Abstract
According to the ‘good genes’ hypothesis, females choose males based on traits that indicate the male's genetic quality in terms of disease resistance. The ‘immunocompetence handicap hypothesis’ proposed that secondary sexual traits serve as indicators of male genetic quality, because they indicate that males can contend with the immunosuppressive effects of testosterone. Masculinity is commonly assumed to serve as such a secondary sexual trait. Yet, women do not consistently prefer masculine looking men, nor is masculinity consistently related to health across studies. Here, we show that adiposity, but not masculinity, significantly mediates the relationship between a direct measure of immune response (hepatitis B antibody response) and attractiveness for both body and facial measurements. In addition, we show that circulating testosterone is more closely associated with adiposity than masculinity. These findings indicate that adiposity, compared with masculinity, serves as a more important cue to immunocompetence in female mate choice.
Keywords: mate choice, good genes, immunocompetence handicap hypothesis, masculinity, adiposity, attractiveness
1. Introduction
Females choose males not only based on the direct benefits they can provide, such as resources, but also because of indirect benefits, such as ‘good genes’ that are passed on to her offspring [1]. In a highly influential study, Hamilton & Zuk [2] found that birds with more striking plumage (males and females) and song (males only) had significantly fewer blood parasites than their less striking counterparts. Folstad & Karter [3] interpreted these condition-dependent cues to be secondary sexual cues. They proposed that exaggerated secondary sexual cues in males indicate genetic quality by showing that the male can contend with the immunosuppressive effects of testosterone [3]. According to the immunocompetence handicap hypothesis (ICHH), secondary sexual cues ‘enables females to assess the status of a potential partner's parasite burden and resistance’ [3]. Thus, in order for females to use cues to immunocompetence in mate choice, a cue needs to be (i) sexually selected, and (ii) significantly related to the immune response. More specifically, the cue should mediate the relationship between immune response and attractiveness.
Masculinity is generally considered to serve as a secondary sexual cue to immunocompetence in human mate choice [4–7]. Nevertheless, studies testing the relationship between masculinity and attractiveness have produced mixed results (for review see [6,8]). Studies have varyingly found a preference for masculinity [9–11], femininity [12,13] or no significant preference for sexual dimorphism [4,14,15]. This inconsistency in masculinity preferences might be partly attributed to a trade-off between the benefits (e.g. genetic quality) and costs (e.g. negative personality attributes) associated with masculinity [12]. The relationship between masculinity and health measurements is also inconsistent across studies. Rhodes et al. [7] found a modest positive association between rated facial masculinity in young adolescent male faces and medically assessed health scores. Similarly, Thornhill & Gangestad [5] showed that men with a higher level of measured facial masculinity report a lower incidence of antibiotics use and respiratory diseases, but not stomach and intestinal infections than less masculine men. By contrast, Lie et al. [14] did not find any significant relationship between facial masculinity and an indirect measure of innate immunity (diversity at the major histocompatibility complex). To our knowledge, no previous study has tested the relationship between masculinity and a direct measure of immunity.
We propose that adiposity could serve as a valid cue to immunocompetence in humans, because it significantly influences attractiveness [8,16–20] and is highly associated with various health measures [20–27]. Several studies found that the body mass index (BMI; weight scaled for height) significantly predicts male and female bodily attractiveness (for review, see [8,16,18,19]). The link between BMI and general health measures has also been well-established. Obese and overweight individuals are at increased risk of developing various diseases [25–27]. More specifically, BMI plays a crucial role in immunity. On one end of the spectrum, malnourished individuals, particularly those with protein-calorie malnutrition, are less immunocompetent than normal weight individuals [22]. On the other, several studies show that obese individuals are also less immunocompetent than their non-obese counterparts [21].
Rated facial adiposity, in turn, significantly predicts facial attractiveness and serves as a robust cue to health, because it is significantly related to both health judgements and actual measures of health (increased respiratory infections, antibiotics use and reduced cardiovascular health [20]). Adolescent facial adiposity judgements are associated with all-cause mortality (particularly heart disease mortality) and several medically assessed chronic conditions in a large longitudinal study (n = 3027 [24]). Adiposity is also highly heritable [28], thus a person with an optimal level of adiposity could potentially provide not only direct, but also indirect benefits to a partner.
In a previous study using the current sample, we found a significant positive association between a direct measure of immune response (antibody response to a hepatitis B vaccine) and facial attractiveness [29]. To determine which facial or body cues underlie the relationship between immune response and attractiveness, we use mediational analysis, a subset of structural equation modelling [30]. The first aim of this study is to test whether masculinity and/or adiposity significantly mediate the relationship between immune response and attractiveness in both the face and body of human males. We selected female raters in the fertile phase of their menstrual cycle to rate the males for attractiveness, because women in the fertile phase are considered to be more attentive to phenotypic cues indicating herein quality [31,32]. Second, we test whether masculinity (and adiposity) is significantly associated with circulating testosterone levels, because a second basic assumption of the ICHH is that secondary sexual cues are positively associated with circulating testosterone levels [3]. Previous work using the current sample showed a significant positive association between circulating testosterone and facial attractiveness [29].
The trade-off between the benefits and costs associated with masculinity could mask a relationship between masculinity and attractiveness. The third aim of the study is, therefore, to test whether the female raters show a consistent preference for sexual dimorphism or whether some women prefer more masculine looking men while other women prefer more feminine looking men.
2. Material and methods
(a). Participants
Sixty-nine Caucasian males (mean age, 23.0; s.d., 3.9; range 19–31), a subsample of 74 males who agreed to have body photographs taken, were recruited from the University and Transportation College of Daugavpils, Latvia. Full colour facial and full body photographs were taken with a Nikon D50 digital camera under standardized conditions, with participants wearing standardized underwear. We measured each participant's percentage body fat (hereafter body adiposity; Omron Body Composition Monitor BF500), a more accurate measure of adiposity than BMI [33]. In addition, we assessed testosterone and anti-HBsAg (hepatitis B antibody) levels from 10 ml of venous blood collected approximately 30 min before, and one month after, a dose of hepatitis B vaccine (Engerix B, Glaxosmithkline) was administered. Blood samples were collected between 9.00 and 11.00. Levels of anti-HBsAg were assessed using enzyme immunoassay (AxSYM, Abbott Laboratories) and commercially available kits (AUSAB, Abbott Laboratories). Testosterone levels were assessed using competitive chemiluminescent enzyme immunoassay with commercially available kits (Immulite2000 Total Testosterone). For a full description of methods see Rantala et al. [29]. Testosterone levels were consistent with pre- and post-vaccination (Cronbach α = 0.90) and were, therefore, averaged for each participant. No participant expressed anti-HBsAg prior to vaccination.
(b). Image ratings
Twenty-nine heterosexual Caucasian women reporting regular menstrual cycles and no use of hormonal contraception from the University of Daugavpils, Latvia (mean age, 20.0; s.d., 1.9) rated the body and facial images for sexual attractiveness on an 11 point Likert scale (−5 = very unattractive, 0 = neutral and +5 = very attractive). The women were selected from a larger group of 94 women, because they were in the fertile phase of their menstrual cycle. The fertile phase was calculated as the 5 days before ovulation and the day of ovulation itself [34]. Ovulation was assumed to occur 14 days before the onset of menses. The method is commonly used in evolutionary psychological studies [32].
The images were also rated for: body and facial masculinity by 20 heterosexual Finnish participants (10 male; mean age, 24.3; s.d., 4.3) on a seven point Likert scale (1 = not masculine, 7 = very masculine), and facial adiposity by 14 heterosexual Latvian women (mean age, 23.6; s.d., 4.1) on an 11 point Likert scale (−5 = very underweight, 0 = normal weight, +5 = very overweight). Images were presented in random order and body images were presented with faces blurred. Inter-rater reliability was high for all ratings (all Cronbach α > 0.93), thus were averaged across raters for all ratings.
(c). Analyses
Descriptive statistics for each measure are reported in the electronic supplementary material, table S1. Body adiposity and all the averaged rating measures were normally distributed (skewness and kurtosis between ±1.2), except anti-HBsAg (skewness and kurtosis greater than 2) and facial attractiveness (kurtosis 1.7), so we performed a Box–Cox transformation and log transformation to normalize the distributions of the respective measures (skewness and kurtosis between ±1.0). All facial and body measurements were linearly related to attractiveness, except body adiposity, which showed a curvilinear relationship, peaking at a body fat percentage of 12 per cent. A squared transformation was used to linearize body adiposity [35]. We tested the direct relationship between antibody response and attractiveness measures using Pearson's correlations, and constructed separate path models for body and facial attractiveness using multiple regression analyses, with attractiveness as dependent variable and adiposity, masculinity and antibody response as independent variables (figure 1). In addition, we used multiple mediator models to test specific indirect effects (i.e. the effect of one potential mediator on the relationship between antibody response and attractiveness, controlling for the other potential mediator). Support for mediation was evaluated using non-parametric bias-corrected bootstrapping analysis—as recommended for small sample sizes [36]—and the more conventional Sobel Test [30,36]. In these bootstrap analyses (10 000 bootstrap samples), mediation is significant if the 95% bias-corrected confidence intervals for the indirect effect do not include 0 [30,36].
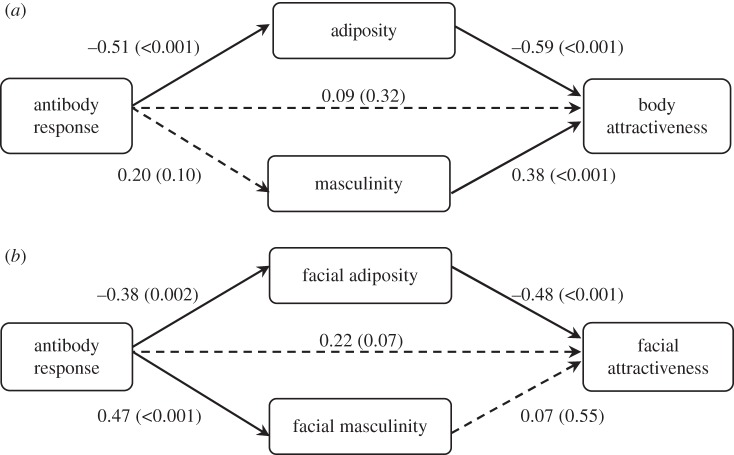
Figure 1.
Path coefficients for (a) body and (b) facial mediation models. Standardized regression coefficients and associated p-values in brackets; n = 69. Dotted lines indicate non-significant coefficients (p > 0.05). Facial attractiveness was log transformed.
To address the second aim, correlations between circulating testosterone and (i) adiposity, and (ii) masculinity, were tested using Pearson's correlation analysis (two-tailed). To address the third aim, we tested the relationship between each female rater's attractiveness judgements and the average masculinity score for each male image using Spearman's rank order correlations (two-tailed), in accordance with Stephen et al. [15]. All analyses were performed in SPSS 20.0 with the addition of a macro for bootstrapping and Sobel test analyses [36]. Data deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.pb7nb.
3. Results
(a). Body measurements
Antibody response was significantly correlated with bodily attractiveness (r69 = 0.47, p < 0.001). In the multiple regression model, body adiposity and masculinity, but not antibody response, significantly predicted bodily attractiveness (figure 1a). Adiposity was also significantly predicted by antibody response, whereas masculinity was not (figure 1a). Adiposity significantly mediated the relationship between antibody response and bodily attractiveness, but masculinity did not (table 1). This finding was consistent for both the bias-corrected bootstrapping analysis and the Sobel test (table 1), indicating that only the indirect effect through adiposity significantly mediates the relationship between antibody response and bodily attractiveness. This pattern of results was also observed after excluding males that did not show any antibody response after hepatitis B vaccination (see the electronic supplementary material, table S2). Moreover, a significant pairwise contrast between the indirect effects showed that the specific indirect effect through adiposity is significantly larger than the specific indirect effect through masculinity. Circulating testosterone levels were significantly correlated with adiposity (r69 = 0.54, p < 0.001), but not masculinity (r69 = 0.21, p = 0.084). The correlation between testosterone and adiposity was significantly stronger than the correlation between testosterone and masculinity (Steiger's Z = 2.32, d.f. = 66, p = 0.015).
Table 1.
Mediation analyses for indirect effects. (n = 69; BC, bias-corrected; s.e., standard error. Effect refers to the specific indirect effect of antibody response on attractiveness through each mediator.)
| effect | BC bootstrap 95% CI |
Sobel test |
||||
|---|---|---|---|---|---|---|
| s.e. | lower | upper | s.e. | p | ||
| body attractiveness | ||||||
| adiposity | 0.16 | 0.05 | 0.09 | 0.27 | 0.04 | <0.001 |
| masculinity | 0.04 | 0.03 | −0.01 | 0.11 | 0.03 | 0.11 |
| total indirect | 0.20 | 0.06 | 0.09 | 0.34 | 0.05 | <0.001 |
| adiposity versus masculinity | 0.12 | 0.05 | 0.04 | 0.22 | 0.05 | 0.010 |
| facial attractiveness | ||||||
| adiposity | 0.05 | 0.02 | 0.02 | 0.09 | 0.02 | 0.015 |
| masculinity | 0.02 | 0.02 | −0.01 | 0.06 | 0.02 | 0.26 |
| total indirect | 0.07 | 0.02 | 0.02 | 0.12 | 0.03 | 0.011 |
| adiposity versus masculinity | 0.03 | 0.03 | −0.02 | 0.08 | 0.03 | 0.30 |
All but one of the female raters (96.6%) showed a positive correlation between body attractiveness judgements and averaged masculinity scores for each male image, a relationship that was significant in 69.0 per cent (e.g. 20/29) of cases (all rs69 ≥ 0.25, p < 0.05). The single negative correlation between masculinity and attractiveness was not significant (rs69 = –0.01, p = 0.95).
(b). Face measurements
Antibody response was significantly correlated with facial attractiveness (r69 = 0.43, p < 0.001), in line with previously reported findings using the current sample [29]. In the multiple regression model, facial adiposity significantly predicted facial attractiveness, whereas facial masculinity and antibody response did not (figure 1b). Both adiposity and masculinity were significantly predicted by antibody response (figure 1b). The electronic supplementary material, figure S2 illustrates the facial features associated with high- and low-antibody response. As with the body measurements, adiposity significantly mediated the relationship between antibody response and facial attractiveness, whereas masculinity did not (see table 1 and the electronic supplementary material, table S2). There was no significant difference between the specific indirect effect through adiposity and the specific indirect effect through masculinity (table 1). Circulating testosterone levels were significantly correlated with adiposity (r69 = 0.52, p < 0.001) and masculinity (r69 = 0.38, p = 0.001). There was no significant difference in the testosterone–adiposity and testosterone–masculinity correlations (Steiger's Z = 1.04, d.f. = 66, p = 0.28).
Most of the female raters (75.9%) showed a positive correlation between facial attractiveness judgements and averaged masculinity scores for each male image, but the relationship was only significant in 24.1 per cent (7/29) of cases (all rs69 ≥ 0.27, p < 0.05). None of the negative correlations between masculinity and facial attractiveness were significant (all rs69 ≥ –0.14, p > 0.05).
4. Discussion
Results show that adiposity is consistently and significantly associated with (i) antibody response, and (ii) attractiveness in both the body and the face of a group of Latvian men. Masculinity, on the other hand, was not significantly related to both attractiveness and antibody response in either the body or the face. Moreover, adiposity is a significant mediator of the relationship between antibody response and attractiveness for both body and face measures, whereas masculinity does not significantly contribute to the relationship above and beyond the contribution of adiposity for either body or face measurements. These findings indicate that women in this study use adiposity, and not masculinity, as a cue to immunocompetence when judging the attractiveness of men.
We should point out two potential caveats to these findings. First, masculinity might be a comparatively better cue to immunocompetence in populations with a higher variance in masculinity and a lower variance in adiposity. Second, other factors, apart from adiposity and masculinity, might also affect the relationship between immune response and attractiveness. However, the direct relationship between antibody response and attractiveness reported here and in Rantala et al. [29] is no longer significant once adiposity and masculinity are controlled for, indicating that these two factors mediate the relationship (at least for the given sample size).
This finding builds on a growing body of evidence showing that labile conditional cues, such as adiposity and skin colour, might be better indicators of mate quality than more stable conditional cues, such as masculinity [4,15,37]. Previous research largely supports the findings presented here in four ways. First, in accordance with our findings, previous work showed that measures of adiposity are significantly associated with attractiveness in the face [20] and the body [8,17]. Second, 4 per cent of men in our sample were underweight, 65 per cent healthy weight and 30.4 per cent overweight or obese according to criteria developed by Gallagher et al. [33]. One would, therefore, expect a negative relationship between adiposity and immunity in our sample, which is indeed what we observed. Our results are, therefore, consistent with previous findings on the role of obesity, and overweight status, on the immune system [21].
Third, although this is the first study, to our knowledge, to test the relationship between masculinity and a direct measure of immunity, previous studies also found mixed results regarding the relationship between masculinity and indirect measures of immunity [5,7,14]. Fourth, in accordance with our results, previous studies found a significant positive association between body masculinity and attractiveness [11]. We found no significant association between facial masculinity and attractiveness, not surprising given that previous studies also found mixed results regarding the relationship between masculinity and attractiveness [6,12,15]. The lack of a significant association between facial masculinity and attractiveness cannot be attributed to women showing opposing individual preferences for masculine and feminine looking male faces. For one, the women in this study were tested in the fertile phase of their menstrual cycle when masculinity preferences are enhanced [9]. Second, we found no evidence that some women strongly preferred feminine, as opposed to masculine, looking faces or bodies in this study.
Although we found that masculinity does not underlie the relationship between immune response and attractiveness, masculinity could still affect sexual selection in other ways. For example, masculine men might simply outcompete their less masculine rivals [38]. Women might also prefer masculine men because of direct benefits they can provide or because of other indirect benefits such as genetic quality in terms of dominance or competitiveness [38,39]. Indeed, Peters et al. [10] did find a positive association between masculinity and mating success.
A second basic assumption of the ICHH is that testosterone is positively associated with cues that indicate mate quality [3]. Although we have no information on the adolescent testosterone levels that would have played a role in the production of secondary sexual traits [40], adult testosterone levels in our study were more closely correlated with adiposity than with masculinity (although not significantly so for facial measures).
In summary, our results show that adiposity, and not masculinity, underlies the relationship between immune response and attractiveness. Compared with masculinity, adiposity is also more strongly associated with circulating testosterone levels. Taken together, these findings highlight the role of adiposity as a cue to male quality in modern human societies.
Acknowledgements
This study was approved by the Ethics in Research Committee of Daugavpils University and the Ethics Committee at the University of Pretoria (EC111010-070).
We thank Jolanta Vrublevska and Inese Gavarane for their help with data collection and the E. Gulbis Laboratory, Daugavpils for biological assays. The authors were funded by the South African National Research Foundation (V.C.), the Academy of Finland (M.J.R.), and Royal Society of Edinburgh International Exchange Program (F.R.M.).
References
- 1.Kirkpatrick M, Ryan MJ. 1991. The evolution of mating preferences and the paradox of the lek. Nature 350, 33–38 10.1038/350033a0 (doi:10.1038/350033a0) [DOI] [Google Scholar]
- 2.Hamilton WD, Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 3.Folstad I, Karter AK. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622 10.1086/285346 (doi:10.1086/285346) [DOI] [Google Scholar]
- 4.Scott IML, Pound N, Stephen ID, Clark AP, Penton-Voak IS. 2010. Does masculinity matter? The contribution of masculine face shape to male attractiveness in humans. PLoS ONE 5, e13585. 10.1371/journal.pone.0013585 (doi:10.1371/journal.pone.0013585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornhill R, Gangestad SW. 2006. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evol. Hum. Behav. 27, 131–144 10.1016/j.evolhumbehav.2005.06.001 (doi:10.1016/j.evolhumbehav.2005.06.001) [DOI] [Google Scholar]
- 6.Rhodes G. 2006. The evolutionary psychology of facial beauty. Annu. Rev. Psychol. 57, 199–226 10.1146/annurev.psych.57.102904.190208 (doi:10.1146/annurev.psych.57.102904.190208) [DOI] [PubMed] [Google Scholar]
- 7.Rhodes G, Chan J, Zebrowitz LA, Simmons LW. 2003. Does sexual dimorphism in human faces signal health? Proc. R. Soc. Lond. B 270, S93–S95 10.1098/rsbl.2003.0023 (doi:10.1098/rsbl.2003.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weeden J, Sabini J. 2005. Physical attractiveness and health in Western societies: a review. Psychol. Bull. 131, 635–653 10.1037/0033-2909.131.5.635 (doi:10.1037/0033-2909.131.5.635) [DOI] [PubMed] [Google Scholar]
- 9.Johnston VS, Hagel R, Franklin M, Fink B, Grammer K. 2001. Male facial attractiveness: evidence for hormone-mediated adaptive design. Evol. Hum. Behav. 22, 251–267 10.1016/S1090-5138(01)00066-6 (doi:10.1016/S1090-5138(01)00066-6) [DOI] [Google Scholar]
- 10.Peters M, Simmons LW, Rhodes G. 2008. Testosterone is associated with mating success but not attractiveness or masculinity in human males. Anim. Behav. 76, 297–303 10.1016/j.anbehav.2008.02.008 (doi:10.1016/j.anbehav.2008.02.008) [DOI] [Google Scholar]
- 11.Little AC, Jones BC, Burriss RP. 2007. Preferences for masculinity in male bodies change across the menstrual cycle. Horm. Behav. 51, 633–639 10.1016/j.yhbeh.2007.03.006 (doi:10.1016/j.yhbeh.2007.03.006) [DOI] [PubMed] [Google Scholar]
- 12.Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, Henzi SP, Castles DL, Akamatsu S. 1998. Effects of sexual dimorphism on facial attractiveness. Nature 394, 884–887 10.1038/29772 (doi:10.1038/29772) [DOI] [PubMed] [Google Scholar]
- 13.Rhodes G, Hickford C, Jeffrey L. 2000. Sex-typicality and attractiveness: are supermale and superfemale faces super-attractive? Brit. J. Psychol. 91, 125–140 10.1348/000712600161718 (doi:10.1348/000712600161718) [DOI] [PubMed] [Google Scholar]
- 14.Lie HC, Rhodes G, Simmons LW. 2008. Genetic diversity revealed in human faces. Evolution 62, 2473–2486 10.1111/j.1558-5646.2008.00478.x (doi:10.1111/j.1558-5646.2008.00478.x) [DOI] [PubMed] [Google Scholar]
- 15.Stephen ID, Scott IML, Coetzee V, Pound N, Perrett DI, Penton-Voak IS. 2012. Cross-cultural effects of color, but not morphological masculinity, on perceived attractiveness of men's faces. Evol. Hum. Behav. 33, 260–267 10.1016/j.evolhumbehav.2011.10.003 (doi:10.1016/j.evolhumbehav.2011.10.003) [DOI] [Google Scholar]
- 16.Symons D. 1995. Beauty is in the adaptations of the beholder: the evolutionary psychology of human female sexual attractiveness. In sexual nature, sexual culture (eds Abramson PR, Pinkerton SD.), pp. 80–120 Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Maisey DS, Vale ELE, Cornelissen PL, Tovée MJ. 1999. Characteristics of male attractiveness for women. Lancet 353, 1500. 10.1016/S0140-6736(99)00438-9 (doi:10.1016/S0140-6736(99)00438-9) [DOI] [PubMed] [Google Scholar]
- 18.Swami V. 2006. The influence of body weight and shape in determining female and male physical attractiveness. In Focus on body mass index and health research (ed. Ferrera LA.), pp. 1–27 New York, NY: Nova Science Publishers [Google Scholar]
- 19.Bateson M, Cornelissen PL, Tovée MJ. 2007. Methodological issues in studies of female attractiveness. In The Body beautiful: evolutionary and socio-cultural perspectives (eds Swami V, Furnham A.), pp. 46–64 Basingstoke, UK: Palgrave Macmillan [Google Scholar]
- 20.Coetzee V, Perrett DI, Stephen ID. 2009. Facial adiposity: a cue to health? Perception 38, 1700–1711 10.1068/p6423 (doi:10.1068/p6423) [DOI] [PubMed] [Google Scholar]
- 21.Samartín S, Chandra RK. 2001. Obesity, overnutrition and the immune system. Nutr. Res. 21, 243–262 10.1016/S0271-5317(00)00255-4 (doi:10.1016/S0271-5317(00)00255-4) [DOI] [Google Scholar]
- 22.Ritz BW, Gardner EM. 2006. Malnutrition and energy restriction differentially affect viral immunity. J. Nutr. 136, 1141–1144 [DOI] [PubMed] [Google Scholar]
- 23.Balkau B, et al. 2007. International day for the evaluation of abdominal obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168 000 primary care patients in 63 countries. Circulation 116, 1942–1951 10.1161/CIRCULATIONAHA.106.676379 (doi:10.1161/CIRCULATIONAHA.106.676379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reither EN, Hauser RM, Swallen KC. 2009. Predicting adult health and mortality from adolescent facial characteristics in yearbook photographs. Demography 46, 27–41 10.1353/dem.0.0037 (doi:10.1353/dem.0.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pi-Sunyer FX. 1993. Medical hazards of obesity. Ann. Intern. Med. 119, 655–660 [DOI] [PubMed] [Google Scholar]
- 26.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. 1999. The disease burden associated with overweight and obesity. J. Am. Med. Assoc. 282, 1523–1529 10.1001/jama.282.16.1523 (doi:10.1001/jama.282.16.1523) [DOI] [PubMed] [Google Scholar]
- 27.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. J. Am. Med. Assoc. 289, 76–79 10.1001/jama.289.1.76 (doi:10.1001/jama.289.1.76) [DOI] [PubMed] [Google Scholar]
- 28.Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henrikson JE, Heitmann BL, Sorensen TI. 2004. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int. J. Obes. 28, 39–48 10.1038/sj.ijo.0802524 (doi:10.1038/sj.ijo.0802524) [DOI] [PubMed] [Google Scholar]
- 29.Rantala MJR, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. 2012. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nat. Commun. 3, 694. 10.1038/ncomms1696 (doi:10.1038/ncomms1696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabachnick BG, Fidell LS. 2007. Structural equation modeling. In Using multivariate statistics, 5th edn., pp. 676–780 Boston, MA: Pearson Education [Google Scholar]
- 31.Gangestad SW, Thornhill R. 1998. Menstrual cycle variation in women's preferences for the scent of symmetrical men. Proc. R. Soc. Lond. B 265, 927–933 10.1098/rspb.1998.0380 (doi:10.1098/rspb.1998.0380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, Minamisawa R. 1999. Menstrual cycle alters face preference. Nature 399, 741–742 10.1038/21557 (doi:10.1038/21557) [DOI] [PubMed] [Google Scholar]
- 33.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. 2000. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 72, 694–701 [DOI] [PubMed] [Google Scholar]
- 34.Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. 1999. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum. Reprod. 14, 1835–1839 10.1093/humrep/14.7.1835 (doi:10.1093/humrep/14.7.1835) [DOI] [PubMed] [Google Scholar]
- 35.Tabachnick BG, Fidell LS. 2007. Cleaning up your act: screening data prior to analysis. In Using multivariate statistics, 5th edn., p. 83 Boston, MA: Pearson Education [Google Scholar]
- 36.Preacher KJ, Hayes AF. 2008. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Meth. 40, 879–891 10.3758/BRM.40.3.879 (doi:10.3758/BRM.40.3.879) [DOI] [PubMed] [Google Scholar]
- 37.Adamo SA, Spiteri RJ. 2009. He's healthy, but will he survive the plague? Possible constraints on mate choice for disease resistance. Anim. Behav. 77, 67–78 10.1016/j.anbehav.2008.09.011 (doi:10.1016/j.anbehav.2008.09.011) [DOI] [Google Scholar]
- 38.Puts DA. 2010. Beauty and the beast: mechanisms of sexual selection in humans. Evol. Hum. Behav. 31, 157–175 10.1016/j.evolhumbehav.2010.02.005 (doi:10.1016/j.evolhumbehav.2010.02.005) [DOI] [Google Scholar]
- 39.Boothroyd LG, Jones BC, Burt DM, Perrett DI. 2007. Partner characteristics associated with masculinity, health and maturity in male faces. Pers. Indiv. Differ. 43, 1161–1173 10.1016/j.paid.2007.03.008 (doi:10.1016/j.paid.2007.03.008) [DOI] [Google Scholar]
- 40.Enlow DH, Hans MG. 1996. Essentials of facial growth. Philadelphia, PA: W. B. Saunders Company [Google Scholar]