay BL, Thomas JA, McGavin GC, Fenchel T, Clarke RT. 2006. Self-similar patterns of nature: insect diversity at local to global scales. Proc. R. Soc. B 273, 1935–1941 10.1098/rspb.2006.3525 (doi:10.1098/rspb.2006.3525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schönrogge K, et al. 2006. Host propagation permits extreme local adaptation in a social parasite of ants. Ecol. Lett. 9, 1032–1040 10.1111/j.1461-0248.2006.00957.x (doi:10.1111/j.1461-0248.2006.00957.x) [DOI] [PubMed] [Google Scholar]
- 8.Smith MA, Rodriguez JJ, Whitfield JB, Deans AR, Janzen DH, Hallwachs W, Hebert PDN. 2008. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology and collections. Proc. Natl Acad. Sci. USA 105, 12 359–12 364 10.1073/pnas.0805319105 (doi:10.1073/pnas.0805319105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas JA, Schönrogge K, Elmes GW. 2005. Specialisations and host associations of social parasites of ants. In Insect evolutionary ecology (eds Fellowes MDE, Holloway GJ, Rolff J.), pp. 479–518 Wallingford, UK: CABI [Google Scholar]
- 10.Smith MA, Woodley NE, Janzen DH, Hallwachs W, Hebert PD. 2006. DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc. Natl Acad. Sci. USA 103, 3657–3662 10.1073/pnas.0511318103 (doi:10.1073/pnas.0511318103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert PD, Penton EH, Burns JM, Janzen DH, Hallwachs W. 2004. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl Acad. Sci. USA 101, 14 812–14 817 10.1073/pnas.0406166101 (doi:10.1073/pnas.0406166101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiemers M, Fiedler K. 2007. Does the DNA barcoding gap exist? A case study in blue butterflies (Lepidoptera: Lycaenidae). Front. Zool. 4, 8. 10.1186/1742-9994-4-8 (doi:10.1186/1742-9994-4-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueshima R, Asami T. 2003. Single-gene speciation by left–right reversal: a land-snail species of polyphyletic origin results from chirality constraints on mating. Nature 425, 679. 10.1038/425679a (doi:10.1038/425679a) [DOI] [PubMed] [Google Scholar]
- 14.Galtier N, Nabholz B, Gle Min S, Hurs GDD. 2009. Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol. Ecol. 18, 4541–4550 10.1111/j.1365-294X.2009.04380.x (doi:10.1111/j.1365-294X.2009.04380.x) [DOI] [PubMed] [Google Scholar]
- 15.Thomas JA, Settele J. 2004. Butterfly mimics of ants. Nature 432, 283–284 10.1038/432283a (doi:10.1038/432283a) [DOI] [PubMed] [Google Scholar]
- 16.Thomas JA, Simcox DJ, Clarke RT. 2009. Successful conservation of a threatened Maculinea butterfly. Science 325, 80–83 10.1126/science.1175726 (doi:10.1126/science.1175726) [DOI] [PubMed] [Google Scholar]
- 17.Settele J, Kühn E. 2009. Insect conservation. Science 325, 41–42 10.1126/science.1176892 (doi:10.1126/science.1176892) [DOI] [PubMed] [Google Scholar]
- 18.Akino T, Knapp JJ, Thomas JA, Elmes GW. 1999. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 266, 1419–1426 10.1098/rspb.1999.0796 (doi:10.1098/rspb.1999.0796) [DOI] [Google Scholar]
- 19.Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K. 2009. Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323, 782–785 10.1126/science.1163583 (doi:10.1126/science.1163583) [DOI] [PubMed] [Google Scholar]
- 20.Als TD, Vila R, Kandul NP, Nash DR, Yen S-H, Hsu Y-F, Mignault AA, Boomsma JJ, Pierce NE. 2004. The evolution of alternative parasitic life histories in large blue butterflies. Nature 432, 386–390 10.1038/nature03020 (doi:10.1038/nature03020) [DOI] [PubMed] [Google Scholar]
- 21.Ugelvig LV, Vila R, Pierce NE, Nash DR. 2011. A phylogenetic revision of the Glaucopsyche section (Lepidoptera: Lycaenidae), with special focus on the Phengaris–Maculinea clade. Mol. Phylogenet. Evol. 61, 237–243 10.1016/j.ympev.2011.05.016 (doi:10.1016/j.ympev.2011.05.016) [DOI] [PubMed] [Google Scholar]
- 22.Sielezniew M, Rutkowski R, Ponikwicka D, Ratkiewicz M, Dziekańska I, Švitra G. 2012. Differences in genetic variability between two ecotypes of endangered myrmecophilous butterfly Phengaris (=Maculinea)alcon–the setting of conservation priorities. Insect Conserv. Divers. 5, 222–236 10.1111/j.1752-4598.2011.00163.x (doi:10.1111/j.1752-4598.2011.00163.x) [DOI] [Google Scholar]
- 23.Thomas JA, Elmes GW, Wardlaw JC. 1990. Polymorphic growth in larvae of the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 265, 1895–1901 10.1098/rspb.1998.0517 (doi:10.1098/rspb.1998.0517) [DOI] [Google Scholar]
- 24.Nash DR, Als TD, Jones GR, Boomsma JJ. 2008. A mosaic of chemical coevolution in a large blue butterfly. Science 319, 88–90 10.1126/science.1149180 (doi:10.1126/science.1149180) [DOI] [PubMed] [Google Scholar]
- 25.IUCN 1990. Red list of threatened animals. Gland, Switzerland: IUCN [Google Scholar]
- 26.Elmes GW, Akino T, Thomas JA, Clarke RT, Knapp JJ. 2002. Interspecific differences in cuticular hydrocarbon profiles of Myrmica ant species are sufficiently consistent to explain host specificity in Maculinea (large blue) butterflies. Oecologia 130, 525–535 10.1007/s00442-001-0857-5 (doi:10.1007/s00442-001-0857-5) [DOI] [PubMed] [Google Scholar]
- 27.Schönrogge K, Wardlaw JC, Peters AJ, Everett S, Thomas JA, Elmes GW. 2004. Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J. Chem. Ecol. 30, 91–107 10.1023/B:JOEC.0000013184.18176.a9 (doi:10.1023/B:JOEC.0000013184.18176.a9) [DOI] [PubMed] [Google Scholar]
- 28.Elmes GW, Wardlaw JC, Schönrogge K, Thomas JA. 2004. Food stress causes differential survival of socially parasitic larvae of Maculinea rebeli (Lepidoptera, Lycaenidae) integrated in colonies of host and non-host Myrmica species (Hymenoptera, Formicidae). Entomol. Exp. Appl. 110, 53–63 10.1111/j.0013-8703.2004.00121.x (doi:10.1111/j.0013-8703.2004.00121.x) [DOI] [Google Scholar]
- 29.Stankiewicz AM, Sielezniew M, Buszko J. 2005. Maculinea alcon and M. rebeli in Poland: distribution, habitats, host ant specificity and parasitoids. In Studies on the ecology and conservation of butterflies in Europe , vol. 2 (eds Settele J, Kühn E, Thomas JA.), pp. 90–93 Sofia, Bulgaria: Pensoft [Google Scholar]
- 30.Hochberg M, Thomas JA, Elmes GW. 1992. The population dynamics of a large blue butterfly, Maculinea rebeli, a parasite of red ant nests. J. Anim. Ecol. 61, 397–409 10.2307/5331 (doi:10.2307/5331) [DOI] [Google Scholar]
- 31.Thomas JA, Elmes GW, Wardlaw JC, Woyciechowski M. 1989. Host specificity among Maculinea butterflies in Myrmica ant nests. Oecologia 79, 452–457 10.1007/BF00378660 (doi:10.1007/BF00378660) [DOI] [PubMed] [Google Scholar]
- 32.Elmes GW, Thomas JA, Wardlaw JC. 1991. Larvae of Maculinea rebeli, a large blue butterfly, and their Myrmica host ants: wild adoption and behaviour in ant nests. J. Zool. 223, 447–460 10.1111/j.1469-7998.1991.tb04775.x (doi:10.1111/j.1469-7998.1991.tb04775.x) [DOI] [Google Scholar]
- 33.Wardlaw JC, Elmes GW, Thomas JA. 1998. Techniques for studying Maculinea butterflies. I. Rearing Maculinea caterpillars with Myrmica ants in the laboratory. J. Insect Conserv. 2, 79–84 10.1023/A:1009648908035 (doi:10.1023/A:1009648908035) [DOI] [Google Scholar]
- 34.Carr MR. 1996. Primer user manual (Plymouth routines in multivariate ecological research), version 4.0b. Plymouth, UK: Plymouth Marine Laboratory [Google Scholar]
- 35.Clarke KR. 1993. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143 10.1111/j.1442-9993.1993.tb00438.x (doi:10.1111/j.1442-9993.1993.tb00438.x) [DOI] [Google Scholar]
- 36.Stewart KEJ, Bourn NAD, Thomas JA. 2001. An evaluation of three quick methods commonly used to assess sward height in ecology. J. Appl. Ecol. 38, 1148–1154 10.1046/j.1365-2664.2001.00658.x (doi:10.1046/j.1365-2664.2001.00658.x) [DOI] [Google Scholar]
- 37.Thomas JA, Elmes GW. 2001. Foodplant niche selection rather than the presence of ant nests explains oviposition patterns in the myrmecophilous butterfly genus Maculinea. Proc. R. Soc. Lond. B 268, 471–477 10.1098/rspb.2000.1398 (doi:10.1098/rspb.2000.1398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radchenko AG, Elmes GW. 2011. Myrmica ants (Hymenoptera: Formicidae) of the Old World. Warsaw, Poland: NODF [Google Scholar]
- 39.Blomquist GJ, Bagnéres AG. 2010. Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 40.Elmes GW, Thomas JA, Hammarstedt O, Munguira MC, Martin J, van der Made JG. 1994. Differences in host-ant specificity between Spanish, Dutch and Swedish populations of the endangered butterfly Maculinea alcon (Schiff.) (Lepidoptera). Zool. Memorabilia 48, 55–68 [Google Scholar]
- 41.Wood CC, Bickham JW, Nelson RJ, Foote CJ, Patton JC. 2008. Recurrent evolution of life history ecotypes in sockeye salmon: implications for conservation and future evolution. Evol. Appl. 1, 207–221 10.1111/j.1752-4571.2008.00028.x (doi:10.1111/j.1752-4571.2008.00028.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tartally A, Nash DR, Lengyel S, Varga Z. 2008. Patterns of host ant use by sympatric populations of M. alcon and M. rebeli in the Carpathian Basin. Insect. Soc. 55, 370–381 10.1007/s00040-008-1015-4 (doi:10.1007/s00040-008-1015-4) [DOI] [Google Scholar]
- 43.Schönrogge K, Barr B, Napper E, Breen J, Gardner MG, Elmes GW, Thomas JA. 2002. When rare species become endangered: cryptic speciation in myrmecophilous hoverflies. Biol. J. Linn. Soc. 75, 291–300 10.1111/j.1095-8312.2002.tb02070.x (doi:10.1111/j.1095-8312.2002.tb02070.x) [DOI] [Google Scholar]