Abstract
The hunting and gathering lifestyle adopted by human ancestors around 2 Ma required a large increase in aerobic activity. High levels of physical activity altered the shape of the human body, enabling access to new food resources (e.g. animal protein) in a changing environment. Recent experimental work provides strong evidence that both acute bouts of exercise and long-term exercise training increase the size of brain components and improve cognitive performance in humans and other taxa. However, to date, researchers have not explored the possibility that the increases in aerobic capacity and physical activity that occurred during human evolution directly influenced the human brain. Here, we hypothesize that proximate mechanisms linking physical activity and neurobiology in living species may help to explain changes in brain size and cognitive function during human evolution. We review evidence that selection acting on endurance increased baseline neurotrophin and growth factor signalling (compounds responsible for both brain growth and for metabolic regulation during exercise) in some mammals, which in turn led to increased overall brain growth and development. This hypothesis suggests that a significant portion of human neurobiology evolved due to selection acting on features unrelated to cognitive performance.
Keywords: brain size, encephalization, physical activity, endurance running, neurotrophins, brain-derived neurotrophic factor
1. Introduction
A wealth of recent studies detail connections between physical activity and neurobiology [1,2]. In particular, aerobic physical activity (APA) generates, and protects new neurons, increases the volume of brain structures and improves cognition in humans and other mammals [2–8]. These neurobiological effects accrue during an individual's lifetime, and a great deal of research has begun to explore the implications of APA for cognitive health [5]. However, recent data also suggest that there is an evolutionary relationship between APA and the brain, including a positive correlation between aerobic capacity and brain size across a wide range of mammals [6]. Here, we review this growing body of evidence suggesting that the relationship between APA and neurobiology exists across evolutionary timescales, and that selection acting on endurance capacity in mammals may have had important effects on the evolution of brain size in these taxa.
In addition to neurobiological effects on mammals in general, this recent work has profound implications for human brain evolution. The human brain is approximately three times larger than expected for our body size, due to increases in several brain components, including the frontal lobe, temporal lobe and cerebellum [9,10]. This major increase in both absolute brain size and brain size relative to body mass occurred during the early evolution of the genus Homo, becoming especially pronounced during the evolution of Homo erectus [9,11–13] (figure 1). Because brain size changes in human evolution are often interpreted in the context of cognition [11], previous hypotheses for increased brain size in hominins have focused on greater social complexity [14] or enhanced ecological demands on cognition [15,16]. However, at the same time as brain size began to increase in the human lineage, aerobic activity levels appear to have changed dramatically [17–20]. Our ancestors, beginning with H. erectus, shifted to a hunting and gathering lifestyle that required higher levels of aerobic activity [21–24], with morphological evidence showing adaptations for increased long-distance trekking and the adoption of endurance running (ER; aerobic running for distances of more than 5 km) as a new hunting method [17,18]. Thus, in addition to reviewing patterns of brain evolution in non-human mammals, we propose the novel hypothesis that selection acting on human locomotor endurance had a measurable effect on the evolution of human brain structure and cognition.
Figure 1.
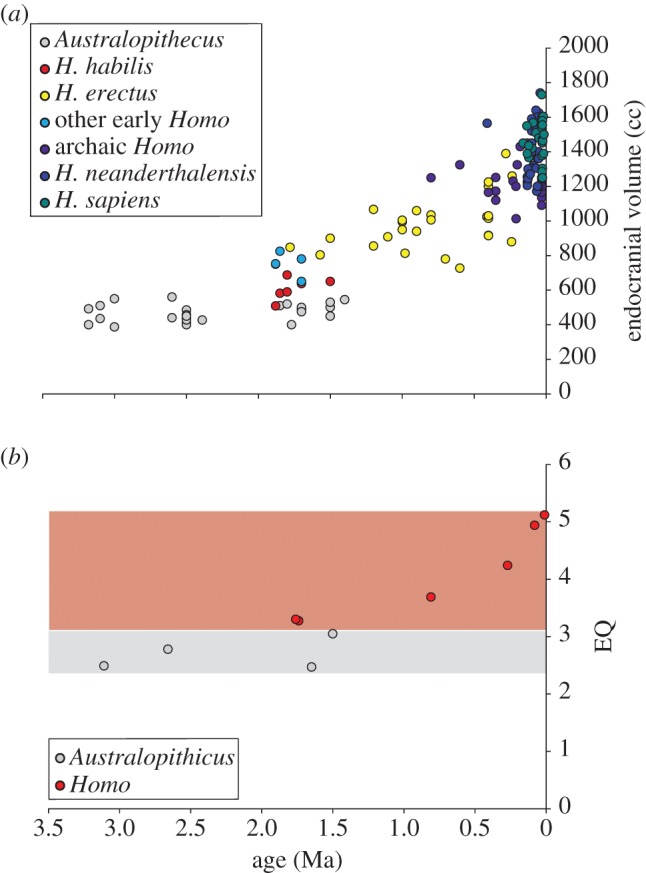
Absolute and relative brain sizes of hominins. (a) Endocranial volumes of hominin skulls from Holloway et al. [12]. (b) Encephalization quotients (EQs) through time for hominin taxa (from Lieberman [9]). EQs are a measure of the difference between actual and expected endocranial volumes. Grey area denotes australopithecine EQs; red area denotes Homo EQs. Ma is millions of years before present.
To explore hypotheses linking physical activity and brain evolution, we begin by reviewing proximate mechanisms that allow APA to alter the adult mammalian brain. We then examine intra- and interspecific studies (as well as artificial selection experiments) that suggest selection acting to improve endurance capacity alters these proximate mechanisms and, in the end, affects neurobiological evolution in mammals that have an evolutionary history of endurance activity (athletic species). Finally, we explore correlations between APA and neurobiology across evolutionary timescales in the human lineage. The purpose of this review is not to suggest that aerobic activity alone is responsible for all aspects of human brain size or cognitive evolution. However, our review suggests that aerobic activity represents a previously unrecognized factor in mammalian neurobiological evolution, and highlights the possibility that non-cognitive selection pressures may have played an important role in the development of the human brain.
2. Effects of aerobic physical activity on the brain: proximate mechanisms
Many studies suggest that APA leads to the formation of new neurons (neurogenesis) in some portions of the adult brain [2–4,7,25–27]. In rodents, voluntary wheel-running produces a three- to fourfold increase in neurons in the dentate gyrus of the hippocampus [2,8]. There is also some limited evidence that neurobiological changes associated with APA occur in other brain regions [2]. For example, there is a trend towards increased neurogenesis, and evidence of gliogenesis (generation of new glia that support neuronal activity) with APA in the frontal cortex of rats [28,29], and neurogenerative activity induced by APA was found in superficial cortical layers and in the motor cortex of rodents [29].
Activity-induced neurogenesis has a major impact on cognitive function and on the size of brain components. For example, performance in memory and spatial learning tasks improves following APA in non-human taxa such as monkeys [30] and rodents [8,31,32]. In humans, aerobic fitness is positively correlated with hippocampal and basal ganglia volume in children and older adults [25,33,34], with grey matter density in the insula of young adults [35], as well as with the amount of grey and white matter in the frontal lobe and other brain areas of older adults [27]. These structural changes across many brain regions appear to have significant functional effects. In school-aged children, fitness levels and participation in higher amounts of physical activity are correlated with improved cognitive function [5,26,36]. In healthy young adults (approx. 22 years of age), both acute and long-term APA improves performance on memory tasks, suggesting enhanced hippocampal function [37]. Finally, several studies have linked APA with either improved cognitive performance (especially executive functions and spatial memory) or a reduction in cognitive decline in older populations [3,38,39].
(a). Neurotrophins and growth factors
Aerobic physical activity (APA) appears to lead to neurogenesis, neuroprotection and cognitive improvements in adults primarily through the upregulation of neurotrophins and growth factors [40]. Neurotrophins are a family of proteins that play a major role in the development and maintenance of new neurons, and regulate neuronal structure and activity [1,2,4,8,32,41]. Brain-derived neurotrophic factor (BDNF) is among the more important neurotrophins involved in APA-induced neurogenesis [1]. Voluntary and forced running in rodents and humans results in a significant upregulation of BDNF, which improves neuronal survival [40,42,43], and APA-induced neurogenesis is diminished when BDNF signalling is blocked using antibodies to its receptor (TrkB) [1].
In addition to BDNF, concentrations of two growth factors—insulin-like growth factor I (IGF-1) and vascular endothelial growth factor (VEGF)—increase with physical activity, and both are known to play a role in neurogenesis [4,44]. For example, infusion of IGF-1 in the periphery leads to increased hippocampal neurogenesis [45], while hippocampal gene transfer of VEGF also enhances neurogenesis [46]. The role of VEGF and IGF-1 in neurogenesis was confirmed in a set of experiments showing that blocking peripheral VEGF and IGF-1 significantly inhibited APA-induced neurogenesis [4,7]. Long-term endurance training increases circulating levels of IGF-1 and VEGF in humans at rest [47,48], indicating that the neuroprotective effects of APA may also be enhanced by increased physical activity over time.
In addition to neurobiological functions, BDNF, IGF-1 and VEGF all have important effects on metabolic pathways, suggesting selection for APA performance may have had pleiotropic effects. First, increases in IGF-1 and VEGF occur peripherally in response to activity to stimulate tissue and vascular system growth and repair, which improves oxygen delivery to muscles and increases aerobic performance [49,50]. For example, VEGF signalling regulates muscle capillary density, which is a major determinant of maximal aerobic capacity across mammals [51,52]. Second, BDNF and IGF-1 facilitate lipid oxidation in muscles [53] and glucose metabolism [1], which are essential for energy regulation during submaximal activity [54]. Peripheral production of neurotrophins and growth factors can lead to effects on the central nervous system (CNS) because they all cross the blood–brain barrier, resulting in correlated peripheral and CNS concentrations [55]. It is possible that growth factors are upregulated in the periphery for their beneficial effects on metabolic and vascular systems, and then cross the blood–brain barrier, triggering neurogenic actions. Because athletic individuals have high resting levels of both IGF-1 and VEGF [47,48], baseline levels of these neurotrophins are important indicators of aerobic performance.
Based on the effects of APA on neurotrophins and growth factors, researchers have suggested two hypotheses for activity-induced adult neurogenesis [56,57]. First, neurotrophins and growth factors may be upregulated in the periphery to support endurance activity (metabolism, vascular growth), with ancillary effects on the brain [56]. Second, links between activity and neurogenesis may create a neurogenic reserve during foraging in novel or complex environments to enhance cognitive engagement during locomotor bouts [56,57]. Indeed, cognitive engagement following APA has an additive effect on neurogenesis in response to physical activity [57], supporting this hypothesis. While these functional hypotheses may explain why APA-induced neurobiological effects accrue during an individual's lifetime, recent evidence suggests that selection acting on neurotrophin and growth factor signalling may have had broader impacts on the evolution of the brain [6]. Below, we detail an evolutionary model that links these proximate mechanisms acting on adults to brain growth and development, and suggests that the evolution of high levels of aerobic activity had an important effect on brain evolution in athletic taxa.
(b). Physical activity and the evolution of brain growth: a model
The proximate mechanisms described above generally affect regional changes in brain structure following physical activity in adults. Here, we propose an evolutionary model where selection in athletic taxa leads to the upregulation of circulating neurotrophins and growth factors, but has pleiotropic effects on brain growth. Research in both humans and animal models supports a strong role for neurotrophins and growth factors in brain development. Experimental work in rodents has shown that deficiencies in neurotrophins and growth factors during development in utero and post-natally lead to smaller overall adult brain sizes [45,58–60], whereas overexpression of IGF-1 leads to significantly larger brain sizes at adulthood [61]. For example, in mice with homozygous inactivation of VEGF, brain size is significantly smaller than controls [62]. Additionally, mice with lower levels of BDNF owing to genetic manipulation grew significantly smaller brains during development [59]. Human data generally support experimental studies in animal models; however, we should interpret these results with some caution because sample sizes are often smaller and studies are observational in nature. In humans, naturally low levels of circulating IGF-1 leads to reduced brain size in offspring [63], and pre-term infants show a strong correlation between circulating IGF-1 concentrations and brain volume measured by MRI [64]. Pre-term human infants also have lower levels of BDNF, which correlates with negative neurodevelopmental outcomes [65].
Based on the proximate mechanisms linking energy regulation and vascular growth during APA with neurotrophins, growth factors and neurogenesis, we propose a model where selection acting to improve APA performance through enhanced metabolic regulation and oxygen transport alters baseline neurotrophin and growth factor signalling (figure 2), as is found in highly athletic subjects [47,48]. This evolutionary change in baseline signalling would lead to increased brain growth during ontogeny in species adapted for endurance activities, effectively linking selection for increased locomotor performance to brain size in some taxa. This is not the only possible way to explain evolutionary links between APA and neurobiology (see §5); however, we believe this model provides an important avenue for generating predictions and testing the overall hypothesis that there is an evolutionary relationship between APA and the brain. Below, we present a wide array of evidence in support of this model, including intraspecific data, interspecific data from a broad sample of mammals and data from evolution experiments that clearly show neurobiological changes when mammals undergo selection for APA performance.
Figure 2.
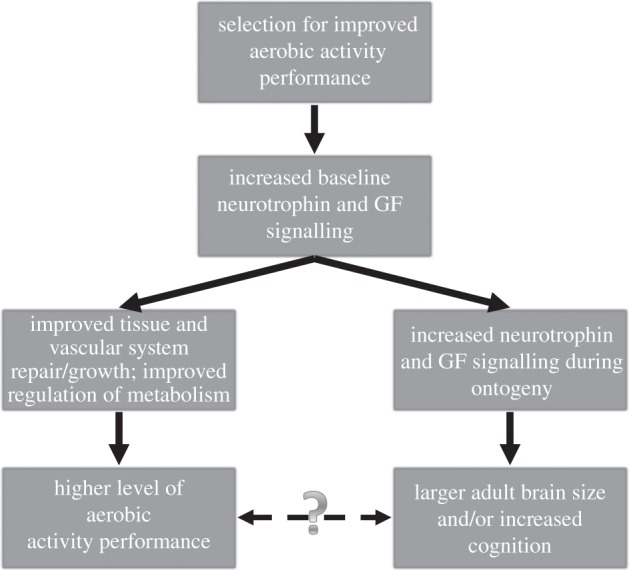
Evolutionary model linking APA and brain size in humans. Solid arrows denote downstream effects of selection for APA performance. Dashed arrow indicates a possible adaptive link between neurobiology and exercise performance. See text for a detailed explanation of this model.
3. Evolutionary links between aerobic physical activity and neurobiology
Intraspecific evidence suggests that population and individual variations in neurotrophins and brain size are strongly correlated with APA performance. First, in rats, variation in the distance run voluntarily on running wheels in different strains is correlated with variation in brain BDNF levels at rest, but not after activity [66]. Importantly, BDNF levels were measured in rats that were not given access to running wheels, suggesting the differences in BDNF levels are not simply plastic responses to physical activity during their lifetimes, but rather reflect differences between strains accumulated over multiple generations [66]. Second, in adult gerbils without prior exercise training, total brain size was positively correlated with maximum aerobic capacity (VO2,max) during voluntary wheel running, a good measure of endurance capacity [67]. Finally, a polymorphism in the human VEGF gene is correlated with higher VO2,max prior to exercise training, as well as with a greater response in VO2,max to exercise training [68], showing that heritable changes in the human genome link APA performance to growth factors. Combined, these studies suggest that if selection were to act on APA performance, it is likely that a change in circulating neurotrophins, growth factors and brain size would occur.
A recent interspecific study supports this evolutionary hypothesis in a wider range of mammalian taxa. Raichlen & Gordon [6] showed, using conventional and phylogenetically controlled analyses, that VO2,max is significantly correlated with brain size across non-human mammals, including rodents, ungulates, canids and felids. In this dataset, animals were not trained over long periods of time, providing further evidence that correlations between aerobic capacity and neurobiology are inherited traits. The relationship between VO2,max and brain size across mammals is likely to be related to differences in VEGF expression in more athletic taxa [6], because VO2,max is positively correlated with the volume of muscle capillary networks across mammals, driven by the need to deliver oxygen during APA [52]. Because skeletal muscle capillarity is regulated by VEGF [51,69], the increased volume of the capillary network found in mammals with high aerobic capacities may be due to evolutionarily altered VEGF signalling.
Thus, both intra- and interspecific data show that variables associated with endurance are correlated with increased neurotrophin and growth factor expression and larger brain size. However, because these analyses are correlational in nature, they do not clearly demonstrate evolutionary mechanisms. To do that, we explore data from artificial selection experiments that show how changes in neurobiology are associated with the evolution of increased exercise capacity in two rodent species.
(a). Artificial selection experiments
Two artificial selection experiments help show exactly how intraspecific variation in neurotrophins can be altered by selection for improved APA performance, and in the end, how these evolutionary events impact neurobiology. In one experiment, researchers selected for high and low VO2,max in rats, and in a second experiment, researchers selected for high amounts of voluntary wheel running in mice. It is important to note that in both evolution experiments, the target of selection was APA rather than the cognitive demands associated with activity.
In rats, selection for high levels of aerobic capacity is associated with evolutionary effects on both VEGF and IGF-1, as predicted by our model. High-aerobic-capacity rats have significantly higher muscle capillary density than low-aerobic-capacity rats, suggesting an evolutionary effect on the VEGF pathway [70]. High-aerobic-capacity rats also have significantly higher baseline levels of IGF-1 compared with low-aerobic-capacity rats [71]. The evolution of high aerobic capacity in rats also affects cognition. High-aerobic-capacity rats perform better on cognitive tasks (e.g. spatial memory) and learn faster than low-aerobic-capacity rats, while performance on motor learning tasks does not differ significantly [72]. In these experiments, VEGF, IGF-1 and cognition were measured in subjects that did not undergo exercise training during their lifetimes, showing that differences between rat groups are not due to plastic changes, but are due to an evolutionary change in each lineage [70,72].
In another artificial selection experiment, mice were selectively bred for high amounts of voluntary wheel-running [73]. Four lines of selected mice voluntarily ran about three times further per day than control lines [73], and this type of selection also increases VO2,max in these mice [74]. Selected mice had higher BDNF levels following several days of voluntary wheel-running compared with control lines [75], and selected mice showed significantly greater cell genesis in the hippocampus when allowed to run, compared with control mice [76]. In addition, two of the selected mouse lines have significantly higher baseline VEGF levels than control mice, which was correlated with higher muscle capillary density (the other two lines showed a strong trend towards higher VEGF levels than controls, but the difference fell just outside statistical significance; p = 0.055) [77]. Just as in the rat evolution experiment, the evolution of ER in mice leads to increased circulating levels of compounds essential for both brain growth and increased aerobic capacity.
Finally, this research group has begun investigating changes in brain architecture and size associated with the evolution of high levels of voluntary wheel-running. Kolb [78,79] and Rhodes et al. [76] found significant differences in the size of brain components in selected mice compared with control mice. Specifically selected mice had significantly larger mid-brain and dentate gyrus volumes compared with control mice [76,78,79], and selected mice had significantly increased non-cerebellar brain masses compared with controls [78,79].
These studies clearly demonstrate how selection for high aerobic capacity and APA frequency can lead to increases in the size of brain structures. Importantly, these studies confirm two essential components of our model, and help demonstrate evolutionary mechanisms suggested by inter- and intraspecific studies detailed above. First, selection acting on either APA frequency or aerobic capacity leads to altered neurotrophin and growth factor signalling. Increased VEGF and IGF-1 at baseline was found in selected groups, and increased BDNF signalling followed APA in selected mice. Second, and most importantly, these evolutionary changes in APA performance are associated with changes in brain size and cognition, showing that selection specifically targeting athletic ability, rather than cognitive performance, can have significant effects on the brain.
4. Human evolution
Through intraspecific data, interspecific comparisons and evolution experiments, we have shown how selection acting on APA performance can affect the mammalian brain. As described earlier, recent work suggests humans may have undergone such selection for increased APA performance [17–20], implying the same evolutionary patterns at work in the brains of athletic mammals may explain some portion of human neurobiological evolution. However, testing this hypothesis is made difficult by the fact that humans have an evolutionary history tied to the primate lineage. Primates are not generally considered endurance athletes, and do not usually engage in high levels of sustained aerobic activity [17]. Thus, human endurance athleticism arose from an evolutionary starting point very different from the athletic abilities of most quadrupedal cursorial mammals. As with other studies of brain size [80], humans, and primates more generally, must be examined in their appropriate phylogenetic context, and not in comparisons with a wide array of non-primate mammals [6]. Unfortunately, physiological measures of athletic performance are not available for other primates, and are unknown for our fossil ancestors. Therefore, we must examine other variables that are related to endurance capacity for evidence of correlated neurobiological and APA evolution in the human lineage.
Bramble & Lieberman [17] suggest several morphological traits that highlight selection acting to increase endurance capacity in general, and specifically ER performance, during human evolution. We compiled data for the two traits with available samples in great apes, humans and hominin taxa: hindlimb length and semicircular canal dimensions. These traits allow us to begin testing the hypothesis that increased brain size during human evolution is, in part, related to a shift to higher levels of aerobic activity. Increased hindlimb length is often considered a hallmark trait for selection acting to generate endurance or cursorial behaviours in mammals [17]. In extant humans, long hindlimbs increase stride length (approx. 1 m longer than a similarly sized quadruped), allowing for high ER speeds and also reduced energy costs of walking and running, important for either long-distance trekking or ER [17]. The semicircular canal system is also a suggested target of selection for ER capabilities in humans [17] because it senses angular rotations of the head, coordinating head, eye and body movements essential to maintaining stability during ER [19,81,82]. The diameter of the canals is tuned to the frequency spectrum of head movements experienced by a particular taxon, with relatively larger canal dimensions strongly associated with animals that engage in faster, more agile locomotor behaviours that cause higher angular accelerations of the head [82]. Spoor et al. [82] showed that humans and H. erectus have increased anterior and posterior semicircular canal radii compared with earlier hominins (australopithecines) and great apes, with larger canals attributed to selection for running and other agile behaviours.
To test the hypothesis that there is an evolutionary association between APA and neurobiology in humans and our ancestors, we explored correlations between size-corrected ER variables (hindlimb length and semicircular canal radii) and encephalization quotients (EQs) in great apes, humans and fossil hominins. EQs quantify the difference between observed brain size and brain size expected for a mammal of similar body mass, and thus provide an important measure of brain size evolution [9]. After controlling for body mass, we found significant positive correlations between EQ and hindlimb length (r = 0.93), anterior (r = 0.77) and posterior semicircular canal radii (r = 0.66) in great apes, fossil hominins and humans (table 1 and figure 3). Importantly, these measures of ER capabilities index different parts of human athletic performance (speed/energy costs versus stability), supporting the idea that athletic ability in general is tied to neurobiological evolution. Although more work is clearly needed to test our hypothesis, this analysis of fossil data serves as important preliminary support for the idea that human aerobic activity capabilities are correlated with the evolutionary increase in brain size seen in fossil hominins.
Table 1.
Brain size, limb length and semicircular canal radii in hominids. Relative hindlimb lengths (RHLs) from Pontzer [83]. EQ from Lieberman [9] calculated as EQ = brain mass/0.59 × body mass0.76 following Martin [84] (EQ for Ardipithecus ramidus calculated from data of Suwa et al. [85], EQ for P. paniscus from species means of Isler et al. [86]). Anterior and posterior semicircular canal radii (ASC and PSC, respectively) from Spoor et al. [82] (ASC and PSC for H. neanderthalensis from Spoor et al. [88]). Body masses for extinct taxa from Lieberman [9] and for extant taxa from Spoor et al. [87].
| species | RHL | EQ | ASC (mm) | PSC (mm) | body mass (kg) |
|---|---|---|---|---|---|
| Ardipithecus ramidus | 0.16 | 1.57 | — | — | — |
| Australopithecus afarensis | 0.19 | 2.49 | — | — | — |
| Australopithecus africanus | 0.19 | 2.78 | 2.40 | 2.60 | 34.00 |
| Australopithecus robustus | — | 3.05 | 2.60 | 2.70 | 36.00 |
| Homo habilis | 0.20 | 3.30 | 2.90 | 2.80 | 39.00 |
| Homo erectus | 0.22 | 3.69 | 3.20 | 3.10 | 61.00 |
| Homo neanderthalensis | 0.21 | 4.94 | 3.02 | 2.88 | 72.00 |
| Homo heidelburgensis | 0.23 | 4.24 | — | — | — |
| Homo floresiensis | 0.18 | 2.95 | — | — | — |
| Homo sapiens | 0.23 | 5.12 | 3.20 | 3.10 | 58.31 |
| Pongo pygmaeus | 0.15 | 1.56 | 2.70 | 2.50 | 56.95 |
| Pan paniscus | 0.17 | 1.87 | 2.60 | 2.50 | 39.10 |
| Pan troglodytes | 0.16 | 2.07 | 2.70 | 2.80 | 44.97 |
| Gorilla gorilla | 0.14 | 1.02 | 2.90 | 3.00 | 120.95 |
Figure 3.
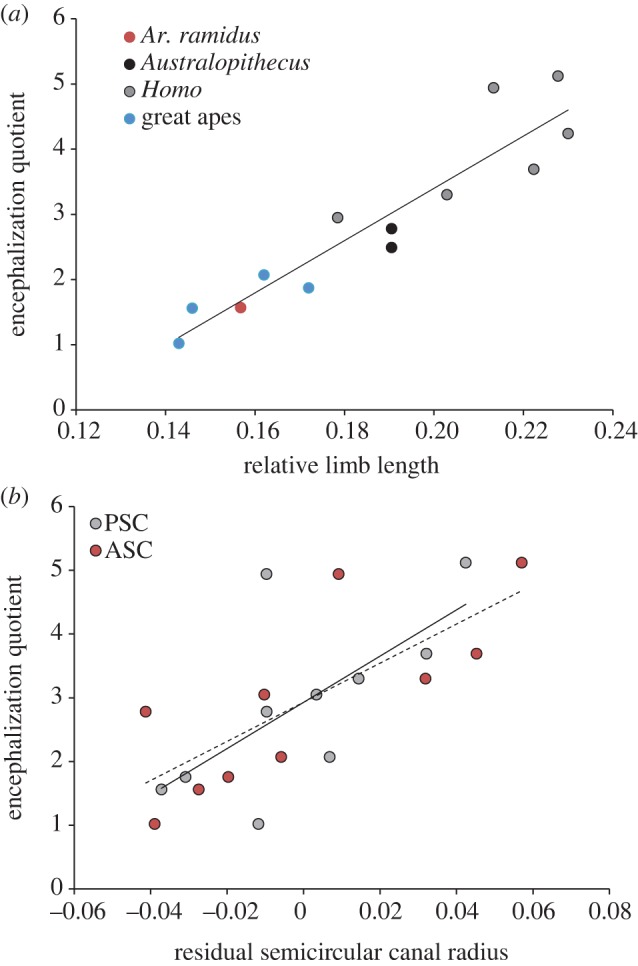
Relationship between encephalization quotient (EQ) and morphology in hominids (extant humans, great apes and fossil hominins). (a) EQs are positively correlated with size-corrected limb length (hindlimb length/body mass1/3; r = 0.93; p < 0.0001). (b) Residuals of semicircular canal radii from body mass are significantly correlated with EQ in hominids (anterior semicircular canal, ASC in red circles, dashed line: r = 0.77, p = 0.005; posterior semicircular canal, PSC in grey circles, solid line: r = 0.66, p = 0.02).
5. Conclusions
This review addressed the novel hypothesis linking the evolution of APA to a change in some portion of mammalian neurobiology. Evidence for this evolutionary relationship includes: (i) intraspecific data linking aerobic capacity, brain size, neurotrophins and growth factors; (ii) interspecific data showing a relationship between aerobic capacity and brain size; and (iii) evolution experiments detailing how selection for aerobic performance alone can affect neurobiology. Based on this body of evidence for mammals, we proposed the novel hypothesis that human neurobiology was influenced by our evolutionary history as endurance athletes. As early as 1.8 Ma, our ancestors began walking and running longer distances than previous hominin taxa [17,19], and morphological adaptations for increased aerobic activity levels during human evolution are correlated with increases in brain size relative to body size across great apes and fossil hominins. This hypothesis is not meant to suggest that other factors were unimportant in the evolution of mammalian brains in general, nor in human brain evolution specifically. Social and ecological selection pressures played an essential role in human neurobiological evolution [10,14,15,89]. However, our review of evidence from humans, fossil hominins, rodents and other mammals suggests that the evolution of increased aerobic capacity had a significant effect on brain evolution in athletic mammals, including humans. In conjunction with other social and ecological selection pressures, this review suggests that human brain evolution was influenced, in part, by the evolution of our active lifestyle.
In exploring these relationships, we presented a model that attempts to explain how selection for aerobic athletic performance can impact the evolution of the mammalian brain; however, this model remains untested. There is much work to be done fleshing out the model described above and in devising experiments to test specific parts of the model. Studies in molecular and developmental biology can test the ontogenetic model described above, while greater emphasis on selection experiments across a wider array of mammals will continue to test our overall model for how selection acting on APA ultimately affects neurobiology. Finally, an increased emphasis on exercise testing in non-human primates can generate a comparative dataset to help examine correlated changes in neurobiology and aerobic capacity in the appropriate phylogenetic context. An expanded comparative dataset is also necessary to quantify the contribution of physical activity to brain evolution, allowing researchers to effectively determine the relative weights of APA and cognitive selection pressures on the human brain. We hope that this review stimulates new areas of research that will also examine alternative hypotheses for evolutionary mechanisms that link the brain and body across a wide array of mammals, including humans.
One area we have not discussed is whether these links are adaptive (i.e. dashed arrow in figure 2). Our hypothesis does not require an adaptive explanation, but we see two possible reasons why neurobiology may be affected by selection for physical activity. In both cases, the true target of selection was likely to be improved foraging performance, which would lead to increased reproductive success through greater access to food energy. First, it is certainly possible that selection acted on these links to establish cognitive capacities capable of increased foraging demands during endurance travel [56]. For example, improved spatial relational memory and planning abilities could aid humans and other mammals during long-distance foraging. This possibility would be supported if more athletic taxa with higher baseline neurotrophin and growth factor levels have greater neurogenesis following a given amount of activity compared with non-athletic taxa. However, the selection experiments described above suggest that the evolution of aerobic activity can affect the brain without cognition being a target of selection. Additionally, an association between aerobic capacity and brain size is present among mammalian taxa that differ widely in their foraging patterns and home ranges, implying any adaptive link between APA and cognition must be general and not specific to a given foraging strategy. While future research may confirm this hypothesis, a more parsimonious explanation, and one best supported by current experimental and comparative data, is that selection improved aerobic capacity by altering the signalling systems responsible for metabolism and oxygen transport (i.e. VEGF, IGF-1 and BDNF). In the end, these altered neurotrophin and growth factor systems led to increased brain size during growth and development. This intriguing possibility implies that some portion of human neurobiology simply evolved as a by-product of the evolution of increased aerobic capacity. Such a suggestion certainly requires more careful testing; however, to our knowledge, this is the first time evidence suggests that a portion of human brain size may have evolved for reasons that have little to do with sensory or cognitive selection pressures.
Thus, this review suggests that humans' evolutionary story may ultimately be more complex than the traditional view of brains over brawn. If our hypothesis is supported by more extensive data, then the evolution of the human mind and cognitive capacity may be the result of multiple selection pressures, some of which act on cognition specifically (e.g. social systems, group size, language or technology), and some of which act on the body and generate cognitive changes as a by-product of improved athletic performance. A complete understanding of the evolution of the human brain in our distant past and in more recent times requires an acknowledgement of both selection and correlated responses, as well as research into each line of evolutionary change.
Acknowledgements
Thanks to Herman Pontzer, Daniel Lieberman, Rebecca Stumpf, Charles Roseman, Steve Leigh, Karl Rosengren, Mark Grabowski, Scott Williams, Petra Jelinek, and Peter Fernandez and four anonymous reviewers for helpful discussions of this project and comments on previous versions of this manuscript. This project was supported by a Wenner Gren Hunt Fellowship to D.A.R. and by the National Science Foundation (BCS 0820270 to D.A.R. and BCS 0962903 to J.D.P.).
References
- 1.Cotman CW, Berchtold NC, Christie LA. 2007. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472 10.1016/j.tins.2007.06.011 (doi:10.1016/j.tins.2007.06.011) [DOI] [PubMed] [Google Scholar]
- 2.van Praag H. 2008. Neurogenesis and exercise: past and future directions. Neuromol. Med. 10, 128–140 10.1007/s12017-008-8028-z (doi:10.1007/s12017-008-8028-z) [DOI] [PubMed] [Google Scholar]
- 3.Colcombe SJ, Kramer AF. 2003. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 14, 125–130 10.1111/1467-9280.t01-1-01430 (doi:10.1111/1467-9280.t01-1-01430) [DOI] [PubMed] [Google Scholar]
- 4.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci 18, 2803–2812 [DOI] [PubMed] [Google Scholar]
- 5.Hillman CH, Erickson KI, Kramer AF. 2008. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 9, 58–65 10.1038/nrn2298 (doi:10.1038/nrn2298) [DOI] [PubMed] [Google Scholar]
- 6.Raichlen DA, Gordon AD. 2011. Relationship between exercise capacity and brain size in mammals. PLoS ONE 6, e20601. 10.1371/journal.pone.0020601 (doi:10.1371/journal.pone.0020601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trejo JL, Carro E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Praag H, Kempermann G, Gage FH. 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 10.1038/6368 (doi:10.1038/6368) [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DE. 2011. The evolution of the human head. Cambridge, MA: Harvard University Press [Google Scholar]
- 10.Schoenemann PT. 2006. Evolution of the size and functional areas of the human brain. Annu. Rev. Anthropol. 35, 379–406 10.1146/annurev.anthro.35.081705.123210 (doi:10.1146/annurev.anthro.35.081705.123210) [DOI] [Google Scholar]
- 11.Holloway RL. 2008. The human brain evolving: a personal retrospective. Annu. Rev. Anthropol. 37, 1–19 10.1146/annurev.anthro.37.081407.085211 (doi:10.1146/annurev.anthro.37.081407.085211) [DOI] [Google Scholar]
- 12.Holloway RL, Broadfield DC, Yuan MS. 2004. The human fossil record, vol. 3. Brain endocasts: the paleoneurological evidence. Hoboken, NJ: Wiley and Sons [Google Scholar]
- 13.Sherwood CC, Subiaul F, Zawidzki TW. 2008. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J. Anatomy 212, 426–454 10.1111/j.1469-7580.2008.00868.x (doi:10.1111/j.1469-7580.2008.00868.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunbar RIM. 2003. The social brain: mind, language, and society in evolutionary perspective. Annu. Rev. Anthropol. 32, 163–181 10.1146/annurev.anthro.32.061002.093158 (doi:10.1146/annurev.anthro.32.061002.093158) [DOI] [Google Scholar]
- 15.Barton RA. 1996. Neocortex size and behavioural ecology in primates. Proc. R. Soc. Lond. B 263, 173–177 10.1098/rspb.1996.0028 (doi:10.1098/rspb.1996.0028) [DOI] [PubMed] [Google Scholar]
- 16.Milton K. 1981. Distribution patterns of tropical plants as an evolutionary stimulus to primate mental development. Am. Anthropol. 83, 534–548 10.1525/aa.1981.83.3.02a00020 (doi:10.1525/aa.1981.83.3.02a00020) [DOI] [Google Scholar]
- 17.Bramble DM, Lieberman DE. 2004. Endurance running and the evolution of Homo. Nature 432, 345–352 10.1038/nature03052 (doi:10.1038/nature03052) [DOI] [PubMed] [Google Scholar]
- 18.Carrier DR. 1984. The energetic paradox of human running and hominid evolution. Curr. Anthropol. 25, 483–495 10.1086/203165 (doi:10.1086/203165) [DOI] [Google Scholar]
- 19.Lieberman DE, Bramble DM, Raichlen DA, Shea JJ. 2009. Brains versus brawn and the evolution of Homo. In The origin of homo (eds Grine F, Leakey REF.), pp. 77–92 New York, NY: Plenum [Google Scholar]
- 20.Lieberman DE, Raichlen DA, Pontzer H, Bramble DM, Cutright-Smith E. 2006. The human gluteus maximus and its role in running. J. Exp. Biol. 209, 2143–2155 10.1242/jeb.02255 (doi:10.1242/jeb.02255) [DOI] [PubMed] [Google Scholar]
- 21.Hilton CE, Meldrum DJ. 2004. Striders, runners, and transporters. In From biped to strider: the emergence of modern human walking, running, and resource transport (eds Meldrum DJ, Hilton CE.), pp. 1–8 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- 22.Kelly RL. 1992. Mobility/sedentism: concepts, archaeological measures, and effects. Annu. Rev. Anthropol. 21, 43–66 10.1146/annurev.an.21.100192.000355 (doi:10.1146/annurev.an.21.100192.000355) [DOI] [Google Scholar]
- 23.Krantz GS. 1968. Brain size and hunting ability in earliest man. Curr. Anthropol. 9, 450–451 10.1086/200927 (doi:10.1086/200927) [DOI] [Google Scholar]
- 24.Malina RM, Little BB. 2008. Physical activity: the present in the context of the past. Am. J. Hum. Biol. 20, 373–391 10.1002/ajhb.20772 (doi:10.1002/ajhb.20772) [DOI] [PubMed] [Google Scholar]
- 25.Chaddock L, Erickson KI, Prakash RS, VanPatter M, Voss MW, Pontifex MB, Raine LB, Hillman CH, Kramer AF. 2010. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev. Neurosci. 32, 249–256 10.1159/000316648 (doi:10.1159/000316648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaddock L, Hillman CH, Buck SM, Cohen NJ. 2011. Aerobic fitness and executive control of relational memory in preadolescent children. Med. Sci. Sports Exerc. 43, 344–349 10.1249/MSS.0b013e3181e9af48 (doi:10.1249/MSS.0b013e3181e9af48) [DOI] [PubMed] [Google Scholar]
- 27.Colcombe SJ, et al. 2006. Aerobic exercise training increases brain volume in aging adults. J. Gerontol. 61A, 1166–1170 [DOI] [PubMed] [Google Scholar]
- 28.Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. 2007. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J. Neurosci. 27, 11 442–11 450 10.1523/JNEUROSCI.2505-07.2007 (doi:10.1523/JNEUROSCI.2505-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehninger D, Kempermann G. 2003. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb. Cortex 13, 845–851 10.1093/cercor/13.8.845 (doi:10.1093/cercor/13.8.845) [DOI] [PubMed] [Google Scholar]
- 30.Rhyu IJ, et al. 2010. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 167, 1239–1248 10.1016/j.neuroscience.2010.03.003 (doi:10.1016/j.neuroscience.2010.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Praag H, Shubert T, Zhao CM, Gage FH. 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 10.1523/JNEUROSCI.1731-05.2005 (doi:10.1523/JNEUROSCI.1731-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaynman S, Ying Z, Gomez-Pinilla F. 2004. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590 10.1111/j.1460-9568.2004.03720.x (doi:10.1111/j.1460-9568.2004.03720.x) [DOI] [PubMed] [Google Scholar]
- 33.Chaddock L, et al. 2010. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 1358, 172–183 10.1016/j.brainres.2010.08.049 (doi:10.1016/j.brainres.2010.08.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson KI, et al. 2011. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 10.1073/pnas.1015950108 (doi:10.1073/pnas.1015950108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters J, Dauvermann M, Mette C, Platen P, Franke J, Hinrichs T, Daum I. 2009. Voxel-based morphometry reveals an association between aerobic capacity and grey matter density in the right anterior insula. Neuroscience 163, 1102–1108 10.1016/j.neuroscience.2009.07.030 (doi:10.1016/j.neuroscience.2009.07.030) [DOI] [PubMed] [Google Scholar]
- 36.Sibley BA, Etnier JL. 2003. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr. Exerc. Sci. 15, 243–256 [Google Scholar]
- 37.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. 2011. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 104, 934–941 10.1016/j.physbeh.2011.06.005 (doi:10.1016/j.physbeh.2011.06.005) [DOI] [PubMed] [Google Scholar]
- 38.Hawkins HL, Kramer AF, Capaldi D. 1992. Aging, exercise, and attention. Psychol. Aging 7, 643–653 10.1037/0882-7974.7.4.643 (doi:10.1037/0882-7974.7.4.643) [DOI] [PubMed] [Google Scholar]
- 39.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. 2010. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 72, 239–252 10.1097/PSY.0b013e3181d14633 (doi:10.1097/PSY.0b013e3181d14633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotman CW, Berchtold NC. 2002. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301 10.1016/S0166-2236(02)02143-4 (doi:10.1016/S0166-2236(02)02143-4) [DOI] [PubMed] [Google Scholar]
- 41.Allen SJ, Dawbarn D. 2006. Clinical relevance of the neurotrophins and their receptors. Clin. Sci. 110, 175–191 10.1042/CS20050161 (doi:10.1042/CS20050161) [DOI] [PubMed] [Google Scholar]
- 42.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. 1995. Exercise and brain neurotrophins. Nature 373, 109. 10.1038/373109a0 (doi:10.1038/373109a0) [DOI] [PubMed] [Google Scholar]
- 43.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. 2008. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 59, 119–132 [PubMed] [Google Scholar]
- 44.Carro E, Nunez A, Busiguina S, Torres-Aleman I. 2000. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aberg ND, Brywe KG, Isgaard J. 2006. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci. World J. 6, 53–80 10.1100/tsw.2006.22 (doi:10.1100/tsw.2006.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao L, Jiao XY, Zuzga DS, Liu YH, Fong DM, Young D, During MJ. 2004. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 36, 827–835 10.1038/ng1395 (doi:10.1038/ng1395) [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson T, Bodin K, Sylven C, Gordon A, Tyni-Lenne R, Jansson E. 2001. Increased expression of VEGF following exercise training in patients with heart failure. Eur. J. Clini. Invest. 31, 362–366 10.1046/j.1365-2362.2001.00816.x (doi:10.1046/j.1365-2362.2001.00816.x) [DOI] [PubMed] [Google Scholar]
- 48.Koziris LP, Hickson RC, Chatterton RTJ, Groseth RT, Christie JM, Goldflies DG, Unterman TG. 1999. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J. Appl. Physiol. 86, 1436–1442 [DOI] [PubMed] [Google Scholar]
- 49.Cappon J, Brasel JA, Mohan S, Cooper DM. 1994. Effect of brief exercise on circulating insulin-like growth factor I. J. Appl. Physiol. 76, 2490–2496 10.1063/1.357607 (doi:10.1063/1.357607) [DOI] [PubMed] [Google Scholar]
- 50.Kraus RM, Stallings HWI, Yeager RC, Gavin TP. 2004. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J. Appl. Physiol. 96, 1445–1450 10.1152/japplphysiol.01031.2003 (doi:10.1152/japplphysiol.01031.2003) [DOI] [PubMed] [Google Scholar]
- 51.Amaral SL, Papanek PE, Greene AS. 2001. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am. J. Physiol. 281, H1163–H1169 [DOI] [PubMed] [Google Scholar]
- 52.Weibel ER, Bacigalupe LD, Schmitt B, Hoppeler H. 2004. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir. Physiol. Neurobiol. 140, 115–132 10.1016/j.resp.2004.01.006 (doi:10.1016/j.resp.2004.01.006) [DOI] [PubMed] [Google Scholar]
- 53.Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. 2009. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 94, 1153–1160 10.1113/expphysiol.2009.048561 (doi:10.1113/expphysiol.2009.048561) [DOI] [PubMed] [Google Scholar]
- 54.Coggan AR. 1991. Plasma-glucose metabolism during exercise in humans. Sports Med. 11, 102–124 10.2165/00007256-199111020-00003 (doi:10.2165/00007256-199111020-00003) [DOI] [PubMed] [Google Scholar]
- 55.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. 2011. Blood BDNF concentrations reflect brain-tissue BDNF across species. Int. J. Neuropsychopharmacol. 14, 347–353 10.1017/S1461145710000738 (doi:10.1017/S1461145710000738) [DOI] [PubMed] [Google Scholar]
- 56.Fabel K, Kempermann G. 2008. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromol. Med. 10, 59–66 10.1007/s12017-008-8031-4 (doi:10.1007/s12017-008-8031-4) [DOI] [PubMed] [Google Scholar]
- 57.Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. 2010. Why and how physical activity promotes experience-induced brain plasticity? Front. Neurosci. 4, 1–9 10.3389/fnins.2010.00189 (doi:10.3389/fnins.2010.00189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. 1995. IGF-I disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14, 717–730 10.1016/0896-6273(95)90216-3 (doi:10.1016/0896-6273(95)90216-3) [DOI] [PubMed] [Google Scholar]
- 59.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. 2006. The disease progression of Mecp2 mutant mice affected by the level of BDNF expression. Neuron 49, 341–348 10.1016/j.neuron.2005.12.027 (doi:10.1016/j.neuron.2005.12.027) [DOI] [PubMed] [Google Scholar]
- 60.Cheng CM, Joncas G, Reinhardt RR, Farrer R, Quarles R, Janssen J. 1998. Biochemical and morphometric analyses show that myelination in the insulin-like growth factor 1 null brain is proportionate to its neuronal composition. J. Neurosci. 18, 5673–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye P, Xing Y, Dai Z, D'Ercole AJ. 1996. In vivo actions of insulin-like growth factor-I (IGF-I) on cerebellum development in transgenic mice: evidence that IGF-I increases proliferation of granule cell progenitors. Dev. Brain Res. 95, 44–54 10.1016/0165-3806(96)00492-0 (doi:10.1016/0165-3806(96)00492-0) [DOI] [PubMed] [Google Scholar]
- 62.Raab S, Beck H, Gaumann A, Yuce A, Gerber H, Plate K, Hammes H, Ferrara N, Breier G. 2004. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 91, 595–605 [DOI] [PubMed] [Google Scholar]
- 63.Woods KA, CamachoHubner C, Savage MO, Clark AJL. 1996. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. New Engl. J. Med. 335, 1363–1367 10.1056/NEJM199610313351805 (doi:10.1056/NEJM199610313351805) [DOI] [PubMed] [Google Scholar]
- 64.Hansen-Pupp I, et al. 2011. Postnatal decrease in circulating insulin-like growth factor-i and low brain volumes in very preterm infants. J. Clin. Endocrinol. Metab. 96, 1129–1135 10.1210/jc.2010-2440 (doi:10.1210/jc.2010-2440) [DOI] [PubMed] [Google Scholar]
- 65.Rao R, Mashburn CB, Mao J, Wadhwa N, Smith GM, Desai NS. 2009. Brain-derived neurotrophic factor in infants <32 weeks gestational age: correlation with antenatal factors and postnatal outcomes. Pediatr. Res. 65, 548–552 10.1203/PDR.0b013e31819d9ea5 (doi:10.1203/PDR.0b013e31819d9ea5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson RA, Mitchell GS. 2003. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain Res. 983, 108–114 10.1016/S0006-8993(03)03039-7 (doi:10.1016/S0006-8993(03)03039-7) [DOI] [PubMed] [Google Scholar]
- 67.Chappell MA, Garland TJ, Robertson GF, Saltzmann W. 2007. Relationships among running performance, aerobic physiology and organ mass in male Mongolian gerbils. J. Exp. Biol. 210, 4179–4197 10.1242/jeb.006163 (doi:10.1242/jeb.006163) [DOI] [PubMed] [Google Scholar]
- 68.Prior SJ, Hagberd JM, Paton CM, Douglass LW, Brown MD, McLenithan JC, Roth SM. 2006. DNA sequence variation in the promoter region of the VEGF gene impacts VEGF gene expression and maximal oxygen consumption. Am. J. Physiol. Heart Circ. Physiol. 290, H1848–H1855 10.1152/ajpheart.01033.2005 (doi:10.1152/ajpheart.01033.2005) [DOI] [PubMed] [Google Scholar]
- 69.Tang K, Breen EC, Gerber HP, Ferrara NMA, Wagner PD. 2004. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol. Genomics 18, 63–69 10.1152/physiolgenomics.00023.2004 (doi:10.1152/physiolgenomics.00023.2004) [DOI] [PubMed] [Google Scholar]
- 70.Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. 2003. Selected contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J. Appl. Physiol. 94, 1682–1688 [DOI] [PubMed] [Google Scholar]
- 71.Bye A, et al. 2008. Gene expression profiling of skeletal muscle in exercise-trained and sedentary rats with inborn high and low VO2,max. Physiol. Genomics 35, 213–221 10.1152/physiolgenomics.90282.2008 (doi:10.1152/physiolgenomics.90282.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wikgren J, Mertikas GG, Raussi P, Tirkkonen R, Ayravainen L, Pelto-Huikko M, Koch LG, Britton SL, Kainulainen H. 2012. Selective breeding for endurance running capacity affects cognitive but not motor learning in rats. Physiol. Behav. 106, 95–100 10.1016/j.physbeh.2012.01.011 (doi:10.1016/j.physbeh.2012.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swallow JG, Carter PA, Garland T., Jr 1998. Artificial selection for increased wheel-running behavior in house mice. Behav. Genet. 28, 227–237 10.1023/A:1021479331779 (doi:10.1023/A:1021479331779) [DOI] [PubMed] [Google Scholar]
- 74.Rezende EL, Garland TJ, Chappell MA, Malisch JL, Gomes FR. 2006. Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability, and the mini-muscle phenotype. J. Exp. Biol. 209, 115–127 10.1242/jeb.01883 (doi:10.1242/jeb.01883) [DOI] [PubMed] [Google Scholar]
- 75.Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. 2003. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience 121, 1–7 10.1016/S0306-4522(03)00422-6 (doi:10.1016/S0306-4522(03)00422-6) [DOI] [PubMed] [Google Scholar]
- 76.Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland TJ, Gage FH. 2003. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav. Neurosci. 117, 1006–1016 10.1037/0735-7044.117.5.1006 (doi:10.1037/0735-7044.117.5.1006) [DOI] [PubMed] [Google Scholar]
- 77.Audet GN, Meek TH, Garland TJ, Olfert IM. 2011. Expression of angiogenic regulators and skeletal muscle capillarity in slectively bred high aerobic capacity mice. Exp. Physiol. 11, 1138–1150 [DOI] [PubMed] [Google Scholar]
- 78.Kolb EM. 2010. Neurobiological and physiological underpinnings of high voluntary wheel running. Riverside, CA: University of California at Riverside [Google Scholar]
- 79.Kolb EM, Rezende EL, Holness L, Radtke A, Lee SK, Obnaus A, Garland TJ. In press Mice selectively bred for high voluntary wheel running have larger midbrains: support for the mosaic model or brain evolution. J. Exp. Biol. [DOI] [PubMed] [Google Scholar]
- 80.Perez-Barberia FJ, Shultz S, Dunbar RIM. 2007. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution 61, 2811–2821 10.1111/j.1558-5646.2007.00229.x (doi:10.1111/j.1558-5646.2007.00229.x) [DOI] [PubMed] [Google Scholar]
- 81.Lieberman DE, Bramble DM, Raichlen DA, Shea JJ. 2007. Endurance running and the tyranny of ethnography: a reply to Pickering and Bunn. J. Hum. Evol. 53, 434–437 10.1016/j.jhevol.2007.01.012 (doi:10.1016/j.jhevol.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 82.Spoor F, Wood B, Zonneveld F. 1994. Implications of early hominid labyrinth morphology for the evolution of human bipedal locomotion. Nature 369, 645–648 10.1038/369645a0 (doi:10.1038/369645a0) [DOI] [PubMed] [Google Scholar]
- 83.Pontzer H. 2006. Locomotor energetics, ranging ecology, and the emergence of the genus Homo. Cambridge, MA: Harvard University Press [Google Scholar]
- 84.Martin RD. 1981. Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature 293, 57–60 10.1038/293057a0 (doi:10.1038/293057a0) [DOI] [PubMed] [Google Scholar]
- 85.Suwa G, Asfaw B, Kono RT, Kubo D, Lovejoy CO, White TD. 2009. The Ardipithecus ramidus skull and its implications for hominid origins. Science 326, 68e61–68e67 [PubMed] [Google Scholar]
- 86.Isler K, Kirk EC, Miller JMA, Albrecht GA, Gelvin BR, Martin RD. 2008. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J. Hum. Evol. 55, 967–978 10.1016/j.jhevol.2008.08.004 (doi:10.1016/j.jhevol.2008.08.004) [DOI] [PubMed] [Google Scholar]
- 87.Spoor F, Garlan TJ, Krovitz G, Ryan TM, Silcox MT, Walker A. 2007. The primate semicircular canal system and locomotion. Proc. Natl Acad. Sci. USA 104, 10 808–10 812 10.1073/pnas.0704250104 (doi:10.1073/pnas.0704250104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spoor F, Hublin JJ, Braun M, Zonneveld F. 2003. The bony labyrinth of Neanderthals. J. Hum. Evol. 44, 141–165 10.1016/S0047-2484(02)00166-5 (doi:10.1016/S0047-2484(02)00166-5) [DOI] [PubMed] [Google Scholar]
- 89.Dunbar RIM, Shultz S. 2007. Evolution of the social brain. Science 1344, 1344–1347 10.1126/science.1145463 (doi:10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]


