Introduction
Vohwinkel syndrome or keratoderma hereditaria mutilans (KHM), first described in 1929, is a very rare hereditary disease belonging to the group of palmoplantar keratodermas [7]. The syndrome is marked by hyperkeratosis of the palms and soles creating a typical honeycomb pattern and a starfish appearance on the extensor surfaces. In addition, constricting bands form on the digits, usually beginning with the fifth digits. Modern genetics have shown KHM results from a missense mutation of the proteins loricrin and connexin 26 [5, 6]. The disease can be sporadic or inherited in an autosomal dominant fashion with variable phenotypic expression. Published data suggest that this disease is more common among Caucasian women, and the disease manifests clinically in the neonatal period and progresses throughout life. The presence of constriction bands on the digits of the hands and feet, leading to progressive strangulation and auto-amputation, known as pseudo-ainhum, is a feature unique to KHM [2]. The disease may be associated with additional features such as alopecia, high-tone hearing loss, nail anomalies, and, in some cases, cleft lip and palate or facial asymmetry [3, 5]. Treatment of the pseudo-ainhum is often necessary to prevent ischemic necrosis of the fingertips.
Relatively few cases have been reported, and results of treatment with various modalities are inconsistent and uncertain. Medical management includes the use of systemic synthetic retinoids [2–4], vitamin A derivatives, which enhance the differentiation and proliferation of epidermal tissues. Retinoids offer moderate success but are often limited by their toxicities and teratogenicity [1, 8]. Surgical treatments described include local tissue rearrangements, local flaps, and skin grafts. Optimal long-term results have not been readily obtainable with any treatment modality [2].
Materials and Methods
A 23-year-old African-American female was identified through our facility with the clinical diagnosis of Vohwinkel syndrome. Her primary complaint was pain in her fingertips due to long-standing constriction bands, the most severe affecting her right ring finger. She suffered auto-amputation of the distal phalanges of the fifth digit on both hands and feet a few years prior (Fig. 1). Angiography of her digital vasculature was ordered to assess the caliber and quality of the digital arteries (Fig. 2). As seen in Fig. 2, both radial and ulnar digital arteries are diminutive beyond the cutaneous constriction. Although this study is not essential for diagnosis or treatment of KHM, assessment of the digital vasculature is helpful in gauging severity of disease, providing appropriate informed consent, and tracking disease progression over time. In the case presented, the angiogram reveals miniscule digital arteries with poor distal fill; however, clinically, the patient exhibited intact sensation and normal capillary refill. Therefore, the angiogram is most valuable for perioperative planning and prognosis.
Fig. 1.
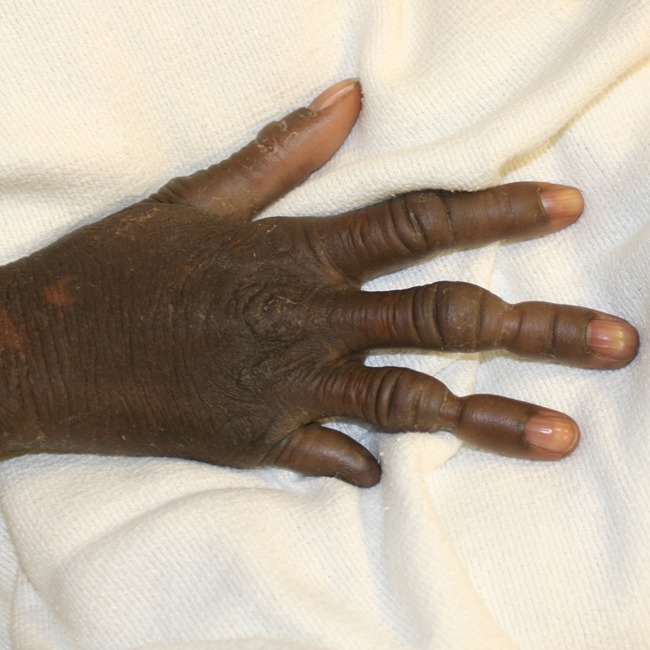
Auto-amputation of the distal phalanges of the fifth digit of the patient’s hand a few years prior
Fig. 2.
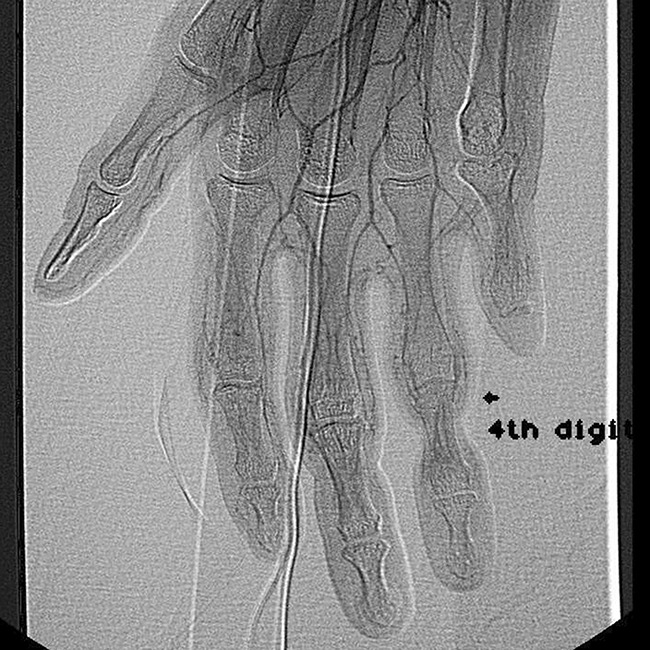
Angiograph of the patient’s digital vasculature
In an operating room under general anesthesia, we excised the constriction band on her right ring finger. As per the primary surgeon’s (YL) preference, both an operating microscope and upper arm tourniquet were used (Fig. 3).
Fig. 3.
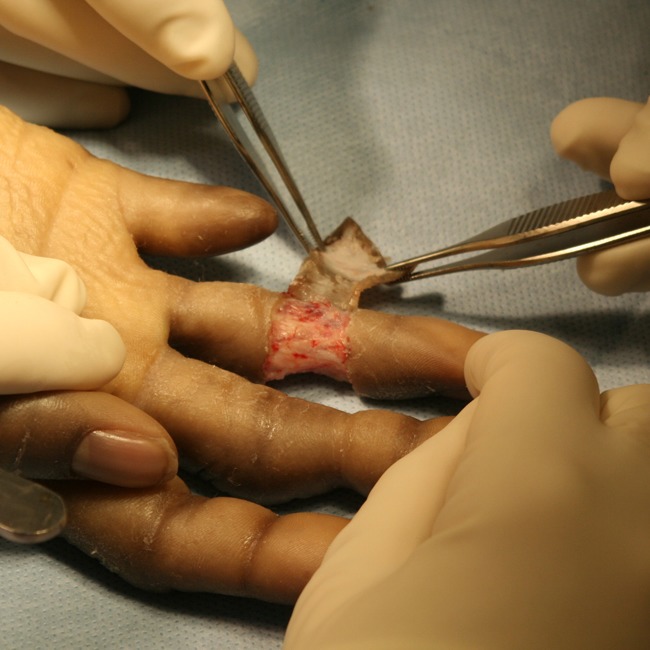
Excision of the constriction band using an operating microscope and upper arm tourniquet
Our surgical treatment included full-thickness excision of the constriction band with identification and preservation of the neurovascular bundles. The wound bed, after excision of the constriction band, appeared well vascularized and bled adequately with release of the tourniquet. Gentle pressure alone was used for hemostasis so as to avoid inadvertent neurovascular injury. The circumferential defect was then covered with a full-thickness skin graft (FTSG) harvested from her groin crease. The dermis of the skin graft was thinned slightly, and the graft was secured circumferentially using chromic suture. A bolster dressing was created with antibiotic-impregnated petroleum gauze and self-adherent elastic wrap. The finger was splinted until the dressing was removed 5 days later. Complete take of the graft to the wound bed suggested adequate vascularity.
Our use of the operating microscope was based on the angiographic findings of diminutive digital vessels and the inherent potential for injury during dissection. However, the case could be performed under standard loupe magnification, as the diseased skin peels free of the underlying structures rather easily. We would, however, recommend having the microscope available for unforeseen difficulties.
The patient returned at 12 months with worsening symptoms in her right long finger, and this was treated similarly. She was followed for 16 months after treatment of the right ring finger and has had no recurrence (Figs. 4 and 5).
Fig. 4.
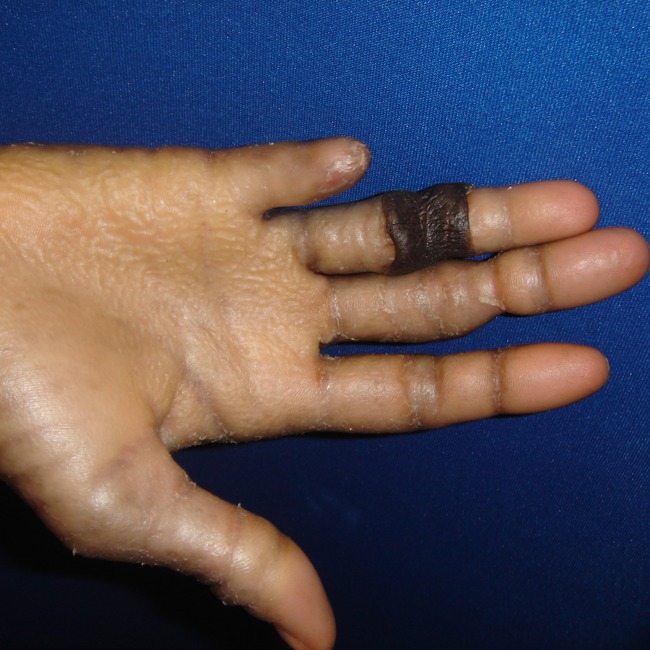
The patient’s right hand 12 months after treatment
Fig. 5.
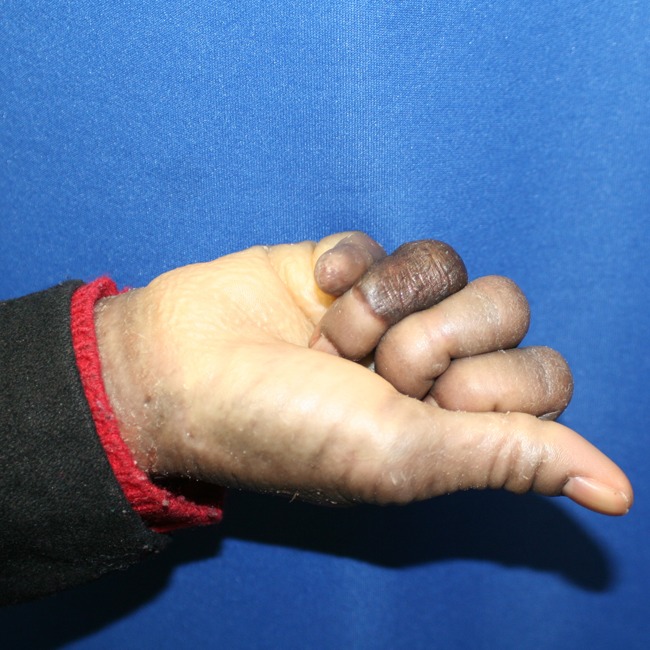
The patient’s right hand 16 months after treatment
A literature review was performed on PubMed to compare the results of other published treatment modalities.
Results
Our patient had successful amelioration of pseudo-ainhum without recurrence of the treated areas after 16 months of follow-up. Other therapies reported in the literature include high-dose synthetic retinoids, Z-plasty, cross-finger flaps, and skin grafting. All published results are case studies. The longest follow-up was of 3 years in a patient treated with Z-plasty; she had recurrence of all constriction bands. A cross-finger flap was reported to be successful at 18 months of follow-up. No single therapy has shown complete success in eradication of constriction banding.
Discussion
The expression of KHM is variable, with the cutaneous manifestations paramount. As the constriction bands threaten digital loss, patients seek therapy from specialists. Although the disease is typically diagnosed clinically, DNA analysis has recently become available; however, its use is extremely limited [9].
Given the extra-cutaneous findings associated with KHM, should a clinician encounter a small child with the diagnosis, referral to an audiologist would be prudent. In addition, children of KHM patients may warrant screening audiometry and close dermatologic surveillance.
Based on published reports, surgical therapy is superior to medical therapy relative to side effects and duration of results. Z-plasty techniques have shown temporary success but long-term failure. Our patient has had successful treatment via full-thickness skin grafting without evidence of recurrence at 16 months of follow-up.
With regard to decision making in surgical treatment of the constriction bands, basic science may help improve outcomes. KHM is a genetic disorder which would require a genetic alteration for cure. Surgical treatments are aimed only at the manifestation of limb-threatening constriction bands. Excision alone should be expected to recur over time given the characteristics of the disease and its tendency toward specific areas of the fingers and toes. We also know that full-thickness skin grafts tend to retain the skin characteristics of the donor site. Therefore, a FTSG may permanently modify the locally diseased skin. To this regard, a remote FTSG may be a better choice than a local FTSG. This same line of reasoning may explain the recurrence seen with the Z-plasty method. Perhaps, the cross-finger flap was successful because the skin on the chosen flap was disease free. Full-thickness skin grafting is promising, but further follow-up is needed to assure no recurrence.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Atabay K, Yavuzer R, Latifoglu O, Ozmen S. Keratoderma hereditarium mutilans (Vohwinkel syndrome): an unsolved surgical mystery. Plast Reconstr Surg. 2001;108(5):1276–1280. doi: 10.1097/00006534-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Bassetto F, Tiengo C, Sferrazza R, et al. Vohwinkel syndrome: treatment of pseudo-ainhum. Int J Dermatol. 2010;49(1):79–82. doi: 10.1111/j.1365-4632.2009.04267.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang Sing Pang A, Oranje A, Vuzevki V, et al. Succesful treatment of keratoderma hereditaria mutilans with an aromatic retinoid. Arch Dermatol. 1981;117:225–228. doi: 10.1001/archderm.117.4.225. [DOI] [PubMed] [Google Scholar]
- 4.Heller EH, Shiffman NJ. Synthetic retinoids in dermatology. Can Med Assoc J. 1985;132:1129–1136. [PMC free article] [PubMed] [Google Scholar]
- 5.Maestrini E, Korge BP, Ocana-Sierra J, et al. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel’s syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- 6.Maestrini E, Monaco AP, McGrath JA, et al. A molecular defect in loricrin, .the major component of the cornified cell envelope, underlies Vohwinkel’s syndrome. Nat Genet. 1996;13:70. doi: 10.1038/ng0596-70. [DOI] [PubMed] [Google Scholar]
- 7.Vohwinkel KH. Keratoderma hereditarium mutilans. Arch Dermtol Syph. 1929;158:354–364. [Google Scholar]
- 8.Wereide K. Mutilating palmoplantar keratoderma successfully treated with etretinate. Acta Derm Venereol. 1984;63:181. [PubMed] [Google Scholar]
- 9.White TW. Functional analysis of human Cx 26 mutations associated with deafness. Brain Res Rev. 2000;32(1):181–183. doi: 10.1016/S0165-0173(99)00079-X. [DOI] [PubMed] [Google Scholar]


