Abstract
Background
The most prominent nonneurological finding in the common compression neuropathy carpal tunnel syndrome (CTS) is fibrosis of the subsynovial connective tissue (SSCT). Recently, a rabbit model of CTS has been developed, based on the hypothesis that SSCT injury and subsequent fibrosis cause nerve compression. The purpose of this study was to evaluate the effects in this model at earlier and later time points than have heretofore been reported.
Methods
Sixty rabbits were operated on and observed at two different time periods: 6 and 24 weeks. Nerve electrophysiology (EP), SSCT histology, and SSCT mechanical properties were assessed.
Results
There was no significant difference in median motor nerve amplitude or latency at either time point. The total cell density in the SSCT was significantly higher at 6 and 24 weeks compared to controls. The mean size of the collagen fibrils in the SSCT was higher 6 and 24 weeks after surgery compared to controls. Both the ultimate load and the total energy absorption of the SSCT were significantly higher at 6 and 24 weeks compared to controls.
Conclusions
In this model, there were signs of SSCT fibrosis and histology changes at 6 weeks, which persist after 24 weeks. Thus, this model leads to sustained SSCT fibrosis, which is one characteristic of human CTS. However, no significant EP changes were found at these two time points, which is in contrast to the findings reported previously for this model at 12 weeks. The significance of the differences in EP findings will be the subject of future studies.
Keywords: Carpal tunnel syndrome, Rabbit animal model, Shear injury, Subsynovial connective tissue
Background
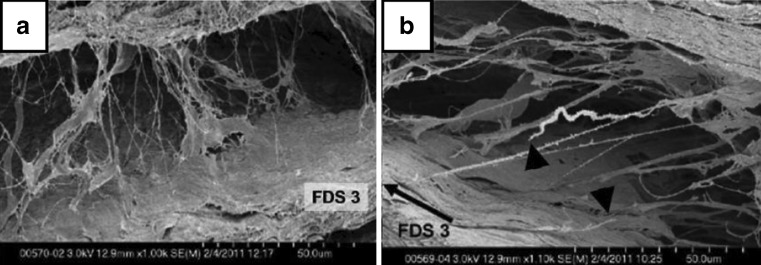
Carpal tunnel syndrome (CTS) is the most common peripheral neuropathy. CTS is caused by compression of the medial nerve within the carpal tunnel. The most prominent nonneurological finding in CTS is fibrosis of the subsynovial connective tissue (SSCT). Guimberteau was one of the first to investigate the SSCT, noting that it consisted of multiple layers, parallel to the tendon, that are interconnected by collagenous fibrils [5]. These layers move sequentially as the tendon moves (Fig. 1).
Fig. 1.
Rabbit SSCT. a Neutral positioned specimen, two SSCT layers connected by loose collagenous fibrils (SEM, ×1.00K); b the black arrow indicates the direction of the FDS3 tendon displacement. Black triangles point to stumps of ruptured fibrils (SEM, ×1.10K). This material is reproduced with permission from John Wiley & Sons, Inc. [11]
Despite extensive research, the mechanism that produces the nerve compression in CTS is still unknown. Previously developed animal models of CTS have mainly focused on the effects of chronic or acute pressure on median nerve physiology, rather than focusing on a biological process that might trigger the nerve compression [6–9]. Recently, a rabbit model of CTS has been developed, based on the hypothesis that SSCT fibrosis causes the nerve compression. In this model, an operative procedure is performed to create an SSCT injury. After 12 weeks of observation, there were signs of SSCT fibrosis similar to CTS in humans, and there was a significant decrease in an electrophysiologic (EP) measure, median motor amplitude [10]. This is an intermediate severity EP finding in clinical CTS; the earliest clinical CTS EP changes, sensory latency and then sensory amplitude, cannot be assessed in the rabbit model.
The initial report of this rabbit model only studied one time point after SSCT injury. The time course was not investigated. Therefore, the purpose of this study was to investigate the effects of this SSCT injury model at earlier and later time points, 6 and 24 weeks after surgery, respectively.
Materials and Methods
A total of 72 female rabbits, weighing 3.2–6.2 kg, were used in this study, which was approved by our Institutional Animal Care and Use Committee. Female rabbits were used because clinical CTS is more common in females [13]. Sixty rabbits had surgery, 30 each observed at 6 and 24 weeks. Twelve rabbits were used as controls; these animals were held in the same living conditions, but no surgery was performed.
Surgical Procedure
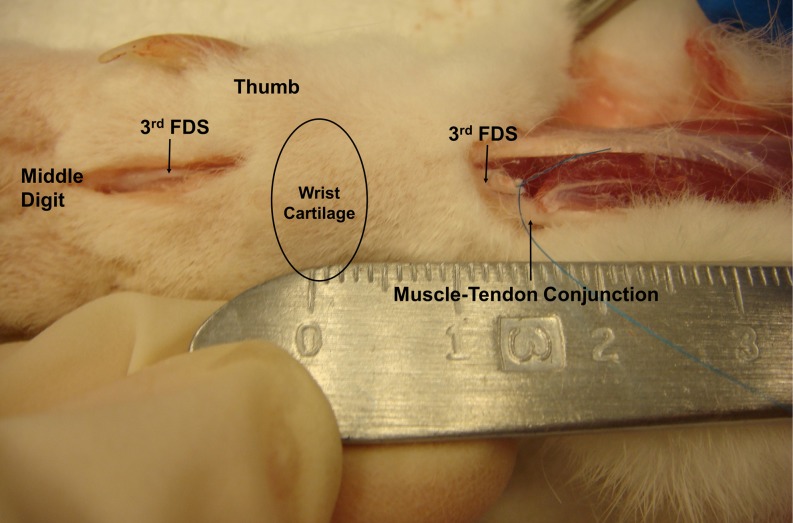
All animals had EP testing on both paws after the induction of anesthesia but before any surgery. One randomly selected paw was operated on in each rabbit. Before surgery, the paws were shaved and scrubbed with povidone–iodine and draped with a sterile cloth. A tourniquet was applied proximal to the elbow. To create an injury to the SSCT, a volar longitudinal incision was made proximal to the carpal tunnel. The carpal tunnel was left intact (Fig. 2). The flexor digitorum superficialis tendon of the middle digit (FDS3) and the flexor carpi ulnaris (FCU) tendon were identified and marked with a 6-0 Prolene (Ethicon, New Brunswick, NJ) suture. The FDS3 was then cut proximal to the marking suture. A volar incision was then made distal to the carpal tunnel, through which the FDS3 was identified and shifted distally for 5 mm, making a tendon loop fixed with 5-0 Ethibond (Ethicon, New Brunswick, NJ) suture distal to the carpal tunnel (Fig. 3). The distance of the FDS3 shift was confirmed by measuring the change in distance of the FDS3 marking suture relative to the suture marker on the FCU. Finally, the skin was closed with sutures of 4-0 Ethibond.
Fig. 2.
Surgical exposure
Fig. 3.
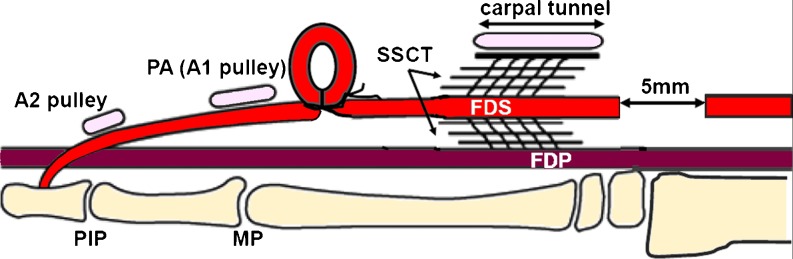
Distal loop shifts tendon proximally to the CT. This material is reproduced with permission from John Wiley & Sons, Inc. [12]
Postoperatively, an Elizabethan collar was applied for 2 weeks to prevent the rabbits from chewing on the operated paws. Full cage activity was allowed until the rabbits were sacrificed.
Evaluation of SSCT and Median Nerve
Thirty rabbits were sacrificed after 6 weeks, and another 30 rabbits were sacrificed after 24 weeks. Prior to sacrifice, all animals had EP testing. After sacrifice, the animals were evaluated by histology, transmission electron microscopy, and mechanical testing. If the distal FDS3 was found to have migrated into the carpal tunnel at the time of sacrifice, the rabbits were excluded from the analysis. The rationale for exclusions was both practical and anatomic. Practically, the mechanical test to determine the force necessary to pull the tendon through the carpal tunnel could not be done if the tendon was already within the tunnel; anatomically, we were concerned that if the tendon had migrated into the carpal tunnel during the study period, it could potentially damage the nerve, thus invalidating the usefulness of the postoperative EP testing and, possibly, the usefulness of the histology measures as well.
Electrophysiology
As noted above, EP testing of the median nerve was performed immediately before and after the surgical procedure and again immediately before sacrifice. During this procedure, we measured the amplitude of compound muscle action potential (CMAP) of the median nerve using pairs of fine subdermal near-nerve stimulating and recording electrodes. The CMAP was recorded from the thenar muscle while stimulating from the forepaw (flexor digitorum superficialis), located 3.0 cm proximal to the recording site. Stimulation was continued until a submaximal response was reached. Both latency and amplitude of the CMAP were measured.
Histology and Transmission Electron Microscopy
In both of the experimental groups, one of the light microscopy specimens was mounted improperly and could not be assessed. Thus, in each group, there were eight specimens for light microscopy and nine for electron microscopy (EM). Immediately after sacrifice, the skin was removed, and the paws were placed in Trump's fixative (1 % glutaraldehyde and 4 % formaldehyde in 0.1 M phosphate buffer, pH 7.2) for 48 h. Then the carpal tunnel was cut out “en bloc,” and this block was sharply cut transversely, 5 mm proximal to the distal edge of the wrist cartilage.
The proximal half was put in formalin and later embedded in paraffin. Five-micrometer cross sections were made through the carpal tunnel, starting on the distal side of the block. Then they were stained with standard hematoxylin and eosin. The sections were blindly evaluated by the second author under a light microscope (BH2, Olympus, Tokyo, Japan). Fibroblasts of the SSCT were measured at magnification ×400 by counting the number of cells at three locations around the FDS3, one palmar and two dorsal. The distal half of the carpal tunnel was used for transmission electron microscopy (TEM). Slices 0.5 mm thick were made using a cryostat. The slices were rinsed with 0.1 M phosphate buffer and then rinsed in three changes of distilled water. The tissues were then dehydrated in progressive concentrations of ethanol and critical-point dried. Finally, they were mounted on copper grids and sputter coated for 90 s with gold–palladium [15]. The size of the collagen fibrils was evaluated at high magnification (×120K) by TEM (Hitachi S-4700 field emission scanning electron microscope) (0–30 kV). The mean diameter of the collagen fibrils was determined using a custom-made software program (KS400 Image Analysis Software from Carl Zeiss, Inc).
Nerve Histology
In order to assess possible demyelination, nerves were prepared according to a protocol described previously [18].
Mechanical Testing
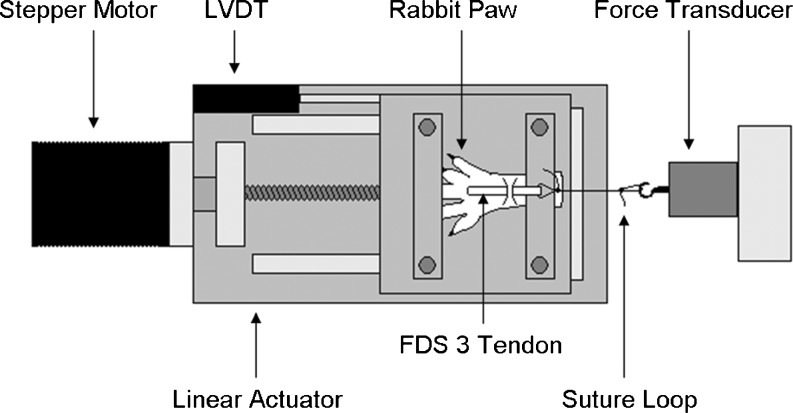
We used a modified method to measure the SSCT mechanical properties, originally described by Yamaguchi [17]. The paws were amputated at the forearm level immediately after sacrifice. The FDS3 was exposed proximal and distal to the carpal tunnel, with the carpal tunnel and SSCT left intact. The paw was then mounted on a custom-made device, and the proximal end of the tendon was connected to a load cell (Fig. 4). Next, the FDS3 was cut distal to the carpal tunnel. Finally, the tendon was pulled proximally at a rate of 0.5 mm/s. The specimen was kept moist throughout testing. The ultimate tensile load and energy absorption were determined. The ultimate tensile load was defined as the maximal load. The energy absorption was defined as the area under the load/displacement curve.
Fig. 4.
Setup mechanical testing
Statistical Analysis
Based on previous studies [10, 18], a sample size of 12 was needed in the mechanical testing group, and 25 were needed for EP testing. We estimated that eight specimens would be needed for histology and noted that the preparations for routine staining, nerve histology, and EM all required different fixation and so could not all be done on the same specimens. After consideration of the sample size number, a total of 30 rabbits were included in each experimental group, while 12 rabbits were used as controls. One-way analysis of variance, followed by a post hoc Tukey–Kramer test, was used to analyze the EP amplitude, EP latency, the mean size of collagen fibrils, the cell density, the energy absorption, and the ultimate load among the three groups (control, 6 and 24 weeks). The EP values after 6 and 12 weeks of observation were compared to the initial values using a paired t test. The results were expressed as mean ± standard deviation. The overall difference was considered significant if p < 0.05. All statistical analyses were performed using JMP software (SAS Institute Inc., Durham, North Carolina).
Results
A total of five rabbits were excluded from each of the two experimental groups because the proximal end of the FDS3 had migrated into the carpal tunnel. The remaining 25 animals were allocated as follows, based on sample size considerations: 12 for mechanical testing, 9 for histology (both light and EM), and 4 for nerve histology.
Electrophysiology (n = 25)
In the 6-week rabbits, the mean initial median motor amplitude was 2.59 ± 0.75 mV, and the mean initial latency was 1.52 ± 0.10 ms. In this same group, the mean amplitude after 6 weeks of observation was 3.00 ± 1.31 mV, and the mean latency was 1.58 ± 0.16 ms. In the 24-week observation group, the mean initial amplitude was 2.57 ± 0.81 mV, and the mean initial latency was 1.57 ± 0.16 ms. After 24 weeks of observation, the mean amplitude was 2.93 ± 0.10 mV, and the mean latency was 1.62 ± 0.14 ms. There was no significant difference in amplitude or latency 6 or 24 weeks after surgery, compared to the initial measurements.
Histology (n = 8)
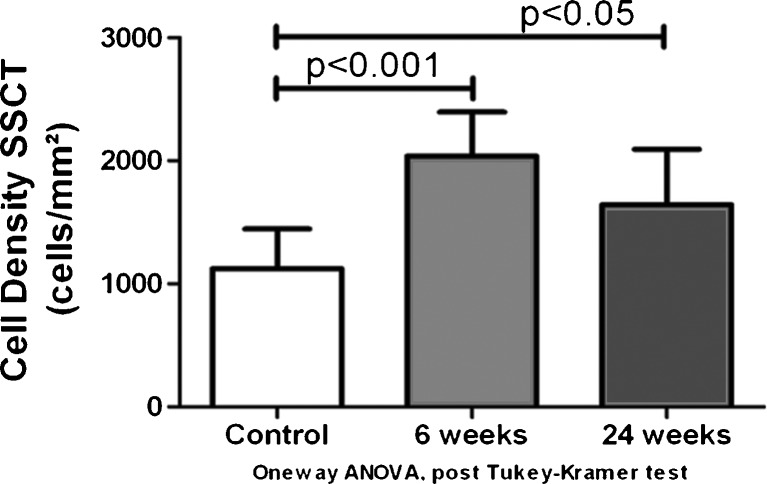
The mean cell density in the control group was 1,124.23 ± 320.90 cells/mm2. After 6 weeks of surgery, the mean cell density was 2,038.62 ± 357.81 cells/mm2. Twenty four weeks after surgery, the mean cell density was 1,643.63 ± 451.22 cells/mm2. The total cell density in the SSCT after 6 weeks of surgery was significantly higher compared to the control population (p < 0.001). The cell density after 24 weeks of SSCT injury was also significantly higher than the cell density in the controls (p < 0.05) (Fig. 5). Although the cell density in the 24-week group was less than the 6-week group, this difference was not statistically significant.
Fig. 5.
Cell density SSCT in the control, after 6 and 12 weeks
Transmission Electron Microscopy (n = 9)
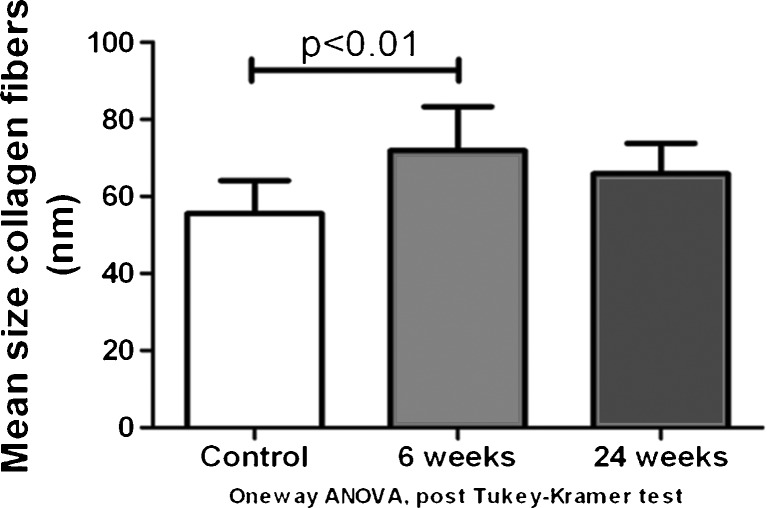
The mean size of the collagen fibrils was 55.55 ± 8.61 nm in the control group, 71.87 ± 11.42 nm 6 weeks after surgery, and 65.90 ± 7.93 nm 24 weeks after surgery. The mean size of the collagen fibrils of the SSCT in the 6-week group was significantly larger than the normal control group (p < 0.01). However, there was no significant difference between the 24-week group and the normal control, or between the 6-week group and the 24-week group (Fig. 6).
Fig. 6.
Mean size of collagen fibrils measured with TEM in the control, after 6 and 12 weeks (×120K)
Nerve Histology (n = 4)
Because the EP studies were normal, we elected not to complete this aspect of the study.
Mechanical Testing (n = 12)
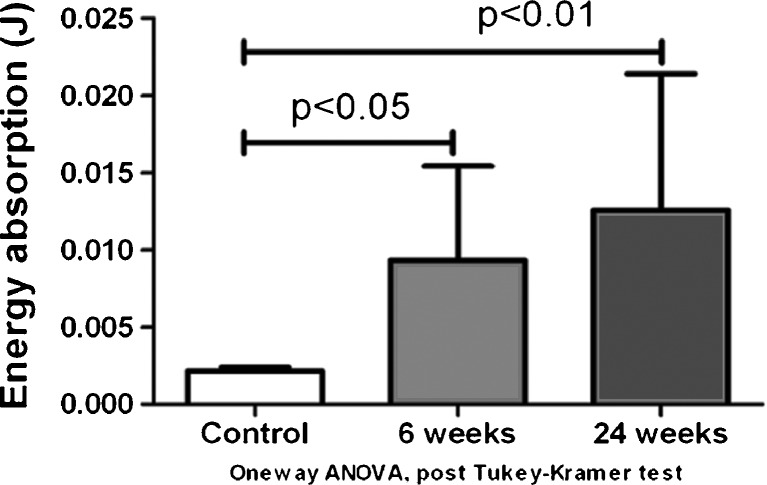
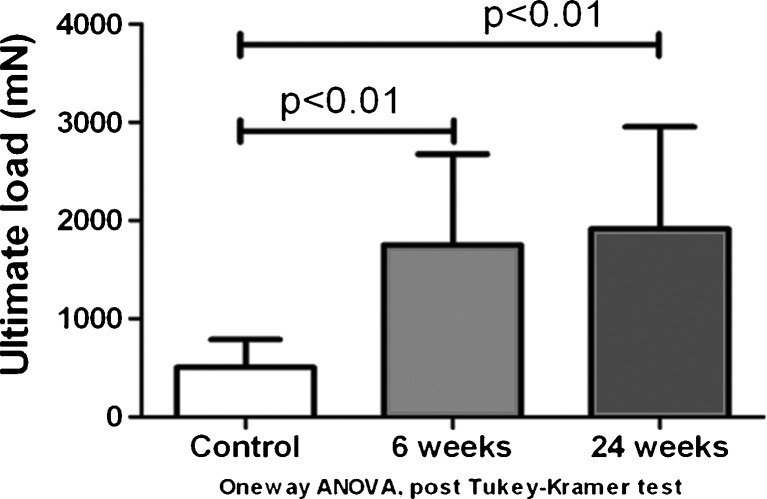
For the mechanical testing, the mean energy absorption during pullout was 2.16 ± 0.86 mJ in the control group, 9.33 ± 6.11 mJ 6 weeks after surgery, and 12.57 ± 8.83 mJ 24 weeks after surgery (Fig. 7). The mean energy absorption of both the 6- and 24-week groups was significantly higher than the control group (p < 0.05), but there was no significant difference between the 6- and 24-week groups (Fig. 8). The mean ultimate load was 508.17 ± 281.45, 1,752.25 ± 925.31, and 1,915.19 ± 1,039.52 mN in the control group, 6-week group, and 24-week group, respectively. The ultimate load of both the 6- and 24-week groups was significantly higher than the control group (p < 0.05). There was no significant difference between the 6- and 24-week groups (Fig. 8).
Fig. 7.
Total energy absorption during pullout of the FDS3 tendon
Fig. 8.
Ultimate load during pullout of the FDS3 tendon
Discussion
Moriya et al. developed a rabbit model that showed signs of SSCT fibrosis similar to CTS in humans, combined with a significant decrease in EP amplitude [10]. One major limitation of this study was the single time point observation, 12 weeks after surgery. The purpose of this study was to investigate the effect of time on the SSCT and the median nerve after a similar surgical procedure.
Another study by Ettema et al. [3] showed that the mean fibroblast density and the mean size of the collagen fibrils in the SSCT in CTS patients were higher compared to control specimens, in a different model of SSCT fibrosis, in which fibrosis was induced by an osmotic injury caused by an injection of 10 % dextrose rather than a surgical insult to the FDS3. We found, like Ettema et al., that the mean cell density in the SSCT was significantly higher, both at 6 and 24 weeks after surgery compared to the control population. We also found that the mean size of the collagen fibrils was higher at 6 and 24 weeks after surgical intervention compared to the controls. These histological and TEM observations suggest that the fibrosis which is created by this surgical insult is not of a temporary nature.
The surgical injury also seems to result in changes to the mechanical properties of the SSCT within the carpal tunnel. Both the total energy and the ultimate load are significantly higher at both time points, compared to the control group. This observation further indicates that the surgical injury causes sustained fibrosis of the SSCT.
In contrast to the previous findings, neither median nerve amplitude nor latency was significantly different at 6 or 24 weeks of observation, compared to the control population. Although this is an important diagnostic factor in human CTS, we believe this is not a reliable finding in the rabbit animal model for several reasons. First, changes in motor nerve function are a relatively late finding in clinical CTS [16]; the most sensitive EP measure is sensory amplitude, but this measure is technically not possible in the rabbit model. In addition, the human carpal tunnel is very rigid, but the rabbit carpal tunnel is much less so. Tung et al. compared the compliance of the transverse carpal ligament in humans and rabbits and found that the rabbit carpal tunnel transverse ligament was much more compliant than the human ligament [14]. Thus, it would take a relatively larger increase in SSCT volume in the rabbit to get a similar compression of the median nerve than in humans.
One of the limitations of this study might be the age of the animals included. CTS appears more often in middle-aged human populations, with a peak incidence of 45–54 years [4], but the mean age of the rabbits used in the current study was 6 months–2 years, which is relatively young, considering a rabbit lifespan of about 10 years. Unfortunately, we were unable to find a supplier of older rabbits, and the costs of housing rabbits for 3–4 years prior to study is prohibitive. We would assume that a traumatic event to the SSCT would heal sooner at younger age and is less likely to lead to sustained fibrosis. Thus, a similar surgical procedure could lead to more pronounced fibrosis, possibly with larger EP changes.
The one-time injury that we created with the surgical procedure might be another limitation, as it is widely accepted (although experimental data are limited) that forceful and repetitive finger movements contribute to the development of CTS [1, 2]. However, our injury was designed so that the advancement of the FDS3 would stretch its surrounding SSCT. We assumed, although currently we cannot prove due to a lack of suitable dynamic in vivo imaging, that normal cage activity would repetitively load the stretched SSCT differently than it would a normal SSCT, and we believe that our data support this hypothesis.
Conclusions
In models that injure the SSCT by either osmotic stress or mechanical stretch, 12 weeks of cage activity results in SSCT fibrosis, with increased cell density, enlarged SSCT collagen diameter, and decreased SSCT mechanical compliance, as well as EP changes which are similar to the findings observed in clinical CTS [16]. The current data suggest that these SSCT changes occur at least as early as 6 weeks post injury and are sustained for at least 24 weeks. Although this reliable animal model of SSCT fibrosis did not result in a reliable median nerve neuropathy, we believe that this relates to the major differences in the way that EP can be assessed in humans as compared to rabbits, and to the differences in human versus rabbit carpal tunnel compliance. We do believe that this model may be useful to investigate the etiology and development of SSCT fibrosis, which we hypothesize to be an important initiator of CTS. This information contributes to the development of a valid animal model for CTS and may have relevance in understanding the pathogenesis of human CTS and offers a possibility to study the effect of CTS interventions biologically, in ways that would not be possible clinically.
Acknowledgments
This study was funded by a grant from NIH (NIAMS AR49823).
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- 1.Amadio PC. Repetitive stress injury. J Bone Joint Surg Am. 2001;83-A:136–137. doi: 10.2106/00004623-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 2.English CJ, Maclaren WM, Court-Brown C, et al. Relations between upper limb soft tissue disorders and repetitive movements at work. Am J Ind Med. 1995;27:75–90. doi: 10.1002/ajim.4700270108. [DOI] [PubMed] [Google Scholar]
- 3.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Gelfman R, Melton LJ, 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33–41. doi: 10.1212/01.wnl.0000338533.88960.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimberteau JC. New ideas in hand flexor tendon surgery. The sliding system, Vascularized flexor tendon transfers. Pessac: Aquitaine Domaine Forestier; 2001. [Google Scholar]
- 6.Gupta R, Rowshan K, Chao T, et al. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187:500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Lim JY, Cho SH, Han TR, et al. Dose-responsiveness of electrophysiologic change in a new model of acute carpal tunnel syndrome. Clin Orthop Relat Res. 2004;427:120–126. doi: 10.1097/01.blo.0000142352.88039.33. [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon SE, Dellon AL, Hudson AR, et al. Chronic nerve compression—an experimental model in the rat. Ann Plast Surg. 1984;13:112–120. doi: 10.1097/00000637-198408000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mackinnon SE, Dellon AL, Hudson AR, et al. A primate model for chronic nerve compression. J Reconstr Microsurg. 1985;1:185–195. doi: 10.1055/s-2007-1007073. [DOI] [PubMed] [Google Scholar]
- 10.Moriya T, Zhao C, Cha SS, et al. Tendon injury produces changes in SSCT and nerve physiology similar to carpal tunnel syndrome in an in vivo rabbit model. Hand. 2011;6:399–407. doi: 10.1007/s11552-011-9356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morizaki Y, et al. The response of the rabbit subsynovial connective tissue to a stress-relaxation test. J Orthop Res. 2012;30(3):443–447. doi: 10.1002/jor.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun YL, et al. Subsynovial connective tissue is sensitive to surgical interventions in a rabbit model of carpal tunnel syndrome. J Orthop Res. 2012;30(4):649–654. doi: 10.1002/jor.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Wild DK, Seligman PJ, et al. The US prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey data. Am J Public Health. 1994;84:1846–1848. doi: 10.2105/AJPH.84.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung WL, Zhao C, Yoshii Y, et al. Comparative study of carpal tunnel compliance in the human, dog, rabbit, and rat. J Orthop Res. 2010;28:652–656. doi: 10.1002/jor.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallis MA, Griffin RL. A routine method for embedding animal tissues in Spurr resin for electron microscopy. J Clin Pathol. 1973;26:77–78. doi: 10.1136/jcp.26.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011;44:597–607. doi: 10.1002/mus.22208. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Osamura N, Zhao C, et al. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2008;41:3519–3522. doi: 10.1016/j.jbiomech.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshii Y, Zhao C, Schmelzer JD, et al. Effects of hypertonic dextrose injections in the rabbit carpal tunnel. J Orthop Res. 2011;29:1022–1027. doi: 10.1002/jor.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]