Abstract
Colour signals are expected to match visual sensitivities of intended receivers. In birds, evolutionary shifts from violet-sensitive (V-type) to ultraviolet-sensitive (U-type) vision have been linked to increased prevalence of colours rich in shortwave reflectance (ultraviolet/blue), presumably due to better perception of such colours by U-type vision. Here we provide the first test of this widespread idea using fairy-wrens and allies (Family Maluridae) as a model, a family where shifts in visual sensitivities from V- to U-type eyes are associated with male nuptial plumage rich in ultraviolet/blue colours. Using psychophysical visual models, we compared the performance of both types of visual systems at two tasks: (i) detecting contrast between male plumage colours and natural backgrounds, and (ii) perceiving intraspecific chromatic variation in male plumage. While U-type outperforms V-type vision at both tasks, the crucial test here is whether U-type vision performs better at detecting and discriminating ultraviolet/blue colours when compared with other colours. This was true for detecting contrast between plumage colours and natural backgrounds (i), but not for discriminating intraspecific variability (ii). Our data indicate that selection to maximize conspicuousness to conspecifics may have led to the correlation between ultraviolet/blue colours and U-type vision in this clade of birds.
Keywords: colour vision, Malurus, adaptive evolution, ultraviolet
1. Introduction
How signals evolve with the sensory system of receivers is a central question in evolutionary biology [1–3]. From an adaptationist standpoint, it is expected that sensory systems and signals are optimally matched in order to maximize performance [4], with signal design complementing the abilities of the sensory system to optimize detection and discrimination. In the case of vision and colour signals, visual sensitivities generally evolve in response to environmental variables (e.g. habitat and food), which leads to subsequent changes in visual signals that suit the new characteristics of the visual system [4]. The rich chromatic palette of birds, for example, may have evolved as a consequence of their highly developed colour vision system, which enables them to discriminate a large diversity of colours [5]. But while avian colour variation is large, variation in the visual sensitivities of birds seems rather low [4], and the links between variation in colour vision and coloration are not well-understood [4]. Colour vision in birds is mediated by four types of cone, which are sensitive to very short (VS cone), short (S cone), medium (M cone) and long (L cone) wavelengths of light, together encompassing a range from near ultraviolet (UV) to red (300–700 nm) [6]. The two main types of visual systems found in birds differ chiefly in their sensitivities to shorter wavelengths due to different sensitivity functions of VS and S cones: the violet-sensitive (V-type) and the ultraviolet-sensitive (U-type) visual system [6]. In general, U-type eyes are better at discriminating or detecting colours [7–10]. It is often assumed that this advantage should be disproportionately larger for colours rich in shorter wavelengths (such as UV or blue) and this should lead to the more frequent evolution of shortwave-rich colours in species with U-type eyes [7,10–12].
While some evidence suggests that shortwave-rich colours are more frequent in bird orders that have species with U-type eyes [11], a recent study on fairy-wrens and allies (Family Maluridae) by Ödeen et al. [12] provides further support for this idea. Fairy-wrens are remarkable among birds not only due to their conspicuous male nuptial colours and high levels of promiscuity [13], but also because, unlike other bird families, they have repeatedly evolved U-type vision from V-type eyes [12]. Character correlation analyses indicate that these shifts in visual system are associated with changes in plumage coloration, and all fairy-wrens with U-type vision exhibit plumage patches rich in shortwave reflectance (UV, violet, blue) [12]. This could suggest that changes in visual sensitivity led to the evolution of these colours. However, since shortwave-rich colours pre-date the evolution of U-type eyes in this genus, Ödeen et al. [12] hypothesize that the presence of shortwave-rich colours facilitated the shift to U-type eyes. Regardless of which came first, two main mechanisms could explain the link between U-type vision and shortwave-rich plumage coloration: U-type eyes may increase the ability of conspecifics to detect shortwave-rich plumage against natural backgrounds (thereby making males with such plumage more conspicuous); and/or U-type eyes may enable them to better discriminate chromatic differences between potential mates or rivals with shortwave-rich plumage [12].
Here we test these two hypotheses by evaluating the performance of V- and U-type vision at (i) detecting contrast between male plumage colours and natural backgrounds (figure 1), and (ii) discriminating intraspecific variability in nuptial coloration (figure 1) [15]. We use psychophysical visual models [16] to place reflectance spectra in the visual space of birds (figure 1) with V- and U-type eyes, and model a large dataset of spectral measurements encompassing male nuptial coloration of 16 out of 17 species of fairy-wrens and emu-wrens (see the electronic supplementary material, table S1). We expect that, in general, U-type eyes should outperform V-type eyes at both of these tasks, and predict that this difference should be larger for colours rich in short-wavelength reflectance. This constitutes the first test of the common assumption that U-type eyes should perform better at detecting or discriminating shortwave-rich colours. In addition, we also tested whether, irrespective of shortwave reflectance, the plumage of U-type species is particularly easy to detect or discriminate by U-type vision. This could also be expected if plumage colours evolve to suit the visual sensitivities of the intended receivers in ways other than changes in short-wavelength reflectance.
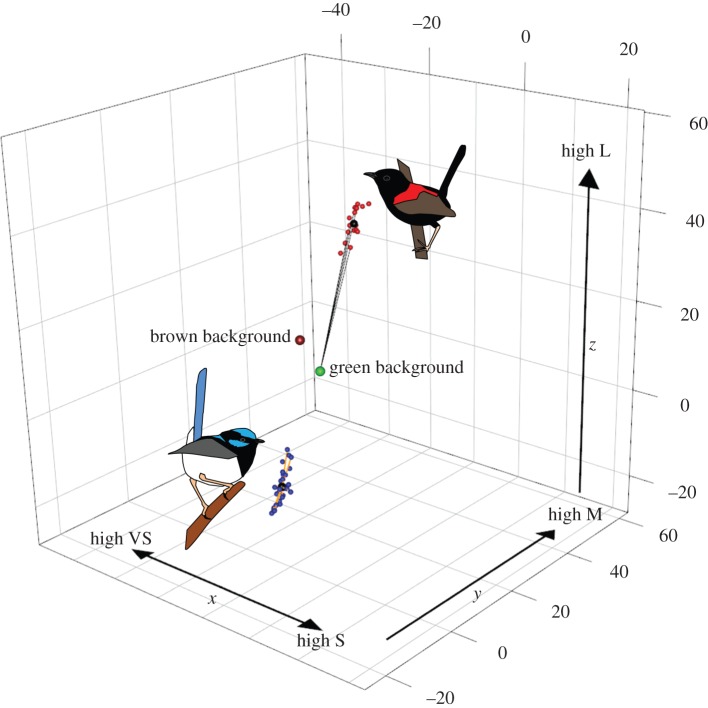
Figure 1.
A visual representation of methods, showing how detectability and chromatic variability were calculated. The graph depicts plumage chromatic variability of the back plumage of two fairy-wren species in the visual space of birds (following [14]), where chromatic variation along the axes is measured in just noticeable differences (jnds). Variation along the z-axis represents stimulation of the L cone relative to the other three cones (M, S, VS); variation along the y-axis represents stimulation of the M cone relative to the S and VS cones; and finally variation along the x-axis represents stimulation of the VS cone relative to the S cone. Red symbols correspond to the red back plumage of M. melanocephalus, while blue symbols correspond to the blue back plumage of M. cyaneus. The black symbols within each cloud correspond to the patch-specific mean. Detectability, the contrast against the background, was computed as the distance between each point and the average green and brown backgrounds, shown here as the lines between red plumage of M. melanocephalus and the average green background (black lines; only a few lines are depicted, for clarity). Intraspecific chromatic variability (inter-individual variation in coloration of homologous plumage patches [15]) was computed as the distance between each point in a sample (plumage patch) and its joint xyz mean, as shown here for the blue plumage of M. cyaneus (orange lines; only a few lines are depicted, for clarity). This procedure was carried out for both U- and V-type visual sensitivities (U-type visual space depicted here).
2. Methods
(a). Reflectance measurements
Reflectance spectra of plumage and backgrounds were collected in a standardized manner using a spectrometer (Avaspec 2048) connected to a pulsed xenon light source (Avalight-XE, Avantes, Eerbek, The Netherlands) through a bifurcated fibre-optic cable fitted at the end, with a plastic cylinder to standardize measuring distance and shield out ambient light. Illumination and recording angles were both 90° [17]. Reflectance was computed relative to a WS-2 white standard using the program Avasoft v. 6.2.1 (Avantes). Reflectance spectra between 300 and 700 nm (which encompass the visual sensitivity of birds [6]) were down-sampled to 5 nm steps and imported into spreadsheets.
We measured plumage reflectance of males in breeding plumage from 345 specimens belonging to 13 species of fairy-wren (Genera Malurus, Clytomyias) and three species of emu-wren (Genus Stipiturus) housed in the collections of the Melbourne Museum and the Australian Wildlife Collection in Canberra. We measured six plumage areas: head, cheek, back (representing upper dorsal plumage, including scapulars), tail (dorsal side only), breast and belly. Some species showed two obviously different ‘back’ colours and we sampled these separately (hence for these species we had two patches of back colour: back 1 and back 2; electronic supplementary material, table S1). Depending on the size of the plumage patch, we took between two and four spectral scans per patch. Since for M. coronatus we did not have enough samples of museum specimens belonging to nuptial males of the same subspecies, we used instead a sample of 65 males in breeding plumage captured in the wild. These birds were measured using the same equipment and protocol in Australian Wildlife Conservancy's Mornington Wildlife Sanctuary, in northwest Australia (17°31′ S, 126°6′ E), between 2005 and 2010. For wild birds, we measured head, back, tail and breast plumage reflectance, and took five spectral scans per patch. A list of species, subspecies and plumage patches measured and their sample sizes are provided in the electronic supplementary material, table S1.
We obtained average reflectance spectra of two types of common backgrounds typically occurring in nature: green (green leaves) and brown (bark, leaf litter, soil; see the electronic supplementary material, figure S1). These were computed from 629 reflectance spectra collected in Mornington Wildlife Sanctuary between 2005 and 2010. These background colours are likely to be broadly representative of the major types of backgrounds encountered by most species (for simplicity, hereafter ‘natural backgrounds’), given the limited variation in reflectance of natural terrestrial backgrounds [18]. Consistent with this, using average green and brown spectra collected in a mixed forest in southern Germany [19] yielded similar results (cf. table S2 with table S3 in the electronic supplementary material).
(b). Colour modelling
Our aim here was to calculate how well U- and V-type eyes perform at two tasks: (i) detecting contrast between male plumage colours and two types of natural backgrounds, and (ii) discriminating intraspecific chromatic variation. Since these tasks are important mainly in the context of females finding or selecting mates, or males finding or assessing rivals, we carried out the analyses separately per subspecies (if more than one was available in our samples). Also, we excluded patches of black plumage from the analyses since they contain little if any chromatic variation [9] (see the electronic supplementary material, table S1 for a list of black patches). Thus, for each taxon, we had data for one to seven different plumage patches, giving 4065 reflectance spectra in total.
Visual modelling was based on the model by Vorobyev & Osorio [8,16] using the formulas described by Cassey et al. [14]. For each reflectance spectrum, we obtained a set of xyz coordinates that determine its position in three-dimensional avian perceptual space [14] (figure 1). Euclidean distances (distanceij = ((xi − xj)2 + (yi − yj)2 + (zi − zj)2)1/2) between points in this space are measured in just noticeable differences (jnds) and it is usually assumed that distances >1jnd are detectable by birds [8,16]. These models require knowledge of the following key parameters: visual sensitivity functions (cone sensitivities, including the effect of the transmission of the ocular medium and filtering by oil droplets), relative abundance of each cone type and the spectrum of irradiant light. The models assume that discrimination is carried out under bright light and that achromatic information is disregarded. Achromatic information in birds is mediated by a different photoreceptor—the double cone [6]—but since its sensitivity has not been shown to be different between birds with V- and U-type eyes we only model chromatic aspects of visual perception here.
Colour vision in birds is mediated by four different types of single cones sensitive to very short (VS), short (S), medium (M) and long (L) wavelengths, and differences between U- and V-type eyes are found mainly in the sensitivity functions of VS and S cones [6]. We used generalized cone sensitivity functions of U- and V-type eyes [20], which already incorporate effects of ocular medium and oil droplets. Cone proportions are needed to determine the signal-to-noise ratio (also called Weber fraction, ωi) for each type of single cone. A Weber fraction of 0.05 is usually used as representative of the L cone [14] and based on this value the ωi of the other cone types can be computed using formula 10 of Vorobyev et al. [8]. Cone proportions are only known for 22 species of birds, which do not include any species of fairy-wren [21]. Based on this limited sample, in general birds have fewer VS cones than S cones, fewer S cones than M cones, and similar quantities of M and L cones [21]. There seem to be no consistent differences between birds with U- versus V-type eyes [10,21]. Given that there is no indication that eye sensitivity functions and cone proportions change in a correlated fashion, and given that our main interest is to assess the performance of both types of cone sensitivity, we decided to use average cone proportions of all 22 known species (0.377 : 0.721 : 1.028 : 1; for VS : S : M : L) for the main analyses. However, we also tested whether the results are robust to variation in cone proportions of U- and V-type eyes (see the electronic supplementary material, text S1 for methods).
Finally, we used three irradiance spectra relevant to fairy-wrens and emu-wrens: standard daylight (D65, open environments), woodland shade (semi-open environments) and forest shade (inside forests) [8,22]. Based on the habitats occupied [12,23], we determined for each species the most probably illuminant or combination of illuminants in their natural environment (see the electronic supplementary material, table S1). If more than one illuminant was considered representative of the natural environment we averaged the results from the corresponding visual models before analyses. The main analysis was carried out on this combined dataset using the ‘correct’ irradiance for each species, but we also repeated the analyses for each irradiance type separately in order to assess the effect of variation in this parameter. All computations were carried out using custom-made scripts in the R environment [24] (scripts available upon request from K.D.).
(c). Data analysis
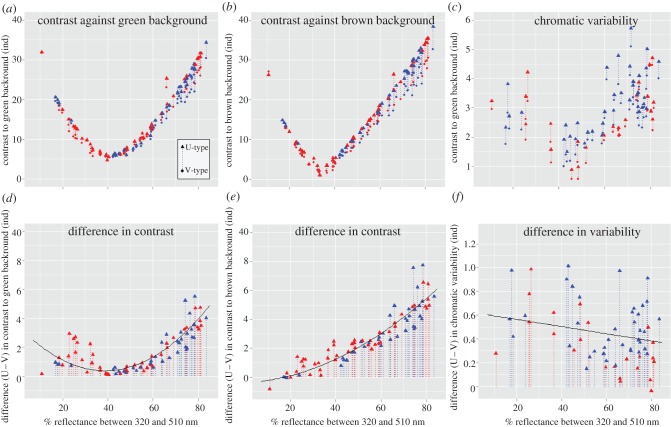
Detected contrast between plumage and natural backgrounds was computed as the Euclidean distance between plumage and each type of natural background (green and brown, figure 1). Chromatic variability was computed using the index described by Delhey & Peters [15], which is the average chromatic (Euclidean) distance of all points in a sample to their joint xyz mean (figure 1). For each plumage patch within each species, we computed the difference in performance of each vision type as the difference in detected contrast and chromatic variability between U- and V-type values (U−V). Positive values indicate better performance of U-type eyes when compared with V-type eyes. For those species with data for more than one subspecies, we averaged the variables separately for each plumage patch. This yielded data for 83 plumage patches of 16 species for the contrast against background analyses, and 61 plumage patches of 12 species for the chromatic variability analysis. Differences in sample size between these analyses stem from the fact that for some species only a few individuals (less than five) could be measured, making it impossible to compute intraspecific variability (species excluded: Clytomyias insignis, Stipiturus mallee, Stipiturus ruficeps, Malurus grayi). In order to compare performance of both eye types, we needed to quantify the level of shortwave reflectance of the plumage. Ödeen et al. [12] defined shortwave-rich colours as those that have more than 60 per cent reflectance in the 320–510 nm range. Given that this is an arbitrary cut-off point (see variation in figure 2), necessary to run their character correlation analyses [12], we preferred to analyse percentage reflectance in the 320–510 nm range as continuous covariate. High values would represent colours rich in UV/blue wavelengths and low values would represent colours rich in red wavelengths. This variable was centred on the mean of the entire sample before analyses [25].
Figure 2.
Comparing the performance of U- and V-type vision. Variation along the horizontal axis represents percentage of reflectance in the 320–510 nm range (see §2), where high values correspond to colours rich in short wavelength reflectance (UV/blue) and low values to colours rich in long wavelength reflectance (red). Upper panels (a–c) depict absolute values of contrast against (a) green and (b) brown backgrounds, and chromatic variability (c, note different scale on y-axis) as computed for V-type eyes (circle at the start of the arrow) and U-type eyes (triangle at the end of arrow). Lower panels (d–f) depict the corresponding differences in performance between U- and V-type eyes (computed as U−V). Red and blue symbols correspond to plumage patches belonging to species with V- and U-type eyes, respectively. Fitted lines are based on results from models in the electronic supplementary material, table S2.
As our data consisted of repeated samples per species (i.e. different plumage patches) with shared ancestry, we accounted for these two sources of non-independence in the same analysis using Bayesian phylogenetic mixed models (BPMM) in the R package ‘MCMCglmm’ [26,27]. These models have been used in other comparative studies [28,29] and phylogenetically controlled meta-analyses [30]. We initially used flat non-informative priors with low degree of belief for all parameters [30] (S. Nakagawa 2012, personal communication). Given that these priors can be informative with small values of posterior distributions (as was the case for our random effects; electronic supplementary material, table S2), we repeated the analyses using parameter-expanded priors [29] (J. Hadfield 2012, personal communication). Results were very similar, and we report results using parameter-expanded priors. R syntax for priors and models are provided in the electronic supplementary material, text S2. We assessed model convergence using the R package ‘coda’ [31]. For each model in electronic supplementary material, table S2, chains were run three times and we tested the convergence of model parameters using the Gelman–Rubin test. Here the potential scale reduction (PSR) factor should be less than 1.1. In all cases PSR < 1.03, indicating model convergence. We used a recent phylogeny of fairy-wrens [32] and set branch length using Grafen's method in the package ‘ape’ [33]. The data used in the main analyses (see the electronic supplementary material, table S2) are provided as electronic supplementary material, data S1.
3. Results
(a). Detected contrast against natural backgrounds
The contrast between plumage colours and two types of natural background (green and brown) was highest for colours rich in reflectance at shorter wavelengths (UV/blue) and colours poor in reflectance at shorter wavelengths (red), while it was lowest for intermediate levels (e.g. brown plumage; figure 2a,b). Overall, detected contrast was higher for U-type eyes: the difference in contrast between U- and V-type eyes was generally positive (figure 2d,e, green background: intercept = 1.167, credible interval CI = 0.345–2.031; brown background: intercept = 2.787, CI = 2.165–3.389; both p < 0.05; full results of models in electronic supplementary material, table S2). Consistent with our prediction, the difference in performance between U- and V-type visual systems was highest for plumage rich in shortwave reflectance (figure 2d,e, green background: percentage shortwave reflectance = 0.085, CI = 0.071–0.099; brown background: percentage shortwave reflectance = 0.103, CI = 0.09–0.117; both p < 0.001). This pattern was curvilinear, as indicated by the significant effect of the squared term (green background: percentage shortwave reflectance2 = 0.0021, CI = 0.0016–0.0026; brown background: percentage shortwave reflectance2 = 0.0008, CI = 0.0003–0.0012; both p < 0.001), and especially in the case of contrast against green background it tended to increase again for plumage poor in shortwave reflectance (figure 2d). After accounting for the effects of percentage shortwave reflectance, the advantage of U- over V-type eyes at detecting contrast was not greater for plumage colours belonging to species with U-type eyes (green background: 0.148, CI = −0.607–0.923; brown background: 0.255, CI = −0.375–0.835; both p > 0.3).
(b). Intraspecific chromatic variability
Intraspecific variability was higher for plumage with high and low levels of shortwave reflectance (figure 2c), resembling the patterns described for contrast (figure 2a,b). In general, U-type eyes could perceive more chromatic variation in conspecific coloration than V-type eyes (e.g. most data points in figure 2f are positive; intercept = 0.543, CI = 0.283–0.822; p = 0.003; full model results in electronic supplementary material, table S2). Against predictions, the advantage of U-type eyes at detecting intraspecific chromatic variability was slightly lower for colours rich in shortwave reflectance (figure 2f; percentage shortwave reflectance = −0.003, CI = −0.007 to −0.0007; p = 0.02). After accounting for the effects of percentage shortwave reflectance, the advantage of U-type eyes was not significantly greater at discriminating intraspecific chromatic variability in the plumage of species with U-type vision (effect = −0.191, CI = −0.460–0.091; p = 0.135).
(c). Effects of using different illuminants and cone proportions
Conclusions were not affected by using different irradiances in the visual models (see the electronic supplementary material, tables S4–S6). Similarly, general patterns were qualitatively similar when we used different cone proportions for U- and V-type eyes. Substantial departures from the general trend were only seen for extreme cone proportions: the advantage of U- over V-type eyes at detecting shortwave-rich colours was offset only in the unusual situation where V-type eyes had much higher abundance of VS and S cones than U-type eyes (see full results in the electronic supplementary material, text S1 and figures S2–S8).
4. Discussion
Here we show that U-type generally outperform V-type cone sensitivities at detecting plumage colours against natural backgrounds and at discriminating intraspecific variability in plumage coloration. This pattern seems general, as it has been shown in other studies [7–10], and is most likely to be due to the more even distribution of U-type cone sensitivities over the visual sensitivity range [8]. On its own, such a pattern cannot explain the correlation between U-type vision and shortwave-rich colours. In order for this to happen, U-type eyes need to perform especially well at detecting or discriminating shortwave-rich colours when compared with other colours. Our results provide the first empirical evidence supporting this idea, since the advantage of U-type eyes at detecting male nuptial plumage colours against two common types of natural background was larger for colours rich in shortwave reflectance (figure 2d,e). Against predictions, however, U-type eyes did not have an advantage at discriminating intraspecific differences in coloration of shortwave-rich plumage (figure 2f). Below we elaborate on these results, and discuss alternative explanations and potential implications.
Like most animals that communicate with visual signals, birds face the trade-off of facilitating detection by conspecifics while avoiding detection by predators [7]. This has sometimes led to the evolution of the so-called private communication channels, which are open mainly/only to conspecifics [34]. In birds, it has been hypothesized that the general advantage of U-type eyes may enable species with such a visual system to communicate using colours that are less visible to birds of prey, which have V-type eyes [7]. This has also been proposed to explain the shifts in visual system in fairy-wrens [12]. According to this idea, the colonization of semi-open environments rich in avian predators by ancestral fairy-wrens facilitated the shifts to visual systems (U-type) and plumage colours (shortwave-rich) that minimize detectability to predators and maximize conspicuousness to conspecifics [12]. At first glance, our results seem to provide support for this hypothesis since the advantage of U- over V-type eyes is larger at detecting shortwave-rich colours (figure 2d,e). However, shortwave-rich fairy-wren colours are nonetheless highly contrasting to both visual systems (figure 2a,b), often in excess of 10 jnds, which may indicate that these colours are always easy to detect [9,35]. Moreover, shifts in visual systems will not affect conspicuousness to predators unless they occur together with changes in coloration. Thus, it seems more parsimonious that, rather than favouring the evolution of different visual sensitivities, high risk of predation should first lead to the evolution of less conspicuous colours.
On the other hand, the association between U-type eyes and shortwave-rich colours may be due to increased success of highly contrasting males either at attracting females or deterring rivals. Detectability is an important component of signal design, and in general the most conspicuous signals are the most successful ones [36], perhaps due to inherent sensory biases [37]. As a result of such sensory biases, arbitrary changes in coloration due to drift followed by Fisher-like Lande–Kirkpatrick mechanisms of sexual selection [38] could result in the further elaboration of already existing [12] shortwave-rich colours in species with U-type eyes more often than in species with V-type eyes. This is because contrast increases more rapidly with higher reflectance at shorter wavelengths for this type of visual system (figure 2d,e). Interestingly, the advantage of U-type eyes seems larger when detecting plumage colours against brown than against green backgrounds (figure 2d,e; compare intercepts for both backgrounds in the electronic supplementary material, table S2). This could indicate that environmental differences in background reflectance can play an important role when it comes to shifts in visual systems or their effects on animal coloration, as has been shown to be the case with variability in irradiance [39]. Based on our results, we predict that species with U-type eyes may inhabit or display in environments rich in brown backgrounds. This hypothesis could be tested by exhaustive sampling of representative backgrounds in the visual environment of each fairy-wren species.
Our results do not support the idea that the association between shortwave-rich plumage colours and U-type eyes is related to increased performance at discriminating intraspecific variability in coloration [12]. Discriminability is another important aspect of signal design [40] and colours that have a signalling function have higher discriminability [15]. Thus, an optimal match between visual system and coloration that maximizes discriminability could favour both signallers and receivers [4,41]. Receivers would benefit by being better able to perceive differences in attractiveness or quality between prospective mates or rivals while honest signallers would benefit by being better able to broadcast their differences in coloration to these receivers. Against predictions, we did not find that the advantage of U-type eyes was higher for shortwave-rich colours; rather there was a slightly higher advantage at discriminating intraspecific variability of shortwave-poor colours (figure 2f). In other words, intraspecific chromatic variability of shortwave-rich plumage colours is not particularly easy to detect for U-type eyes. Thus, this explanation cannot account for the correlation between shortwave-rich colours and U-type eyes.
To conclude, our study reveals that the increased conspicuousness of shortwave-rich colours to U-type eyes may explain the correlation between plumage coloration and visual system in fairy-wrens and allies [12]. Whether these shifts in visual system were favoured by the presence of shortwave-rich plumage colours or whether visual shifts led to further elaboration of UV/blue colours cannot be established from our data. Irrespective of the order of events, however, our results constitute the first unequivocal confirmation that U-type eyes perform better when dealing with shortwave-rich colours. Given that shifts in bird visual sensitivities are more common than previously expected [42], future studies could address the generality of our findings in other clades of birds. Additionally, our supplemental analyses (see the electronic supplementary material, text S1 and figures S2–S8) suggest that very large differences in cone proportions between U- and V-type eyes could also lead to substantial variation in perceptual abilities. More research on interspecific variability in cone proportions is needed to fully establish the extent of this variation and its effect on visual sensitivities. Assessing the importance of different sources of sensory variation is essential to understand its role in shaping the diversity of animal colours.
Acknowledgements
We thank L. Revell, E. Paradis, J. Hadfield and S. Nakagawa for help with the comparative analyses, L. Joseph, R. Palmer and W. Longmore for access to museum collections under their care, T.-L. Gluckman, A. Taysom, T. Detto and L. Aplin for help with reflectance measurements, S. Legge (AWC) for access to Mornington Wildlife Sanctuary, B. Kempenaers, B. Wong and two anonymous reviewers for comments on the manuscript, and V. Delhey for help with R coding. This project was funded by the Max Planck Society (Minerva grant to A.P.), and the Australian Research Council (FT110100505 to A.P. and DE120102323 to K.D.).
References
- 1.Endler J. A., Basolo A. L. 1998. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420 10.1016/S0169-5347(98)01471-2 (doi:10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- 2.Seehausen O., et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626 10.1038/nature07285 (doi:10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 3.Bybee S. M., Yuan F., Ramstetter M. D., Llorente-Bousquets J., Reed R. D., Osorio D., Briscoe A. D. 2012. UV photoreceptors and UV-yellow wing pigments in Heliconius butterflies allow a color signal to serve both mimicry and intraspecific communication. Am. Nat. 179, 38–51 10.1086/663192 (doi:10.1086/663192) [DOI] [PubMed] [Google Scholar]
- 4.Osorio D., Vorobyev M. 2008. A review of the evolution of animal colour vision and visual communication signals. Vision Res. 48, 2042–2051 10.1016/j.visres.2008.06.018 (doi:10.1016/j.visres.2008.06.018) [DOI] [PubMed] [Google Scholar]
- 5.Stoddard M. C., Prum R. O. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052 10.1093/beheco/arr088 (doi:10.1093/beheco/arr088) [DOI] [Google Scholar]
- 6.Hart N. S., Hunt D. M. 2007. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, 7–26 10.1086/510141 (doi:10.1086/510141) [DOI] [PubMed] [Google Scholar]
- 7.Håstad O., Victorsson J., Ödeen A. 2005. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl Acad. Sci. USA 102, 6391–6394 10.1073/pnas.0409228102 (doi:10.1073/pnas.0409228102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorobyev M., Osorio D., Bennett A. T. D., Marshall N. J., Cuthill I. C. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633 10.1007/s003590050286 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 9.Schaefer H. M., Schaefer V., Vorobyev M. 2007. Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? Am. Nat. 169, S159–S169 10.1086/510097 (doi:10.1086/510097) [DOI] [PubMed] [Google Scholar]
- 10.Avilés J. M., Soler J. J. 2009. Nestling colouration is adjusted to parent visual performance in altricial birds. J. Evol. Biol. 22, 376–86 10.1111/j.1420-9101.2008.01655.x (doi:10.1111/j.1420-9101.2008.01655.x) [DOI] [PubMed] [Google Scholar]
- 11.Mullen P., Pohland G. 2008. Studies on UV reflection in feathers of some 1000 bird species: are UV peaks in feathers correlated with violet-sensitive and ultraviolet-sensitive cones? Ibis 150, 59–68 10.1111/j.1474-919X.2007.00736.x (doi:10.1111/j.1474-919X.2007.00736.x) [DOI] [Google Scholar]
- 12.Ödeen A., Pruett-Jones S., Driskell A. C., Armenta J. K., Håstad O. 2012. Multiple shifts between violet and ultraviolet vision in a family of passerine birds with associated changes in plumage coloration. Proc. R. Soc. B 279, 1269–1276 10.1098/rspb.2011.1777 (doi:10.1098/rspb.2011.1777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingma S., Hall M., Segelbacher G., Peters A. 2009. Radical loss of an extreme extra-pair mating system. BMC Ecol. 9, 15. 10.1186/1472-6785-9-15 (doi:10.1186/1472-6785-9-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassey P., Ewen J., Blackburn T., Hauber M., Vorobyev M., Marshall N. 2008. Eggshell colour does not predict measures of maternal investment in eggs of Turdus thrushes. Naturwis senschaften 95, 713–721 10.1007/s00114-008-0376-x (doi:10.1007/s00114-008-0376-x) [DOI] [PubMed] [Google Scholar]
- 15.Delhey K., Peters A. 2008. Quantifying variability of avian colours: are signalling traits more variable? PLoS ONE 3, e1689. 10.1371/journal.pone.0001689 (doi:10.1371/journal.pone.0001689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson S., Prager M. 2006. Quantifying colors. In Bird coloration (eds Hill G. E., McGraw K.), pp. 41–89 Cambridge, MA: Harvard University Press [Google Scholar]
- 18.Osorio D., Bossomaier T. R. J. 1992. Human cone-pigment spectral sensitivities and the reflectances of natural surfaces. Biol. Cyber. 67, 217–222 10.1007/BF00204394 (doi:10.1007/BF00204394) [DOI] [PubMed] [Google Scholar]
- 19.Delhey K., Roberts M., Peters A. 2010. The carotenoid-continuum: carotenoid-based plumage ranges from conspicuous to cryptic and back again. BMC Ecol. 10, 13. 10.1186/1472-6785-10-13 (doi:10.1186/1472-6785-10-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endler J. A., Mielke P. W. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 10.1111/j.1095-8312.2005.00540.x (doi:10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 21.Hart N. 2001. Variations in cone photoreceptor abundance and the visual ecology of birds. J. Comp. Physiol. A 187, 685–697 10.1007/s00359-001-0240-3 (doi:10.1007/s00359-001-0240-3) [DOI] [PubMed] [Google Scholar]
- 22.Endler J. A. 1993. The color of light in forests and its implications. Ecol. Monogr. 63, 2–27 [Google Scholar]
- 23.Rowley I., Russell E. 1997. Fairy-wrens and grasswrens. Oxford, UK: Oxford University Press [Google Scholar]
- 24.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See www.R-project.org. [Google Scholar]
- 25.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Meth. Ecol. Evol. 1, 103–113 10.1111/j.2041-210X.2010.00012.x (doi:10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 26.Hadfield J. D. 2010. MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J. Stat. Soft. 33, 1–22 [Google Scholar]
- 27.Hadfield J. D., Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508 10.1111/j.1420-9101.2009.01915.x (doi:10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 28.Cornwallis C. K., West S. A., Davis K. E., Griffin A. S. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972 10.1038/nature09335 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 29.Longdon B., Hadfield J. D., Webster C. L., Obbard D. J., Jiggins F. M. 2011. Host phylogeny determines viral persistence and replication in novel hosts. PLoS Pathog. 7, e1002260. 10.1371/journal.ppat.1002260 (doi:10.1371/journal.ppat.1002260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horváthová T., Nakagawa S., Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. B 279, 163–70 10.1098/rspb.2011.0663 (doi:10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plummer M., Best N., Cowles K., Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7–11 [Google Scholar]
- 32.Driskell A. C., Norman J. A., Pruett-Jones S., Mangall E., Sonsthagen S., Christidis L. 2011. A multigene phylogeny examining evolutionary and ecological relationships in the Australo-papuan wrens of the subfamily Malurinae (Aves). Mol. Phyl. Evol. 60, 480–485 10.1016/j.ympev.2011.03.030 (doi:10.1016/j.ympev.2011.03.030) [DOI] [PubMed] [Google Scholar]
- 33.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 34.Cummings M. E., Rosenthal G. G., Ryan M. J. 2003. A private ultraviolet channel in visual communication. Proc. R. Soc. Lond. B 270, 897–904 10.1098/rspb.2003.2334 (doi:10.1098/rspb.2003.2334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens M., Cuthill I. C. I. C. 2007. Hidden messages: are ultraviolet signals a special channel in avian communication? BioScience 57, 501. 10.1641/B570607 (doi:10.1641/B570607) [DOI] [Google Scholar]
- 36.Endler J. A. 2000. Evolutionary implications of the interaction between animal signals and the environment. In Animal signals: signalling and signal design in animal communication (eds Espmark Y., Amundsen T., Rosenqvist G.), pp. 11–46 Trondheim, Norway: Tapir Academic Press [Google Scholar]
- 37.Ryan M. J., Keddy-Hector A. 1992. Directional patterns of female mate choice and the role of sensory biases. Am. Nat. 139, 4–35 10.1086/285303 (doi:10.1086/285303) [DOI] [Google Scholar]
- 38.Prum R. O. 2010. The Lande–Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for meaning, honesty, and design in intersexual signals. Evolution 64, 3085–100 10.1111/j.1558-5646.2010.01054.x (doi:10.1111/j.1558-5646.2010.01054.x) [DOI] [PubMed] [Google Scholar]
- 39.Cummings M. E. 2007. Sensory trade-offs predict signal divergence in surfperch. Evolution 61, 530–545 10.1111/j.1558-5646.2007.00047.x (doi:10.1111/j.1558-5646.2007.00047.x) [DOI] [PubMed] [Google Scholar]
- 40.Guilford T., Dawkins M. S. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14 10.1016/S0003-3472(05)80600-1 (doi:10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 41.Briscoe A. D., Bybee S. M., Bernard G. D., Yuan F., Sison-Mangus M. P., Reed R. D., Warren A. D., Llorente-Bousquets J., Chiao C.-C. 2010. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl Acad. Sci. USA 107, 3628–3633 10.1073/pnas.0910085107 (doi:10.1073/pnas.0910085107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ödeen A., Håstad O., Alström P. 2011. Evolution of ultraviolet vision in the largest avian radiation—the passerines. BMC Evol. Biol. 11, 313. 10.1186/1471-2148-11-313 (doi:10.1186/1471-2148-11-313) [DOI] [PMC free article] [PubMed] [Google Scholar]