Abstract
Alien plants are a growing threat to the Galápagos unique biota. We evaluated the impact of alien plants on eight seed dispersal networks from two islands of the archipelago. Nearly 10 000 intact seeds from 58 species were recovered from the droppings of 18 bird and reptile dispersers. The most dispersed invaders were Lantana camara, Rubus niveus and Psidium guajava, the latter two likely benefiting from an asynchronous fruit production with most native plants, which facilitate their consumption and spread. Lava lizards dispersed the seeds of 27 species, being the most important dispersers, followed by small ground finch, two mockingbirds, the giant tortoise and two insectivorous birds. Most animals dispersed alien seeds, but these formed a relatively small proportion of the interactions. Nevertheless, the integration of aliens was higher in the island that has been invaded for longest, suggesting a time-lag between alien plant introductions and their impacts on seed dispersal networks. Alien plants become more specialized with advancing invasion, favouring more simplified plant and disperser communities. However, only habitat type significantly affected the overall network structure. Alien plants were dispersed via two pathways: dry-fruited plants were preferentially dispersed by finches, while fleshy fruited species were mostly dispersed by other birds and reptiles.
Keywords: exotics, frugivory, mutualistic interactions, oceanic islands, ornithochory, saurochory
1. Introduction
Globally, invasive species rank among the most serious threats to native biodiversity and as such they become a major driver of global change [1]. Their ravaging effects are nowhere more powerful than on oceanic islands [2], where ecologically ‘naive’ species evolved under low selective pressure from higher trophic-level species (e.g. herbivores and parasites) [3].
Even in remote archipelagos, such as the Galápagos, Mauritius and Hawaii, alien plants already outnumber native species [4–6]. Many of these aliens have high invasion rates owing to the long distance dispersal of their seeds [7]. Seed dispersal may be particularly decisive when frugivores include the fruit of invasive plants into their diets and consequently facilitate their establishment and spread [8].
Most oceanic islands, particularly remote ones, have low species diversity, and some animal groups are more poorly represented than plant species [3], i.e. the ratio between animal and plant species tends to be lower on islands than on continents [9]. This can magnify the effect of anthropogenic shifts in the assemblage of frugivores, affecting seed dispersal and influencing overall vegetation structure and ecosystem functioning [10]. Knowledge of seed dispersal processes can thus prove crucial to understanding the dynamics of plant invasions and the planning of effective conservation strategies, such as the control or eradication of fleshy fruited weeds [11]. There are increasing efforts to collect information on the potential dispersers of invasive plant species [12] and on the plants dispersed by introduced animals [13]. However, rigorous information on the entire species assemblages of plants, seed dispersers and their interactions is still rarely available [14]. Even when such studies exist, they have mainly considered birds, with reptiles and mammals receiving much less attention [15]. In order to make an unbiased community-level assessment of seed dispersal, all animals that include fruits or seeds in their diets should be considered simultaneously [16].
Much ecosystem functioning is founded on species interactions [17], and it is through the network of interactions that disturbances cascade through biological communities [18]. In recent years, this growing realization has lifted the focus of conservation efforts from species to ecosystems [1,19]. While there is an increasing number of studies documenting different aspects of the dispersal of native and invasive plants by frugivores [10,20], the consequences of the integration of alien plants into seed dispersal networks has been poorly explored [21]. By contrast, several studies have evaluated the impact of alien plants upon pollination networks. These have produced different results, with some studies detecting changes in the structure of pollination networks [22,23], while others have not [24,25]. These results suggest that the effect of alien species is system-dependent. In some cases, the disruptive effects of alien plants can be detected at the network-level, whereas in others, changes are more subtle and take place at the level of individual species [25]. Here, we report on the results of a study on the impact of alien plant species upon plant-seed disperser networks on the Galápagos Islands, simultaneously considering network- and species-level effects.
The Galápagos Islands and their unique biodiversity are seriously threatened by alien invasive plants [26]. These may affect native species directly, but repercussions may also ripple off throughout the entire ecological network of an island or the archipelago without necessarily leading to the local extinction of native species [27]. We suggest that such a disturbance scenario can be better understood by a network approach; however, our knowledge on seed dispersal networks in the archipelago is still very limited [28]. In this study, we analyse the temporal patterns of fruit production, which set the template for frugivory, and the links connecting fruiting plants and their seed dispersers.
There are four objectives in our study: (i) Evaluate the synchrony in the fruiting phenology of the most abundant native and alien plants. We hypothesize that the benefit resulting from seed dispersers might be higher for alien species that produce fruits in periods of native fruit shortage. (ii) Evaluate the extent to which alien plants infiltrate the seed dispersal network and the structural consequences of that integration at the species and network levels. We hypothesized that the linkage pattern in invaded sites would become more generalized, given that alien species tend to be attractive to many frugivores [29]. (iii) Assess the relative importance of different fruit-eating species as seed dispersers. (iv) Evaluate the existence of preferred ‘invasion routes’ taken by animal-dispersed fleshy- and dry-fruited alien plants into the Galápagos seed dispersal systems.
2. Material and methods
(a). Study site
The Galápagos lie on the equator in the Eastern Pacific, ca. 960 km west of South America (see the electronic supplementary material, appendix A). This young volcanic archipelago, 0.5–4 Myr, [30] is composed of 13 islands larger than 10 km2 and numerous islets.
The archipelago has two seasons: a hot/wet season prevails from January to May, corresponding to the fruiting period of most plants, while a cold/dry season occurs from June to December [31]. During the dry season, a permanent drizzle (or garúa) allows the development of a permanently humid habitat in the highest part of the tallest islands, whereas the lowland zone of all the islands is markedly dry [31].
The late establishment of permanent human settlements in the archipelago, as recent as the nineteenth century, delayed the onset of anthropogenic habitat degradation [32]. However, alien species rapidly took their toll and changed extensive areas of the archipelago [33]. According to the Charles Darwin Foundation checklist [34], the Galápagos flora consists of 557 native vascular plant species (of which 32% are endemic), and more than 825 alien species. Among the most problematic invasive plants are the fleshy-fruited Psidium guajava (guava) and Rubus niveus (blackberry), which have severely altered the composition and structure of some of the natural ecosystems, particularly in the humid zone [33].
(b). Experimental design
Data were collected from eight sites using a hierarchical design (see the electronic supplementary material, appendix A) including the two most human-populated islands (Santa Cruz and San Cristóbal), the two most representative vegetation types (dry lowland and humid highland) and two levels of invasion (‘native’ and ‘invaded’). The classification of the invasion level was first made based on the visual estimation of native and introduced plant cover and further confirmed by counting all native and introduced fruits produced in each site. The eight sites were sampled with equal effort between March 2010 and February 2011. During the main fruiting season (February–July) each site was visited twice per month, while the same sites were visited once per month in the cold/dry season. Data from each site were pooled and used to build year-round seed dispersal networks. Quantitative seed dispersal networks were based on the analysis of faecal samples from birds, the giant tortoise and lava lizards. Interaction frequency was quantified as the number of droppings from each animal species containing at least one intact seed of each plant species. Bird faecal samples were collected during 18 ringing sessions with mist nets at each site. In each session, mist nets were opened at sunrise and remained open for 6 consecutive hours. Captured birds were left up to 30 min in ringing bags to defaecate. Intact seeds in droppings were identified under a dissecting microscope by comparison with the reference collection in the Charles Darwin Foundation. Reptile droppings were collected along one fixed 50 × 2 m linear transect in each plot and seed identification was performed with the same methodology. Overall, 2879 droppings were collected: 2293 from 15 bird species and 586 from three reptile species.
To document fruiting phenology, the abundance of fleshy fruits was estimated for each plot by monthly counts of all ripe fruits within a swathe of vegetation of 1 m either side of a fixed 50 m linear transect.
(c). Species interaction patterns
We explored the effects of level of invasion, plant origin, island and habitat on the following species-level parameters: linkage level (or degree), plant specialization (d′) and species strength. Linkage level is the number of disperser species per plant. Plant specialization (d′) as suggested by Blüthgen et al. [35] is a measure of the selectivity of a species that takes into account surrogates of overall plant availability for their interactions partners. Species strength of plants and dispersers, suggested by Bascompte et al. [36], is the sum of each species' dependencies and reflects the importance of each species to the other ‘trophic’ level. Throughout the text, we used species strength as a proxy of the dispersers importance to plants.
(d). Network structure
We tested whether the integration of alien plants affected six common network descriptors: connectance, weighed nestedness based on overlap and decreasing fills (WNODF), plants' niche overlap, dispersers' generality, robustness against extinction of dispersers and weighted interaction evenness (see Dormann et al. [37] and references therein for detailed descriptions of all parameters and their implementation).
(e). Data analyses
Species- and network-level parameters were calculated using the statistical package Bipartite v. 1.16 [37] for R [38]. Species-level descriptors did not achieve normality after transformations and were included in generalized linear models with the most appropriate error distribution (normal, gamma or Poisson) and correspondent link function. Four explanatory variables were included as fixed factors in the model: island, habitat, level of invasion and plant origin. Network-level descriptors were transformed and included in general linear models with three fixed factors (island, habitat and level of invasion). Network size was used as a covariate in all models, as it is known to influence most network descriptors [39]. All models were fitted using SPSS v. 17.
The existence of ‘preferred’ dispersal routes was evaluated with two-way χ2 contingency tables to test for independence between the frequency of occurrence of seeds of dry- and fleshy-fruited plants in the droppings of the following disperser groups: Galápagos finches, other birds and reptiles. While there were other possible ways to group the dispersers after phylogenetic and/or functional traits, these three broad categories allows us to detect overall trends between finches and non-finch birds which are known to have quite distinct feeding behaviour [28,40,41] and distinguish these from non-flying dispersers.
3. Results
Intact seeds were retrieved from 498 bird (22%) and 208 reptile (36%) droppings. Only droppings from the birds Lateralus spilonotus, Zenaida galapagoensis and Coccyzus melacoryphus did not have any intact seed.
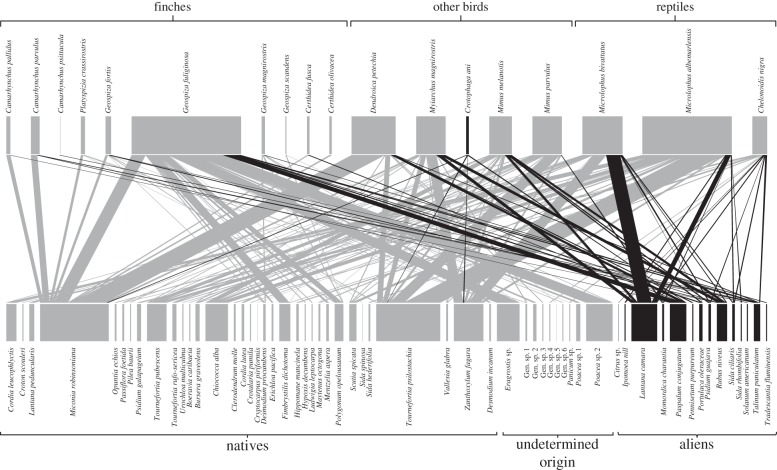
A total of 9159 intact seeds from 58 plant species were retrieved from the droppings, revealing a total of 144 interactions with the 18 dispersers. The overall seed dispersal network is shown in figure 1 and site-specific networks in electronic supplementary material, appendix B. Identified plants included 33 (57%) natives and 14 (24%) alien species. The remaining 19 per cent could not be identified to species-level. Of all identified seeds, 8019 (88%) were from natives and only 447 (5%) were from aliens. This result, however, is influenced by the high abundance of the very small seeds of Miconia robinsoniana (n = 5781 seeds; 63.1% of all seeds found), which was mainly dispersed by the yellow warbler (Dendroica petechia). However, even excluding the seeds of this species, the majority (66%) were native, compared with 13 per cent alien and 21 per cent of undetermined origin. Nevertheless, intact alien seeds were found in droppings of 15 of the 18 seed dispersers. Among the serious invasive species, seeds of R. niveus were dispersed by six bird species (mainly Myiarchus magnirostris and Mimus melanotis); Lantana camara was mainly dispersed by the two lizard species, and to a minor extent by Myiarchus magnirostris and Mimus melanotis; finally, P. guajava was dispersed by the bird Mimus melanotis, the lizard Microlophus bivattatus, and the giant tortoise Chelonoidis nigra (see the electronic supplementary material, appendix C).
Figure 1.
Graphical representation of the overall seed dispersal network. Native species are shaded in light grey and alien species in black.
(a). Level of invasion and fruiting phenology
Fruit production was highly variable among sites (see the electronic supplementary material, appendix D). The total number of fleshy fruits counted at each site over the whole year ranged from 480 to 34 654 (mean = 11 178). The proportion of alien fruits was nearly 100-fold higher in the invaded than in the native site across all pairs (table 1), supporting the a priori experimental design. Although the proportion of alien fruits at native sites was consistently small, there was much variation in level of invasion among invaded sites (0.3%, 42.1%, 65.5% and 96.1%, respectively), with a higher level of invasion on San Cristóbal (see the electronic supplementary material, appendix E).
Table 1.
Mean values of the network descriptors for island, habitat and level of invasion. p (w/size) reflects the significance of each variable when network size is included in the model. Significant differences for α = 0.05 are marked with asterisk (*).
| island |
habitat |
invasion |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean |
mean |
mean |
invasion x island |
invasion x habitat |
||||||||||||
| S. Cruz | S. Cristóbal | p | p (w/size) | humid | dry | p | p (w/size) | invaded | native | p | p (w/size) | p | p (w/size) | p | p (w/size) | |
| network size | 144.8 | 164.5 | 0.800 | 59.8 | 249.5 | 0.003* | 144.0 | 165.3 | 0.512 | 0.718 | 0.655 | |||||
| % alien fruits | 11.3 | 41.0 | 0.004* | 0.056 | 34.6 | 17.6 | 0.012* | 0.242 | 51.7 | 0.6 | 0.001* | 0.030* | 0.005* | 0.059 | 0.009* | 0.078 |
| P - plant species | 17.0 | 18.5 | 0.867 | 0.665 | 7.8 | 27.8 | 0.002* | 0.411 | 16.0 | 19.5 | 0.274 | 0.222 | 0.592 | 0.829 | 1.000 | 0.302 |
| D - disperser species | 8.3 | 8.3 | 1.000 | 0.787 | 7.5 | 9.0 | 0.070 | 0.649 | 8.3 | 8.3 | 1.000 | 0.675 | 0.814 | 0.466 | 0.178 | 0.247 |
| unique interactions | 28.0 | 31.8 | 0.768 | 0.738 | 15.3 | 44.5 | 0.000* | 0.085 | 28.3 | 31.5 | 0.303 | 0.442 | 0.628 | 0.300 | 0.932 | 0.647 |
| connectance | 0.23 | 0.23 | 0.932 | 0.611 | 0.28 | 0.18 | 0.033* | 0.593 | 0.25 | 0.21 | 0.251 | 0.399 | 0.370 | 0.172 | 0.164 | 0.190 |
| weighted nestedness | 21.22 | 21.14 | 0.992 | 0.611 | 28.15 | 14.21 | 0.014* | 0.871 | 23.26 | 19.11 | 0.282 | 0.456 | 1.000 | 0.214 | 0.276 | 0.347 |
| robustness dispersers extinction | 0.44 | 0.45 | 0.822 | 0.981 | 0.41 | 0.48 | 0.003* | 0.369 | 0.43 | 0.45 | 0.112 | 0.216 | 0.443 | 0.478 | 0.138 | 0.220 |
| plant niche overlap | 0.30 | 0.40 | 0.349 | 0.305 | 0.40 | 0.31 | 0.498 | 0.959 | 0.35 | 0.35 | 0.996 | 0.938 | 0.232 | 0.646 | 0.917 | 0.976 |
| dispersers generality | 3.95 | 4.30 | 0.815 | 0.960 | 2.46 | 5.80 | 0.001* | 0.084 | 3.76 | 4.49 | 0.127 | 0.192 | 0.663 | 0.451 | 0.870 | 0.938 |
| interaction evenness | 0.80 | 0.83 | 0.328 | 0.330 | 0.79 | 0.84 | 0.226 | 0.700 | 0.82 | 0.81 | 0.755 | 0.590 | 0.659 | 0.270 | 0.442 | 0.591 |
The peak of the fruiting season was reached in May and ranged from April to August for most species, although some species such as Scutia spicata set fruit earlier (figure 2; electronic supplementary material, appendix F). Most common native plants had sequential fruiting peaks with a large overlapped in fruit production. However, two common alien invasive species fruited mostly asynchronously with the main peak of native fruit production: R. niveus in February and P. guajava in July–August.
Figure 2.
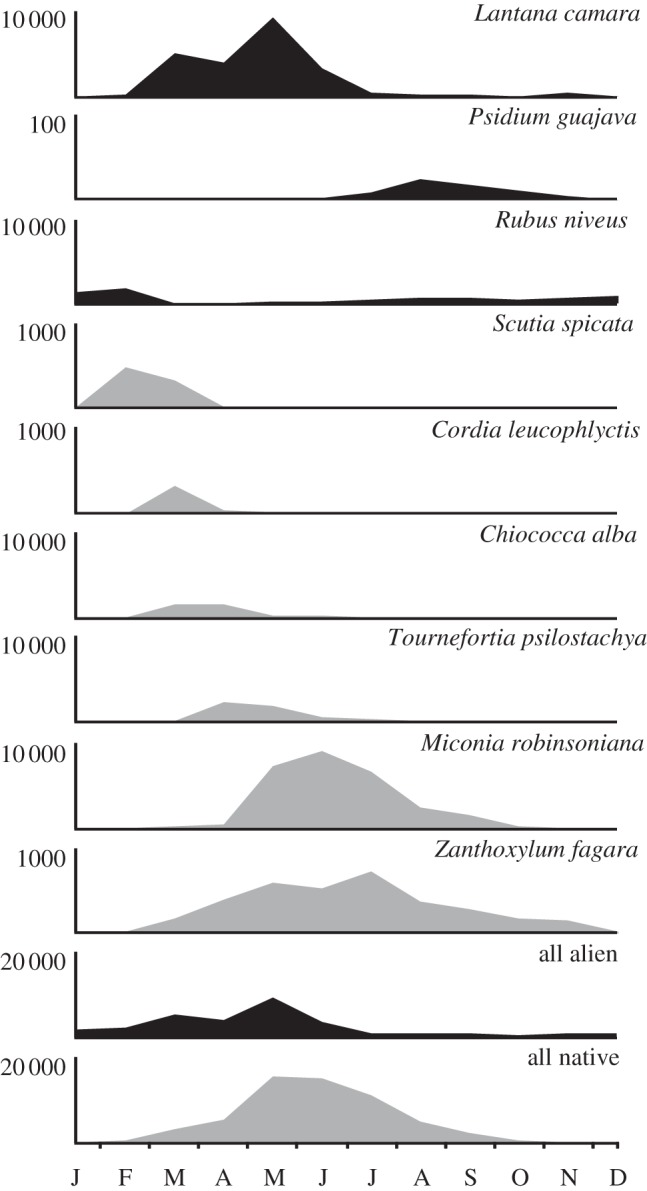
Number of ripe fleshy fruits of the most abundant species counted along monthly linear transects in the eight study sites between March 2010 and February 2011. Alien species are represented in grey and native species in black. Note the different scales on the y-axis (values indicate the scale maximum).
(b). Species interaction patterns
On Santa Cruz, native plants tended to have more disperser species than aliens, while the opposite was found on San Cristóbal (figure 3 and table 2).
Figure 3.
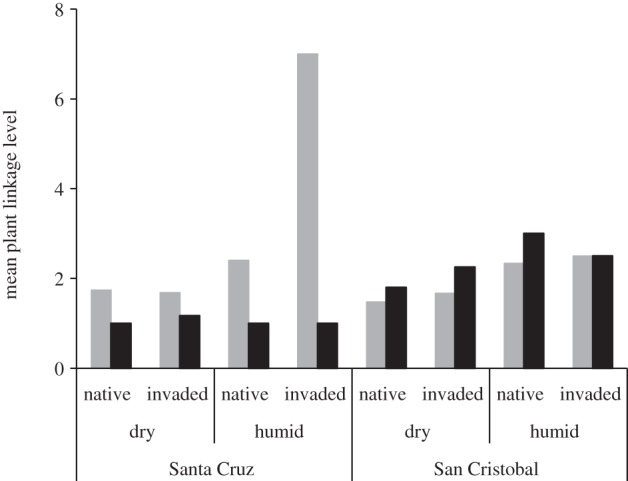
Mean number of disperser species for native (grey bars) and alien (black bars) plants (i.e. plant linkage level or degree) at each study site.
Table 2.
Mean parameter values for the plant species-level descriptors for island, habitat and level of invasion. Significant differences for α = 0.05 are marked with asterisk (*).
| island |
habitat |
invasion |
plant origin |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean |
mean |
mean |
mean |
island x invasion | island x origin | invasion x origin | |||||||||
| S. Cruz | S. Cristóbal | p | dry | humid | p | native | invaded | p | native | alien | p | p | p | p | |
| plant linkage level | 1.65 | 1.72 | 0.228 | 1.60 | 1.97 | 0.024* | 1.62 | 1.77 | 0.223 | 1.83 | 1.72 | 0.105 | 0.760 | 0.010* | 0.864 |
| plant specialization (d') | 0.26 | 0.29 | 0.212 | 0.26 | 0.31 | 0.044* | 0.28 | 0.26 | 0.885 | 0.26 | 0.25 | 0.726 | 0.113 | 0.156 | 0.032* |
| plant strength | 0.49 | 0.45 | 0.009* | 0.32 | 0.97 | 0.000* | 0.42 | 0.52 | 0.105 | 0.58 | 0.36 | 0.007* | 0.222 | 0.000* | 0.156 |
On average, plants from the humid zone showed a higher degree of specialization (d′), i.e. a higher selectivity among possible dispersers, and also a higher strength than species from the dry zone (table 2). Invaded sites had lower levels of specialization (d′) for native plants but greater levels for aliens (figure 4), implying that natives become less selective on their dispersers as invasion progresses, whereas aliens become more selective. Moreover, alien plants showed higher linkage level than natives on the most invaded San Cristóbal, while the opposite occurred on Santa Cruz (figure 3).
Figure 4.
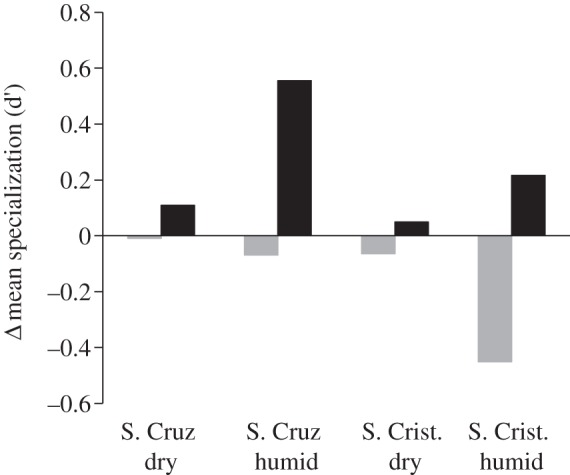
Effect of invasion on the variation of the specialization index (d′) proposed by Blüthgen et al. [35] for native and alien plants. Positive bars indicate an increase in d′ with invasion, while negative bars represent a decrease. Grey columns indicate native plants; black columns indicate changes in alien plants.
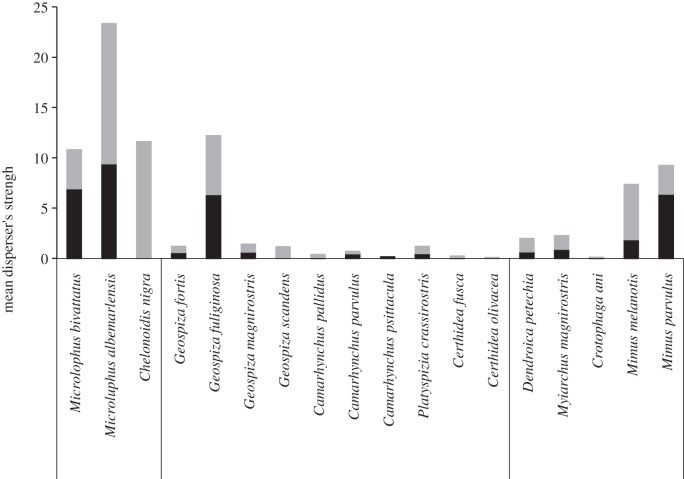
Reptiles, particularly the two species of lava lizards, were the most important dispersers for the plant community in terms of their strength (figure 5). Among birds, the small ground finch (Geospiza fuliginosa), followed by the two endemic mocking birds (Mimus spp.), showed the highest strength.
Figure 5.
Mean species strength of seed dispersers in native (grey bars) and invaded (black bars) sites. The disperser strength, suggested by Bascompte et al. [36], reflects the importance of each species for the plant community.
(c). Network structure
As suggested from the visual inspection of the networks (see the electronic supplementary material, appendix B), the overall network structure was quite similar between islands and between native and invaded sites (table 1; electronic supplementary material, appendix E). Regarding habitat, however, networks in the humid zone were simpler in structure than in the dry zone and were usually dominated by two or three very common interactions (see the electronic supplementary material, appendix B). Thus, habitat had a strong effect on network structure, affecting most network descriptors. However, when network size was included as a covariate in the model, none of the parameters was significantly affected, indicating a high correlation between network size and most descriptors. Similarly, none of the network descriptors was significantly affected by level of invasion or by the interaction between this variable and either of the two other explanatory variables (table 1).
(d). Invasion routes
Overall, 28 plant species with dry fruits and 24 species with fleshy fruits (excluding undetermined species) had their seeds dispersed by 18 animals. In our analysis, we operated with three disperser types: (i) 10 species of finch, (ii) five other bird species, and (iii) three species of reptiles (the giant tortoise and two lava lizards).
Different seed disperser types dispersed significantly different fruit types (fleshy versus dry; χ2 = 18.17, d.f. = 2, p < 0.001). Dry fruits were dispersed by finches more than expected by their overall proportion (expected 11.3, observed 17; χ2 = 6.21, d.f. = 1, p = 0.013), and fleshy fruits were dispersed by ‘other birds’ (expected 11.1, observed 18; χ2 = 8.04, d.f. = 1, p = 0.005) and reptiles (expected 16.2, observed 22; χ2 = 3.93, d.f. = 1, p = 0.047), more often than expected (see details in electronic supplementary material, appendix I).
On average, the number of disperser types used by native plants (1.53) was slightly lower than for aliens (1.80). A high proportion of plants (n = 29; 56%) appeared to be consumed by only one type of disperser type, whereas only four species were consumed by all three types. These four plants included two dry-fruited aliens Portulaca oleraceae and Talinum paniculatum, the fleshy-fruited invader R. niveus and the fleshy-fruited native Tournefortia psilostachya (see the electronic supplementary material, appendix J).
4. Discussion
Despite the serious threats posed by alien species in the Galápagos, with ca 60 per cent of the flora being alien and over 100 invasive species [6], the proportion of alien plants in the seed dispersal networks is still relatively low (24% of the species; 17% of the droppings; 5% of the seeds). Although comparable data are not available for most oceanic archipelagos, this invasion level is considerably lower than that of a similar study in the Azores ca 60 per cent (R. Heleno 2012, unpublished data). Nevertheless, four of the five top invasive plants in the Galápagos have fleshy fruits and we found three of these (R. niveus, L. camara and P. guajava) well integrated into the seed dispersal networks.
(a). The role of different vertebrates as seed dispersers
Lava lizards (Microlophus albermarlensis in S. Cruz and Microlophus bivattatus in San Cristóbal) were quantitatively the most important seed dispersers of all. The seven endemic Microlophus species are abundant in the dry zone of all main islands (except on Darwin and Wolf), which is the most common habitat type in the archipelago [42]. Although lizards are important seed dispersers on other oceanic islands [43], their role as seed dispersers in the Galápagos had not been previously evaluated. In the present study, the two species of lava lizards dispersed 972 intact seeds (present in 190 droppings; mean = 5.1 (min = 1, max = 24) seeds/dropping) of 27 plant species, especially T. psilostachya, Zanthoxylum fagara, Chiococca alba and L. camara (figure 1). The attractiveness of Z. fagara fruits is puzzling, considering the almost complete absence of fruit pulp and the sharp spines protecting fruiting branches. Notwithstanding, these fruits were among the most consumed food items of lizards and flycatchers, resulting in the dispersal of many (n = 246) intact seeds. We hypothesize that such consumption might be related to the antifungal properties recently identified in Z. fagara fruits [44].
Although giant tortoises were present at only one of our study sites, this species also played an important role as seed disperser (i.e. high species strength). Thus, the extirpation of tortoises must have had a negative impact upon seed dispersal in general [45]. The current re-introduction of tortoises to several islands is likely to have important consequences for the population dynamics of many species and should be monitored carefully [46].
Our results also support previous findings [41,47] that ground finches act mainly as seed predators, and not so much as dispersers, of L. camara. Instead, we found 100 intact seeds of this species in the droppings of lava lizards (n = 38), mockingbirds (n = 5), flycatcher (n = 8) and giant tortoise (n = 1), which clearly shows that animals are actively involved in the dispersal of this invasive shrub.
Among birds, G. fuliginosa was the most important disperser, followed by the two mocking bird species from each island (Mimus parvulus in S. Cruz and Mimus melanotis in San Cristóbal). However, when considered together, these two species exceeded the importance of G. fuliginosa, which occurs on both islands. Geospiza fuliginosa dispersed a high number of intact seeds by virtue of its high abundance (c. 37% of all bird captures; electronic supplementary material, appendix H) and wide distribution in the dry and humid zones, but many seeds were physically destroyed [41]. By contrast, mockingbirds, mostly restricted to the dry zone, appeared to act more as legitimate dispersers than as seed predators.
The important contribution of insectivores for seed dispersal (particularly Myiarchus magnirostris and D. petechia) (figure 1) [48] suggests that the effectiveness of the Galápagos dispersers is better described as a gradient from poor to good dispersers than by the typically assumed dichotomy between legitimate seed dispersers and non-dispersers, akin to the distribution described by Heleno et al. [49] in the Azores (and see also Nogales et al. [50]). The only introduced bird in the Galápagos, Crotophaga ani, has been suggested to play an important role in facilitating plant invasions [48,51]. Even if based on a small number of droppings (n = 12), we found little evidence for this effect, as 99.7 per cent of the 329 seeds retrieved from its droppings were from native species (Miconia robinsoniana, T. psilostachya and Z. Fagara) even if alien fruits were present on the collection sites.
(b). Historical factors driving a delayed integration of invaders
San Cristóbal was colonized by humans earlier than Santa Cruz, which translated into an earlier arrival of invasive plants [6,52]. This might explain the stronger integration of alien plants in the San Cristóbal networks. Although Santa Cruz has now more naturalized species, as a consequence of its exponential human population growth [32], many of these aliens have not yet become widely spread [6], and may consequently be still poorly infiltrated into the local seed dispersal networks. It is thus likely that seed dispersal networks in Santa Cruz will develop along the same trajectory as that seen in San Cristóbal. Two of the most invasive species, R. niveus and L. camara, are especially widespread and abundant in San Cristóbal compared with Santa Cruz. This might actually lead to the higher number of dispersers of alien plants observed in the former, and supports the hypothesis of a delayed integration of alien plants in Santa Cruz.
(c). Native and alien fruiting phenology and invasion routes
Fruiting phenology is an important constraint of plant-disperser interactions [53], although knowledge regarding fruiting phenology of most Galápagos plants is very limited. The pattern described here of sequential ripening of native fruits in the Galápagos is compatible with an inter-specific strategy to avoid satiation of dispersers, in line with what has been suggested for asynchronous fruit ripening within conspecific plants [54]. Similarly, the asynchronous fruit production of R. niveus and P. guajava in relation to most native species is likely to offer a competitive advantage to these aliens, as the abundance of seed dispersers in periods of native fruit shortage might be an important mechanism assisting alien expansion. Although our study is a first step in the understanding of fruit-frugivore dynamics in the archipelago, such a hypothesis deserves further attention.
Alien plant species invaded the seed dispersal networks along one of two pathways: the dry-fruited and the fleshy-fruited routes. The invasion along the dry-fruited route was facilitated by finches, whereas the invasion along the fleshy-fruited route was facilitated by other bird species, lizards and the giant tortoise (see the electronic supplementary material, appendix J). We found almost no exception to this pattern.
(d). Species interaction patterns
Disperser specialization was lower in the dry than in the humid zone, which we attribute to the more diverse vegetation in the former, as it offers a higher variety of resources to frugivores. Moreover, disperser strength was, on average, higher in the humid than in the dry zone, reflecting a greater importance of each disperser species for humid communities, with less disperser species. Our findings showed that alien plants tend to disperse more seeds by means of fewer dispersers (i.e. becoming more specialized) as invasion progresses, while native plants show the opposite pattern. We attribute these results to the selective pressure that alien plants may exert on the frugivore community, starting off by being dispersed by generalists but favouring the dispersers that are most effective in consuming their fruits and displacing those that are more dependent on native resources, from highly invaded sites. The end result might thus be a simplified plant community, which would tend to promote a less diverse community of dispersers [21].
(e). Network structure
Overall, network topology did not vary much between islands or with the level of invasion, although differences between native and invaded sites were easily perceived and quantified in the field. It is possible that seed dispersal networks behave like phase-shift systems, i.e. highly resilient to intermediate levels of disturbance and then suddenly breaking down irreversibly, as suggested for trophic [55] and pollination networks [56]. If that is the case, our data suggest that this phase-shift threshold has not yet been reached in any of the studied sites; however, the networks on San Cristóbal are at a more advanced stage of degradation.
The dry and humid zones in the Galápagos are markedly different, and differences in their seed dispersal systems were also expected. This expectation was largely confirmed as most network and species level descriptors evaluated differed significantly between dry and humid habitats (table 1). Such differences were largely explained on the basis of network size, with much larger, diverse networks in the dry lowlands. Miconia robinsoniana, the characteristic tree in the humid habitats (also known as the Miconia zone), was the only species found to be dispersed into all habitats, suggesting that the species distribution is not limited by seed dispersal. Hence, this species might respond well to the on-going control of alien species in the humid zone by the Galapagos National Park.
(f). Concluding remarks
Despite the advanced plant invasions in Galápagos, the level of integration of alien seeds into seed dispersal networks is still relatively moderate. Lava lizards were found to be the most important dispersers, at least quantitatively, moving the seeds of 27 species. The large representation of granivorous and insectivorous birds is reflected in an overall low frequency of occurrence of intact seeds in droppings. Nevertheless, two insectivorous species (Myiarchus magnirostris and D. petechia) showed an unexpectedly high contribution to the overall seed dispersal process.
By fruiting outside the main native fruiting season, the aliens R. niveus and P. guajava might benefit from an unsaturated disperser community to assist their spread. Alien plants were found to become more specialized during the invasion process, favouring more simplified plant and disperser communities. Sites on San Cristóbal were at a more advanced stage of invasion, which translated into a higher integration of alien fruits in the seed dispersal networks. This suggests a time-lag between the establishment of alien plants and their impacts on the structure and function of seed dispersal networks. Alien plants tend to be integrated into seed disperser networks via two preferred routes: dry-fruited species such as grasses and herbs tend to be dispersed by Galápagos finches, mostly granivorous, whereas fleshy-fruited plants are mainly dispersed by other birds and reptiles, particularly lava lizards.
Acknowledgements
We are grateful to the Fundación BBVA (Spain) for financing this project and to the Charles Darwin Foundation and the Galápagos National Park (Ecuador) for crucial logistical support in the archipelago. We thank Catarina Heleno, Denise Meribeth, Patricia Jaramillo and Susana Chamorro for their help in the field and in the laboratory, to Carsten Dormann for his help in the data analysis and also the Portuguese ringing Scheme (CEMPA/ICNB) for providing bird rings.
References
- 1.Millennium Ecosystem Assessment Board, 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press [Google Scholar]
- 2.Sax DF, Gaines SD. 2008. Species invasions and extinction: the future of native biodiversity on islands. Proc. Natl Acad. Sci. USA 105, 11 490–11 497 10.1073/pnas.0802290105 (doi:10.1073/pnas.0802290105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittaker RJ, Fernández-Palacios JM. 2007. Island biogeography: ecology, evolution, and conservation, 401 p. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Olesen JM, Eskildsen LI, Venkatasamy S. 2002. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Divers. Distributions 8, 181–192 10.1046/j.1472-4642.2002.00148.x (doi:10.1046/j.1472-4642.2002.00148.x) [DOI] [Google Scholar]
- 5.Brockie RE, Loope LL, Usher MB, Hamann O. 1988. Biological invasions of island nature reserves. Biol. Conserv. 44, 9–36 10.1016/0006-3207(88)90003-1 (doi:10.1016/0006-3207(88)90003-1) [DOI] [Google Scholar]
- 6.Trueman M, Atkinson R, Guezou A, Wurm P. 2010. Residence time and human-mediated propagule pressure at work in the alien flora of Galapagos. Biol. Invas. 12, 3949–3960 10.1007/s10530-010-9822-8 (doi:10.1007/s10530-010-9822-8) [DOI] [Google Scholar]
- 7.Nathan R. 2006. Long-distance dispersal of plants. Science 313, 78. 10.1126/science.1124975 (doi:10.1126/science.1124975) [DOI] [PubMed] [Google Scholar]
- 8.Nathan R, Muller-Landau HC. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 15, 278–285 10.1016/S0169-5347(00)01874-7 (doi:10.1016/S0169-5347(00)01874-7) [DOI] [PubMed] [Google Scholar]
- 9.González-Castro A, Traveset A, Nogales M. 2012. Seed dispersal interactions in the Mediterranean Region: contrasting patterns between islands and mainland. J. Biogeogr. 39, 1938–1947 10.1111/j.1365-2699.2012.02693.x (doi:10.1111/j.1365-2699.2012.02693.x) [DOI] [Google Scholar]
- 10.Milton SJ, Wilson JRU, Richardson DM, Seymour CL, Dean WRJ, Iponga DM, Proches S. 2007. Invasive alien plants infiltrate bird-mediated shrub nucleation processes in arid savanna. J. Ecol. 95, 648–661 10.1111/j.1365-2745.2007.01247.x (doi:10.1111/j.1365-2745.2007.01247.x) [DOI] [Google Scholar]
- 11.Gosper CR, Stansbury CD, Vivian-Smith G. 2005. Seed dispersal of fleshy-fruited invasive plants by birds: contributing factors and management options. Divers. Distributions 11, 549–558 10.1111/j.1366-9516.2005.00195.x (doi:10.1111/j.1366-9516.2005.00195.x) [DOI] [Google Scholar]
- 12.Padrón B, Nogales M, Traveset A, Vilà M, Martínez-Abraín A, Padilla DP, Marrero P. 2011. Integration of invasive Opuntia spp. by native and alien seed dispersers in the Mediterranean area and the Canary Islands. Biol. Invas. 13, 831–844 10.1007/s10530-010-9872-y (doi:10.1007/s10530-010-9872-y) [DOI] [Google Scholar]
- 13.Linnebjerg JF, Hansen DM, Bunbury N, Olesen JM. 2010. Diet composition of the invasive red-whiskered bulbul Pycnonotus jocosus in Mauritius. J. Trop. Ecol. 26, 347–350 10.1017/S0266467409990617 (doi:10.1017/S0266467409990617) [DOI] [Google Scholar]
- 14.Buckley YM, et al. 2006. Management of plant invasions mediated by frugivore interactions. J. Appl. Ecol. 43, 848–857 10.1111/j.1365-2664.2006.01210.x (doi:10.1111/j.1365-2664.2006.01210.x) [DOI] [Google Scholar]
- 15.Mello M, Marquitti F, Guimarães PR, Kalko E, Jordano P, Aguiar Md. 2011. The missing part of seed dispersal networks: structure and robustness of bat–fruit interactions. PLoS ONE 6, e17395. 10.1371/journal.pone.0017395 (doi:10.1371/journal.pone.0017395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donatti CI, Guimarães PR, Galetti M, Pizo MA, Marquitti FMD, Dirzo R. 2011. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol. Lett. 14, 773–781 10.1111/j.1461-0248.2011.01639.x (doi:10.1111/j.1461-0248.2011.01639.x) [DOI] [PubMed] [Google Scholar]
- 17.Duffy JE, Carinale BJ, France KE, McIntyre PB, Thébault E, Loreau M. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538 10.1111/j.1461-0248.2007.01037.x (doi:10.1111/j.1461-0248.2007.01037.x) [DOI] [PubMed] [Google Scholar]
- 18.Heleno RH, Ceia RS, Ramos JA, Memmott J. 2009. The effect of alien plants on insect abundance and biomass: a food web approach. Conserv. Biol. 23, 410–419 10.1111/j.1523-1739.2008.01129.x (doi:10.1111/j.1523-1739.2008.01129.x) [DOI] [PubMed] [Google Scholar]
- 19.Tylianakis JM, Laliberté E, Nielsen A, Bascompte J. 2010. Conservation of species interaction networks. Biol. Conserv. 143, 2270–2279 10.1016/j.biocon.2009.12.004 (doi:10.1016/j.biocon.2009.12.004) [DOI] [Google Scholar]
- 20.Kawakami K, Mizusawa L, Higuchi H. 2009. Re-established mutualism in a seed-dispersal system consisting of native and introduced birds and plants on the Bonin Islands, Japan. Ecol. Res. 24, 741–748 10.1007/s11284-008-0543-8 (doi:10.1007/s11284-008-0543-8) [DOI] [Google Scholar]
- 21.Spotswood EN, Meyer J-Y, Bartolome JW. 2012. An invasive tree alters the structure of seed dispersal networks between birds and plants in French Polynesia. J. Biogeogr. 39, 2007–2020 10.1111/j.1365-2699.2012.02688.x (doi:10.1111/j.1365-2699.2012.02688.x) [DOI] [Google Scholar]
- 22.Memmott J, Waser NM. 2002. Integration of alien plants into a native flower–pollinator visitation web. Proc. R. Soc. Lond. B 269, 2395–2399 10.1098/rspb.2002.2174 (doi:10.1098/rspb.2002.2174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aizen MA, Morales CL, Morales JM. 2008. Invasive mutualists erode native pollination webs. PLoS Biol. 6, 396–403 10.1371/journal.pbio.0060031 (doi:10.1371/journal.pbio.0060031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padrón B, Traveset A, Biedenweg T, Díaz D, Nogales M, Olesen JM. 2009. Impact of alien plant invaders on pollination networks in two archipelagos. PLoS ONE 4, e6275. 10.1371/journal.pone.0006275 (doi:10.1371/journal.pone.0006275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilà M, Bartomeus I, Dietzsch AC, Petanidou T, Steffan-Dewenter I, Stout JC, Tscheulin T. 2009. Invasive plant integration into native plant-pollinator networks across Europe. Proc. R. Soc. B 276, 3887–3893 10.1098/rspb.2009.1076 (doi:10.1098/rspb.2009.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magee J, McMullen CK, Reaser JK, Spitzer E, Struve S, Tufts C, Tye A, Woodruff G. 2001. Green invaders of the Galapagos islands. Science 294, 1279–1280 10.1126/science.294.5545.1279c (doi:10.1126/science.294.5545.1279c) [DOI] [PubMed] [Google Scholar]
- 27.Jäger H, Kowarik I, Tye A. 2009. Destruction without extinction: long-term impacts of an invasive tree species on Galapagos highland vegetation. J. Ecol. 97, 1252–1263 10.1111/j.1365-2745.2009.01578.x (doi:10.1111/j.1365-2745.2009.01578.x) [DOI] [Google Scholar]
- 28.Heleno R, Blake S, Jaramillo P, Traveset A, Vargas P, Nogales M. 2011. Frugivory and seed dispersal in the Galápagos: what is the state of the art? Integr. Zool. 6, 110–128 10.1111/j.1749-4877.2011.00236.x (doi:10.1111/j.1749-4877.2011.00236.x) [DOI] [PubMed] [Google Scholar]
- 29.Renne IJ, Barrow WC, Randall LAJ, Bridges WC. 2002. Generalized avian dispersal syndrome contributes to Chinese tallow tree (Sapium sebiferum, Euphorbiaceae) invasiveness. Divers. Distributions 8, 285–295 10.1046/j.1472-4642.2002.00150.x (doi:10.1046/j.1472-4642.2002.00150.x) [DOI] [Google Scholar]
- 30.White WM, McBirney AR, Duncan RA. 1993. Petrology and geochemistry of the Galapagos islands—portrait of a pathological mantle plume. J. Geophys. Res. Solid Earth 98, 19 533–19 563 10.1029/93JB02018 (doi:10.1029/93JB02018) [DOI] [Google Scholar]
- 31.Trueman M, d'Ozouville N. 2010. Characterizing the Galapagos terrestrial climate in the face of global climate change. Galapagos Res. 67, 26–37 [Google Scholar]
- 32.Tye A. 2006. Can we infer island introduction and naturalization rates from inventory data? Evidence from introduced plants in Galápagos. Biol. Invas. 8, 201–215 10.1007/s10530-004-3574-2 (doi:10.1007/s10530-004-3574-2) [DOI] [Google Scholar]
- 33.Guézou A, Trueman M, Buddenhagen CE, Chamorro S, Guerrero AM, Pozo P, Atkinson R. 2010. An extensive alien plant inventory from the inhabited areas of Galapagos. PLoS ONE 5, e10276. 10.1371/journal.pone.0010276 (doi:10.1371/journal.pone.0010276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaramillo P, Guézou A, Mauchamp A, Tye A. 2012. CDF checklist of Galapagos flowering plants. In Charles Darwin Foundation Galapagos species checklist (eds Bungartz F, Herrera H, Jaramillo P, Tirado N, Jímenez-Uzcategui G, Ruiz D, Guézou A, Ziemmeck F.). Puerto Ayora, Galapagos: Charles Darwin Foundation; (http://checklistsdatazonedarwinfoundationorg/) [Google Scholar]
- 35.Blüthgen N, Menzel F, Blüthgen N. 2006. Measuring specialization in species interaction networks. BMC Ecol. 6, 9. 10.1186/1472-6785-6-9 (doi:10.1186/1472-6785-6-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bascompte J, Jordano P, Olesen JM. 2006. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433 10.1126/science.1123412 (doi:10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- 37.Dormann C, Gruber B, Frund J. 2008. Introducing the bipartite package: analysing ecological networks. R news 8, 8–11 [Google Scholar]
- 38.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 39.Dormann CF, Fründ J, Blüthgen N, Gruber B. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open J. Ecol. 2, 7–24 10.2174/1874213000902010007 (doi:10.2174/1874213000902010007) [DOI] [Google Scholar]
- 40.Guerrero AM. 2002. El rol de las aves en el proceso de dispersión de semillas de plantas nativas e introducidas en Santa Cruz, Galápagos. Quito: Universidad Central del Ecuador [Google Scholar]
- 41.Guerrero AM, Tye A. 2009. Darwin's finches as seed predators and dispersers. Wilson J. Ornithol. 121, 752–764 10.1676/09-035.1 (doi:10.1676/09-035.1) [DOI] [Google Scholar]
- 42.Tye A. 2006. Restoration of the vegetation of the Dry Zone in Galapagos. Lyonia 9, 29–50 [Google Scholar]
- 43.Olesen J, Valido A. 2003. Lizards as pollinators and seed dispersers: an island phenomenon. Trends Ecol. Evol. 18, 177–181 10.1016/s0169-5347(03)00004-1 (doi:10.1016/s0169-5347(03)00004-1) [DOI] [Google Scholar]
- 44.Prieto JA, Patino OJ, Delgado WA, Moreno JP, Cuca LE. 2011. Chemical composition, insecticidal, and antifungal activities of fruit essential oils of three Colombian Zanthoxylum species. Chilean J. Agric. Res. 71, 73–82 10.4067/S0718-58392011000100009 (doi:10.4067/S0718-58392011000100009) [DOI] [Google Scholar]
- 45.Blake S, Wikelski M, Cabrera F, Guezou A, Silva M, Sadeghayobi E, Yackulic CB, Jaramillo P. 2012. Seed dispersal by Galápagos tortoises. J. Biogeogr. 39, 1961–1972 10.1111/j.1365-2699.2011.02672.x (doi:10.1111/j.1365-2699.2011.02672.x) [DOI] [Google Scholar]
- 46.Hansen DM, Donlan CJ, Griffiths CJ, Campbell KJ. 2010. Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions. Ecography 33, 272–284 10.1111/j.1600-0587.2010.06305.x (doi:10.1111/j.1600-0587.2010.06305.x) [DOI] [Google Scholar]
- 47.Carrión-Tacuri J, Berjano R, Guerrero G, Figueroa E, Tye A, Castillo JM. 2012. Predation on seeds of invasive Lantana camara by Darwin's finches in the Galapagos islands. Wilson J. Ornithol. 124, 338–344 10.1676/11-111.1 (doi:10.1676/11-111.1) [DOI] [Google Scholar]
- 48.Guerrero AM, Tye A. 2011. Native and introduced birds of Galapagos as dispersers of native and introduced plants. Ornitol. Neotrop. 22, 207–217 [Google Scholar]
- 49.Heleno RH, Ross G, Everard A, Ramos JA, Memmott J. 2011. On the role of avian seed predators as seed dispersers. Ibis 153, 199–203 10.1111/j.1474-919X.2010.01088.x (doi:10.1111/j.1474-919X.2010.01088.x) [DOI] [Google Scholar]
- 50.Nogales M, Heleno R, Traveset A, Vargas P. 2012. Evidence for overlooked mechanisms of long-distance seed dispersal to and between oceanic islands. New Phytol. 194, 313–317 10.1111/j.1469-8137.2011.04051.x (doi:10.1111/j.1469-8137.2011.04051.x) [DOI] [PubMed] [Google Scholar]
- 51.Connett L, Guézou A, Herrera HW, Carrión V, Parker PG, Deem SL. In press. Gizzard contents of the smooth-billed ani, Crotophaga ani (L.), in the Galapagos Islands, Ecuador. Galapagos Res. [Google Scholar]
- 52.Restrepo A, et al. 2012. Impacts of climate variability and human colonization on the vegetation of the Galápagos Islands. Ecology 93, 1853–1866 10.1890/11-1545.1 (doi:10.1890/11-1545.1) [DOI] [PubMed] [Google Scholar]
- 53.Encinas-Viso F, Revilla TA, Etienne RS. 2012. Phenology drives mutualistic network structure and diversity. Ecol. Lett. 15, 198–208 10.1111/j.1461-0248.2011.01726.x (doi:10.1111/j.1461-0248.2011.01726.x) [DOI] [PubMed] [Google Scholar]
- 54.Thompson JN, Willson MF. 1979. Evolution of temperate fruit–bird interactions—phenological strategies. Evolution 33, 973–982 10.2307/2407660 (doi:10.2307/2407660) [DOI] [PubMed] [Google Scholar]
- 55.Duffy JE. 2002. Biodiversity and ecosystem function: the consumer connection. Oikos 99, 201–219 10.1034/j.1600-0706.2002.990201.x (doi:10.1034/j.1600-0706.2002.990201.x) [DOI] [Google Scholar]
- 56.Memmott J, Waser NM, Price MV. 2004. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 10.1098/rspb.2004.2909 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]