Abstract
We investigated whether among-sibling differences in the phenotypes of juvenile fish were systematically related to the position in the egg mass where each individual developed during oogenesis. We sampled eggs from the front, middle and rear thirds of the egg mass in female brown trout of known dominance rank. In the resulting juveniles, we then measured traits that are related to individual fitness: body size, social status and standard metabolic rate (SMR). When controlling for differences among females in mean egg size, siblings from dominant mothers were initially larger (and had a lower mass-corrected SMR) if they developed from eggs at the rear of the egg mass. However, heterogeneity in the size of siblings from different positions in the egg mass diminished in lower-ranking females. Location of the egg within the egg mass also affected the social dominance of the resulting juvenile fish, although the direction of this effect varied with developmental age. This study provides the first evidence of a systematic basis for among-sibling differences in the phenotypes of offspring in a highly fecund organism.
Keywords: maternal effects, unpredictable environments, maternal fitness
1. Introduction
Environmental effects on mothers can lead to variations in their growth, condition and physiological state that can be transmitted to offspring via non-genetic resources provided to eggs [1]. There are numerous examples in the literature demonstrating that phenotypic differences among juveniles are influenced by environmental conditions that affect the state of the mother [2–4]. For example, the pattern or extent of maternal investment can depend on the mother's social environment or physiological state. In oviparous vertebrates, a mother's interactions with conspecifics can influence the size and composition of her eggs [5,6]. Such variation can have implications for maternal fitness because egg size and composition can influence offspring growth, survival and behaviour [1,7].
Mothers can also adjust the phenotypes of sibling offspring. For example, in many species of birds, differences in the sizes of the first- and last-laid eggs within a clutch have been interpreted as a maternal tool for matching the size of the brood to local environmental conditions [8]. Recent evidence from teleost fish and marine invertebrates demonstrates that within-clutch variation in offspring phenotype increases when environments are unpredictable [9], suggesting that variation among siblings may be a bet-hedging mechanism. However, alternative hypotheses for within-clutch variation, excluding bet-hedging scenarios, have not been examined in more fecund vertebrates, such as teleost fishes. In many species of fish, embryos, larvae and juveniles receive little or no parental care, meaning that mothers can influence the development of their young only through their initial investment in each egg. Furthermore, in some species, such as the salmonids, females spawn large clutches of similarly sized eggs almost simultaneously. The nests may be spread over some distance, or laid sequentially in an upstream direction and in close proximity to each other, to form a ‘redd’ [10,11]. In fishes, if the position of the eggs in the abdominal cavity correlates with laying order, then eggs nearest the ovipositor will be deposited first and possibly in a separate nest(s) from those further away from the ovipositor. Given that the number of eggs deposited by females in successive nests can decline throughout oviposition [10,12], the degree of competition among siblings may vary over the sequence of nests produced by the mother if those nests are well separated. Hence, the relative survival and growth of siblings may be related to the distribution of their phenotypes in the egg mass prior to spawning.
The performance of juvenile salmonid fish in streams is typically influenced by a dominance/territoriality-based social structure [13]. Two phenotypic traits primarily determine juvenile success in these systems. First, larger eggs give rise to larger juveniles that have a survival advantage under poor growth conditions [14]. Second, competitive ability is positively related to standard metabolic rate (SMR) [15]. SMR is the lowest rate of energy use when measured in an inactive, post-absorptive ectotherm and corrected for temperature [16]; two- to threefold variation in SMR has been reported among sibling salmonids (reviewed by Burton et al. [17]). Juvenile salmonids with higher SMRs can process food faster and so potentially gain a further growth advantage through their ability to feed more frequently [18]. Thus, in productive environments (e.g. abundant food or low competition) juveniles with a relatively high SMR are more likely to be competitively dominant, allowing them to gain productive territories and grow faster [19,20]. However, in poorer conditions (for example, where competition among siblings is intense), a high SMR may be of no advantage if gains in food intake are unable to offset the higher ‘maintenance costs’ of this phenotype, leading to no relationship between SMR/dominance and growth [17,21].
Several hypotheses attempt to explain why there may be advantages in such large within-family variation in SMR of juvenile salmonids. Such variation among siblings may confer maternal heterogeneous advantage, whereby family survival is increased if siblings comprise a range of specialists (with different SMRs) that can exploit the variety of habitats evident in natural streams [22]. Alternatively, variation in sibling SMR may enable bet-hedging in unpredictable environments [9], or comprise a dispersal mechanism to produce dominant fish that remain near the nest and subordinates that lose encounters and relocate [23]. Here we test whether the mean phenotype of juvenile trout differs according to the position in the egg mass where individual eggs were located before spawning. In addition, because investment in offspring can vary according to the social environment of the mother, we test whether this relationship may be modulated by maternal dominance rank.
2. Material and methods
(a). Maternal dominance ranking and crosses
We used an experimental approach to determine how maternal dominance and position of eggs in the ovary during development affect juvenile phenotype. Stock for this experiment came from clutches of eggs taken from 12 female brown trout (Salmo trutta). To ensure that these females covered a broad spectrum of dominance ranks, the sampled clutches were selected from a larger pool of 34 spawning female trout that had been randomly spread between four tanks. Female trout are largely non-feeding in the weeks prior to spawning. However, during this time, they prefer habitats with overhead cover [23]. Thus we quantified relative dominance using a serial removal technique by recording priority of access to a single shelter in otherwise bare tanks. The shelter was a PVC pipe (40 cm long and 15 cm diameter), fitted with a passive integrated transponder detector that monitored the presence and identity of tagged females residing within it.
For each selected fish, separate batches of eggs were obtained from the front, middle and rear third of the egg mass (by dissection, position relative to the mother's head), and then fertilized using milt from a single male for each batch. We assume that the spatial configuration of eggs within the ovaries is maintained from vitellogenesis (the main period of egg growth, where the developing eggs are bound within a cellular sheath known as the follicle layer) through to ovulation (after the follicle layer of each ovary has broken down, allowing the eggs to lie loose in the abdominal cavity anterior to the ovipositor), and so predicts the order in which the eggs will be laid. Subsamples of eggs were taken from each female and preserved in buffered formalin for later counting to determine individual egg weight. Unfortunately, we did not preserve a subsample of eggs that was specific to each region of the egg mass because of the need to ensure we had sufficient numbers of surviving offspring for the behavioural experiments. Details of animal husbandry and the dominance ranking procedure are presented in the electronic supplementary material.
(b). Measurement of offspring phenotypes
Representative egg mass values (referred to hereafter as ‘egg sizes’) were estimated by counting the number of eggs in a weighed subsample (range 3.9–11.8 g) from each female. To test whether position within the egg mass influenced subsequent offspring body size, 10 juveniles from each egg mass position per family were killed and preserved in 5 per cent buffered formalin at the onset of independent feeding, but before they were given exogenous food. The preserved juveniles were subsequently weighed (to 0.0001 g).
Open flow respirometry was employed to investigate whether position within the egg mass influenced offspring SMR at the time of first feeding. The protocol followed that of Burton et al. [24], with minor modifications. SMR data were obtained for eight to nine juveniles from each egg mass position for each of 12 families (n = 301 juveniles in total). After being screened for metabolic rate, the juveniles were allocated into family triads containing one sibling from each egg mass position. Each triad was then placed in a compartment of a re-circulating stream, as described by Burton et al. [24]. The relationship between position within the egg mass and subsequent offspring behaviour was assessed according to the protocols described by Burton et al. [24]. Briefly, we assessed the relative social status of the three individuals in each tank compartment by monitoring their ability to compete for food and territory space, together with the outcome of any overt aggressive interactions. These three indicators of social status are hereafter referred to as competitive ability, territory quality and aggression. Behavioural data were obtained for eight juveniles from each egg mass position for each of 12 families (n = 288 total; n = 96 triads; data collected over the first 7 weeks of juvenile life). See the electronic supplementary material for further details of the rearing and phenotypic measurements of offspring.
(c). Data analysis
To analyse relationships between egg mass position and subsequent offspring phenotypes, we fitted linear mixed-effect (LME) models, with family as a random factor, egg mass position (front, middle or rear) as a fixed categorical variable and maternal dominance rank as a continuous variable. Details of specific models are outlined below. The relationship between juvenile body mass at the first feeding stage of development and egg mass position was analysed with egg size as an additional explanatory variable (including all two way interactions). This analysis omitted data from one of the families, because one female produced too few eggs to enable retention of a preserved egg sample.
The relationship between metabolic rate (MR) of the juveniles and position within the egg mass was modelled with individual activity level during respirometry measurement, water temperature and age (days since the first feeding stage of development) at the time of measurement as additional continuous variables (including the two-way interactions between age, maternal dominance rank and egg mass position). Activity level, as visually observed during respirometry, was included here to account for potential inflation of MR with increased activity and hence to assess SMR. Respirometry batch (i.e. date of SMR measurement) was included as an additional random variable to account for potential among-batch differences in MR. Prior to analysis, rates of SMR were corrected for effect of body mass by calculating the residuals from a regression of SMR on body mass (both values log-transformed).
Measurements of juvenile aggression, territory quality and competitive ability were summarized using principal component analysis (PCA; see the electronic supplementary material). This resulted in a single principal component (PC1) summarizing all three behaviours as an index of juvenile social status. Individual PC scores were then analysed in an LME model comparing egg mass positions with family as a random variable and juvenile body mass, age (days since first feeding) and residual SMR as a continuous variable (including all two-way interactions). See the electronic supplementary material for full details of statistical analysis.
3. Results
Egg size, maternal dominance rank and position within the egg mass all influenced the size of juvenile salmon. Overall, egg size had a positive effect on juvenile body size (parameter estimate±s.e.: 0.62±0.18, t-value = 3.50, p < 0.001; figure 1a). However, the strength of this effect was contingent upon the location within the mother's egg mass from which the egg had originated (hereafter referred to as ‘egg mass position’). In females that produced small eggs, egg mass position had little effect on juvenile body size. However, as the female's mean egg size increased, juveniles originating from the middle part of her egg mass were larger than those from the front and the rear of the egg mass (parameter estimates±s.e. for egg size × egg mass position interaction: middle juveniles versus front juveniles, 0.57±0.17, t-value = 3.33, p < 0.01; middle juveniles versus rear juveniles, 0.62±0.17, t-value = 3.66, p < 0.001; figure 1a and electronic supplementary material, table S3).
Figure 1.
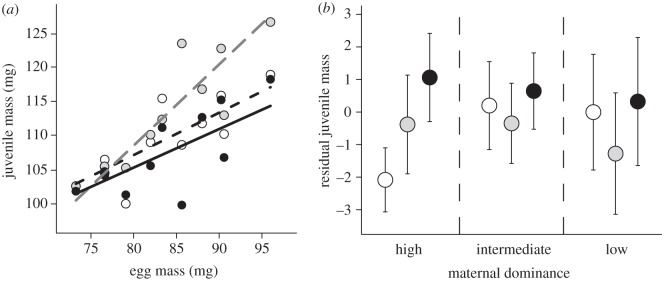
Juvenile body mass at the first feeding stage of development in relation to (a) egg mass (mean value per family) and (b) maternal dominance. In both cases, juvenile mass depends on the position within the egg mass (front, middle or rear) from which the juveniles originated. In (a), lines are the predicted values for each egg mass position from the final LME model (see electronic supplementary material, table S3 for statistical analysis). White circles/dashed black line, front of egg mass; grey circles/dashed grey line, middle of egg mass; black circles/solid black line, rear of egg mass. The predicted values are based on a female of average dominance (2.95). Juvenile mass data are mean family values (n = 10 per egg mass position). In (b), points are mean residual values (± s.e.) averaged by egg mass position across females of different dominance status: white circles, front of egg mass; grey circles, middle of egg mass; black circles, rear of egg mass. High dominance, mothers ranked between 1.0 and 2.0 (n = 4); intermediate dominance, mothers ranked between 2.5 and 3.0 (n = 4); low dominance, mothers ranked between 4.0 and 5.5 (n = 3). Residual juvenile mass values were derived from an LME model with family as a random factor and mean family egg mass × egg mass position interaction as explanatory variables, because the relationship between juvenile mass and egg mass differed among sections of the egg mass (see electronic supplementary material, table S3). Maternal dominance effects have been plotted categorically to aid visual interpretation.
Maternal dominance rank also influenced juvenile body size, independently of egg size, although the strength and direction of this effect was dependent on egg mass position. As maternal dominance decreased, the mass of juveniles from the middle part of the egg mass decreased relative to that of juveniles from the front and the rear of the egg mass (parameter estimates±s.e. for maternal dominance × egg mass position interaction: middle juveniles versus front juveniles, −1.82±0.78, t-value = −2.33, p < 0.05; middle juveniles versus rear juveniles, −2.06±0.78, t-value = −2.63, p < 0.01; figure 1b and electronic supplementary material, table S3). In the full sample of spawning female trout, maternal dominance ranks were not associated with their body size (general linear model: F1,23 = 1.62, p = 0.22), nor were the size of eggs produced by individual females related to their body size or dominance rank (sequential removal of terms in general linear model: body size, F1,20 = 0.51, p = 0.48: maternal dominance rank, F1,21 = 1.09, p = 0.31). Together these results indicate that both dominant females and females that laid large eggs (irrespective of their own body size) produced offspring that differed in body size depending upon the position within the egg mass where those individuals developed.
Standard metabolic rate (SMR) values were log-linearly related (figure 2a) to the individual's body mass according to the equation log SMR = 0.847 × (log body mass mg) − 1.366 (r2 = 0.44, n = 301, p < 0.0001). After correction for the effect of body mass, both maternal dominance rank and egg mass position influenced the standard metabolism of juveniles. Juvenile offspring of dominant mothers had relatively higher metabolic rates if they originated from the front and middle parts of the egg mass (figure 2b). However, as maternal social status decreased, this trend reversed: juveniles from the rear section of the egg mass of subordinate mothers had higher SMRs than those from the front and middle of the egg mass (parameter estimates±s.e. for maternal dominance × egg mass position interaction: rear juveniles versus front juveniles, 0.02±0.01, t-value = 2.47, p < 0.05; middle juveniles versus rear juveniles, −0.02±0.01, t-value = −2.72, p < 0.01; figure 2b and electronic supplementary material, table S4).
Figure 2.
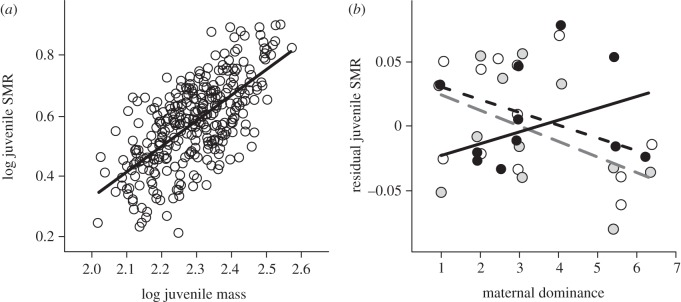
Standard metabolic rate (SMR) in relation to (a) body mass of juvenile trout (log values shown for both variables), and (b) maternal dominance and the position within the egg mass from which the juveniles originated. In (a), line represents the predicted values from the LME model describing the relationship between SMR and body mass. In (b) values are residuals from the regression shown in (a) and lines are the predicted values for each egg mass position from the final LME model (see electronic supplementary material, table S4 for statistical analysis). White circles/dashed black line, front of egg mass; grey circles/dashed grey line, middle of egg mass; black circles/solid black line, rear of egg mass.
Contrary to our findings for juvenile body size and SMR, juvenile social status (defined as PC1) was unrelated to maternal dominance rank, and nor was it influenced by mass-corrected SMR or juvenile size (see the electronic supplementary material, table S5). However, social status was significantly influenced by the position in the egg mass from which juveniles originated, but the direction of this effect changed as juveniles grew older (figure 3). The first and last measurements of SMR on replicate batches of fish covered a time span of 32 days, so that the age (since the start of exogenous feeding) of the fish in each triad ranged from 18 to 49 days. During the earliest batches (age = 18–20 days), juveniles from the rear of the egg mass had higher social status than those from the front and middle sections. Among juveniles of intermediate ages (age = 34–36 days), individuals from the front of the egg mass had the highest social status, but by 7 weeks of age, dominance in offspring increased from front to middle to rear positions in the egg mass (parameter estimates±s.e. for age × egg mass position interaction: middle juveniles versus front juveniles, 0.03±0.02, t-value = 2.04, p < 0.05; middle juveniles versus rear juveniles, 0.05±0.02, t-value = 3.08, p < 0.01; figure 3 and electronic supplementary material, table S5). In summary, social status of juveniles from the front of the egg mass was approximately equivalent over time, whereas social status of juveniles from the middle and rear of the egg mass, respectively, tended to increase and decrease with juvenile age.
Figure 3.
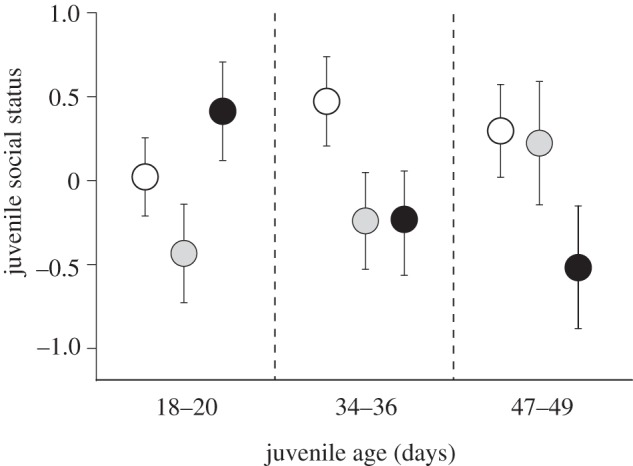
Relationship between social status of juvenile trout and position within the egg mass from which the juveniles originated is dependent on juvenile age (n = 15–18 juveniles per egg mass position in each age category). White circles, front of egg mass; grey circles, middle of egg mass; black circles, rear of egg mass. Data are mean (± s.e.) principal component scores (PC1), derived from a PCA of the aggression, competitive ability and territory quality measures of behaviour averaged across all 12 families for each egg mass position (see the electronic supplementary material). Although age is treated as a continuous variable in the analyses (see the electronic supplementary material, table S5), effects have been plotted at discrete time periods to aid visual interpretation. Behavioural data were obtained for the same juveniles that were measured for SMR (see electronic supplementary material, table S5 for statistical analysis).
4. Discussion
This study provides the first evidence for systematic differences in the phenotypes of offspring within egg batches in a highly fecund species. Our study also shows that within an egg batch, maternal influences may be expressed as differences in offspring size, behaviour and physiology. Differences in the body size, energy metabolism and social status of siblings were partly attributable to the location within the egg batch in which they developed as eggs. However, when considering juvenile size and SMR, the strength and direction of the effect of egg mass position was related to the dominance rank of the mother, suggesting that ecological factors, such as environmental conditions or competitor densities experienced by mothers, can also influence how egg mass position affects the development of offspring phenotypes. Our results also show that the relationship between egg size and subsequent juvenile size is modulated by egg mass position, and that the effects of position within the egg mass on subsequent offspring social status can change with juvenile age.
Steroid hormones are likely mediators of sibling differences within egg batches because studies across a range of vertebrate taxa, including fish, have shown that maternal hormone levels are under environmental influence, are transferred to eggs, and affect many offspring traits, including growth [25,26], physiology [27,28] and behaviour [29,30]. Preliminary evidence indicates that concentrations of maternally derived cortisol are higher among eggs from the anterior part of the ovary in trout [31]. Thus, female fish could theoretically produce the range of phenotypes reported here among siblings via the differential transfer of hormones to eggs, dependent on position within the egg mass. However, it is unlikely that the current results can be attributed solely to hormonal effects. For example, the largest differences in juvenile size with respect to position within the egg mass were observed in females that produced the largest eggs. Although egg hormones can have strong effects on juvenile growth, systematic differences in egg size among regions of the egg mass represent a more plausible explanation in this study because egg size explains more than 70 per cent of the variation in the size of juvenile fishes [32]. While we did not collect egg size data from each region of the egg mass, within-female variation in egg size is generally low in salmonids: less than 3 per cent of the variation in egg size is due to differences within females [11]. However, within-female variation in egg size may be increased in some situations (e.g. captive rearing [14]). This suggests that certain environments may result in a systematic component to the provisioning of individual eggs (in terms of composition or size) among females that produce large eggs or differ in dominance status.
In contrast to studies of Atlantic salmon, juvenile social status was not associated with body size or mass-corrected SMR [20,33]. This finding suggests that the relationship between egg mass position and juvenile social status is not mediated through an altered programming of these traits. Our results also indicate that ontogenetic changes in juvenile behaviour are related to their developmental position within the ovary, since the relative social status of juveniles from the front, middle and rear parts of the ovary changed with age. If siblings are provisioned differentially according to their position within the ovary, it is possible that variation in their social rank may be expressed at different stages of ontogeny. We observed a general decrease in social status with juvenile age for offspring that developed from eggs at the rear of the egg mass compared with a general increase in social status for offspring resulting from eggs from the middle of the egg mass during the same time period. If eggs from different regions of the egg mass are spawned into closely grouped nests, such a pattern might reduce competition among siblings by generating subordinate dispersers that later exhibit more dominant behaviour and establish territories remote from the nest site.
In general, the results of this study suggest that investment in offspring may vary among mothers of different social rank. Differences in offspring among regions of the egg mass were evident in dominant mothers, whereas subordinate mothers seem to spread differences in offspring phenotypes more uniformly across the egg mass. This difference might reflect an energy cost that can be accommodated by dominant but not subordinate mothers. Conversely, bet-hedging hypotheses have suggested that within-clutch variation may be a constraint resulting from an inability of mothers to allocate resources evenly among siblings [34]. Thus, if dominant mothers are required to expend more energy in maintaining their social rank they might have less energy available for reproduction and be constrained to invest in a certain manner across the egg mass. Evidence from birds shows that mothers can be constrained in their investment in individual offspring because the last-laid egg is often smaller and more poorly provisioned, and the resulting chicks have a lower probability of survival [35]. Nevertheless, we found no evidence that dominant mothers in our experiment were in poorer physiological condition than subordinates. Indeed, these females probably benefitted from reduced metabolic rates owing to their greater access to shelter [36]. It is also possible that spawning options vary with female dominance, which is reflected in different investment strategies within the egg mass. Adult brown trout are known to be very cautious, and overhead cover (e.g. submerged logs, undercut banks) is a critical habitat requirement for their spawning. For example, it has been reported that over 80 per cent of nests are located within 1.5 m of cover [37]. In the closely related Atlantic salmon, female choice of nesting location may be influenced by aggression from other nesting females [10]. It is likely, therefore, that dominant females acquire preferential access to spawning areas with close proximity to cover. If dominant mothers are better able to determine where (and when) they spawn their nests (for example, in a sequence clumped near overhead cover), they may benefit from greater differences in egg/offspring traits between nests. Indeed, recent empirical evidence shows that enhanced diversity in phenotypes (and genotypes) within populations may increase the number of juveniles that survive and the amount of biomass produced [22,37] (but see [38] for an examination of this issue, though limited to egg size effects). This has led to suggestions that a similar principle may apply within clutches as a bet-hedging mechanism against environmental uncertainty and to match differences in offspring to spatial variation in their environment [39]. Conversely, subordinate mothers may have no access to preferred nesting sites, be less likely to place their nests within close proximity to each other and hence have less to gain from encouraging dispersal among their offspring.
In summary, we show the first evidence of a systematic component to the distribution of within-clutch heterogeneity in offspring size, behaviour and physiology in a highly fecund species. Furthermore, we show that these differences can reflect maternal dominance rank and egg size. Variation in the composition of eggs (e.g. egg hormone concentrations, relative lipid content, presence of antioxidants) from different regions of the ovary warrants further investigation as a mechanistic explanation for the results presented here. Overall, our study suggests that strategies for investment in offspring may vary among mothers of different social rank, and that the results of different investment strategies might change with ontogeny during early juvenile development. More broadly, our results are consistent with the hypothesis that mothers can enhance their fitness by programming the phenotypes of their offspring during egg development.
Acknowledgements
We thank M. S. Miles at Almondbank hatchery and G. Law at the University of Glasgow for their assistance with procedures and fish husbandry. We also thank T. G. G. Groothuis from Groningen University for helpful comments on the initial design of the experiment, P. Rycroft for building the PIT-tag-detecting shelters and two anonymous referees whose comments greatly improved a previous version of this manuscript. T.B. was funded by a University of Glasgow Postgraduate Scholarship and ORSAS award, and M.O.H. by an NERC grant to N.B.M., J.D.A. and T. G. G. Groothuis, and through the ARC Centre of Excellence for Coral Reef Studies. This experiment was authorized by licences from the UK Home Office. Original data are available at the DRYAD depository: http://dx.doi:10.5061/dryad.cj48b.
References
- 1.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 2.Gagliano M, McCormick MI. 2006. Maternal condition influences phenotypic selection on offspring. J. Anim. Ecol. 76, 174–182 10.1111/j.1365-2656.2006.01187.x (doi:10.1111/j.1365-2656.2006.01187.x) [DOI] [PubMed] [Google Scholar]
- 3.Warner DA, Lovern MB, Shine R. 2007. Maternal nutrition affects reproductive output and sex allocation in a lizard with environmental sex determination. Proc. R. Soc. B 274, 883–890 10.1098/rspb.2006.0105 (doi:10.1098/rspb.2006.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verboven N, Monaghan P, Evans DM, Schwabl H, Evans N, Whitelaw C, Nager RG. 2003. Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the lesser black-backed gull (Larus fuscus). Proc. R. Soc. Lond. B 270, 2223–2232 10.1098/rspb.2003.2496 (doi:10.1098/rspb.2003.2496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanvez A, Parisot M, Chastel O, Leboucher G. 2008. Does maternal social hierarchy affect yolk testosterone deposition in domesticated canaries? Anim. Behav. 75, 929–934 10.1016/j.anbehav.2007.08.006 (doi:10.1016/j.anbehav.2007.08.006) [DOI] [Google Scholar]
- 6.Verboven N, Evans NP, D'Alba L, Nager RG, Blount JD, Surai PF, Monaghan P. 2005. Intra-specific interactions influence egg composition in the lesser black-backed gull (Larus fuscus). Behav. Ecol. Sociobiol. 57, 357–365 10.1007/s00265-004-0862-x (doi:10.1007/s00265-004-0862-x) [DOI] [Google Scholar]
- 7.Groothuis TGG, Muller W, von Engelhardt N, Carere C, Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 8.Slagsvold T, Sandvik J, Rofstad G, Lorentsen Ö, Husby M. 1984. On the adaptive value of intraclutch egg-size variation in birds. Auk 101, 685–697 10.2307/4086895 (doi:10.2307/4086895) [DOI] [Google Scholar]
- 9.Crean AJ, Marshall DJ. 2009. Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Phil. Trans. R. Soc. B 364, 1087–1096 10.1098/rstb.2008.0237 (doi:10.1098/rstb.2008.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming IA. 1996. Reproductive strategies of Atlantic salmon: ecology and evolution. Rev. Fish Biol. Fisheries 6, 379–416 10.1007/BF00164323 (doi:10.1007/BF00164323) [DOI] [Google Scholar]
- 11.Jonsson B, Jonsson N. 2011. Ecology of Atlantic salmon and brown trout: habitat as a template for life histories. Dordrecht, The Netherlands: Springer Science+Business Media BV [Google Scholar]
- 12.de Gaudemar B, Schroder SL, Beall EP. 2000. Nest placement and egg distribution in Atlantic salmon redds. Environ. Biol. Fishes 57, 37–47 10.1023/A:1007562508973 (doi:10.1023/A:1007562508973) [DOI] [Google Scholar]
- 13.Metcalfe NB. 1998. The interaction between behavior and physiology in determining life history patterns in Atlantic salmon (Salmo salar). Can. J. Fisheries Aquatic Sci. 55, 93–103 10.1139/d98-005 (doi:10.1139/d98-005) [DOI] [Google Scholar]
- 14.Einum S, Fleming IA. 1999. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc. R. Soc. Lond. B 266, 2095–2100 10.1098/rspb.1999.0893 (doi:10.1098/rspb.1999.0893) [DOI] [Google Scholar]
- 15.McCarthy ID. 2001. Competitive ability is related to metabolic asymmetry in juvenile rainbow trout. J. Fish Biol. 59, 1002–1014 10.1111/j.1095-8649.2001.tb00167.x (doi:10.1111/j.1095-8649.2001.tb00167.x) [DOI] [Google Scholar]
- 16.McNab BK. 2002. The physiological ecology of vertebrates. Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- 17.Burton T, Killen SS, Armstrong JD, Metcalfe NB. 2011. What causes intra-specific variation in resting metabolic rate and what are its ecological consequences?. Proc. R. Soc. B 278, 3465–3473 10.1098/rspb.2011.1778 (doi:10.1098/rspb.2011.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millidine KJ, Armstrong JD, Metcalfe NB. 2009. Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proc. R. Soc. B 276, 2103–2108 10.1098/rspb.2009.0080 (doi:10.1098/rspb.2009.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy ID. 2000. Temporal repeatability of relative standard metabolic rate in juvenile Atlantic salmon and its relation to life history variation. J. Fish Biol. 57, 224–238 10.1111/j.1095-8649.2000.tb00788.x (doi:10.1111/j.1095-8649.2000.tb00788.x) [DOI] [Google Scholar]
- 20.Metcalfe NB, Taylor AC, Thorpe JE. 1995. Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim. Behav. 49, 431–436 10.1006/anbe.1995.0056 (doi:10.1006/anbe.1995.0056) [DOI] [Google Scholar]
- 21.Reid D, Armstrong JD, Metcalfe NB. 2011. Estimated standard metabolic rate interacts with territory quality and density to determine the growth rates of juvenile Atlantic salmon. Funct. Ecol. 25, 1360–1367 10.1111/j.1365-2435.2011.01894.x (doi:10.1111/j.1365-2435.2011.01894.x) [DOI] [Google Scholar]
- 22.Griffiths SW, Armstrong JD. 2001. The benefits of genetic diversity outweigh those of kin association in a territorial animal. Proc. R. Soc. Lond. B 268, 1293–1296 10.1098/rspb.2001.1660 (doi:10.1098/rspb.2001.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong JD, Kemp PS, Kennedy GJA, Ladle M, Milner NJ. 2003. Habitat requirements of Atlantic salmon and brown trout in rivers and streams. Fisheries Res. 62, 143–170 10.1016/S0165-7836(02)00160-1 (doi:10.1016/S0165-7836(02)00160-1) [DOI] [Google Scholar]
- 24.Burton T, Hoogenboom MO, Armstrong JD, Groothuis TGG, Metcalfe NB. 2011. Egg hormones in a highly fecund vertebrate: do they influence offspring social structure in competitive conditions? Funct. Ecol. 25, 1379–1388 10.1111/j.1365-2435.2011.01897.x (doi:10.1111/j.1365-2435.2011.01897.x) [DOI] [Google Scholar]
- 25.Eising CM, Eikenaar C, Schwabl H, Groothuis TGG. 2001. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. Lond. B 268, 839–846 10.1098/rspb.2001.1594 (doi:10.1098/rspb.2001.1594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick MI. 1999. Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia 118, 412–422 10.1007/s004420050743 (doi:10.1007/s004420050743) [DOI] [PubMed] [Google Scholar]
- 27.Sloman KA. 2010. Exposure of ova to cortisol pre-fertilisation affects subsequent behaviour and physiology of brown trout. Horm. Behav. 58, 433–439 10.1016/j.yhbeh.2010.05.010 (doi:10.1016/j.yhbeh.2010.05.010) [DOI] [PubMed] [Google Scholar]
- 28.Tobler M, Nilsson JÅ, Nilsson JF. 2007. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol. Lett. 3, 408–410 10.1098/rsbl.2007.0127 (doi:10.1098/rsbl.2007.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller W, Dijkstra C, Groothuis TGG. 2009. Maternal yolk androgens stimulate territorial behaviour in black-headed gull chicks. Biol. Lett. 5, 586–588 10.1098/rsbl.2009.0283 (doi:10.1098/rsbl.2009.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uller T, Olsson M. 2006. Direct exposure to corticosterone during embryonic development influences behaviour in an ovoviviparous lizard. Ethology 112, 390–397 10.1111/j.1439-0310.2006.01164.x (doi:10.1111/j.1439-0310.2006.01164.x) [DOI] [Google Scholar]
- 31.Suter HC. 2002. The effects of maternal steroids on individual variation in juvenile salmonids. PhD thesis, University of Glasgow, Glasgow, UK [Google Scholar]
- 32.Chambers RC, Leggett WC. 1996. Maternal influences on variation in egg sizes in temperate marine fishes. Am. Zool. 36, 180–196 10.1093/icb/36.2.180 (doi:10.1093/icb/36.2.180) [DOI] [Google Scholar]
- 33.Cutts CJ, Metcalfe NB, Taylor AC. 1999. Competitive asymmetries in territorial juvenile Atlantic salmon, Salmo salar. Oikos 86, 479–486 10.2307/3546652 (doi:10.2307/3546652) [DOI] [Google Scholar]
- 34.Einum S, Fleming IA. 2004. Environmental unpredictability and offspring size: conservative versus diversified bet-hedging. Evol. Ecol. Res. 6, 443–455 [Google Scholar]
- 35.Groothuis TGG, Eising CM, Blount JD, Surai P, Apanius V, Dijkstra C, Muller W. 2006. Multiple pathways of maternal effects in black-headed gull eggs: constraint and adaptive compensatory adjustment. J. Evol. Biol. 19, 1304–1313 10.1111/j.1420-9101.2005.01072.x (doi:10.1111/j.1420-9101.2005.01072.x) [DOI] [PubMed] [Google Scholar]
- 36.Millidine KJ, Armstrong JD, Metcalfe NB. 2006. Presence of shelter reduces maintenance metabolism of juvenile salmon. Funct. Ecol. 20, 839–845 10.1111/j.1365-2435.2006.01166.x (doi:10.1111/j.1365-2435.2006.01166.x) [DOI] [Google Scholar]
- 37.Ellers J, Rog S, Braam C, Berg MP. 2011. Genotypic richness and phenotypic dissimilarity enhance population performance. Ecology 92, 1605–1615 10.1890/10-2082.1 (doi:10.1890/10-2082.1) [DOI] [PubMed] [Google Scholar]
- 38.Einum S, Fleming IA. 2004. Does within-population variation in egg size reduce intraspecific competition in Atlantic Salmon, Salmo salar? Funct. Ecol. 18, 110–115 10.1111/j.1365-2435.2004.00824.x (doi:10.1111/j.1365-2435.2004.00824.x) [DOI] [Google Scholar]
- 39.Armstrong JD, Millidine KJ, Metcalfe NB. 2011. Ecological consequences of variation in standard metabolism and dominance among salmon parr. Ecol. Freshw. Fish 20, 371–376 10.1111/j.1600-0633.2011.00486.x (doi:10.1111/j.1600-0633.2011.00486.x) [DOI] [Google Scholar]


