The commentary by Rosa et al., argues that while a V3-like area (VLP), termed VLP, is present in the V3v region immediately adjacent to the ventral half of V2; dorsally, a V3d is not found along the dorsal half of V2 in any primate species. Instead, the dorsal region (which represents the lower visual field) of the VLP is displaced more anteriorly. In place of a V3d, they contend that both an upper and lower field representation begin immediately adjacent to a portion of dorsal V2, forming the upper and lower quadrants of the dorsomedial visual area (DM). While in the Lyon & Connolly [1] review we also support the existence of DM, the key difference with Rosa and co-workers is that our DM is displaced by a V3d. This V3d forms a mirror reversal of the lower field representation in V2 and is approximately half the width. The view presented in the Rosa et al.'s commentary is based on retinotopic maps from Rosa's group and connection patterns from Angelucci's group, with all of the work being conducted in marmoset monkeys. As we presented in the original review not only does a large body of anatomical and mapping data in several species other than marmoset support the Lyon & Connolly interpretation of a V3d [2–15], the original work that led Jon Kaas and me to the re-interpretation of a V3d in place of DM was with marmosets as well [16].
Why does the Lyon & Kaas interpretation differ from Rosa and co-workers? As explained in the Lyon & Connolly [1] review, microelectrode maps can be reinterpreted to support a V3d. While the presence of the upper field representation for cells found just beyond the dorsal V2 border is used by Rosa as evidence for a DM, the receptive fields in these examples actually include a substantial portion of the lower visual field and therefore can just as well be used to support the horizontal meridian representation of a V3d. We agree with Rosa's commentary that this observed retinotopic progression at the outer border of V2 is consistent with what one would expect the emergence of the upper field representation in DM to resemble, but it is not conclusive as it is also the pattern one would expect for a narrow V3d. Furthermore, while microelectrode mapping can provide detail at the level of individual neurons, reconstructions of electrode tracks using coronal sections make it more difficult to translate the pattern onto a two-dimensional cortical surface. As noted in our review, techniques such as intrinsic signal optical imaging of the cortical surface in lisencephalic primates and fMRI in macaques and humans provide a better global view for evaluating retinotopic patterns, and the evidence overwhelmingly favours a V3d, not only for the limited studies in New World monkey [9,17,18] and prosimian galago [19], but also in the extensive work on Old World macaques and humans [1,2,4,20–23] (see fig. 5 in the original review [1]). Thus, possible aberrations from microelectrode mapping such as the three horizontal meridians, two of which are entirely separated from each other, and the two entirely separated foveal representations in DM portrayed by Rosa and co-workers (see fig. 1 in their commentary), are not found using intrinsic signal imaging. Rather, imaging shows a clear lower field only representation along the outer V2 border with a single horizontal meridian (along the outer border of V2) and a single foveal representation, all consistent with a V3d [9] (for more details, see figs 3 and 4 in the original review [1]).
The new anatomical evidence from Jeffs et al. [24] highlighted in the commentary does provide more detailed connectivity patterns than previously published as they used up to as many as seven tracers in dorsal V2 across a narrow region, as well as several injections in DM within individual marmosets. By contrast, my work on marmosets made only one tracer injection per case into dorsal V2 or DM, and up to five injections per case into V1, but these were spread over a wider area [16]. While Jeffs et al. [24] interpret the superbly detailed results as supporting an area DM in place of a V3d the inability to accurately determine, the outer border of V2 from ambiguous cytochrome oxidase (CO) architecture and potential damage caused by several injections being made at or near the V3d region leaves this conclusion in doubt.
As explained in the initial review [1] and in my publications with Jon Kaas, we have maintained that the primary discrepancy between Rosa's, and now Jeffs' work, and ours is that a narrow V3d, about half the width of V2, is found between V2d and DM. An overestimate of the outer border of dorsal V2 will result in a larger V2 that effectively consumes V3d within it, leading to the interpretation that V3 is not present. Thus, the difference in interpretation is not due to the fact that only four to five tracers were used for our injections into marmoset V1 [16] and up to seven tracers were used in V2 by Jeffs et al. [24], it is rather that we interpret the outer border of V2 to be in a relatively different location, rostrocaudally. The provided CO stains in Jeffs are particularly useful for determining the V1/V2 border and the MT/MTc complex [24]. However, in their examples, it is difficult to identify with much certainty the outer border of V2; this border largely seems to be estimated and is much larger at the widest portion of dorsal V2, approximately 4 mm in their fig. 6 [24], than the 2.5–3.0 mm range found in Lyon & Kaas [16]. This larger estimate likely places V3d at the outer 1 mm of Jeffs' dorsal V2. In support of this, an injection near the outer region of their V2 actually exhibits a V3d-like characteristic pattern of connections. This can be seen in figs 3 and 5 of Jeffs (blue tracer), where a V3d-like pattern of label is even acknowledged by them. This injection densely labels the outer border of dorsal V2, and in my opinion, the inner border of V3d, but that pattern is not represented in summary figures of Jeffs et al. [24] nor in the summary figures in the commentary of Rosa et al.
In fig. 7 of Jeffs et al. [24] the overall pattern of many of the tracers does seem to emphasize a VLP/VLA border much more so than a V3d region, which is quite intriguing. However, my concern is that the large fast blue injection that is placed right where I would have located V3d, because I would have the outer V2 border at least a millimetre caudally, may mask some of the V3d pattern of label. By contrast, injections made in dorsal V1 in my own work with Kaas leave the V3d region totally unperturbed and show clear and robust labelling in both V3d and the VLP/VLA-like region. This is found in marmosets (see fig. 3 in Lyon & Kaas [16]) and in several other primate species [6–8]. For example, as shown in figure 1, where three tracer injections were made in relatively close proximity in V1 of a New World squirrel monkey [8], the yellow-outlined DLc and DLr region (caudal and rostral subdivisions of the dorsal lateral area; a region that may be homologous to V4 in macaques [7,25]) is in the relative location of VLP/VLA portrayed by Rosa and co-workers and has a pattern of label that looks very similar to that shown for VLP/VLA in fig. 7 of Jeffs et al. [24]. Yet, in addition to this VLP/VLA-like label, there is also substantial label in the expected location of V3d along the outer border of most of dorsal V2 (figure 1).
Figure 1.
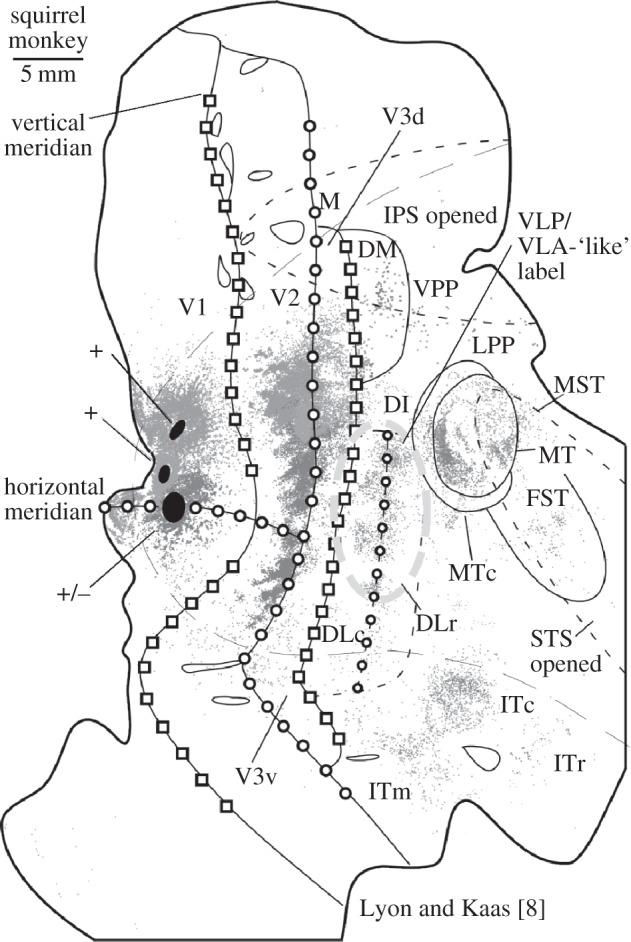
Connection patterns following tracer injections across adjacent locations in V1 of a squirrel monkey. Adapted from Lyon & Kaass [8]. The yellow highlighted region shows a connection similar to that the VLP/VLA label shown from the high-resolution tracer mapping by Jeffs et al. [24]. However, the point of showing this example is that robust labelling is also present in V3d; unlike the results from Jeffs et al., which report little if any V3d-like connectivity patterns. As discussed in the text, such a difference in results is likely due to different interpretations of the width of dorsal V2 and to potential damage of the V3d region by placing so many injections in the immediate area.
It is also worth noting that for some of the tracer injections in fig. 7 of Jeffs et al. [24] (see fig. 8 as well) a V3d pattern is actually visible if one uses a smaller 2.5 or 3 mm estimate of the width of V2. For example, the green and red tracer patterns of label just anterior to the injection sites are in what I would consider to be V3d; however, the pattern of label that could be seen in V3d is somewhat obfuscated by the large blue injection site that is probably in V3d. The V3d location of the blue injection seems clear because all blue label in V1 is in the lower visual field representation, and that the horizontal meridian in ventral V2 and what I would consider V3v are also labelled (which would be expected from an injection on the horizontal meridian, as shown previously by Jeffs et al. [26]). If, on the other hand, the blue injection is partially overlapping the upper field representation of DM, as portrayed in the figure, blue label should be in the upper quadrants of V1, V2 and MT, but this is not clearly supported by connection patterns that show label in either the lower field representation of V1 or tightly along the horizontal meridian in MT and V2.
In summary, it seems likely that differences in the localization of the outer border of dorsal V2 is the primary reason for the very different conclusions regarding V3d and DM following the analysis of connection patterns [6–8,16,24]. Even while more anatomical studies would certainly help in evaluating whether or not there is a DM or a V3d immediately adjacent to the outer border of dorsal V2, the preponderance of evidence from imaging of retinotopic maps in monkeys and humans [1] is overwhelmingly in favour of V3d. Nevertheless, future studies, combining both imaging and connectional approaches in the same animal, can perhaps finally resolve the controversy over the third tier of primate visual cortex, allowing us to turn our full attention to controversies concerning the fourth tier [25,27–29].
Footnotes
The accompanying comment can be viewed at http://dx.doi.org/doi:10.1098/rspb.2012.1372.
References
- 1.Lyon D. C., Connolly J. D. 2012. The case for primate V3. Proc. R. Soc. B 279, 625–633 10.1098/rspb.2011.2048 (doi:10.1098/rspb.2011.2048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer A. A., Press W. A., Logothetis N. K., Wandell B. A. 2002. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J. Neurosci. 22, 10 416–10 426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cragg B. G. 1969. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 9, 733–747 10.1016/0042-6989(69)90011-X (doi:10.1016/0042-6989(69)90011-X) [DOI] [PubMed] [Google Scholar]
- 4.Fize D., Vanduffel W., Nelissen K., Denys K., Chef d'Hotel C., Faugeras O., Orban G. A. 2003. The retinotopic organization of primate dorsal V4 and surrounding areas: a functional magnetic resonance imaging study in awake monkeys. J. Neurosci. 23, 7395–7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattass R., Sousa A. P., Gross C. G. 1988. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 8, 1831–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyon D. C., Kaas J. H. 2002. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav. Evol. 59, 114–129 10.1159/000064159 (doi:10.1159/000064159) [DOI] [PubMed] [Google Scholar]
- 7.Lyon D. C., Kaas J. H. 2002. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron 33, 453–461 10.1016/S0896-6273(02)00580-9 (doi:10.1016/S0896-6273(02)00580-9) [DOI] [PubMed] [Google Scholar]
- 8.Lyon D. C., Kaas J. H. 2002. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J. Comp. Neurol. 449, 281–297 10.1002/cne.10297 (doi:10.1002/cne.10297) [DOI] [PubMed] [Google Scholar]
- 9.Lyon D. C., Xu X., Casagrande V. A., Stefansic J. D., Shima D., Kaas J. H. 2002. Optical imaging reveals retinotopic organization of dorsal V3 in New World owl monkeys. Proc. Natl Acad. Sci. USA 99, 15 735–15 742 10.1073/pnas.242600699 (doi:10.1073/pnas.242600699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schira M. M., Tyler C. W., Breakspear M., Spehar B. 2009. The foveal confluence in human visual cortex. J. Neurosci. 29, 9050–9058 10.1523/JNEUROSCI.1760-09.2009 (doi:10.1523/JNEUROSCI.1760-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sereno M. I., Dale A. M., Reppas J. B., Kwong K. K., Belliveau J. W., Brady T. J., Rosen B. R., Tootell R. B. 1995. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893 10.1126/science.7754376 (doi:10.1126/science.7754376) [DOI] [PubMed] [Google Scholar]
- 12.Sousa A. P., Pinon M. C., Gattass R., Rosa M. G. 1991. Topographic organization of cortical input to striate cortex in the Cebus monkey: a fluorescent tracer study. J. Comp. Neurol. 308, 665–682 10.1002/cne.903080411 (doi:10.1002/cne.903080411) [DOI] [PubMed] [Google Scholar]
- 13.Tootell R. B., Mendola J. D., Hadjikhani N. K., Ledden P. J., Liu A. K., Reppas J. B., Sereno M. I., Dale A. M. 1997. Functional analysis of V3A and related areas in human visual cortex. J. Neurosci. 17, 7060–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Essen D. C., Newsome W. T., Maunsell J. H., Bixby J. L. 1986. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J. Comp. Neurol. 244, 451–480 10.1002/cne.902440405 (doi:10.1002/cne.902440405) [DOI] [PubMed] [Google Scholar]
- 15.Zeki S. M. 1969. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 14, 271–291 10.1016/0006-8993(69)90110-3 (doi:10.1016/0006-8993(69)90110-3) [DOI] [PubMed] [Google Scholar]
- 16.Lyon D. C., Kaas J. H. 2001. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J. Neurosci. 21, 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaskan P. M., Lu H. D., Dillenburger B. C., Kaas J. H., Roe A. W. 2009. The organization of orientation-selective, luminance-change and binocular-preference domains in the second (V2) and third (V3) visual areas of New World owl monkeys as revealed by intrinsic signal optical imaging. Cereb. Cortex 19, 1394–1407 10.1093/cercor/bhn178 (doi:10.1093/cercor/bhn178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X., Bosking W., Sary G., Stefansic J., Shima D., Casagrande V. 2004. Functional organization of visual cortex in the owl monkey. J. Neurosci. 24, 6237–6247 10.1523/JNEUROSCI.1144-04.2004 (doi:10.1523/JNEUROSCI.1144-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan R. H., Baldwin M. K., Jermakowicz W. J., Casagrande V. A., Kaas J. H., Roe A. W. 2012. Intrinsic signal optical imaging evidence for dorsal V3 in the prosimian galago (Otolemur garnettii). J. Comp. Neurol. 10.1002/cne.23154 (doi:10.1002/cne.23154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sereno M. I., Tootell R. B. 2005. From monkeys to humans: what do we now know about brain homologies? Curr. Opin. Neurobiol. 15, 135–144 10.1016/j.conb.2005.03.014 (doi:10.1016/j.conb.2005.03.014) [DOI] [PubMed] [Google Scholar]
- 21.Tootell R. B., Tsao D., Vanduffel W. 2003. Neuroimaging weighs in: humans meet macaques in ‘primate’ visual cortex. J. Neurosci. 23, 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Essen D. C. 2004. Organization of visual areas in macaque and human cerebral cortex In The visual neurosciences (eds Chalupa L. M., Werner J. S.), pp. 507–521 Cambridge, MA: MIT Press [Google Scholar]
- 23.Wandell B. A., Dumoulin S. O., Brewer A. A. 2007. Visual field maps in human cortex. Neuron 56, 366–383 10.1016/j.neuron.2007.10.012 (doi:10.1016/j.neuron.2007.10.012) [DOI] [PubMed] [Google Scholar]
- 24.Jeffs J., Federer F., Ichida J. M., Angelucci A. 2012. High-resolution mapping of anatomical connections in marmoset extrastriate cortex reveals a complete representation of the visual field bordering dorsal V2. Cereb. Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon D. C., Rabideau C. 2012. Lack of robust LGN label following transneuronal rabies virus injections into macaque area V4. J. Comp. Neurol. 520, 2500–2511 10.1002/cne.23050 (doi:10.1002/cne.23050) [DOI] [PubMed] [Google Scholar]
- 26.Jeffs J., Ichida J. M., Federer F., Angelucci A. 2009. Anatomical evidence for classical and extra-classical receptive field completion across the discontinuous horizontal meridian representation of primate area V2. Cereb. Cortex 19, 963–981 10.1093/cercor/bhn142 (doi:10.1093/cercor/bhn142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepniewska I., Collins C. E., Kaas J. H. 2005. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb. Cortex 15, 809–822 10.1093/cercor/bhh182 (doi:10.1093/cercor/bhh182) [DOI] [PubMed] [Google Scholar]
- 28.Tootell R. B., Hadjikhani N. 2001. Where is ‘dorsal V4’ in human visual cortex? Retinotopic, topographic and functional evidence. Cereb. Cortex 11, 298–311 10.1093/cercor/11.4.298 (doi:10.1093/cercor/11.4.298) [DOI] [PubMed] [Google Scholar]
- 29.Ungerleider L. G., Galkin T. W., Desimone R., Gattass R. 2008. Cortical connections of area V4 in the macaque. Cereb. Cortex 18, 477–499 10.1093/cercor/bhm061 (doi:10.1093/cercor/bhm061) [DOI] [PubMed] [Google Scholar]


