Some of the areas that form the primate visual cortex have been defined with precision, including the first (V1) and second (V2) visual areas. However, the organization of the cortex located immediately rostral to V2 (the ‘third-tier’ areas [1]) remains contentious. Recently, Lyon & Connolly [2] concluded that evidence obtained in several primate species supports the hypothesis that an elongated third visual area (V3), forming a complete map of the visual field, occupies this region. The existence of a V3-like area in the primate brain is not a matter of contention [3,4]. However, we disagree with the conclusions of Lyon & Connolly regarding the location and extent of this area. Here, we argue that studies which mapped in detail the receptive fields (RFs) [1,5,6] and anatomical connections [4,7] of cortex immediately rostral to the dorsal half of V2, particularly in New World monkeys, demonstrate the existence of a dorsomedial area (DM) in this location. According to this scheme, a V3-like area, which is less extensive than that proposed by Lyon & Connolly, occupies only the lateral and ventral aspects of the third-tier cortex. To help make our arguments clearer, we refer to this as the ventrolateral posterior area (VLP) [3].
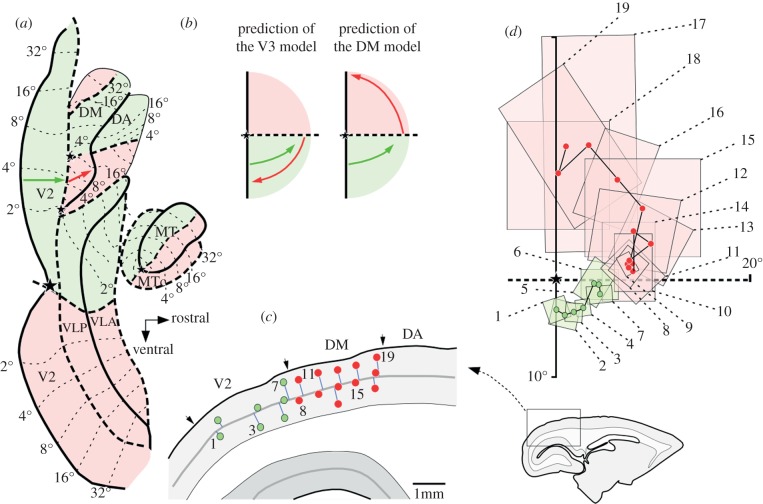
In occipital cortex, each area forms a topographic map of the visual field. One central prediction of Lyon & Connolly's scheme is that cells in the dorsal half of V3 always have RFs representing the lower half of the visual field. Specifically (their fig. 1d), sampling neurons along any sequence of sites starting near the border of dorsal V2, and moving rostrally (red arrow in our figure 1a), should reveal RFs that move from near the horizontal meridian (HM) of the visual field towards the vertical meridian (VM), in the lower half of the visual field. Contrary to this prediction, electrophysiological recordings in parts of the cortex adjacent to dorsal V2 in marmoset and owl monkeys have consistently revealed RFs that drift towards the upper VM, as shown in figure 1. This evidence, spanning more than three decades of research from various laboratories, makes it unlikely that ‘V3’ inserts between V2 and DM in these species; instead, DM is directly adjacent to V2. These recording sites were separated, at most, by a few hundred micrometres, not leaving room for an intervening lower VM representation. Indeed, there is no evidence for even a partial lower quadrant representation between V2 and DM, either in this sequence or in many others illustrated in previous studies of marmoset and owl monkeys [1,5–7,9]. As argued in detail elsewhere, this interpretation is also compatible with physiological evidence in Cebus monkeys [10], and provides an equally plausible interpretation for the available electrophysiological and imaging data obtained in macaques [8].
Figure 1.
Electrophysiological evidence of a representation of the upper visual field in the cortex immediately rostral to dorsal V2. (a) Schematic of ‘unfolded’ marmoset extrastriate cortex, with the locations of areas indicated [8]. Pink-shaded areas indicate upper visual field representations, and green-shaded areas lower field representations. Thick solid and dashed contours mark representations of the VM and HM, respectively, at areal borders, and stars mark representations of the centre of the fovea. Thin-dashed lines and numbers indicate iso-eccentricity lines. The arrows represent caudorostral rows of recording sites in V2 (green) and cortex rostral to V2 (red). (b) The predicted trajectories of RFs of neurons recorded at these sites (arrows projected on the visual field), according to Lyon & Connolly's proposed scheme [2] and to a model whereby DM is adjacent to V2. (c) Parasagittal section showing the location of recording sites in area V2 (green) and cortex rostral to V2 (red). The recording sites are numbered from caudal to rostral according to their projection to cortical layer 4. Arrows indicate histological transitions. The inset shows the location of the part of the section that was magnified to show the recording sites. (d) Receptive fields (rectangles) and receptive field centres (circles) obtained in these sites.
Lyon & Connolly regard the evidence for an upper quadrant representation bordering V2 as ‘unconvincing’, because RFs of cells immediately rostral to V2 are centred near the HM. However, given that borders between visual areas represent congruent regions of the visual field, this is to be expected from either V3 or DM; as explained above, the most relevant criterion for determining which of these areas adjoins V2 is whether RFs recorded from progressively more rostral sites drift towards the lower or upper visual field (figure 1b). Nonetheless, the authors offer a reanalysis of data obtained in marmosets (their fig. 4), in which some recording sites originally attributed to DM [5] appear as reassigned to ‘V3’. In doing so, they disregard many data points in the original publication, and subsequent studies, which show that RFs in this contended region never move towards the lower field [1,4–8]. Thus, invoking a narrow ‘V3’ in this region is unparsimonious. In the same figure, Lyon & Connolly offer a re-interpretation of the extent of DM, which has its putative borders moved rostrally, to overlap partially with what we consider a different area (the dorsoanterior area, DA). This proposal disregards differences in RF size, myeloarchitecture and connections between DM and DA [4–7]. Finally, as a counterpoint to the electrophysiological evidence, the authors refer to a study using optical imaging in owl monkeys [11], which could not detect any activity in the cortex rostral to V2 following stimulation of the upper visual field. Here, it is important to consider that electrophysiological recordings provide direct functional evidence of the sectors of the visual field being represented by neurons, as opposed to indirect measurements based on blood flow; the latter are subject to significant artefacts, which can lead to false-negative and false-positive results, depending on the spatial relationship of neurons to blood vessels [12]. This basic point needs to be taken into consideration in situations where results provided by these techniques disagree (see Rosa et al. [13] for further discussion). In summary, the view that the physiological maps obtained in New World monkeys are ‘either incorrect or have been misinterpreted’ [2] remains open to debate.
Connections between visual areas occur between neuronal populations representing corresponding regions of visual field [14]. Thus, mapping connections arising from an area with a well-characterized visuotopic map is a powerful method for revealing topographic organization in the cortex. However, most of the neuroanatomical data offered in support of Lyon & Connolly's hypothesis in New World monkeys derive from studies involving sparsely distributed tracer injections [15,16]. This experimental design cannot, by itself, resolve cortical organization, because the topography of resulting label is insufficient to constrain the location of areal boundaries. For example, all that can be inferred from a few injections in upper quadrant V1 is that a representation of the upper quadrant is likely to exist near dorsal V2, but this cannot resolve whether this representation adjoins V2 or V3, given that borders can be drawn in ways that are consistent with either scheme (see the electronic supplementary material). Unambiguous answers require closely spaced injections of many distinguishable neuroanatomical tracers across the full width of an area, and systematic analysis of the topography of resulting label in V1 and other well-characterized areas. Recently, Jeffs et al. [4] used this approach in a study of the marmoset (figure 2). Rows of injections spanning the lateral part of putative area DM confirmed that its border with V2 represents the HM. Crucially, cortical sites progressively more anterior to this border formed connections with a topographical sequence of sites in the upper quadrant representation of V1 (figure 2a). Furthermore, rows of injections spanning the full width of dorsal V2 produced three rows of retrograde label, each mirroring the injection site sequence. Two of these rows (1 and 3, figure 2b) bordered dorsal V2, consistent with the existence of two areas in the third-tier cortex (DM and VLP). Significantly, projections to these areas originated from neurons located in different layers of V1 [4].
Figure 2.
Anatomical evidence in marmoset for an upper visual field representation and two distinct visual areas bordering dorsal V2: schematics of unfolded and flattened visual cortex showing, for two example cases, the location of injection sites (coloured circles with black outline) and patches of labelled neurons (filled ovals). (a) Label in V1 following injections in the cortex rostral to V2, and (b) label in V1 and in other areas following injections in dorsal V2. For clarity, label in several other areas is omitted (complete plots of cell label throughout visual cortex for these two cases can be found in [4]). The insets on the right of each panel show visual field maps of the injection sites (outlined in black) and resulting V1 label (shaded colour regions). Other conventions are as in figure 1. (a) Row of four different tracer injections in the upper visual field representation of DM. All injections produced label in upper field V1 (positive symbols) and the blue injection also in lower field V1 (negative symbols). This demonstrates that the injections were located in an upper visual field region, with the blue injection straddling the border with a lower visual field region. Had this been the border between V3 and DM, label from the blue injection would have been located at the VM representation in dorsal V1 (expected location shown by the blue arrow). Instead, V1 label was located at the HM, indicating the injection was located at the V2/DM border. (b) Rows of tracer injections across the full width of dorsal V2. In this case, seven different tracers were injected, but for clarity of illustration here we only show results from four injection sites (see Jeffs et al. [4] for complete data). The topography of V1 label indicates that the injections were confined to dorsal V2, spanning its full width, from the VM (orange) to the HM (green) representations. These injections produced two label reversals abutting dorsal V2 (marked as 1 and 3, red numbers), consistent with the existence of two areas in this region. A third reversal (marked as 2) was located 1.5 mm anterior to reversal 1, and its location and topography suggest it was located in area DA (see figure 1 for a map of DA). According to Lyon & Connolly's parcellation scheme, reversal 2 should directly abut reversal 1, and its label sequence should mirror that of reversal 1.
In comparison, the anatomical evidence in Old World monkeys is less straightforward. Fig. 2a of Lyon & Connolly shows a tracer injection in macaque ventral V1 that failed to label cortex immediately anterior to dorsal V2, consistent with the idea that the upper quadrant representation of DM does not border V2. However, one of the two additional cases illustrated in the original study [17] can be interpreted as consistent with the existence of an upper quadrant representation adjoining dorsal V2 (their fig. 3), whereas the other is inconclusive (showing no label in dorsal cortex anterior to V2, from an injection in ventral V1). At the very least, this indicates that more work is required, particularly in view of the reported variability of the putative borders and topographic organization of macaque ‘V3’. In contrast, given the location of the disputed cortex on the exposed dorsal surface of the brain, studies in marmosets have allowed more extensive and higher-density anatomical and electrophysiological mapping of the dorsal third-tier cortex than in macaques. As demonstrated above, these data are inconsistent with Lyon & Connolly's scheme.
Finally, these authors offer a comparative argument, which appears grounded on the expectation that data obtained in different primate species would be likely to replicate each other, and the assumption that DM is equivalent to area V3a, described in macaques and humans. Both of these premises should be taken with caution. First, it is known that cortical areas do not expand uniformly as a function of brain size, and that the exact spatial relationship between homologous areas varies [8]. Second, the homology between V3a and DM is questionable. In this context, it is significant that another cortical area identified in humans and macaques, V6, is similar to DM in forming a complete visual field representation [18], and receiving a strong projection from V1, which originates primarily from layer IVb [4,7]. V6 borders dorsal V2, and population-averaged maps of the human visual cortex show a clear upper field representation in this location, without an intervening lower quadrant representation [19]. Thus, rather than arguing against the existence of DM, the neuroimaging data suggest that this area becomes relatively smaller, and progressively confined to the cortical midline, as a function of expansion of the cortex. In summary, data in New World primates support a model whereby two areas share the cortex immediately rostral to dorsal V2. We hope that the earlier-mentioned arguments will prompt more detailed and hypothesis-driven studies of the third-tier cortex in Old World primates, aimed at clarifying the relationship between V2, DM/V6 and adjacent areas.
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rspb.2012.1994.
References
- 1.Allman J. M., Kaas J. H. 1975. The dorsomedial cortical visual area: a third-tier area in the occipital lobe of the owl monkey (Aotus trivirgatus). Brain Res. 100, 473–487 10.1016/0006-8993(75)90153-5 (doi:10.1016/0006-8993(75)90153-5) [DOI] [PubMed] [Google Scholar]
- 2.Lyon D. C., Connolly J. D. 2012. The case for primate V3. Proc. R. Soc. B 279, 625–633 10.1098/rspb.2011.2048 (doi:10.1098/rspb.2011.2048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosa M. G. P., Manger P. R. 2005. Clarifying homologies in the mammalian cerebral cortex: the case of the third visual area (V3). Clin. Exp. Pharmacol. Physiol. 32, 327–339 10.1111/j.1440-1681.2005.04192.x (doi:10.1111/j.1440-1681.2005.04192.x) [DOI] [PubMed] [Google Scholar]
- 4.Jeffs J., Federer F., Ichida J., Angelucci A. In press. High-resolution mapping of anatomical connections in marmoset extrastriate cortex reveals a complete representation of the visual field bordering dorsal V2. Cereb. Cortex. (doi:10.1093/cercor/bhs088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosa M. G. P., Schmid L. M. 1995. Visual areas in the dorsal and medial extrastriate cortices of the marmoset. J. Comp. Neurol. 359, 272–299 10.1002/cne.903590207 (doi:10.1002/cne.903590207) [DOI] [PubMed] [Google Scholar]
- 6.Sereno M. I., McDonald C. T., Allman J. M. 1994. Analysis of retinotopic maps in extrastriate cortex. Cereb. Cortex 4, 601–620 10.1093/cercor/4.6.601 (doi:10.1093/cercor/4.6.601) [DOI] [PubMed] [Google Scholar]
- 7.Rosa M. G. P., Palmer S. M., Gamberini M., Burman K. J., Yu H.-H., Reser D. H., Bourne J. A., Tweedale R., Galletti C. 2009. Connections of the dorsomedial visual area: pathways for early integration of dorsal and ventral streams in extrastriate cortex. J. Neurosci. 29, 4548–4563 10.1523/JNEUROSCI.0529-09.2009 (doi:10.1523/JNEUROSCI.0529-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosa M. G. P., Tweedale R. 2005. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Phil. Trans. R. Soc. B 360, 665–691 10.1098/rstb.2005.1626 (doi:10.1098/rstb.2005.1626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krubitzer L. A., Kaas J. H. 1993. The dorsomedial visual area of owl monkeys: connections, myeloarchitecture, and homologies in other primates. J. Comp. Neurol. 334, 497–528 10.1002/cne.903340402 (doi:10.1002/cne.903340402) [DOI] [PubMed] [Google Scholar]
- 10.Rosa M. G. P., Piñon M. C., Gattass R., Sousa A. P. B. 2000. Third-tier ventral extrastriate cortex in the New World monkey, Cebus apella. Exp. Brain Res. 132, 287–305 10.1007/s002210000344 (doi:10.1007/s002210000344) [DOI] [PubMed] [Google Scholar]
- 11.Lyon D. C., Xu X., Casagrande V. A., Stefansic J. D., Shima D., Kaas J. H. 2002. Optical imaging reveals retinotopic organization of dorsal V3 in New World owl monkeys. Proc. Natl Acad. Sci. USA 99, 15 735–15 742 10.1073/pnas.242600699 (doi:10.1073/pnas.242600699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iadecola C. 2002. Intrinsic signals and functional brain mapping: CAUTION, blood vessels at work. Cereb. Cortex 12, 223–224 10.1093/cercor/12.3.223 (doi:10.1093/cercor/12.3.223) [DOI] [PubMed] [Google Scholar]
- 13.Rosa M. G. P., Palmer S. M., Gamberini M., Tweedale R., Piñon M. C., Bourne J. A. 2005. Resolving the organization of the New World monkey third visual complex: the dorsal extrastriate cortex of the marmoset (Callithrix jacchus). J. Comp. Neurol. 483, 164–191 10.1002/cne.20412 (doi:10.1002/cne.20412) [DOI] [PubMed] [Google Scholar]
- 14.Angelucci A., Levitt J. B., Lund J. S. 2002. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Prog. Brain Res. 136, 373–388 10.1016/S0079-6123(02)36031-X (doi:10.1016/S0079-6123(02)36031-X) [DOI] [PubMed] [Google Scholar]
- 15.Lyon D. C., Kaas J. H. 2001. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J. Neurosci. 21, 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyon D. C., Kaas J. H. 2002. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J. Comp. Neurol. 449, 281–297 10.1002/cne.10297 (doi:10.1002/cne.10297) [DOI] [PubMed] [Google Scholar]
- 17.Lyon D. C., Kaas J. H. 2002. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron 33, 453–461 10.1016/S0896-6273(02)00580-9 (doi:10.1016/S0896-6273(02)00580-9) [DOI] [PubMed] [Google Scholar]
- 18.Pitzalis S., Sereno M. I., Committeri G., Fattori P., Galati G., Patria F., Galletti C. 2010. Human V6: the medial motion area. Cereb. Cortex 20, 411–424 10.1093/cercor/bhp112 (doi:10.1093/cercor/bhp112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sereno M. I., Lutti A., Weiskopf N., Dick F. 2012. Mapping the human cortical surface by combining quantitative T1 with retinotopy. Cereb. Cortex 10.1093/cercor/bhs213 (doi:10.1093/cercor/bhs213) [DOI] [PMC free article] [PubMed] [Google Scholar]