Abstract
Recent global change has had a substantial influence on the distribution of organisms, and many species are currently expanding their ranges. To evaluate the underlying processes, long-term data with good geographic resolution are essential. One important but generally overlooked data source is offered by the taxon-specific national catalogues of first provincial records that are kept in many countries. Here, we use such data to quantify trait-based influences on range expansion in Swedish butterflies and moths between 1973 and 2010. Of 282 species meeting pre-defined quality criteria, 170 expanded their northern range margin, with a mean expansion rate of 2.7 km per year. The analyses demonstrate that habitat and diet generalists, forest species and species active during warm conditions have expanded their ranges more rapidly than other species. Notably, range expansion in diet specialists was positively related to a nitrogen-favoured larval diet, an effect not found among oligo- or polyphagous species. In contrast to the general view, this shows that specialist species can undergo rapid range expansion. We suggest that increased areas of nitrogen-rich habitat, and increased availability of a nitrogen-favoured diet, are among the most important drivers of range expansions, potentially having far-reaching consequences for a wide variety of organisms.
Keywords: butterfly, climate change, habitat availability, moth, species trait, Sweden
1. Introduction
Recent global change has had a substantial influence on the distribution and abundance of organisms and, as a consequence, many species are currently expanding their ranges [1–4]. This has led to altered composition and interactions of plant and animal communities, as well as an increased impact on ecosystems from alien species [5–7]. The ability to predict how different species cope with global change and the consequences for ecosystem functioning have therefore become a great challenge among ecologists [3,6,8]. Future ranges of species are often modelled over larger scales using climate data [9–11], but surprisingly few studies have explicitly related expansion to resource availability such as habitat and food.
In addition to climate, range expansions are potentially critically affected by species traits related to resource use, dispersal and reproductive capacity [12–14]. However, the relative importance of such traits involved in range expansion is rarely explicitly quantified (cf. [2,3,15]). In general, resources such as habitat and diet might influence range expansion both directly and indirectly, by offering more resources to generalists than to specialists [15–19]. This may be increasingly important as large areas of natural habitats are being affected by intensified agriculture and forestry, or transformed into urbanized areas. Interestingly, a resource that has recently increased markedly in availability is nitrogen-rich habitat [20,21]. Species inhabiting nitrogen-rich habitat therefore might have a higher potential to expand their ranges. Further, large body size [22,23] and high temperature during the adult flight period increase dispersal capacity, and might therefore increase range expansions [24–26]. Finally, a high reproductive capacity is related to a relatively longer flight period, which might also favour increased range expansion [27].
To reveal underlying processes of climate-change-related range expansion patterns, studies can be conducted over latitudinal or elevational gradients using a study group with long-term distribution data and high geographical resolution. Sweden extends across 1600 km in north–south direction (from latitude 55° to 69°) and therefore offers a highly suitable model system for studying northern range expansions. Lepidoptera is a suitable taxon for exploring relations between range expansion and species traits, because the feeding spectrum of Lepidoptera is highly diverse, they respond quickly to climatic and environmental changes, and there is a solid and robust knowledge on their ecology and distribution. Here, we explore the northern range expansion of Swedish Lepidoptera between 1973 and 2010 in relation to their species traits. We predict a greater range expansion in species exhibiting the following traits: generalists with regard to habitat and dietary width; association with a nitrogen-favoured diet; adult activity period during warm conditions; large body size; and high reproductive capacity.
2. Material and methods
(a). Study area and study species
The northern range expansion in Swedish Lepidoptera between 1973 and 2010 was analysed in relation to their species traits. We restricted the analyses to butterflies and macro-moths (see the electronic supplementary material, table S1) to ensure that distribution and traits of the analysed taxa were sufficiently well known at the time the study started. Taxa that had been split during the period (n = 21) were excluded from the analyses. We further applied the following criteria to minimize the risk of data bias: (i) only species distributed in the provinces up to and including the 60th latitude in 1973 were considered, as a wide distribution in northern Sweden at the time the study started would have constrained the options for further range expansion by limiting the number of provinces left to expand into; (ii) only species reproducing in Sweden were included, as vagrant species would have biased the data otherwise; and (iii) documentation of all records was critically examined, and two cases that were insufficiently documented were excluded from the analyses. After this procedure, 282 species meeting the pre-defined quality criteria remained (see the electronic supplementary material, table S1). Systematics and taxonomy follow Karsholt & Razowski [28].
(b). Range expansion
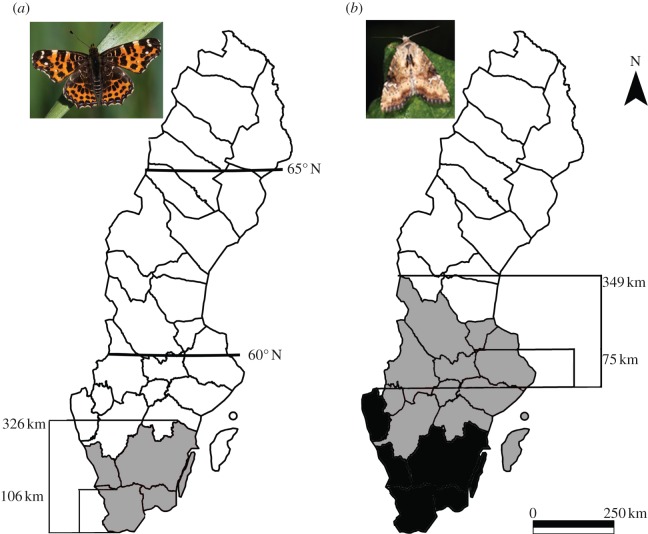
Range expansion distances were measured on a provincial level. In our main approach, range expansion was measured as the distance (km) between the northern range limit of the provinces occupied in 1973 and the range limit in 2010 (figure 1). For species that colonized Sweden during the study period, we used the southern limit of the colonized province as the northern range margin in the analyses (figure 1). Geographical limits of each of the 30 Swedish provinces were extracted from maps available in the Swedish National Atlas [29]. To ensure robustness of the statistical analyses, we used two additional measures of range expansion. First, we measured range expansion in the same way as described earlier, except that we used the southern margin of the northernmost occupied province in 2010 (figure 1). Second, each species was categorized into three expansion classes: no change, moderate expansion (0–150 km) and large expansion (more than 150 km).
Figure 1.
Northern range expansion in Sweden between 1973 and 2010 in (a) Araschnia levana (map butterfly) and (b) Apamea scolopacina (slender brindle). The distribution in 1973 is shown in black, the area colonized up to and including 2010 in grey. In the main approach, northern range expansion was measured as the difference between the northern range of the province where the species occurred in 2010 and the northern range of the province where the species occurred in 1973; in the alternative approach the southern range of the province where the species occurred in 2010 was used (see A. scolopacina). The expansion of species recorded as new for Sweden during the study period was measured as the difference between the northern range of the province where the species occurred in 2010, and the southern range of the province where the species was first recorded (see A. levana).
(c). Analysed traits
(i). Traits related to resource use
We classified each species according to habitat niche, using three classes: species from open habitats (grasslands and other open areas; n = 94), species from forest habitats (n = 98) and habitat generalists (n = 90). We classified the larval dietary width into three classes: specialist species, which feed mainly on a single plant species (n = 76); oligophagous species, which feed on few plant species (less than six or restricted to a particular plant genus per family; n = 102); and generalist species, which feed on several different plant species (six or more) or genera (n = 104). Information of habitats and diets was extracted from Emmet [30], Skou [31,32], Svensson [33] and Huldén et al. [34]. When such information was not consistent in the literature, the information stated in Huldén et al. [34] was used because it is based on extensive studies in Finland, adjacent to Sweden. In order to detect whether species are associated with different soil nutrient conditions, we applied the Ellenberg N indicator value [35] of each species's larval diet. This value refers to the association of a nitrogen-favoured diet for each species, from 1 (low) to 8 (high). For oligo- and polyphagous species, we used the mean of Ellenberg N indicator values from their main diets.
(ii). Traits related to dispersal
We classified body size as the wingspan (mm), according to Skou [31,32] and Emmet [30]. We arbitrarily decided to use male size, but because male and female size are strongly correlated [36], this is unlikely to affect our results. We categorized species according to the mean daytime temperature during the adult activity period [26]. Species in which the mean daytime temperature was above 16°C were classified as ‘warm’ species (n = 169), while species active during other periods of the year were classified as ‘cold’ species (n = 113). In the study area, the period for ‘warm’ species normally ranges from 20 July to 10 September [37].
The following taxonomic groups were included: butterflies (n = 18), Geometridae (n = 89), Noctuidae (n = 124) and ‘other macro-moths’ (n = 51; see the electronic supplementary material, table S1).
(iii). Traits related to reproductive capacity
We used the average length of the flight period in weeks in southern Sweden [33] as a proxy for the reproductive potential of each species. This was because reproduction is strongly related to the adult lifespan of a species [22]. For species with two generations, we summed the flight periods. No multivoltine species occur in the study area.
(d). Statistical analyses
In our main approach, we performed a general linear model to analyse northern range expansion (km) in relation to resource-related traits (habitat niche, larval dietary width and nitrogen-favoured diet), dispersal-related traits (body size, temperature during adult activity period and taxonomic group) and reproductive capacity, as independent variables. Potentially, analyses may be biased if there is an over-representation of certain trait states in some of the taxonomic groups. However, trait states were rather evenly distributed among the taxonomic groups (see the electronic supplementary material, tables S1 and S2). To assess the relative strength of support for the models, given the chosen parameters, we used Akaike's information criterion (AIC), including all possible two-way interactions. Models with ΔAIC < 2 in relation to the top model may be considered to have equal strength [38]. Thus, we performed model averaging to circumvent the problem of competing models. This method takes the parameter estimates of all selected models and calculates average estimates, where each model's contribution is proportional to its weight. In the first of the two additional analyses, we performed the analysis in the same way as described above, using the alternative measure of northern range expansion. In the second additional analysis, we performed a multinomial logistic regression, using the same predictor variables of range expansion as in the main approach. In this analysis, the group of species categorized as 'no change’ was used as the comparison group. The statistical analyses were conducted using the package MuMin in the R v. 2.15.1 software environment [39].
3. Results
Of the 282 analysed species, 170 (60.3%) expanded their northern range margin. The mean northern expansion distance was 101 km, corresponding to a yearly expansion rate of 2.7 km (maximum distance 850 km; electronic supplementary material, table S1).
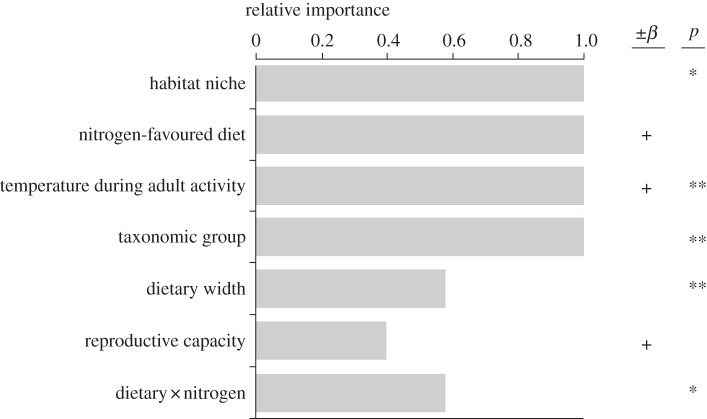
Species traits related to resource use and dispersal were consistently the most important variables predicting range expansion, and were included in the top-ranked models (figure 2; electronic supplementary material, table S3). Interestingly, the interaction term between diet width and a nitrogen-favoured larval diet specifically demonstrates that range expansion is strongly and positively associated with increasing Ellenberg N indicator values of the larval diet in specialist species (figure 3). In oligophagous and generalist species, range expansion was independent of Ellenberg N indicator values of the diet. Further, habitat generalists and species associated with forests expanded their ranges more than species associated with open habitats (87 per cent and 50 per cent, respectively; figure 4a).
Figure 2.
Relative importance of traits for northern range expansion. Bar length is proportional to relative importance values. Plus and minus symbols indicate the sign of the slope (β), and asterisks the significance level: *p < 0.05 and **p < 0.01, in the general linear models.
Figure 3.
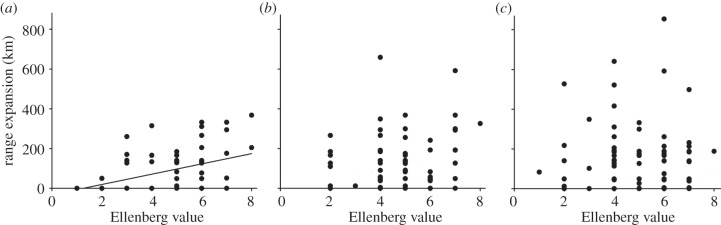
Range expansion is increasing with increased nitrogen-favoured diet, as indicated by a significant interaction in (a) monophagous species, an effect not found in (b) oligophagous or (c) polyphagous species. Linear regressions: monophagous species, p < 0.001; oligophagous species, p = 0.172; and polyphagous species, p = 0.454.
Figure 4.
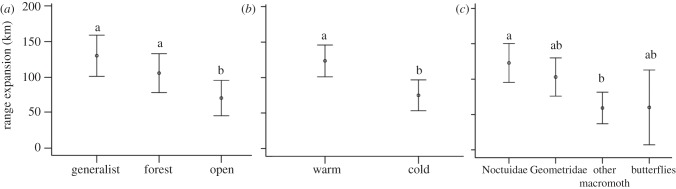
Range expansion in relation to (a) habitat niche, (b) temperature during adult activity period and (c) taxonomic group. Raw mean values and error bars (95% CI) not sharing the same letters are significantly different at p < 0.05.
The dispersal-related trait ‘temperature during adult activity period’ had a relative importance of 1.0 and entered the top-ranked model (figure 2; electronic supplementary material, table S3). Species active during warm conditions expanded their ranges 66 per cent more than species active during cold conditions (figure 4b). Taxonomic group also had a relative importance of 1.0 and entered all top-ranked models. Noctuidae expanded their ranges 105 per cent more than butterflies and ‘other macro-moths’ (figure 4c). Further, reproductive capacity was less important for predicting range expansion, and had a relative importance of 0.40 (figure 2). Range expansion was not related to body size. In the two additional analyses, the results remained qualitatively similar for important variables in the main approach (see the electronic supplementary material, table S3).
4. Discussion
(a). Traits related to resource use
We found that species specialized on nitrogen-favoured diet, habitat generalists and species associated with forests had a more rapid range expansion than species exhibiting other trait states. A rapid range expansion in specialists on nitrogen-favoured diet is in contrast to the general view assuming diet specialists as losers, and diet generalists as winners, because of global change impacts [18,40,41]. This finding is, to our knowledge, the first to show that certain specialist species are favoured by global change over larger scales, and across a species-rich taxon.
Habitat generalists and forest species had a greater range expansion than species associated with open habitats. In fact, forests cover 75 per cent of the land area in Sweden, while open areas cover less than 5 per cent (arable fields excluded) and are decreasing [29]. Thus, habitat generalists and forest species have a lot of habitats available into which they could expand. On the other hand, range expansion is more limited among species associated with open habitats, owing to small and fragmented areas of their habitat [16]. Already in 2004, substantial eutrophication was observed in approximately 50 per cent of habitats within the European Union [20]. Further, our results are consistent with, and supported by, the fact that increased nitrogen deposition has favoured plant species associated with nitrogen-rich habitats [42–45]. Thus, both an increased area of nitrogen-rich habitats and an increased availability of nitrogen-favoured diet appear to directly influence range expansion in Lepidopterans. We therefore suggest that increased availability of nitrogen-rich habitat is one of the most important drivers of range expansions. Indeed, species of nitrogen-poor habitats, having low Ellenberg N indicator values (category 1, n = 5; or category 2, n = 10), showed a very low range expansion (figure 3a; electronic supplementary material, table S1). Our result is in agreement with Angert et al. [13], who called for an inclusion of habitat availability and not only climate data in predictions of range expansions.
(b). Traits related to dispersal and reproductive potential
Species active during warm conditions expanded their ranges more than species active during cold conditions. High temperature minimizes the energy required for movements, shown for different organisms, and is therefore related to increased dispersal capacity [6,26,46]. Range expansion was greater in Noctuidae than in other taxonomic groups. This difference may be explained by a higher dispersal capacity in Noctuidae, possibly because they are more robust [26,47]. However, body size per se had no importance for predicting range expansion. Several studies have indicated larger-sized species to be more mobile than smaller-sized species [22,23,48] (i.e. they should have a greater potential for range expansions). However, other studies do not find any relationship between body size and mobility, and, in fact, some studies suggest that body size might reflect a link to resource use [49,50].
There was a trend that range expansion increased with increasing reproductive capacity. This result is in agreement with Hill et al. [27], who showed a greater range expansion in species with high reproductive capacity. In these species, the larger number of offspring may increase the possibilities for range expansion. Further, because we used the length of flight period as a proxy for reproductive capacity, there is an increased possibility of facing suitable conditions for range expansions during the adult lifespan, which may also contribute to the explanation of this result.
(c). Range expansion
Our estimate of mean northern expansion rate (2.7 km per year) lies well within the range of other expansion estimates. In three global meta-analyses across a large number of taxa, the mean northern expansion rate was 0.6–2.0 km per year [1–3], whereas studies on European butterflies report an expansion of 0.3–5.7 km per year [4,15,51]. Even though our estimate of range expansion is in accordance with other studies, it may be argued that our measure of range expansion may be biased because of the time period chosen and the spatial resolution. First, the study period was chosen because 1973 was the year when high-quality, validated yearly updates of Swedish new provincial records were initiated. Second, it is never possible to detect the most northern population of a species, and it is critical to distinguish range expansion from extra-limital records owing to undocumented populations [52]. Therefore, it is appropriate to use provinces as the resolution of range expansions. Unfortunately, we were not able to analyse declining species, because they are less studied and provincial species losses are unfortunately rarely reported. Hence, it is very difficult to get reliable data on contractions compared with expansions.
5. Conclusions
We highlight that species traits related to resource use and dispersal are important for the prediction of range expansions. Our results suggest that specialists on nitrogen-favoured diet, habitat generalists, forest species and species with adult activity during warm conditions are likely to go farther, and be the winners in future landscapes. We suggest that an increased availability of nitrogen-rich habitat and nitrogen-favoured diet are among the most important drivers for range expansions. Similar processes underlying range expansion are likely to be in action in many organism groups, and constitute an important future line of research.
Acknowledgements
We acknowledge the work of all recorders who collected the data on which this study is based. Thanks to Oliver Schweiger for statistical advice, to two anonymous reviewers for valuable comments on an earlier draft of this manuscript and to Hans Karlsson for permission to use the photo on A. scolopacina. P.-E.B. was funded by Linnaeus University, L.B.P. by Lund University and the Swedish Environmental Protection Agency, N.R. by University of Gävle, and M.F. by FORMAS and the European Commission Framework Programme (FP) 7 via the Integrated Project STEP (grant no. 244090).
References
- 1.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 2.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 12, 450–455 10.1111/j.1365-2486.2006.01116.x (doi:10.1111/j.1365-2486.2006.01116.x) [DOI] [Google Scholar]
- 3.Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 10.1126/science.1206432 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 4.Devictor V, et al. 2012. Differences in the climatic debts of birds and butterflies at a continental scale. Nat. Clim. Change 2, 121–124 10.1038/nclimate1347 (doi:10.1038/nclimate1347) [DOI] [Google Scholar]
- 5.Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH. 2004. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303, 1879–1881 10.1126/science.1095046 (doi:10.1126/science.1095046) [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 7.Huang DC, Haack RA, Zhang RZ. 2011. Does global warming increase establishment rates of invasive alien species? A centurial time series analysis. PLoS ONE 6, e24733. 10.1371/journal.pone.0024733 (doi:10.1371/journal.pone.0024733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devictor V, Julliard R, Couvet D, Jiguet F. 2008. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B 275, 2743–2748 10.1098/rspb.2008.0878 (doi:10.1098/rspb.2008.0878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes L. 2000. Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61 10.1016/S0169-5347(99)01764-4 (doi:10.1016/S0169-5347(99)01764-4) [DOI] [PubMed] [Google Scholar]
- 10.McCarty JP. 2001. Ecological consequences of recent climate change. Conserv. Biol. 15, 320–331 10.1046/j.1523-1739.2001.015002320.x (doi:10.1046/j.1523-1739.2001.015002320.x) [DOI] [Google Scholar]
- 11.Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 12.Hill JK, Collingham YC, Thomas CD, Blakeley DS, Fox R, Moss D, Huntley B. 2001. Impacts of landscape structure on butterfly range expansion. Ecol. Lett. 4, 313–321 10.1046/j.1461-0248.2001.00222.x (doi:10.1046/j.1461-0248.2001.00222.x) [DOI] [Google Scholar]
- 13.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689 10.1038/35102054 (doi:10.1038/35102054) [DOI] [PubMed] [Google Scholar]
- 14.Schweiger O, Heikkinen RK, Harpke A, Hickler T, Klotz S, Kudrna O, Kühn I, Pöyry J, Settele J. 2012. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob. Ecol. Biogeogr. 21, 88–99 10.1111/j.1466-8238.2010.00607.x (doi:10.1111/j.1466-8238.2010.00607.x) [DOI] [Google Scholar]
- 15.Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K. 2009. Species traits explain recent range shifts of Finnish butterflies. Glob. Change Biol. 15, 732–743 10.1111/j.1365-2486.2008.01789.x (doi:10.1111/j.1365-2486.2008.01789.x) [DOI] [Google Scholar]
- 16.Warren MS, et al. 2001. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414, 65–69 10.1038/35102054 (doi:10.1038/35102054) [DOI] [PubMed] [Google Scholar]
- 17.Hill JK, Thomas CD, Fox R, Telfer MG, Willis SG, Asher J, Huntley B. 2002. Responses of butterflies to twentieth century climate warming: implications for future ranges. Proc. R. Soc. Lond. B 269, 2163–2171 10.1098/rspb.2002.2134 (doi:10.1098/rspb.2002.2134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotiaho JS, Kaitala V, Komonen A, Päivinen J. 2005. Predicting the risk of extinction from shared ecological characteristics. Proc. Natl Acad. Sci. USA 102, 1963–1967 10.1073/pnas.0406718102 (doi:10.1073/pnas.0406718102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattila N, Kaitala V, Komonen A, Päivinen J, Kotiaho JS. 2011. Ecological correlates of distribution change and range shift in butterflies. Insect Conserv. Divers. 4, 239–246 10.1111/j.1752-4598.2011.00141.x (doi:10.1111/j.1752-4598.2011.00141.x) [DOI] [Google Scholar]
- 20.EEA 2007. Air pollution in Europe 1990–2004. Copenhagen, Denmark: European Environment Agency [Google Scholar]
- 21.Öckinger E, Hammarstedt O, Nilsson SG, Smith HG. 2006. The relationship between local extinctions of grassland butterflies and increased soil nitrogen levels. Biol. Conserv. 128, 564–573 10.1016/j.biocon.2005.10.024 (doi:10.1016/j.biocon.2005.10.024) [DOI] [Google Scholar]
- 22.Öckinger E, et al. 2010. Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol. Lett. 13, 969–979 10.1111/j.1461-0248.2010.01487.x (doi:10.1111/j.1461-0248.2010.01487.x) [DOI] [PubMed] [Google Scholar]
- 23.Sekar S. 2012. A meta-analysis of the traits affecting dispersal ability in butterflies: can wingspan be used as a proxy? J. Anim. Ecol. 81, 174–184 10.1111/j.1365-2656.2011.01909.x (doi:10.1111/j.1365-2656.2011.01909.x) [DOI] [PubMed] [Google Scholar]
- 24.Summerville KS, Conoan CJ, Steichen RM. 2006. Species traits as predictors of lepidopteran composition in restored and remnant tallgrass prairies. Ecol. Appl. 16, 891–900 10.1890/1051-0761(2006)016[0891:STAPOL]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[0891:STAPOL]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 25.Sparks TH, Dennis RLH, Croxton PJ, Cade M. 2007. Increased migration of Lepidoptera linked to climate change. Eur. J. Entomol. 104, 139–143 [Google Scholar]
- 26.Betzholtz P-E, Franzén M. 2011. Mobility is related to species traits in noctuid moths. Ecol. Entomol. 36, 369–376 10.1111/j.1365-2311.2011.01281.x (doi:10.1111/j.1365-2311.2011.01281.x) [DOI] [Google Scholar]
- 27.Hill JK, Griffiths HM, Thomas CD. 2011. Climate change and evolutionary adaptations at species’ range margins. Annu. Rev. Entomol. 56, 143–159 10.1146/annurev-ento-120709-144746 (doi:10.1146/annurev-ento-120709-144746) [DOI] [PubMed] [Google Scholar]
- 28.Karsholt O, Razowski J. 1996. The Lepidoptera of Europe: a distributional checklist. Stenstrup, Denmark: Apollo Books [Google Scholar]
- 29.SNA. 1996. The national atlas of Sweden. See http://www.sna.se/ .
- 30.Emmet AM. 1991. Life history and habits of the British Lepidoptera. In The moths and butterflies of Great Britain and Ireland, vol. 7, part 2 (eds Emmet AM, Heath J.), pp. 61–203 Colchester, UK: Harley Books [Google Scholar]
- 31.Skou P. 1991. Nordens ugler: håndbog over de i Danmark, Norge, Sverige, Finland og Island forekommende arter af Herminiidae og Noctuidae (Lepidoptera). Stenstrup, Denmark: Apollo Books [Google Scholar]
- 32.Skou P. 1984. Nordens målere: håndbog over de danske og fennoskandiske arter af Drepanidae og Geometridae (Lepidoptera). Copenhagen, Denmark: Fauna Bøger & Apollo Bøger [Google Scholar]
- 33.Svensson I. 1993. Fjärilskalender. Kristianstad, Sweden: Self-published [Google Scholar]
- 34.Huldén L, Albrecht A, Itämies J, Malinen P, Wettenhovi J. 2000. Atlas of Finnish macrolepidoptera. Helsingfors, Finland: Lepidopterologiska sällskapet i Finland [Google Scholar]
- 35.Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D. 1991. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 18, 9–166 [Google Scholar]
- 36.Komonen A, Grapputo A, Kaitala V, Kotiaho JS, Päivinen J. 2004. The role of niche breadth, resource availability and range position on the life history of butterflies. Oikos 105, 41–54 10.1111/j.0030-1299.2004.12958.x (doi:10.1111/j.0030-1299.2004.12958.x) [DOI] [Google Scholar]
- 37.Alexandersson H. 2002. Temperatur och nederbörd i Sverige 1860–2001. Meteorologi 104. Norrköping, Sweden: SMHI [Google Scholar]
- 38.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: A practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 39.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 40.Steffan-Dewenter I, Tscharntke T. 2000. Butterfly community structure in fragmented habitats. Ecol. Lett. 3, 449–456 10.1111/j.1461-0248.2000.00175.x (doi:10.1111/j.1461-0248.2000.00175.x) [DOI] [Google Scholar]
- 41.Nilsson SG, Franzén M, Jonsson E. 2008. Long-term land-use changes and extinction of specialised butterflies. Insect Conserv. Divers. 1, 197–207 10.1111/j.1752-4598.2008.00027.x (doi:10.1111/j.1752-4598.2008.00027.x) [DOI] [Google Scholar]
- 42.Stevens CJ, Dise NB, Mountford JO, Gowing DJ. 2004. Impact of nitrogen deposition on the species richness of grasslands. Science 303, 1876–1879 10.1126/science.1094678 (doi:10.1126/science.1094678) [DOI] [PubMed] [Google Scholar]
- 43.McClean CJ, van den Berg LJL, Ashmore MR, Preston CD. 2011. Atmospheric nitrogen deposition explains patterns of plant species loss. Glob. Change Biol. 17, 2882–2892 10.1111/j.1365-2486.2011.02462.x (doi:10.1111/j.1365-2486.2011.02462.x) [DOI] [Google Scholar]
- 44.Pierik M, van Ruijven J, Bezemer TM, Geerts RHEM, Berendse F. 2011. Recovery of plant species richness during long-term fertilization of a species-rich grassland. Ecology 92, 1393–1398 10.1890/10-0210.1 (doi:10.1890/10-0210.1) [DOI] [PubMed] [Google Scholar]
- 45.Wallis de Vries MF, van Swaay CAM, Plate CL. 2012. Changes in nectar supply: a possible cause of widespread butterfly decline. Curr. Zool. 58, 384–391 [Google Scholar]
- 46.Hoegh-Guldberg O. 2005. Marine ecosystems and climate change. In Climate change and biodiversity (eds Lovejoy TE, Hannah L.), pp. 256–271 New Haven, CT: Yale University Press [Google Scholar]
- 47.Kristensen NP, Scoble MJ, Karsholt O. 2007. Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity. Zootaxa 1688, 699–747 [Google Scholar]
- 48.Nieminen M, Rita H, Uuvana P. 1999. Body size and migration rate in moths. Ecography 22, 697–707 10.1111/j.1600-0587.1999.tb00519.x (doi:10.1111/j.1600-0587.1999.tb00519.x) [DOI] [Google Scholar]
- 49.Loder N, Gaston KJ, Warren PH, Arnold HR. 1998. Body size and feeding specificity: macrolepidoptera in Britain. Biol. J. Linn. Soc. 63, 121–139 10.1111/j.1095-8312.1998.tb01642.x (doi:10.1111/j.1095-8312.1998.tb01642.x) [DOI] [PubMed] [Google Scholar]
- 50.Franzén M, Schweiger O, Betzholtz P-E. 2012. Species-area relationships are controlled by species traits. PLoS ONE 7, e37359. 10.1371/journal.pone.0037359 (doi:10.1371/journal.pone.0037359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parmesan C, et al. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583 10.1038/21181 (doi:10.1038/21181) [DOI] [Google Scholar]
- 52.Frey JK. 2009. Distinguishing range expansions from previously undocumented populations using background data from museum records. Divers. Distrib. 15, 183–187 10.1111/j.1472-4642.2008.00552.x (doi:10.1111/j.1472-4642.2008.00552.x) [DOI] [Google Scholar]