Abstract
Key conceptual issues about speciation go unanswered without consideration of non-mutually exclusive factors. With tests based on speciation theory, we exploit the island distribution and habitat differences exhibited by the Caribbean cricket Amphiacusta sanctaecrucis, and with an analysis of divergent ecological selection, sexually selected differentiation and geographical isolation, address how these different factors interact. After testing for divergent selection by comparing neutral genetic and morphological divergence in one ecological (mandible shape) and one sexual (male genitalia shape) trait, we examine whether ecological or sexual selection is the primary mechanism driving population divergence. We find that all three factors—isolation, ecological and sexual selection—contribute to divergence, and that their interaction determines the stage of completeness achieved during the speciation process, as measured by patterns of genetic differentiation. Moreover, despite the striking diversity in genitalic shapes across the genus Amphiacusta, which suggests that sexual selection drives speciation, the significant differences in genitalia shape between forest habitats revealed here implies that ecological divergence may be the primary axis of divergence. Our work highlights critical unstudied aspects in speciation—differentiating the cause from the consequence of divergence—and suggests avenues for further disentangling the roles of natural and sexual selection in driving divergence in Amphiacusta.
Keywords: sexual selection, natural selection, genetic drift, speciation, geometric morphometrics
1. Introduction
A primary difficulty with testing mechanisms of speciation empirically arises from the inherent challenge of interpreting patterns of divergence. Evidence of local adaptation and divergent selection in promoting species differentiation is widely acknowledged [1], and theoretical models detail the processes underlying speciation [2]. Nevertheless, the processes, and specifically the pace and order in which ecological and sexual divergence accumulates, is much less clear in empirical studies. Ecological divergence, for example, may be a cause or consequence of speciation. Local adaptation may arise only after reproductive isolation leads to a cessation of gene flow, as opposed to driving speciation itself [3]. Likewise, when species differ in both ecological and sexual characters, it is notoriously difficult to distinguish between character divergence that is a cause or a result of species divergence [4–6]. Such distinctions are critical for understanding not only the drivers of speciation, but also for understanding why speciation remains incomplete in many cases (see review, [7]). For example, as the driver of speciation, selective differences cannot withstand the dilution effects of gene flow and adaptive divergence will remain relatively ephemeral unless selection is strong [8]. By contrast, if ecological differences are a consequence of speciation, where reproductive isolation evolved in some other context, adaptive divergence will accumulate irrespective of the strength of selection.
Here we investigate the relative contributions of ecological and sexual divergence, as well as differentiation associated with geographical isolation, on species divergence in flightless Caribbean crickets (genus Amphiacusta). Archipelago systems afford a natural scenario for studying the role of genetic drift in species divergence, while also providing an evolutionary arena where selection contributes to the speciation process [9].
Spatial isolation and limited vagility have indeed played a demonstrable role in the diversification of Amphiacusta (Gryllidae: Phalangopsinae) across the Greater Antilles. Phylogenetic analyses show that the diversification history is dominated by inter-island diversification, with closely related species distributed across different islands [10]. Yet, incidences of intra-island speciation (i.e. sister taxa occur on the same island) raise the question about the role of selection in species divergence, although whether ecological or sexual selection is a cause or consequence of divergence is unclear [10]. The restriction of this highly diverse genus (more than 80 species) to the Caribbean islands, and individual species to wet or dry primary forest, or to damp caves [11], suggests that local adaptation is important. However, Amphiacusta taxa are also characterized by conspicuous divergence in male genitalia [11,12], divergence that is largely concentrated in the C-sclerite, a portion inserted directly into the female genital tract during mating that exhibits unparalleled differentiation compared with other morphological traits both in the diversity of shape variation and magnitude of divergence among species. Extreme divergence in genitalia, especially in the absence of other extensive morphological variation, is generally taken to be a sign of sexual selection operating, either by female choice or male–male competition for fertilization opportunity, on male genitalia shape [13,14], and comparative studies lend support to such a hypothesis ([15]; for reviews, see [14,16,17]).
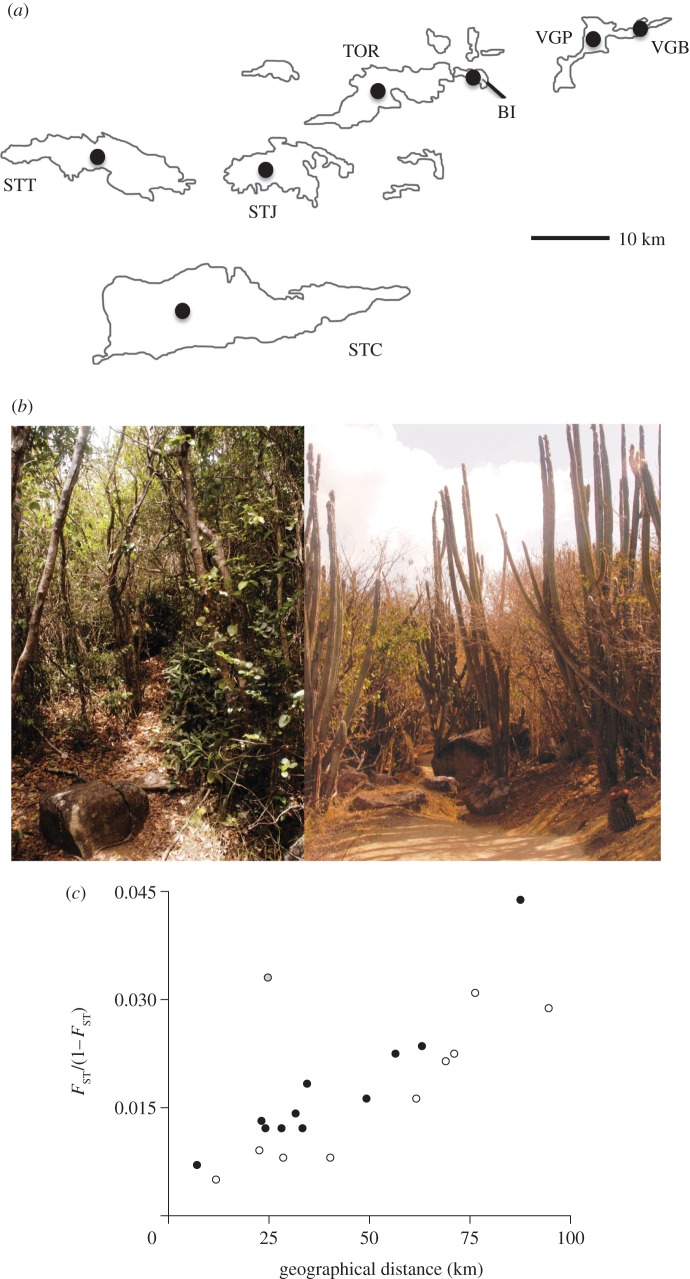
Such divergence in ecological and sexual characters not only characterizes different species of Amphiacusta, but is also paralleled in divergence patterns within species whose populations are distributed across multiple islands (including the focal taxon of this study Amphiacusta sanctaecrucis). In the Virgin Islands, A. sanctaecrucis are restricted to wet tropical forest. However, on two islands (i.e. Beef Island and Virgin Gorda; figure 1a) that are characterized by nearby mountains sufficiently high to create a rain-shadow effect, this species inhabits two very different habitats: a wet or a dry tropical forest (figure 1b).
Figure 1.
Populations of Amphiacusta are restricted to two different habitats within the Virgin Islands and display a pattern of genetic isolation-by-distance. (a) Map of the Virgin Islands with the location of sampled populations marked by black circles and labelled with population designations. (b) Photographs illustrating the two very different habitats in which Amphiacusta occur—either wet tropical forests or dry (desert-like) forests. (c) A significant pattern of isolation by distance across islands is evident (Mantel test: r = 0.756, p = 0.0056). There was also a significant correlation between genetic distance and habitat type when controlling for the effect of geographical distance (partial Mantel test: r = 0.767, p = 0.028). FST-values for wet versus wet forest comparisons are in white, for wet versus dry forest comparisons are in black, and for dry versus dry comparisons are in grey. STC, Saint Croix; STT, Saint Thomas; STJ, Saint John; TOR, Tortola; BI, Beef Island; VGP, Virgin Gorda Peak; VGB, Virgin Gorda Baths.
We exploit this situation to consider a set of non-mutually exclusive, and potentially interacting, factors—divergent selection associated with different ecological habitats, sexually selected differentiation and geography—that may contribute to species divergence in Amphiacusta. Specifically, we approach the question of how different factors might interact with three testable hypotheses. First, we can test for evidence of divergent selection by comparing observed patterns of morphological divergence (QST or PST) to a background level of divergence established from patterns of neutral genetic divergence (FST), as proposed by Wright [18,19].
Differentiation in the mandibles and male genitalia (figure 2) is quantified and used as a proxy for the presence of divergent selection [7]. Studies on the functional morphology of insect mandibles have identified their ecological relevance [20], including in Orthoptera more generally, where their chewing ability appears to be under selection [21]. Likewise, genitalia are a good proxy for sexual selection [13]; genitalic characters not only show species-level divergence in Amphiacusta but have also been shown to mediate reproductive success in other taxa [13,14].
Figure 2.
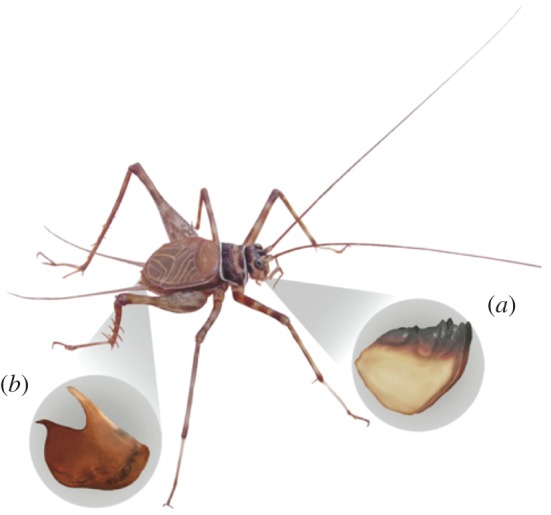
Traits included in our analysis of phenotypic divergence. As a proxy for divergent selection associated with ecological and sexual selection, phenotypic differences were quantified in (a) the mandibles, and (b) the male genitalia (specifically, the C-sclerite, which is inserted into the female reproductive tract during copulation), respectively. Shape divergence among populations of Amphiacusta sanctaecrucis in the genitalia and mandibles were quantified using geometric morphometric techniques (see §2).
Second, selection and drift acting together will produce greater divergence than drift operating alone, because ecological divergence will lead to greater reductions in gene flow than occasioned solely by geographical isolation. Thus, if ecological differences contribute to divergence, there should be a correlation between neutral genetic divergence and ecological differences between populations. Lastly, we dissect the roles of ecological and sexual processes in species divergence. Because A. sanctaecrucis inhabits both wet and dry forests, we can ask whether there is evidence of differentiation in mandible shape between crickets inhabiting dry forests and crickets inhabiting wet forests. Furthermore, if species divergence is driven by ecological selection, and divergence in sexual characters is a consequence (not the driver) of species divergence, then we would expect to see differentiation in shape in male genitalia between dry and wet forest populations. On the other hand, if there is no significant effect of habitat on species divergence, populations may exhibit differences in genitalia shape independent of the consequences of ecological selection.
We make these comparisons among the five wet forest island populations and two dry forest populations of A. sanctaecrucis. By making comparisons among adjacent island populations (figure 1a), we can control for the effects of the timing of colonization on our analyses of divergence. Moreover, by studying population-level divergence, as opposed to species differences, we also avoid the confounding problems of comparisons in which post-speciational differences obscure the processes that drive species divergence [7,22].
2. Material and methods
Specimens of A. sanctaecrucis were collected from a population from a wet tropical forest habitat on each of five Virgin Islands and from the two islands (Virgin Gorda and Beef Island) that had dry forest habitats, for a total of seven populations (figure 1). Only adult males (the adult stage in males is unambiguous because only mature males have wings) were used for calculating phenotypic divergence because the morphological characters most subject to sexual selection are the male genitalia.
(a). Genetic data and analysis
Neutral genetic differentiation was quantified with microsatellites in a total of 176 individuals across each of the populations: Beef Island (BI; n = 16), Saint Croix (STC; n = 26), Saint John (STJ; n = 31), Saint Thomas (STT; n = 37), Tortola (TOR; n = 25), Virgin Gorda Peaks (VGP; n = 30) and Virgin Gorda Baths (VGB; n = 11). Genomic DNA was extracted from the femur of each individual using a Qiagen DNeasy kit. Nine dinucleotide microsatellites were isolated using the protocol outlined in Glenn & Schable [23]. The clone sequences used to generate microsatellites are in Genbank (accessions JX987471-479). Individuals were genotyped for nine loci (AS15, AS17, AS18, AS19, AS29, AS44, AS54, AS69 and AS70). Microsatellites were amplified in a 10 μl reaction containing 5.7 μl H2O, 1.0 μl 10X buffer minus MgCl2, 0.3 μl MgCl2, 0.5 μl of each 10 μM primer, 0.4 μl bovine serum albumin, 0.6 μl of 2.5 mM dNTPs, and 0.04 μl Taq polymerase (Invitrogen) under the following conditions: 2 min of initial denaturation at 94°C and then 35 cycles of 94°C for 15 s, 30 s from 50°C to 56°C, 30 s at 72°C and a final extension of 4 min at 72°C. PCR products were genotyped on an ABI Model 3730 sequencer with a standard of ROX 500 (ABI). Alleles were scored using GeneMarker v. 1.70 (Soft Genetics).
The program Micro-Checker [24] was used to confirm that there were not null-alleles. However, one microsatellite, AS18, was not included in the analyses because of a departure from Hardy–Weinberg expectations (based on 100 000 permutations in Arlequin v. 2; [25]) with p-values Bonferroni corrected for multiple tests. For eight loci, Weir & Cockerham's [26] FST-values were calculated among pairs of populations in Fstat v. 2.9.3.2 [27] with 95% CIs determined from 1000 bootstrap replicates. We used the program IBDWS [28], which performs Mantel tests between genetic and geographical distance matrices, to examine whether populations exhibit isolation by distance. To examine whether habitat type influenced genetic divergence as well, we also performed partial Mantel tests between genetic distance and geographical distance, with habitat comparison as a co-variable. All Mantel tests above were performed with 10 000 permutations. Except where otherwise stated, all statistical tests were performed in R v. 2.15.0.
(b). Morphological data and analysis
Phenotypic differentiation of the mandibles and genitalia were quantified in the 153 sampled males using geometric morphometrics: BI (n = 14), STC (n = 23), STJ (n = 25), STT (n = 29), TOR (n = 21), VGP (n = 27) and VGB (n = 14). Digital images of the mandibles and genitalia (figure 2) were taken with a Leica DC300 dissecting scope and compiled from a standardized set (i.e. the same focal depth per specimen and magnification) of multiple focal planes using Discovery-Pro and Scope-Pro Plus imaging software. Multiple images (n = 5) were taken of each structure for each individual to account for potential errors arising from the digitizing procedure; analyses (discussed below) are based on the average across the five replicates per individual. Femur length (as a measure of body size) was measured with standardized digital images taken with a Leica DFC320 dissecting scope using Image-Pro Plus software (Media Cybernetics). Nine landmarks were digitized to measure shape variation of the mandibles, and five landmarks and 74 sliding semi-landmarks to measure shape variation in the genitalia (specifically, the C-sclerite) [29,30] using TPSDig v. 1.44 software [31].
A generalized Procrustes analysis was performed to control for variation owing to the size and orientation of the images using TPSRelw v. 1.44 [31]. The resulting variables, or partial warps (PWs), represent the location of each sample relative to the mean Procrustes configuration, and are statistically independent from one another [32]. Shape variation and analyses of population differentiation were conducted separately for the mandibles and genitalia.
We used the summary statistic PST (with PWs as input) to quantify population differentiation and test for drift-induced versus selective divergence in mandible and genitalia shape. PST-values are comparable with FST-values and were estimated as
where 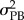 is the between group variance (i.e. between populations),
is the between group variance (i.e. between populations), 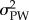 the within group variance (i.e. within populations) of a phenotypic trait and h2 is the heritability. Note that PST is used when QST cannot be estimated (e.g. in field studies that lack heritability estimates) [33–37]. We compared the degree of trait divergence (PST) with the degree of neutral genetic divergence (FST) at eight nuclear loci. Following Wright [18,19], if trait differences among populations are the result of divergent selection, PST > FST; if traits are evolving under similar conditions on different islands, they will exhibit stabilizing selection, PST < FST; and if they are the result of stochastic, drift-induced divergence, then PST ≈ FST [36,37].
the within group variance (i.e. within populations) of a phenotypic trait and h2 is the heritability. Note that PST is used when QST cannot be estimated (e.g. in field studies that lack heritability estimates) [33–37]. We compared the degree of trait divergence (PST) with the degree of neutral genetic divergence (FST) at eight nuclear loci. Following Wright [18,19], if trait differences among populations are the result of divergent selection, PST > FST; if traits are evolving under similar conditions on different islands, they will exhibit stabilizing selection, PST < FST; and if they are the result of stochastic, drift-induced divergence, then PST ≈ FST [36,37].
The inclusion of environmental variance in this measure means that care must be taken in its interpretation. This study focuses on morphological traits that typically exhibit high additive genetic variance [38–42]. A range of heritability values (from 0.1 to 0.6) spanning those commonly reported in the literature for both ecological characters exhibiting patterns of local adaptation [43,44] and traits under sexual selection [45–48] were considered in our calculation of PST to guard against misinterpretations. Results of statistical analyses presented in the text are based on a heritability scalar of 0.6, but all tests are robust to heritability scalar used to calculate PST (i.e. the pattern remains significant for values of h2 from 0.1 to 0.9). Note that the choice to present results based on h2 = 0.6 is conservative for comparing PST and FST-values, given that smaller heritability scalars result in a greater difference between PST and FST (see the electronic supplementary material, figure S1). The 95% CIs were calculated for each PST-value by bootstrapping of 2000 replicates for each trait using R (see the electronic supplementary material).
A MANOVA was used to assess whether population affiliation and habitat type independently explained a significant portion of the variation in mandible and genitalia shape. Canonical variate analysis (CVA) was used to examine whether there were significant differences in mandible and genitalia shape between wet and dry habitats, as well as to visualize how individuals from these habitats were ordinated in morphospace. The significance of the Mahalanobis distance (D2) between habitat was assessed via permutation tests with 10 000 replicates. We used PWs to examine differences in mandible shape and relative warps (RWs) to investigate differences in genitalia shape, because a high variable to sample size ratio makes MANOVA impossible and can distort CVA, exaggerating differences. RWs are equivalent to principal components, are independent variables and their use reduces the dimensionality of the data. We then used discriminant function analysis (DFA) to assess the accuracy of the canonical variates for assigning individuals to wet and dry habitats. CVA and DFA of mandible PWs were performed in MorphoJ v. 1.05b. CVA and DFA of genitalia RWs were performed with the lda function of the ‘MASS’ package in R. Morphological and microsatellite data for this paper are deposited in Dryad: doi:10.5061/dryad.8b490.
3. Results
A significant relationship between the level of neutral genetic divergence, as measured by FST from eight microsatellites, and the geographical distance among islands, shows an isolation-by-distance effect (Mantel test: r = 0.756, p = 0.0056; figure 1c). This effect is exemplified by the very high FST-values between A. sanctaecrucis from Saint Croix compared with other Virgin Island populations (see the electronic supplementary material, table S1). In addition to being geographically separated from the other islands, the geological history of Saint Croix is one of continual isolation, despite dramatic changes in sea level during the Pleistocene, whereas the other Virgin Islands experienced periods of connectedness in the past [49]. Additionally, there was a significant correlation between genetic distance and habitat type when controlling for the effect of geographical distance (partial Mantel test: r = 0.767, p = 0.028).
(a). Tests for divergent selection
To test for evidence of selective divergence, the background level of divergence established from patterns of neutral genetic divergence (FST) was compared with the level of population differentiation in selected characters (as measured from the FST analogue for morphological characters, PST) [50,51]. This comparison showed that both mandibular and genitalic divergence among populations was significantly greater than patterns of neutral genetic divergence, both globally and for nearly all population pairwise comparisons (see figure 3 and the electronic supplementary material, table S1). There was also no significant correspondence between pairwise FST and mandible PST (Mantel test: r = 0.162 and p = 0.28) or genitalia PST (Mantel test: r = −0.041 and p = 0.47). To guard against the inflation of PST-values via differential wear patterns associated with dietary differences in A. sanctaecrucis from dry versus wet forests, the geometric morphometric analyses were repeated, excluding landmarks associated with the mandibular teeth; the results were the same. Because calculations of PST require a heritability scalar, we examined the robustness of this conclusion across a broad range of heritability values (i.e. from 0.1 to 0.9) (see the electronic supplementary material, figure 1), and showed that PST-values remain elevated compared with FST-values. Even though we do not have heredity estimates for the characters (the study was conducted with wild-caught animals), this range of heredity values considered span those observed in other taxa for morphological traits, including those associated with ecological differentiation [42–44,52], and specifically for studies on insect genitalia [45,46]. There is no significant correspondence between population pairwise mandibular and genitalia PST-values (Mantel test: r = 0.556 and p = 0.079).
Figure 3.
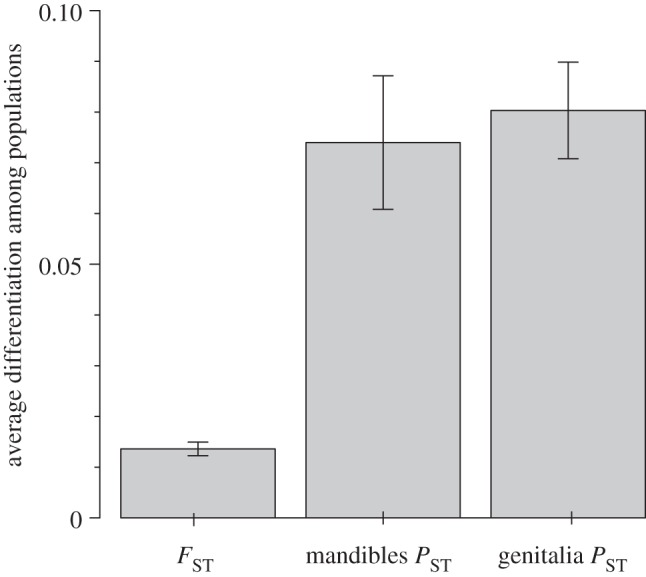
Comparison of average phenotypic and genetic divergence across populations. The average PST-value across populations for both the mandibles and genitalia is significantly greater than the average FST-value across populations. See the electronic supplementary material, table S1 for details about individual population comparisons, and figure S1 which demonstrates that this conclusion is robust to a broad range of heredity scalars (i.e. from 0.1 to 0.9) used to calculate PST.
Populations differed significantly in both mandible shape (MANOVA, Wilks λ = 0.226, F6141 = 3.115, p = 0.0001) and genitalia shape (MANOVA, Wilks λ = 0.046, F6135 = 3.70, p = 0.0001). Habitat type, wet or dry forest, also explained a significant portion of the variation in these structures (mandibles: MANOVA, Wilks λ = 0.635, F1146 = 6.466, p = 0.0001; genitalia: MANOVA, Wilks λ = 0.599, F1141 = 4.043, p = 0.0001). CVA also revealed highly significant differences in mandible shape between dry- and wet-habitat populations (D2 = 2.2037, p < 0.0001), and DFA correctly assigned 130 of 149 samples (87.2%), with the majority (17 of 19) of incorrect assignments being those from wet habitats incorrectly categorized as dry-habitat individuals. Similarly, CVA revealed significant differences in genitalia shape between dry and wet habitat populations (D2 = 2.04, p < 0.0001) and 83.1 per cent of samples were correctly assigned to wet or dry forest, with errors being almost entirely of dry habitat crickets incorrectly assigned to wet habitat (17 of 24). Altogether, there is significant separation in morphospace for both mandibles and genitalia between dry and wet forests, albeit with some overlap (figure 4).
Figure 4.
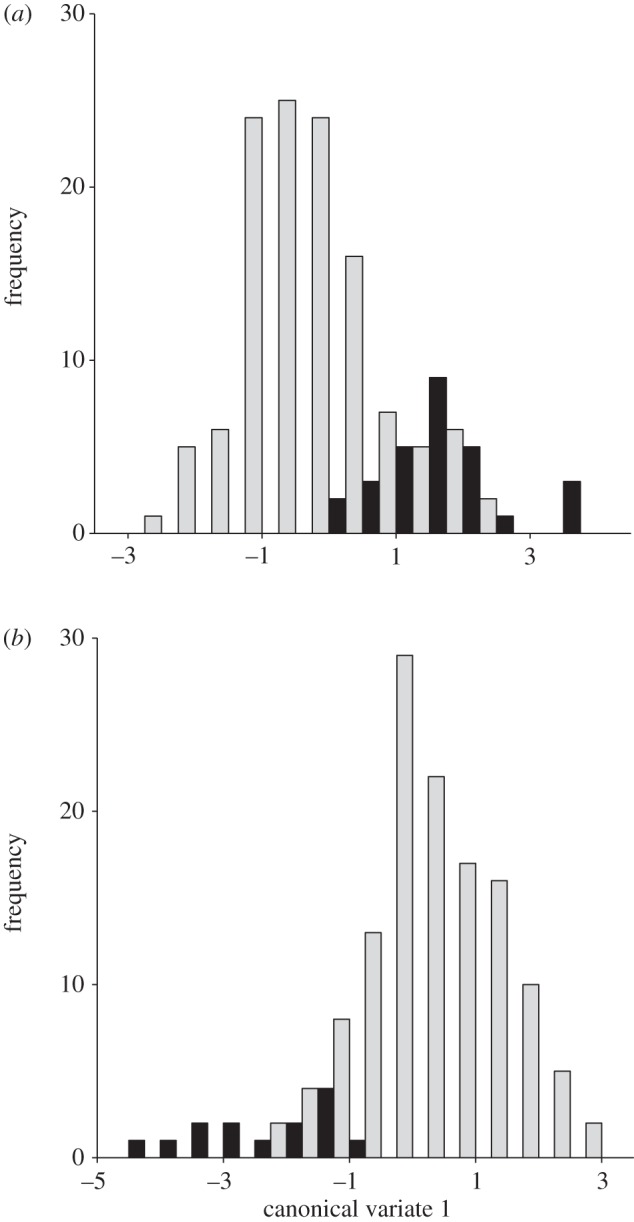
Ordination of wet and dry forest individuals along the first canonical axis for (a) mandibles and (b) genitalia. The Mahalanobis distance (D2) between wet (grey bars) and dry (black bars) populations is significant for both structures (p < 0.0001).
4. Discussion
Evidence for ecological or sexual selection mediating population divergence continues to accumulate [4,53–55]; however, distinguishing between which of these factors has played the primary, and which the secondary, role can be a difficult task [5–7]. We demonstrate that isolated island populations of A. sanctaecrucis are diverging in both an ecological trait (mandible shape) and sexual trait (male genitalia). The evidence suggests that the divergence in these traits is due primarily to selection, rather to than drift. First, both traits exhibit elevated PST-values relative to neutral expectations (see figure 3 and electronic supplementary material, table S1). Second, there is a significant relationship between neutral divergence and habitat, after controlling for geographical distance. Moreover, although values of PST may be confounded by a species demographic history (e.g. a correlation between FST and PST; [42]), the lack of a significant correlation between patterns of neutral divergence and phenotypic divergence in both the mandibles and genitalia indicates that this is not the case here. Thus, our findings indicate that it is not just geographical isolation, but that ecological differences also contributed to species divergence.
We acknowledge that FST-values can have multiple interpretations, such as being indicative of the timing of island colonization [56]. However, this explanation does not appear to fit our data. Rather, the lack of correlation between FST and PST suggests that the contribution of ecological differences to species morphological divergence has arisen through reductions in gene flow that are greater than predicted by geographical isolation alone [57]. The significant influence of habitat on FST supports this interpretation of the pattern of neutral divergence between islands.
To dissect the relative roles of ecological and sexual selection in species divergence, and specifically, the issue of separating cause and consequence, we tested whether mandible and genitalia shape vary not only by population but also with respect to habitat type. If species divergence is driven by ecological selection, such that divergent sexual characters are a consequence (not the cause) of species divergence, we would expect sexually selected traits to vary with respect to ecology. MANOVA and CVA results confirm that genitalia are divergent on an axis of forest type, suggesting the tantalizing possibility that ecological selection is the primary cause, and sexual selection the consequence, of divergence. However, several important caveats need to be addressed.
First, population differences in mandible and genitalia shape may be the result of plastic responses to different environments; a common garden experiment is necessary to address this possibility. Second, although sexual selection is generally invoked to explain divergence in male genitalia, another explanation for the convergent pattern of divergence in mandibular and genitalic shape in A. sanctaecrucis is that these traits are pleiotropically linked [58]. It is difficult to see why genitalia should be especially likely to be affected by pleiotropy [16], and little evidence for pleiotropic evolution of genitalia has emerged to support this hypothesis (but see [59,60]); however, selection on one or more loci that affect cuticular growth and shape could result in similar patterns of evolution for each. (We note there is no significant correspondence between population pairwise mandibular and genitalia PST-values, or between the first relative warps (equivalent to the first principal components) of mandible and genitalia shape (N = 130, r2 = 0.103, p = 0.445), suggesting that rates of divergence between the two phenotypic traits are not coupled.) Furthermore, the pattern of divergence in genitalia and mandibles suggest the intriguing possibility that both represent a ‘magic’ trait, i.e. traits that are pleiotropically linked, under divergent selection and that simultaneously cause reproductive isolation via non-random mating [61]. Future research into the underlying genetic basis of mandibular and genitalic shape will be necessary to fully address these competing hypotheses.
Another possibility is that environmental variance is responsible for the pattern of genitalic divergence with respect to habitat differences. Divergence in sexually selected characters influenced by the ecological environment [62,63] include traits where the perception of acoustic or visual displays by females or interacting males may be affected by the habitat of the presenting male [53]. We consider it unlikely, however, that the genitalic differentiation among populations inhabiting different ecological habitats is a direct consequence of the environmental differences per se. The shape of the male genitalia is not a character that would exhibit such ecological dependence because it is a character subject to post-copulatory selection (i.e. the environment in this case is the female reproductive tract; [13]). Although body size differences associated with environmental differences among populations could in principle contribute to genitalic differences among populations, we did not detect any relationship between genitalia and body size, as measured by leg length (N = 123, linear regression, r2 = 0.0004, p = 0.8231). Moreover, the effect of size was removed in the geometric morphometric analyses prior to quantifying the amount of genitalic divergence among populations (see §2). These reasons lead us to doubt an a priori reason to expect an association between the strength of sexual selection and the ecological environment.
Rather, the pattern of genitalia shape divergence in the genus is suggestive of sexual selection. The most notable feature of Amphiacusta, except its extreme species richness, is the extreme divergence in genitalia shape with little apparent divergence in other morphological traits [11,12]. In addition, there is some evidence of correlated changes in shape between male and female genitalia [11], although this has yet to be rigorously demonstrated, owing to the difficulties of studying female genitalia, which are soft-bodied structures that do not preserve well. Finally, the mating behaviour of Amphiacusta pronauta (sister species to A. sanctaecrucis) [64] and A. sanctaecrucis (L. L. Knowles & E. Oneal 2007, personal observation) suggests a functional role for the C-sclerite, as after a lengthy mating (approx. 15 min), males remove their spermatophore from the female's genital tract after mating and consume it, behaviour that suggests the attempted removal of rivals' sperm [65].
Focusing on the level of genetic differentiation as an indicator of the completeness of speciation (i.e. ranging from population divergence to post-speciational divergence; [7]), the degree of isolation conferred by all three factors—geography, divergent ecological selection and sexual selection—can be considered. Our results indicate that isolation confers some genetic differentiation, but that ecological selection in different habitats has increased the genetic differentiation beyond that expected merely by drift. Ecological selection appears to be primary, perhaps rendering sexual selection less effective as a consequence of lower levels of gene flow (i.e. greater reproductive isolation) between populations from dissimilar habitats.
Lastly, the FST-value between the two dry forest habitats is exceptionally high (figure 1), suggesting that they indeed are the most reproductively isolated. The accumulation of greater genitalic divergence between populations from dissimilar habitats resulting from the greater reproductive isolation driven by ecological divergence predicts that populations from the dry forest should represent the highest level of completeness towards speciation (considering that a progression towards speciation occurs in stages [7]). Altogether, our study demonstrates that non-mutually exclusive factors influencing divergence can be considered together and their effects separated from one another. Finally, despite previous evidence suggesting that sexual selection is the primary driver of species divergence in the genus Amphiacusta [10], we have uncovered evidence that, when geographical isolation permits, ecological divergence precedes sexual divergence among populations of flightless crickets confined to the Virgin Islands.
Acknowledgements
We are grateful to Daniel Otte and Dan Swanson for assistance in the collection of samples. We also thank Qixin He, David Polly and Eladio Marquez for their helpful advice on data analysis, Qixin He and Tim Connallon for fruitful discussions on this research, and Diarmaid O'Foighil and John Willis for helpful comments on the manuscript. Financial support was provided by Rackham Graduate School, the Hinsdale Scholarship (University of Michigan Museum of Zoology) and Society of Systematic Biology grants to E.O., and a National Science Foundation grant (DEB-07-15487) to L.L.K.
References
- 1.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Gavrilets S. 2003. Models of speciation: what have we learned in 40 years? Evolution 57, 2197–2215 10.1111/j.0014-3820.2003.tb00233.x (doi:10.1111/j.0014-3820.2003.tb00233.x) [DOI] [PubMed] [Google Scholar]
- 3.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates [Google Scholar]
- 4.Nosil P, Crespi BJ. 2004. Does gene flow constrain adaptive divergence or vice versa? A test using ecomorphology and sexual isolation in Timema cristinae walking-sticks. Evolution 58, 102–112 10.1111/j.0014-3820.2004.tb01577.x (doi:10.1111/j.0014-3820.2004.tb01577.x) [DOI] [PubMed] [Google Scholar]
- 5.Arnegard ME, McIntyre PB, Harmon LJ, Zelditch ML, Crampton WGR, Davis JK, Sullivan JP, Lavoué S, Hopkins CD. 2010. Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am. Nat. 176, 335–356 10.1086/655221 (doi:10.1086/655221) [DOI] [PubMed] [Google Scholar]
- 6.Labonne J, Hendry AP. 2010. Natural and sexual selection giveth and taketh away reproductive barriers: models of population divergence in guppies. Am. Nat. 176, 26–39 10.1086/652992 (doi:10.1086/652992) [DOI] [PubMed] [Google Scholar]
- 7.Nosil P, Harmon L, Seehausen O. 2009. Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156 10.1016/j.tree.2008.10.011 (doi:10.1016/j.tree.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 8.Hendry AP, Taylor EB, McPhail JD. 2002. Adaptive divergence and the balance between selection and gene flow: lake and stream stickleback in the misty system. Evolution 56, 1199–1216 10.1111/j.0014-3820.2002.tb01432.x (doi:10.1111/j.0014-3820.2002.tb01432.x) [DOI] [PubMed] [Google Scholar]
- 9.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830–836 10.1038/nature07893 (doi:10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 10.Oneal E, Otte D, Knowles LL. 2010. Testing for biogeographic mechanisms promoting divergence in Caribbean crickets (genus Amphiacusta). J. Biogeogr. 37, 530–540 10.1111/j.1365-2699.2009.02231.x (doi:10.1111/j.1365-2699.2009.02231.x) [DOI] [Google Scholar]
- 11.Otte D, Perez-Gelabert D. 2009. Caribbean crickets. Ann Arbor, MI: Orthopterists’ Society [Google Scholar]
- 12.Desutter-Grandcolas L, Otte D. 1997. Revision of the West Indian genus Amphiacusta Saussure, 1874, with descriptions of twenty new species (Orthoptera: Grylloidea: Phalangopsidae). Ann. Soc. Entom. Fr. 33, 101–128 [Google Scholar]
- 13.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press [Google Scholar]
- 14.Eberhard WG. 2010. Rapid divergent evolution of genitalia: theory and data updated. In The evolution of primary sexual characters in animals (eds Leonard JL, Córdoba-Aguilar A.), pp. 40–78 Oxford, UK: Oxford University Press [Google Scholar]
- 15.Arnqvist G. 1998. Comparative evidence for the evolution of genitalia by sexual selection. Nature 395, 784–786 10.1038/31689 (doi:10.1038/31689) [DOI] [Google Scholar]
- 16.Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93 10.1016/j.tree.2003.11.012 (doi:10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- 17.Eberhard WG. 2011. Experiments with genitalia: a commentary. Trends Ecol. Evol. 26, 17–21 10.1016/j.tree.2010.10.009 (doi:10.1016/j.tree.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 18.Wright S. 1931. Evolution in the Mendelian populations. Genetics 16, 97–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright S. 1932. The roles of mutation, inbreeding, crossbreeding, and selection in evolution. In Proc. of the 6th Int. Congress of Genetics, vol. 1 (ed. Jones DF.) pp. 356–366 Menasha, WI: Brooklyn Botanic Garden. [Google Scholar]
- 20.Labandeira CC. 1997. Insect mouthparts: ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Syst. 28, 153–93 10.1146/annurev.ecolsys.28.1.153 (doi:10.1146/annurev.ecolsys.28.1.153) [DOI] [Google Scholar]
- 21.Bernays EA. 1991. Evolution of insect morphology in relation to plants. Phil. Trans. R. Soc. B 333, 257–264 10.1098/rstb.1991.0075 (doi:10.1098/rstb.1991.0075) [DOI] [Google Scholar]
- 22.Coyne JA, Orr HA. 1989. Patterns of speciation in Drosophila. Evolution 43, 362–381 10.1111/j.1095-8312.2009.01345.x (doi:10.1111/j.1095-8312.2009.01345.x) [DOI] [PubMed] [Google Scholar]
- 23.Glenn TC, Schable NA. 2005. Isolating microsatellite DNA loci. Methods Enzymol. 395, 202–222 10.1016/S0076-6879(05)95013-1 (doi:10.1016/S0076-6879(05)95013-1) [DOI] [PubMed] [Google Scholar]
- 24.van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P. 2004. Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 4, 535–538 10.1111/j.1471-8286.2004.00684.x (doi:10.1111/j.1471-8286.2004.00684.x) [DOI] [Google Scholar]
- 25.Schneider S, Roessli D, Excoffier L. 2000. Arlequin: a software for population genetics data analysis, v. 2.000, Genetics and Biometry Laboratory, Department of Anthropology, University of Geneva [Google Scholar]
- 26.Weir BS, Cockerham CC. 1984. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 10.2307/2408641 (doi:10.2307/2408641) [DOI] [PubMed] [Google Scholar]
- 27.Goudet J. 1995. Fstat (version 1.2): a computer program to calculate F-statistics. Heredity 86, 385–486 [Google Scholar]
- 28.Jensen JL, Bohonak AJ, Kelly ST. 2005. Isolation by distance, web service. BMC Genet. 6, 13. v. 3.23, See http://ibdws.sdsu.edu/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bookstein FL. 1997. Landmark methods for forms without landmarks: localizing group differences in outline shape. Med. Image Anal. 1, 225–243 10.1016/S1361-8415(97)85012-8 (doi:10.1016/S1361-8415(97)85012-8) [DOI] [PubMed] [Google Scholar]
- 30.Adams DC, Rohlf FJ, Slice DE. 2004. Geometric morphometrics: ten years of progress following the ‘revolution’. Ital. J. Zool. 71, 5–16 10.1080/11250000409356545 (doi:10.1080/11250000409356545) [DOI] [Google Scholar]
- 31.Rohlf FJ. 2004. Tps series. Stony Brook, NY: Department of Ecology and Evolution, State University of New York; (http://life.bio.sunysb.edu/morph/) [Google Scholar]
- 32.Zelditch ML, Swiderski DL, Sheets DH, Fink WL. 2004. Geometric Morphometrics for Biologists. Amsterdam, The Netherlands: Elsevier Academic Press [Google Scholar]
- 33.Merilä J. 1997. Quantitative trait and allozyme divergence in the greenfinch (Carduelis chloris, Aves: Fringillidae). Biol. J. Linn. Soc. 61, 243–266 [Google Scholar]
- 34.Storz JF. 2002. Contrasting patterns of divergence in quantitative traits and neutral DNA markers: analysis of clinal variation. Mol. Ecol. 11, 2537–2551 10.1046/j.1365-294X.2002.01636.x (doi:10.1046/j.1365-294X.2002.01636.x) [DOI] [PubMed] [Google Scholar]
- 35.Saint-Laurent R, Legault M, Bernatchez L. 2003. Divergent selection maintains high gene flow between sympatric rainbow smelt ecotypes (Osmerus mordax Mitchill). Mol. Ecol. 12, 315–330 10.1046/j.1365-294X.2003.01735.x (doi:10.1046/j.1365-294X.2003.01735.x) [DOI] [PubMed] [Google Scholar]
- 36.Leinonen T, Cano JM, Mäkinen H, Merilä J. 2006. Contrasting patterns of body shape and neutral genetic divergence in marine and lake populations of threespine sticklebacks. J. Evol. Biol. 19, 1803–1812 10.1111/j.1420-9101.2006.01182.x (doi:10.1111/j.1420-9101.2006.01182.x) [DOI] [PubMed] [Google Scholar]
- 37.Wójcik AM, Polly PD, Sikorski MD, Wójcik JM. 2006. Selection in a cycling population: differential response among skeletal traits. Evolution 60, 1925–1935 [PubMed] [Google Scholar]
- 38.Morrow EH, Gage MJG. 2001. Artificial selection and heritability of sperm length in Gryllus bimaculatus. Heredity 87, 356–362 10.1046/j.1365-2540.2001.00921.x (doi:10.1046/j.1365-2540.2001.00921.x) [DOI] [PubMed] [Google Scholar]
- 39.Schluter D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766–1774 10.2307/2410734 (doi:10.2307/2410734) [DOI] [PubMed] [Google Scholar]
- 40.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates [Google Scholar]
- 41.Santos M. 2002. Genetics of wing size asymmetry in Drosophila buzzatii. J. Evol. Biol. 15, 720–734 10.1046/j.1420-9101.2002.00450.x (doi:10.1046/j.1420-9101.2002.00450.x) [DOI] [Google Scholar]
- 42.Houle D. 1992. Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanckenhorn WU. 2002. The consistency of quantitative genetic estimates in field and laboratory in the yellow dung fly. Genetics 114, 171–182 10.1023/A:1015181516827 (doi:10.1023/A:1015181516827) [DOI] [PubMed] [Google Scholar]
- 44.Manier MK, Seyler CM, Arnold SJ. 2007. Adaptive divergence within and between ecotypes of the terrestrial garter snake, Thamnophis elegans, assessed with FST-QST comparisons. J. Evol. Biol. 20, 1705–1719 10.1111/j.1420-9101.2007.01401.x (doi:10.1111/j.1420-9101.2007.01401.x) [DOI] [PubMed] [Google Scholar]
- 45.Simmons LW, House CM, Hunt J, García-González F. 2009. Evolutionary response to sexual selection in male genital morphology. Curr. Biol. 19, 1442–1446 10.1016/j.cub.2009.06.056 (doi:10.1016/j.cub.2009.06.056) [DOI] [PubMed] [Google Scholar]
- 46.Higgins SL, Hosken DJ, Wedell N. 2009. Phenotypic and genetic variation in male genitalia in the seedbug, Lygaeus equestris (Heteroptera). Biol. J. Linn. Soc. Lond. 98, 400–405 10.1111/j.1095-8312.2009.01292.x (doi:10.1111/j.1095-8312.2009.01292.x) [DOI] [Google Scholar]
- 47.Arnqvist G, Thornhill R. 1998. Evolution of animal genitalia: patterns of phenotypic and genotypic variation and condition dependence of genital and non-genital morphology in water striders (Heteroptera: Gerridae: Insecta). Genet. Res. 71, 193–212 10.1017/S0016672398003279 (doi:10.1017/S0016672398003279) [DOI] [Google Scholar]
- 48.Mühlhäuser C, Blanckenhorn W. 2004. The quantitative genetics of sexual selection in the dung fly Sepsis cynipsea. Behaviour 141, 327–341 10.1163/156853904322981888 (doi:10.1163/156853904322981888) [DOI] [Google Scholar]
- 49.Bard E, Hamelin B, Fairbanks RG. 1990. U-Th ages obtained by mass spectrometry in corals from Barbados: sea level during the past 130 000 years. Nature 346, 456–458 10.1038/346456a0 (doi:10.1038/346456a0) [DOI] [Google Scholar]
- 50.Leinonen T, O'Hara RB, Cano JM, Merilä J. 2008. Comparative studies of quantitative trait and neutral marker divergence: a meta-analysis. J. Evol. Biol. 21, 1–17 [DOI] [PubMed] [Google Scholar]
- 51.Brommer JE. 2011. Whither PST? The approximation of QST by PST in evolutionary and conservation biology. J. Evol. Biol. 24, 1160–1168 10.1111/j.1420-9101.2011.02268.x (doi:10.1111/j.1420-9101.2011.02268.x) [DOI] [PubMed] [Google Scholar]
- 52.Grant PR, Grant BR. 1995. Predicting microevolutionary responses to directional selection on heritable variation. Evolution 49, 241–251 10.2307/2410334 (doi:10.2307/2410334) [DOI] [PubMed] [Google Scholar]
- 53.Boughman JW. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411, 944–948 10.1038/35082064 (doi:10.1038/35082064) [DOI] [PubMed] [Google Scholar]
- 54.Hoekstra HE, Drumm KE, Nachman MW. 2004. Ecological genetics of adaptive color polymorphism in pocket mice: geographic variation in selected and neutral genes. Evolution 58, 1329–1341 [DOI] [PubMed] [Google Scholar]
- 55.Boul KE, Funk WC, Darst CR, Cannatella DC, Ryan MJ. 2007. Sexual selection drives speciation in a Amazonian frog. Proc. R. Soc. B 274, 399–406 10.1098/rspb.2006.3736 (doi:10.1098/rspb.2006.3736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clegg SM, Degnan SM, Kikkawa J, Moritz C, Estoup A, Owens IPF. 2002. Genetic consequences of sequential founder events by an island-colonizing bird. Proc. Natl Acad. Sci. USA 99, 8127–8132 10.1073/pnas.102583399 (doi:10.1073/pnas.102583399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nosil P, Crespi BJ. 2006. Ecological divergence promotes the evolution of cryptic reproductive isolation. Proc. R. Soc. B 273, 991–997 10.1098/rspb.2005.3359 (doi:10.1098/rspb.2005.3359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayr E. 1963. Animal species and evolution. Cambridge, MA: Harvard University Press [Google Scholar]
- 59.Arnqvist G, Thornhill R, Rowe R. 1997. Evolution of animal genitalia: morphological correlates of fitness components in a water strider. J. Evol. Biol. 10, 613–640 10.1007/s000360050045 (doi:10.1007/s000360050045) [DOI] [Google Scholar]
- 60.Schäfer MA, Routtu J, Vierira J, Hoikkala A, Ritchie MG, Schlötterer C. 2011. Multiple quantitative trait loci influence intra-specific variation in genital morphology between phylogenetically distinct lines of Drosophila montana. J. Evol. Biol. 24, 1879–1886 10.1111/j.1420-9101.2011.02316.x (doi:10.1111/j.1420-9101.2011.02316.x) [DOI] [PubMed] [Google Scholar]
- 61.Servedio MR, Van Doorn GS, Kopp M, Frame AM, Nosil P. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26, 389–397 [DOI] [PubMed] [Google Scholar]
- 62.Cocroft RB, Rodríguez RL, Hunt RE. 2009. Host shifts and signal divergence: mating signals covary with host use in a complex of specialized plant-feeding insects. Biol. J. Linn. Soc. Lond. 99, 60–72 10.1111/j.1095-8312.2009.01345.x (doi:10.1111/j.1095-8312.2009.01345.x) [DOI] [Google Scholar]
- 63.Knowles LL, Hernandez BB, Markow TA. 2005. Non-antagonistic interactions between the sexes revealed by the ecological consequences of reproductive traits. J. Evol. Biol. 18, 156–161 10.1098/rsbl.2006.0565 (doi:10.1098/rsbl.2006.0565) [DOI] [PubMed] [Google Scholar]
- 64.Alexander RD, Otte D. 1967. The evolution of genitalia and mating behavior in crickets (Gryllidae) and other Orthoptera. Misc. Pub. Mus. Zool. Univ. Mich. 133, 1–62 [Google Scholar]
- 65.Ono T, Siva-Jothy MT, Kato A. 1989. Removal and subsequent ingestion of rivals’ semen during copulation in a tree cricket. Physiol. Ent. 14, 195–202 10.1111/j.1365-3032.1989.tb00952.x (doi:10.1111/j.1365-3032.1989.tb00952.x) [DOI] [Google Scholar]