Abstract
Although many species form socially monogamous pair bonds, relevant neural mechanisms have been described for only a single species, the prairie vole (Microtus ochrogaster). In this species, pair bonding is strongly dependent upon the nonapeptides oxytocin (OT) and vasopressin, in females and males, respectively. Because monogamy has evolved many times in multiple lineages, data from additional species are required to determine whether similar peptide mechanisms modulate bonding when monogamy evolves independently. Here we test the hypothesis that OT-like receptor activation is required for pair bond formation in the socially monogamous zebra finch (Taeniopygia guttata). Males and females were administered chronic intracerebroventricular infusions of saline or an OT receptor antagonist and were observed twice daily for 3 days in a colony environment. A variety of affiliative, aggressive and other behaviours were quantified. The antagonist produced significant and selective effects on pair bonding (latency to pair; number of sessions paired; stable pairing) and the associated behaviour of allopreening. Importantly, findings for males follow the trends of females; this yields main effects of treatment in two-way ANOVAs, although within-sex analyses are significant only for females. These data provide evidence for both convergent evolution and species diversity in the neuroendocrine mechanisms of pair bonding.
Keywords: monogamy, pair bond, oxytocin, finch, mesotocin, VT3
1. Introduction
Social monogamy has independently evolved in many vertebrate taxa, including fish [1], birds [2], mammals [3] and even invertebrates [4]. However, the underlying neural mechanisms are well known for only a single species of vole [5], and thus it remains to be determined whether the independent evolution of monogamy in different species is most often the result of selection on different physiological mechanisms or whether neural circuits that play conserved roles in affiliation may tend to evolve in parallel ways to support monogamous behaviour.
The nine amino acid neuropeptides (nonapeptides), such as oxytocin and vasopressin (OT and VP, respectively), are good candidates as modulators of pair bonding because they exert widespread influences on affiliative behaviours across a variety of vertebrate classes, in addition to modulating many aspects of physiology, including vasoconstriction, hydromineral balance and smooth muscle contractions (reviewed in [6,7]). All vertebrate nonapeptides are evolutionarily derived from arginine vasotocin (VT). The VT gene is thought to have duplicated in early fishes [8], giving rise to a clade of OT-like peptides and VT (in non-mammalian taxa) and either lysine or arginine VP in mammals. The OT clade includes isotocin (e.g. fish) and mesotocin (MT; Ile8-OT; e.g. amphibians, reptiles, birds and some non-eutherian mammals) [9]. Both the structure and functions of these peptides are highly conserved across vertebrates, with only one amino acid residue differing between VT and OT [10].
Despite the broad effects of nonapeptides on affiliation, evidence that these peptides act within the brain to promote pair bonding is limited to the prairie vole (Microtus ochrogaster) [11–13]. Copulation is typically required for pair bond formation in this species, although intracerebroventricular (i.c.v.) infusion of OT or VP facilitates pair bond formation in the absence of mating [12,13], and does so in both males and females [11]. Nonetheless, antagonist experiments demonstrate that endogenous OT primarily mediates pair bonding in females via actions at OT receptors (OTRs) in the nucleus accumbens (NAcc) [14], whereas endogenous VP primarily mediates pair bonding in males via actions at V1a receptors (V1aRs) in the ventral pallidum [15], and also via V1aRs and OTRs in the lateral septum (LS) [16]. Notably, the closely related montane vole (Microtus montanus), a promiscuous species that does not form pair bonds, exhibits significantly lower levels of OTR expression in the NAcc [17] and lower V1aR expression in the ventral pallidum [18]. Experimental overexpression of V1aR in the ventral pallidum of typically polygamous meadow voles (Microtus pennsylvanicus) through the use of viral vectors promotes the formation of a partner preference [19]. This ability to induce a change in partner preference behaviour suggests that even small modifications to nonapeptide systems may have profound effects on mating systems.
Importantly, given that monogamy has evolved independently many times and in multiple vertebrate groups, the predictive value of results obtained from a single species may be limited. Indeed, while the role of OT and VP in prairie vole pair bonding is clear, evidence from other species has thus far been negative. For instance, in a monogamous cichlid (Amatitlania nigrofasciata), administrations of a nonapeptide antagonist initially reduce affiliative behaviour towards a potential partner and decrease aggression towards neighbours, but do not prevent the formation of pair bonds, and antagonist treatment has no effect on affiliation or mate guarding in established pairs [20]. Similarly, administrations of OT and OT receptor antagonist (OTA) to marmosets (Callithrix penicillata) produce significant effects on partner-directed affiliative behaviours, but have no effect on the ability to form a pair bond or on subsequent partner preference [21]. A recent study of eight Peromyscus mouse species further shows that the monogamous species lack multiple V1aR characteristics that distinguish prairie voles from their non-monogamous congeners [22], and chronic central infusions of a VP V1 antagonist cocktail do not impair pair bond formation in male zebra finches [23]. However, VT may influence finch behaviour not only via V1a-like receptors, but also by binding to the oxytocic VT3 receptor, as known for VP actions at septal OTRs in prairie voles [16]. The VT3 receptor appears to bind both VT and MT in songbirds [24], and is thus a good candidate as a mediator of pair bonding.
Oxytocic systems (isotocin, MT and OT) influence numerous affiliation processes in vertebrates that could be co-opted during the evolution of monogamy. For instance, isotocin promotes social approach in goldfish (Carassius auratus) [25] and administration of a non-selective nonapeptide antagonist reduces intersexual affiliation in the convict cichlid [20]. In zebra finches, flocking behaviour and preferences for familiar social partners are reduced, following administration of an OTA [26] that probably binds to the oxytocic VT3 receptor [27]. Furthermore, variation in affiliative behaviour between pairs in tamarins (Saguinus oedipus) is correlated with variation in urinary OT levels [28]. The hypothesis that pre-existing peptide functions provide a framework for the evolution of monogamy is strongly suggested by findings in prairie voles: whereas endogenous OT does not mediate pair bonding in males, exogenous OT can nonetheless induce pair bond formation, suggesting the presence of neural mechanisms that can be co-opted during the evolution of monogamy.
Here we test the hypothesis that central signalling at oxytocic receptors is essential for pair bonding in the socially monogamous zebra finch. Pair bond formation in zebra finches is readily quantified based on the display of selective affiliation between partners such as side-by-side perching (‘clumping’), following, allopreening and co-occupation of a nest cup. Consistent with this hypothesis, recent findings in zebra finches demonstrate that peripheral injections of OTA impair pair bonding and male courtship [29]. However, nonapeptides may influence many behavioural processes by acting in the periphery, and indeed we have found that peripheral injections of OTA virtually abolish nesting behaviour in female zebra finches (replicated three times and at different doses), whereas intraventricular infusions produce absolutely no effect (J.D.K. & J.L.G. 2011, unpublished data), as also shown in the present study. Similarly, it is possible that many or most effects of exogenous OT on human cognition and behaviour are mediated by peripheral feedback rather than direct action in the brain [30]. Thus, because i.c.v. infusions of OTA do not influence courtship song in male zebra finches [31], as is observed with peripheral injections [29], we predicted that chronic infusions of OTA into the brain would impair pair bonding in male and female zebra finches housed in a colony environment, but would not reduce song. These two predictions are supported, suggesting that oxytocic receptors mediate zebra finch behaviour by acting in both the brain (in the case of bonding) and the periphery (in the case of song).
2. Material and methods
(a). Animals and housing
Prior to testing, subjects were housed in a cage 1 m wide (43 cm height × 36 cm diameter) with same-sex conspecifics, and provided ad libitum access to food and water on a 14 L : 10 D photoperiod with lights on at 07.00. All procedures were conducted in a humane manner and in compliance with federal and institutional guidelines.
(b). Surgery
Subjects (35 males and 26 females) were stereotaxically implanted with a guide cannula directed at the caudal aspect of the right lateral ventricle. Individuals were anaesthetized with isoflurane vapour delivered at 1.5 to 3.5 per cent of a compressed air flow. A single 26-gauge guide cannula (Plastics One, Roanoke, VA) with a 4.6 mm extension beyond the pedestal was inserted 3.1 mm rostral, 1.7 mm right lateral and 2.6 mm deep from the anterior pole of the cerebellum at a 21° angle towards medial. The guide cannula was adhered to the skull with dental cement (Stoelting Co., Wood Dale, IL) and veterinary-grade cyanoacrylate glue (3M, Saint Paul, MN). Injection cannulae projected 1 mm beyond the guide cannula. Following surgery, subjects were returned to their home cage and given a minimum of 5 days recovery.
We initiated twice-daily infusions of either 0.5 µl saline vehicle or vehicle containing 250 ng of a highly selective OTA (desGly-NH2,d(CH2)5[Tyr(Me)2,Thr4] ornithine VT) [32], as in previous studies [26]. Testing was conducted in a between-subjects design such that each individual received only one of the treatments. The first round of infusions started at 17.00 on the day before subjects were transferred to the colony testing cages. Subsequent infusion rounds were started at 08.00 (lights-on) and 17.00 daily. Each round of infusions required approximately 30 min, and behavioural observations were conducted in the order of infusion.
(c). Behavioural observations
After AM infusions on the second day, four focal-sex birds (one to two subjects per condition plus one to two uncannulated stimulus birds) and five opposite-sex stimulus birds were placed in a colony testing cage (1.2 m width × 40 cm height × 36 cm diameter). A total of 21 testing cages were established, 18 of which contained subjects with accurate i.c.v. placements (eight female and 10 testing cages). Nine of these cages contained a single i.c.v. subject: five contained one subject from each condition; four cages were imbalanced across treatments (e.g. two control and one OTA).
Colony testing cages contained four plastic nesting cups (one in each of the four corners of the cage). Food, water dishes and nesting material were placed in the centre of the cage floor. Subjects were individually observed for 10 min following morning infusions and just prior to afternoon infusions for a total of six observation periods over 3 days. Observations were made from behind a curtain blind and dictated vocally into a digital recorder for later transcription.
Affiliative behaviours recorded were greet, follow, directed song, undirected song and allopreen. We additionally recorded the amount of time spent in nest cups, the number of nest items (burlap strings) picked up and the number of nest items carried to the nest. Pair bond status was determined based on selective affiliation—primarily following the partner, clumping, allopreening and co-occupation of a nest cup, as in previous studies [23]. Aggressive behaviours recorded were peck, beak fence, threaten and displace. The sex of the individual receiving an aggressive behaviour was recorded to allow separate analyses according to sex. Finally, we recorded other behaviours such as beak wipe, eat, drink and preen [23,33].
(d). Histology
Subjects were infused with 0.5 μl of India ink, euthanized with isoflurane and perfused with 0.1 M phosphate-buffered saline followed by 10 per cent formalin. Brains were post-fixed overnight, sunk in 30 per cent sucrose and sectioned on a cryostat at a thickness of 100 μm. Cannula placements were verified by the presence of ink in the ventricle, as well as the location of the cannula tract in the tissue.
(e). Statistics
Statistical analyses were performed using Statview v. 5.0. With the exception of pair bond status, all data are presented as units of behaviour per minute of time off the nest [23,33]. Aggression data for session 1 and sessions 2–6 were first analysed separately, because although aggression is initially focused on mate acquisition, it is later associated with nest defence, and previous data show that aggression is modulated differentially across these contexts [23]. However, based on the lack of significant effects, all sessions are pooled for the aggression analyses reported here. χ2-tests were performed to determine if an individual was stably paired at the end of testing, defined as being paired to the same individual for at least two sessions, and for the presence or absence of allopreening and following. Other behavioural data were analysed by ANOVA, with sex and treatment as between-subjects variables. This allows direct comparisons of the sexes, but because we obtained main effects of treatment in our pairing analyses that appeared to be driven primarily by females, we additionally conducted within-sex t-tests in order to establish the relative strength of OTA effects in males and females.
3. Results
(a). Cannula locations
A total of 16 males and 14 females exhibited i.c.v. cannula placements. Placements in seven additional birds penetrated the septum, but most of these extended to the midline or even further, creating heavy damage (precluding statistical analysis). An additional 11 males (five control and six OTA) and six females (one control and five OTA) exhibited cannula placements that fell slightly short of the lateral ventricle and were thus located in the medial telencephalon, primarily in the striatum. The latter birds were included in our analyses, as described below.
(b). Pairing behaviour
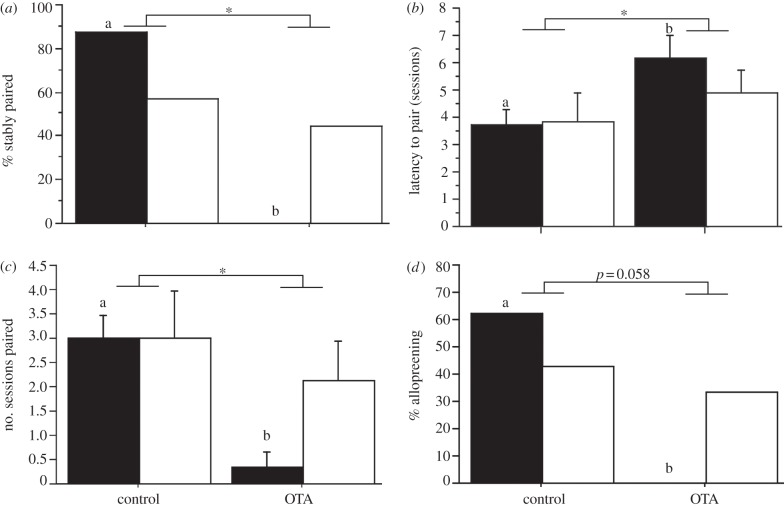
Analyses of i.c.v. data show that subjects administered OTA were less likely to be stably paired (i.e. for two continuous sessions) at the end of testing (χ2 = 6.533, p = 0.011; figure 1a). In addition, OTA subjects were paired for fewer sessions (F1,1,26 = 5.671, p = 0.025; figure 1b) and exhibited a longer latency to pair (F1,1,26 = 4.356, p = 0.047; figure 1c). No significant sex × treatment interactions were observed, although there were trends for larger treatment effects in females. Thus, in order to assess within-sex effects, we conducted t-tests separately for males and females, and found that OTA-treated females were less likely to be stably paired (p = 0.001), paired for fewer sessions (p < 0.001) and exhibited a longer latency to pair (p = 0.024) than control females, whereas no such effects were observed in males (p = 0.614, 0.500 and 0.442, respectively).
Figure 1.
Administration of oxytocin receptor antagonist (OTA) to male and female zebra finches significantly (a) decreases the percentage of pair-bonded individuals that are stably paired (i.e. for two continuous sessions at the end of testing), (b) increases latency to pair, (c) decreases number of sessions paired and (d) decreases the percentage of individuals allopreening. n = 8 control females, 6 treatment females, 7 control males and 9 treatment males; *p < 0.05; χ2 analysis in (a) and (d) with sexes pooled and mixed-model ANOVA in (b) and (c). Significant within-sex comparisons are indicated by different letters above the bars. Black bars denote female, open bars denote male.
Because much of the behaviour that is used to assess pairing status actually occurs within the nest cups (and sharing a nest cup is itself a prime indicator of pairing), we often cannot see into the nest well enough for behavioural quantification, particularly once the dome of the nest is constructed (sometimes by the second observation session). We therefore exclude behaviours during nest time from our analyses, and because this results in low counts for bouts of allopreening, we analysed this behaviour based on presence/absence using χ2 analyses. A near-significant reduction is observed in OTA-treated subjects when the sexes are pooled (χ2 = 3.589, p = 0.058), but as with the other measures of pairing behaviour, separate analyses for each sex yield significant results for females only (figure 1d). Thus, 62.5 per cent of control females engaged in allopreening outside of the nest, whereas 0 per cent of OTA females were observed allopreening with a partner (χ2 = 5.833, p = 0.015). The values for males were 42.9 per cent and 33.3 per cent, respectively (χ2 = 0.152, p = 0.696). The behaviour of ‘following’, which is more commonly observed in males, was exhibited by a comparable percentage of OTA and control subjects, regardless of whether the sexes were pooled or analysed separately (all p > 0.700).
Notably, pairing behaviour following telencephalic infusions of OTA does not differ significantly from that of control subjects that were given i.c.v. infusions of saline, but does differ significantly from subjects that were given i.c.v. infusions of OTA. Thus, relative to OTA subjects that were infused into the telencephalon, the i.c.v. OTA subjects showed a decrease in sessions paired (F1,1,22 = 4.956, p = 0.035) and an increase in the latency to pair (F1,1,22 = 5.042, p = 0.034), and were less likely to be stably paired at the end of testing (χ2 = 7.543, p = 0.006). However, no significant differences were observed for allopreening outside of the nest (χ2 = 0.864, p = 0.352).
Given this pattern of results, we replicated our analyses with an expanded pool of ‘control’ subjects that included i.c.v. control subjects, as well as all subjects with telencephalic placements (both saline- and OTA-treated subjects). The pattern of results is virtually identical to that obtained in comparisons of i.c.v. control and i.c.v OTA subjects, although these analyses were of relatively higher power owing to the larger number of subjects. Thus, relative to the expanded pool of control subjects, the i.c.v. OTA subjects showed a decrease in sessions paired, an increase in the latency to pair and were less likely to be stably paired at the end of testing (all p < 0.01). However, within-sex analyses again show significant effects in females (sessions paired, latency to pair and stably paired; all p < 0.01) but not in males (all p > 0.08). Seventy-nine per cent of control females were stably paired at the end of testing, whereas 0 per cent of the i.c.v. OTA females were paired. Results for males were 72 per cent and 44 per cent, respectively. In a mixed-sex analysis, allopreening was not significantly altered by treatment (χ2 = 0.079, p = 0.778). However, OTA-treated females exhibited a near-significant deficit (χ2 = 3.673, p = 0.055), whereas OTA-treated males did not (χ2 = 1.935, p = 0.164).
(c). Aggression, courtship and other behaviours
Results for aggression, courtship, feeding and other behaviours are shown in table 1 for subjects with i.c.v. cannula placements. In addition to the analyses for all sessions combined, aggression data were analysed separately for sessions 1 and sessions 2–6, and separately by the sex of the bird receiving aggression, but no significant effects on aggression are observed in these analyses (all p > 0.20). For simplicity, table 1 presents data for all aggressive behaviours in a pooled analysis, with both sexes in the model. No significant within-sex effects were observed (all p > 0.05).
Table 1.
Treatment and sex × treatment effects of OTA for non-pairing behaviours in subjects with i.c.v. cannula placements (all p > 0.05).
| behaviour | control female (mean ± s.e.m.) | control male (mean ± s.e.m.) | OTA female (mean ± s.e.m.) | OTA male (mean ± s.e.m.) | treatment |
sex × treatment |
||
|---|---|---|---|---|---|---|---|---|
| F1,1,26 | p-value | F1,1,26 | p-value | |||||
| total same-sex aggression | 0.54 ± 0.22 | 1.73 ± 0.76 | 0.202 ± 0.10 | 1.31 ± 0.48 | 0.623 | 0.437 | 0.006 | 0.938 |
| total aggression | 0.91 ± 0.24 | 2.37 ± 0.91 | 0.421 ± 0.21 | 1.95 ± 0.60 | 0.611 | 0.442 | 0.003 | 0.960 |
| directed song (males only) | — | 0.34 ± 0.09 | — | 0.80 ± 0.12 | 2.132 | 0.156 | — | — |
| undirected song (males only) | — | 0.01 ± 0.01 | — | 0.08 ± 0.04 | 1.691 | 0.205 | — | — |
| greet | 0.03 ± 0.03 | 0.02 ± 0.01 | 0.004 ± 0.004 | 0.02 ± 0.01 | 0.956 | 0.337 | 0.857 | 0.363 |
| beak wipe | 0.21 ± 0.09 | 0.30 ± 0.10 | 0.10 ± 0.05 | 0.23 ± 0.04 | 1.411 | 0.246 | 0.073 | 0.790 |
| feeding movements | 2.22 ± 0.51 | 1.16 ± 0.48 | 1.50 ± 0.37 | 1.19 ± 0.25 | 0.693 | 0.413 | 0.844 | 0.367 |
| drinks | 0.06 ± 0.02 | 0.07 ± 0.04 | 0.07 ± 0.03 | 0.05 ± 0.01 | 0.036 | 0.851 | 0.501 | 0.485 |
| preen | 0.09 ± 0.02 | 0.03 ± 0.01 | 0.10 ± 0.02 | 0.03 ± 0.01 | 0.090 | 0.766 | 0.031 | 0.861 |
| time in nest (s) | 808.9 ± 200.6 | 354.6 ± 136.2 | 469.5 ± 190.2 | 911.4 ± 278.1 | 0.233 | 0.634 | 3.952 | 0.058 |
Similarly, mixed-sex comparisons between the i.c.v. OTA subjects and the expanded control group (i.e. including cannula placements in the telencephalon) yield no significant results. No significant within-sex effects were observed for the listed behaviours, with the exception of directed song, which tended to be facilitated by OTA treatment (p = 0.047). However, one OTA subject fell 5.7 s.d. above the mean, and following the exclusion of this subject the effect was no longer significant (p = 0.075). Finally, OTA treatment produced a near-significant reduction in aggression in females (p = 0.054).
4. Discussion
The present study demonstrates that chronic OTA administrations reduce pair bonding in a colony environment while leaving other behaviours unaffected. Within-sex analyses demonstrate sex-specific effects only for females, however, because no significant sex × treatment interactions are obtained, and because males exhibit weak trends in the direction of females, we cannot conclude that oxytocic receptors are completely irrelevant to male pairing behaviour. These findings are only partially consistent with the effects of peripheral OTA administration, which produce deficits in both pair bonding and male courtship [29], suggesting that peripheral injections may influence behaviour via both central and peripheral mechanisms. Indeed, OTA-treated males in the present study tended to sing more courtship songs than controls, and previous experiments likewise show that central administrations of OTA and MT produce no significant effects on male song [31]. The present effects are also partially consistent with known mechanisms of pair bonding in prairie voles, although there are probably substantial differences in the sites of action, which will be discussed below.
Because autoradiographic data show that the distribution of OTA binding sites in birds is virtually identical to that of VT3 mRNA expression [26,27,34], it is likely that the OTA effects observed here are mediated via the oxytocic VT3 receptor. Notably, competitive binding experiments show that MT and VT have a comparable ability to displace radiolabelled OTA [34], and, similarly, mammalian nonapeptides are also somewhat promiscuous in their receptor binding. Despite this promiscuity, OTA effects on zebra finch social preferences are reversed with administration of MT, but not VT [26], perhaps owing to differential binding of VT and MT to the three nonapeptide receptor types present in the brain.
In female prairie voles, pair bonding is strongly dependent upon OT binding at OTRs in the NAcc [14], whereas bonding in males is dependent upon VP activation of V1aRs in the ventral pallidum and the LS [15,16]. In both cases, local or i.c.v. infusions of peptide induce pair bonding in the absence of mating [11,14,16]. In contrast, acute i.c.v. infusions of VT, MT, OTA or a V1a antagonist in zebra finches do not influence partner preference after a single night of cohabitation [31]. However, these studies differ from the vole studies in numerous important respects, including the use of acute rather than chronic infusions. In addition, the partner preference assay that was employed (a simple two-choice test) may not be particularly sensitive in the highly gregarious zebra finch, given that these birds naturally affiliate with many individuals. Thus, in the present experiments, we employed chronic OTA infusions and directly assessed pair bond formation, rather than employing a partner preference assay as a proxy measure.
The design of this experiment is modelled on that of a previous study in which colony-housed male zebra finches were given chronic i.c.v. infusions of a V1a antagonist, which produced no effect on pair bonding [23]. However, nonapeptides may potentially influence pair bonding via actions at multiple other receptor types, including the VT1 receptor (a V2b receptor [35]), which is distributed in numerous brain areas [27], and the OT-like VT3 receptor. Because the VT3 receptor appears to be promiscuous [34], the OTA effects observed in the present study may reflect the actions of endogenous MT, VT or both.
Although behavioural effects of OTA administration in zebra finches are consistent with those in female prairie voles, the sites of action are probably somewhat different. As described above, pair bonding in female prairie voles is critically dependent on OTR activation in the NAcc [14], but zebra finches have no detectable VT3 expression in this region [27]. Zebra finches also exhibit no VT4 (V1aR) expression in the ventral pallidum [27], the site most extensively studied in relation to pair bonding in male voles. However, VP promotes pair bond formation in male prairie voles by binding to both OTRs and V1aRs in the LS [16], and the zebra finch VT3 receptor is heavily expressed in the LS [26,27,34]. Administration of either OTA or a V1a antagonist into the LS blocks pair bond formation in male prairie voles, whereas administration of VP induces pair bond formation in the absence of mating [16]. Importantly, the effect of exogenous VP is blocked by OTA administration [16], demonstrating that VP promotes pair bonding at least in part via the OTR. Thus, in combination with the known promiscuity of the VT3, these vole findings suggest that VT and/or MT may promote pair bonding in zebra finches via VT3 receptors in the LS.
In addition to influencing pair bond formation, nonapeptide receptors in the LS promote social investigation and paternal care in prairie voles [26], maternal care in mice [36], and preferences for familiar individuals and larger groups in zebra finches [26,37]. The densities of oxytocic-binding densities are also significantly higher in the LS of gregarious finch species relative to territorial species, particularly in the dorsal LS [26]. Perhaps directly relevant to pair bonding are the consistent findings that septal nonapeptide receptors mediate social discrimination and recognition, as demonstrated in numerous studies in rodents. VP V1aR knockout mice fail to reduce their chemoinvestigation of a familiar individual that is presented repeatedly in a habituation–disinhibition paradigm, and fail to increase investigation upon introduction of a novel animal [38]. This deficit is reversed by upregulation of V1aR in the LS using a viral vector [38]. OT administration into the LS also facilitates social recognition in rats [39], whereas knockout mice lacking OT or OTR expression are profoundly impaired [40,41].
Thus, given (i) the numerous social functions of septal nonapeptide receptors, (ii) the species-specific distributions of OTA-binding sites (and greater density in gregarious birds), (iii) the close proximity of the LS to the ventricular infusion site in the present study, and (iv) the essential role of septal nonapeptide receptors in social recognition, we hypothesize that i.c.v. OTA infusions compromise social recognition capabilities by binding to VT3 receptors in the LS, and thereby reduce the ability to form a stable bond.
The LS is only one part of the known social recognition circuitry and is downstream of another potentially viable target of OTA, the medial amygdala. OT administration into the medial amygdala of OT knockout mice rescues social recognition, and, similarly, OTA injections into the medial amygdala of wild-type mice prevent the typical reduction in olfactory investigation with familiar social partners [42]. This effect is not due to general memory impairments, as no deficits are observed in tests of non-social memory [40]. Because the medial amygdala in birds (formerly nucleus taeniae [43,44]) exhibits at least a modest level of OTA binding [34], the amygdala could be another site of action that by itself, or together with the LS, mediates the effects of OTA observed here.
Although the present experiment demonstrates that endogenous activation of oxytocic receptors is necessary for pair bonding in zebra finches, it remains to be determined whether activation of these receptors is sufficient to induce bonding. If the present results reflect an impact on broader social functions, such as social recognition, rather than effects on bonding-specific processes, peptide actions are likely to be necessary but not sufficient to induce pairing. Unfortunately, it may be very difficult to detect the facilitation of pair bond formation by administrations of MT or VT, because zebra finches tend to pair bond rapidly (sometimes immediately upon introduction), and the bond is not mating-induced, as in prairie voles. The mean latency to pair bond in the present study for control individuals (figure 1) is only slightly higher than 24 h, and numerous subjects paired well before that.
Finally, because monogamy has evolved independently in numerous vertebrate taxa, generalizations about relevant mechanisms cannot be made until experiments are conducted in a much larger sample of species. Indeed, although nonapeptides modulate affiliation in virtually every species that has been studied, nonapeptide effects on pair bonding have been demonstrated only in prairie voles and zebra finches [5,29] (present study). For instance, despite significant effects on other aspects of affiliation, neither OT nor OTA administrations influence the establishment of pair bonds or subsequent partner preference in marmosets [21]. Similarly, injections of a nonapeptide receptor antagonist reduce affiliative behaviours during pair bond formation in the monogamous convict cichlid, but do not impair the ability to form a pair bond [20]. Thus, nonapeptides modulate pair bonding in only two of the four species in which relevant experiments have been performed, suggesting caution in the extension of findings from any given study to additional species.
In conclusion, the present experiment demonstrates that endogenous activation of oxytocic receptors in the brain is necessary for the natural formation of pair bonds in zebra finches, as has previously been shown in prairie voles. This similarity across taxa suggests two hypotheses, which are not mutually exclusive. First, OTRs are necessary for pair bonding owing to conserved effects on broader processes such as social recognition, which may be mediated by conserved projections to the medial amygdala and LS. Alternatively, different circuitries may have evolved to support pair bonding in voles and zebra finches. However, it is important to note that the distributions of nonapeptide cell bodies and axonal projections are very similar in birds and mammals, suggesting that common mechanisms may have evolved to support pair bonding in both taxa.
References
- 1.Whiteman EA, Côté IM. 2004. Monogamy in marine fishes. Biol. Rev. 79, 351–375 10.1017/S1464793103006304 (doi:10.1017/S1464793103006304) [DOI] [PubMed] [Google Scholar]
- 2.Adkins-Regan E, Tomaszycki M. 2007. Monogamy on the fast track. Biol. Lett. 3, 617–619 10.1098/rsbl.2007.0388 (doi:10.1098/rsbl.2007.0388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clutton-Brock TH. 1989. Review lecture: mammalian mating systems. Proc. R. Soc. Lond. B 236, 339–372 10.1098/rspb.1989.0027 (doi:10.1098/rspb.1989.0027) [DOI] [PubMed] [Google Scholar]
- 4.Wittenberger JF, Tilson RL. 1980. The evolution of monogamy: hypotheses and evidence. Annu. Rev. Ecol. Syst. 11, 197–232 10.1146/annurev.es.11.110180.001213 (doi:10.1146/annurev.es.11.110180.001213) [DOI] [Google Scholar]
- 5.Lim MM, Young LJ. 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 50, 506–517 10.1016/j.yhbeh.2006.06.028 (doi:10.1016/j.yhbeh.2006.06.028) [DOI] [PubMed] [Google Scholar]
- 6.Goodson JL, Thompson RR. 2010. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 20, 784–794 10.1016/j.conb.2010.08.020 (doi:10.1016/j.conb.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 7.Insel TR. 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–779 10.1016/j.neuron.2010.03.005 (doi:10.1016/j.neuron.2010.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acher R, Chauvet J, Chauvet MT. 1995. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 395, 615–627 [PubMed] [Google Scholar]
- 9.Goodson JL. 2008. Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 170, 3–15 10.1016/S0079-6123(08)00401-9 (doi:10.1016/S0079-6123(08)00401-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acher R, Chauvet J. 1988. Structure, processing and evolution of the neurohypophysial hormone–neurophysin precursors. Biochimie 70, 1197–1207 10.1016/0300-9084(88)90185-X (doi:10.1016/0300-9084(88)90185-X) [DOI] [PubMed] [Google Scholar]
- 11.Cho MM, DeVries AC, Williams JR, Carter CS. 1999. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 113, 1071–1079 10.1037/0735-7044.113.5.1071 (doi:10.1037/0735-7044.113.5.1071) [DOI] [PubMed] [Google Scholar]
- 12.Williams JR, Insel TR, Harbaugh CR, Carter CS. 1994. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J. Neuroendocrinol. 6, 247–250 10.1111/j.1365-2826.1994.tb00579.x (doi:10.1111/j.1365-2826.1994.tb00579.x) [DOI] [PubMed] [Google Scholar]
- 13.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548 10.1038/365545a0 (doi:10.1038/365545a0) [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Wang ZX. 2003. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544 10.1016/S0306-4522(03)00555-4 (doi:10.1016/S0306-4522(03)00555-4) [DOI] [PubMed] [Google Scholar]
- 15.Lim MM, Young LJ. 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45 10.1016/j.neuroscience.2003.12.008 (doi:10.1016/j.neuroscience.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Curtis JT, Wang Z. 2001. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 115, 910–919 10.1037/0735-7044.115.4.910 (doi:10.1037/0735-7044.115.4.910) [DOI] [PubMed] [Google Scholar]
- 17.Insel TR, Shapiro LE. 1992. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl Acad. Sci. USA. 89, 5981–5985 10.1073/pnas.89.13.5981 (doi:10.1073/pnas.89.13.5981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel T, Wang Z, Ferris C. 1994. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci. 14, 5381–5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757 10.1038/nature02539 (doi:10.1038/nature02539) [DOI] [PubMed] [Google Scholar]
- 20.Oldfield RG, Hofmann HA. 2011. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol. Behav. 102, 296–303 10.1016/j.physbeh.2010.11.022 (doi:10.1016/j.physbeh.2010.11.022) [DOI] [PubMed] [Google Scholar]
- 21.Smith AS, Ågmo A, Birnie AK, French JA. 2010. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 57, 255–262 10.1016/j.yhbeh.2009.12.004 (doi:10.1016/j.yhbeh.2009.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner LM, Young AR, Römpler H, Schöneberg T, Phelps SM, Hoekstra HE. 2010. Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol. Biol. Evol. 27, 1269–1278 10.1093/molbev/msq013 (doi:10.1093/molbev/msq013) [DOI] [PubMed] [Google Scholar]
- 23.Kabelik D, Klatt JD, Kingsbury MA, Goodson JL. 2009. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm. Behav. 56, 101–107 10.1016/j.yhbeh.2009.03.017 (doi:10.1016/j.yhbeh.2009.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeyens DA, Cornett LE. 2006. The cloned avian neurohypophysial hormone receptors. Comp. Biochem. Physiol. B 143, 12–19 10.1016/j.cbpb.2005.09.012 (doi:10.1016/j.cbpb.2005.09.012) [DOI] [PubMed] [Google Scholar]
- 25.Thompson RR, Walton JC. 2004. Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav. Neurosci. 118, 620–626 10.1037/0735-7044.118.3.620 (doi:10.1037/0735-7044.118.3.620) [DOI] [PubMed] [Google Scholar]
- 26.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. 2009. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325, 862–866 10.1126/science.1174929 (doi:10.1126/science.1174929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, Maney DL. 2011. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology 152, 4865–4881 10.1210/en.2011-1394 (doi:10.1210/en.2011-1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. 2010. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm. Behav. 58, 614–618 10.1016/j.yhbeh.2010.06.014 (doi:10.1016/j.yhbeh.2010.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen A, Tomaszycki ML. 2012. Oxytocin antagonist treatments alter the formation of pair relationships in zebra finches of both sexes. Horm. Behav. 62, 113–119 10.1016/j.yhbeh.2012.05.009 (doi:10.1016/j.yhbeh.2012.05.009) [DOI] [PubMed] [Google Scholar]
- 30.Churchland PS, Winkielman P. 2012. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm. Behav. 61, 392–399 10.1016/j.yhbeh.2011.12.003 (doi:10.1016/j.yhbeh.2011.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodson JL, Lindberg L, Johnson P. 2004. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm. Behav. 45, 136–143 10.1016/j.yhbeh.2003.08.006 (doi:10.1016/j.yhbeh.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 32.Chini B, Manning M, Guillon G. 2008. Affinity and efficacy of selective agonists and antagonists for vasopressin and oxytocin receptors: an ‘easy guide’ to receptor pharmacology. Prog. Brain Res. 170, 513–517 10.1016/S0079-6123(08)00438-X (doi:10.1016/S0079-6123(08)00438-X) [DOI] [PubMed] [Google Scholar]
- 33.Goodson JL, Eibach R, Sakata J, Adkins-Regan E. 1999. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla). Behav. Brain Res. 101, 167–180 [PubMed] [Google Scholar]
- 34.Leung CH, Goode CT, Young LJ, Maney DL. 2009. Neural distribution of nonapeptide binding sites in two species of songbird. J. Comp. Neurol. 513, 197–208 10.1002/cne.21947 (doi:10.1002/cne.21947) [DOI] [PubMed] [Google Scholar]
- 35.Ocampo Daza D, Lewicka M, Larhammar D. 2012. The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, inclucing two distinct V2 subtypes. Gen. Comp. Endocrinol. 175, 135–143 10.1016/j.ygcen.2011.10.011 (doi:10.1016/j.ygcen.2011.10.011) [DOI] [PubMed] [Google Scholar]
- 36.Curley JP, Jensen CL, Franks B, Champagne FA. 2012. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 61, 454–461 10.1016/j.yhbeh.2012.01.013 (doi:10.1016/j.yhbeh.2012.01.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. 2011. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm. Behav. 60, 12–21 10.1016/j.yhbeh.2011.01.012 (doi:10.1016/j.yhbeh.2011.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. 2005. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47, 503–513 10.1016/j.neuron.2005.06.031 (doi:10.1016/j.neuron.2005.06.031) [DOI] [PubMed] [Google Scholar]
- 39.Popik P, Vos PE, Van Ree JM. 1992. Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav. Pharmacol. 3, 351–358 10.1097/00008877-199208000-00011 (doi:10.1097/00008877-199208000-00011) [DOI] [PubMed] [Google Scholar]
- 40.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. 2000. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288 10.1038/77040 (doi:10.1038/77040) [DOI] [PubMed] [Google Scholar]
- 41.Lee H-J, Caldwell HK, Macbeth AH, Tolu SG, Young WS. 2008. A conditional knockout mouse line of the oxytocin receptor. Endocrinology 149, 3256–3263 10.1210/en.2007-1710 (doi:10.1210/en.2007-1710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson JN, Aldag JM, Insel TR, Young LJ. 2001. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 21, 8278–8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22 10.1016/j.yhbeh.2005.02.003 (doi:10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuenzel WJ, Medina L, Csillag A, Perkel DJ, Reiner A. 2011. The avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 1424, 67–101 10.1016/j.brainres.2011.09.037 (doi:10.1016/j.brainres.2011.09.037) [DOI] [PMC free article] [PubMed] [Google Scholar]