Abstract
Background:
Enteric fever is caused by the serotypes Salmonella Typhi, Salmonella Paratyphi A, Salmonella Paratyphi B and Salmonella Paratyphi C. After emergence of multidrug resistant Salmonellae Ciprofloxacin, a fluorquinolone antibiotic was the first-line therapy. Treatment failure was observed with Ciprofloxacin soon and such strains showed in-vitro resistance to Nalidixic acid. Recent reports suggest re-emergence of Chloramphenicol sensitive strains and increasing Nalidixic acid resistance. This study is aimed at detecting the current trend in the antibiogram of Salmonella isolates from blood culture in coastal Karnataka, with an emphasis on antibiotic susceptibility of Nalidixic acid and Chloramphenicol and evaluate, if there is a need to modify the strategies in the antibiotic therapy for enteric fever.
Materials and Methods:
Blood samples received for culture in the laboratory between June 2009 and August 2011 was cultured in Brain Heart infusion broth, bile broth or in a commercial BACTEC culture media. The growth from blood cultures were processed for identification and antibiotic susceptibility as per standard methods. Antibiotic susceptibility for Ampicillin, Trimethoprim-sulphamethoxazole, Chloramphenicol, Ciprofloxacin, Ceftriaxone and Nalidixic acid were noted.
Results:
Out of 9053 blood culture specimens received, Salmonella was isolated from 103 specimens. There were 85 Salmonella Typhi isolates, 16 Salmonella Paratyphi A and two Salmonella Paratyphi B. Salmonella Typhi and Salmonella Paratyphi A showed the highest resistance to Nalidixic acid. Salmonella Typhi showed highest susceptibility to Ceftriaxone and Salmonella Paratyphi A to trimethoprim-sulphamethoxazole and Chloramphenicol. Two isolates were multidrug resistant. One Salmonella Paratyphi A was resistant to Ceftriaxone.
Conclusion:
Routine screening of Nalidixic acid susceptibility is practical to predict fluorquinolone resistance in Salmonella and preventing therapeutic failure while treating with it. It is worthwhile to consider replacing fluorquinolones with Chloramphenicol or Ceftriaxone as the first line of therapy for enteric fever. Periodic analysis of Salmonella antibiogram should be done to formulate the best possible treatment strategies.
Keywords: Ceftriaxone, Chloramphenicol, Nalidixic acid, resistance, Salmonella
INTRODUCTION
Enteric fever is one of the commonest and widely distributed foodborne illnesses worldwide, responsible for thousands of deaths. The source of infection is contaminated food of animal origin, mainly poultry food and also green vegetables contaminated from manure. The usual route of transmission is faeco-oral. There are 2501 serotypes of Salmonella identified up to 2004.[1] World health organization estimated that typhoid fever accounts for 21.7 million illnesses and paratyphoid fever accounts for 5.4 million cases each year.[2] Enteric fever is caused by the serotypes Salmonella Typhi (S. Typhi), Salmonella Paratyphi A (S.Para A), Salmonella Paratyphi B (S Para B) and Salmonella Paratyphi C (S.Para C). Enteric fever includes typhoid and paratyphoid fever. Enteric fever is endemic in India and S.Typhi and S.Para A being the commonest. S.Para B and S.Para C infections are rare in India. Apart from India, S.Para A is prevalent in other Asian countries, Eastern Europe and South America. S.Para B is prevalent in Western Europe, North America, Britain and S.Para C in Eastern Europe and Guyana.[3]
Chloramphenicol (CHL) was considered as the drug of choice for therapy since its introduction.[4] In India, CHL resistant typhoid fever epidemic appeared for the first time in Calicut, Kerala State, in the year 1972. These strains were susceptible to Ampicillin (AMP) and Trimethoprim-sulphamethoxazole (TMP-SMZ) initially.[2,3] Multidrug resistant (MDR) Salmonellae, resistant to CHL, AMP and TMP-SMZ, emerged in late 1980s and early 1990s.[4] With development of MDR Salmonellae Ciprofloxacin (CIP), a fluorquinolone antibiotic was used as the first-line therapy for enteric fever.[5] Other fluorquinolones (FLUQ) like Ofloxacin, Pefloxacin, Levofloxacin were also used for therapy. Treatment failure with CIP was reported within few years of its introduction.[2] Widespread and irrational uses of FLUQ in human and animal therapeutics lead to decreased susceptibility and resistance to this class of drug.[2,5] Isolates having in-vitro resistance to Nalidixic acid have shown reduced susceptibility to CIP and therefore has been used as a reliable indicator to detect FLUQ resistance.[2,6,7] Ceftriaxone (CEF) is a popular choice for treating enteric fever now. Recent reports suggests re-emergence of CHL sensitive strains in previously resistant areas and increase in NAL resistance.[2,5,6,8–10] This study is aimed at detecting the current trend in the antibiogram of Salmonella isolates from blood culture in coastal Karnataka. It also aims at detecting the antibiotic susceptibility of NAL and CHL and evaluate, if there is a need to modify the strategies in the antibiotic therapy for enteric fever.
MATERIALS AND METHODS
This prospective study was done in Father Muller Medical College Hospital, Mangalore City, Karnataka State, India. Blood samples received in the laboratory between June 2009 and August 2011, from both in-patients and out-patients were included in the study. During this period, 9053 blood samples were received for aerobic bacterial culture, from suspected enteric fever and other infections. Blood culture was done in Brain Heart infusion broth and bile broth for general ward patients and in a commercial BACTEC Aerobic Plus blood culture (Becton and Dickinson, BACTEC 9120 system, U.S.A) for private ward patients as per hospital policy. The blood culture samples received in brain heart infusion broth and bile broth were processed in the laboratory as per standard procedures and those received in BACTEC bottles were processed as per manufacturer's recommendations.[11,12] The cultures were incubated at 37°C for ten days before declaring negative.
Bile broth and Brain heart infusion cultures were subcultured every 24 hours on blood agar and MacConkey agar media. BACTEC cultures were subcultured on Blood and MacConkey agar, only after the machine flagged positive. Growth from blood and MacConkey agar media were processed for identification and antibiotic susceptibility as per standard methods.[7,11,13] Salmonella isolates were identified by recommended biochemical reactions and the identification was further confirmed by slide agglutination tests with a commercial Salmonella antisera. (Denka Seiken Co. Pvt. Ltd, Niigata, Japan)[12,13] All Salmonella isolates were subjected to antibiotic susceptibility test by Kirby-Bauer disc diffusion method and interpreted as per Clinical laboratory Standards Institute (CLSI) guidelines.[7] Though several antibiotics were tested in-vitro, susceptibility was reported only for AMP, TMP-SMZ, CHL, CIP, CEF and NAL, unless isolates were found resistant to these antibiotics.
Minimum inhibitory concentration (MIC) for CEF was determined by agar dilution method, for one S.Para A isolate.[12] A commercial pure form of CEF antibiotic powder was used for performing the MIC (HiMedia Laboratories, Mumbai, India). MIC was not done for the other isolates and other antibiotics. Screening and phenotypic confirmatory test for extended spectrum beta-lactamase (ESBL) detection was also done for the same isolate using a commercial Ceftazidime and Ceftazidime-clavulanic acid antibiotic discs (HiMedia Laboratories, Mumbai, India) and interpreted as per CLSI guidelines.[7]
RESULTS
Out of 9053 blood culture specimens received, Salmonella was isolated from 103 (1.44%) specimens grown either in bile broth, brain heart infusion broth or BACTEC culture. Out of the 103 Salmonella isolates, 85 (82.52%) were S.Typhi, 16 (15.53%) isolates were S.Para A and two isolates (1.94%) were S.Para B. The antibiotic susceptibility for AMP, TMP-SMZ, CHL, CIP, CEF and NAL are illustrated in Table 1.
Table 1.
Antibiogram of the 103 Salmonella isolates from blood culture
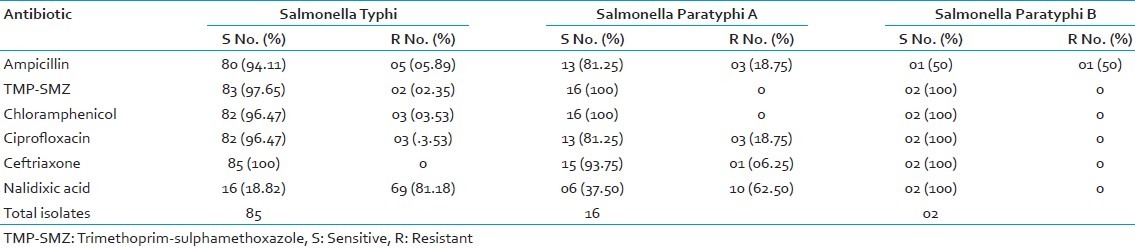
S.Typhi showed the highest resistance to NAL (81.18%) and highest susceptibility to CEF (100%). S.Para A showed highest resistance to NAL (62.5%) and highest susceptibility to TMP-SMZ and CHL (100%). S.Para B were 100% sensitive to TMP-SMZ, CHL, CIP, CEF, NAL and 50% resistant to AMP. Except one isolate of S.Para B, none of the isolates were susceptible to all antibiotics tested.
Two isolates (1.94%) were MDR strains i.e., resistant to AMP, TMP-SMZ and CHL and both isolates were S.Typhi. Both MDR strains were resistant to NAL, sensitive to CEF and one was resistant to CIP. There were 83 (97.65%) isolates of non-MDR Salmonella Typhi. None of the S.Para A and S.Para B isolates were MDR.
One isolate of S.Para A was resistant to CEF, AMP, CIP and NAL. It was sensitive to TMP-SMZ and CHL. The MIC value for CEF was 256 μg/ml for this isolate and it was also positive for ESBL production. This isolate was susceptible to piperacillin-tazobactam, cefoperazone-sulbactum, Imipenem and Meropenem.
DISCUSSION
Enteric fever in India is commonly caused by S.Typhi and S.Para A, with a ratio of 10:1 cases, respectively.[3] In recent years there is an increase in isolation of S.Para A, which was observed in our study also.[10,14] One study showed at least 1.5 times higher isolation of S.Para A against S.Typhi.[15] Out of 103 Salmonella isolates, 16 isolates (15.53%) were S.Para A, which is 5.3 isolates of S.Typhi for every S.Para A isolate. We isolated only two (1.94%) S.Para B and they are known to be very rare in India.[3]
CHL, which was the drug of choice for enteric fever acquired resistance within few years of its introduction.[4] CHL resistant Salmonellae were initially susceptible to other first-line antibiotics like AMP and TMP-SMZ. Soon MDR Salmonellae, resistant to CHL, AMP and TMP-SMZ emerged in late 1980s and early 1990s.[2,3,4] In our study CHL showed a very good susceptibility of 97.65% against S.Typhi and 100% against S.Para A and S.Para B. The other first-line drugs i.e., AMP and TMP-SMZ also showed excellent susceptibility [Table 1]. Some studies done in India and other countries, points to an increase in MDR Salmonellae.[2,16,17] In India maximum MDR Salmonellae were seen in Central India (71.32%) and least in South India (55.2%). In contrast few studies show decrease in MDR strains i.e., 4-7%.[4,5] Only two isolates (1.94%) of Salmonella were MDR in our study and both isolates were S.Typhi. There was no MDR S.Para A or S.Para B. Re-emergence of susceptibility to CHL and other first-line drugs in previously resistant areas have been reported in studies done earlier.[2,4,10] The decreased use of first-line antibiotics in treating Salmonella and other infections could be the likely reason for this. However insufficient old data limited our study in comparing if there was a re-emergence of susceptibility to first-line drugs in our area.
After MDR Salmonella emerged FLUQ like CIP became the drug of choice for treating enteric fever. Others FLUQ like ofloxacin, levefloxacin and pefloxacin were also used. Due to irrational use of FLUQ, the antibiotic became less susceptible for therapy showing increasing MIC values of 0.125-1 μg/ml and even resistant with MIC value of >32 μg/ml.[4,5,18] Treatment failure, which is lack of defervescence even after seven days of therapy with CIP therapy, was observed due to reduced susceptibility to CIP.[2,5] Isolates having in-vitro resistance to Nalidixic acid (NAL), a quinolone antibiotic, have reduced susceptibility to Ciprofloxacin and it has been used as a reliable indicator to predict possibility of therapeutic failures during FLUQ therapy.[2,5,6,7,8,9] An increase in NAL resistance and decreased susceptibility or resistance to FLUQ has been reported in many studies.[2,5,6,8,9,10] While one study by Stevenson et al., showed as little as 2.3% NAL resistance, another study by Kumar et al., showed as high as 96% NAL resistance.[6,9] In our study S.Typhi showed 81.18% resistance to NAL, while S.Para A showed 62.5% resistance [Table 1]. Clinical follow up was not done for these cases, to see if FLUQ was used for therapy and if they responded. Many clinicians in India believe that the efficacy of CIP for treating enteric fever has decreased over the years.[18] It's not just the increased use of FLUQ in clinical practise but also an increased use of FLUQ in veterinary medicine, particularly in chicken and turkey has contributed largely in increasing resistance to NAL and FLUQ, due to consumption of such poultry meat.[6,19]
As FLUQ caused treatment failure due to reduced susceptibility, third generation Cephalosporins like CEF and Cefixime have gained popularity and used widely now.[5] Other antibiotics like fourth generation cephalopsorins, azithromycin, Tigecycline and Carbepemens have also been used for therapy. One isolate of S.Para A was resistant to CEF with an MIC of 256 μg/ml. As per CLSI guidelines an MIC of ≥64 μg/ml is interpreted as resistant.[7] The same isolate was also an ESBL producer. The patient from whom this strain was isolated was a case of carcinoma pyriform fossa, with metastasis to cervical lymph nodes, sepsis and multiorgan failure. This patient was on cefotaxime during the course of illness and expired on 13th day of admission. The blood culture was requested a day before patient's death and it was positive two days later. Hence specific treatment for Salmonella could not be initiated. It was unclear if the patient expired as a direct consequence of resistant S.Para A infection or other complications arising from malignancy metastasis. Salmonellae are known to cause infections in immune compromised and we suspect complications due S.Para A infection is the likely cause of death in this patient.[20] Although CEF has showed the best susceptibility in our study and in other studies, there are sporadic reports of CEF resistant and ESBL producing Salmonella emerging.[2,5] However we believe that CEF may remain susceptible for longer period as compared to FLUQ or CHL, as it an intravenous drug and may not be used frequently.
CONCLUSION
Routine screening of Nalidixic acid susceptibility would be practical to predict fluorquinolone resistance in Salmonella and preventing therapeutic failure while treating with it. It is worthwhile to consider replacing fluorquinolones with Chloramphenicol or Ceftriaxone as the first line of therapy for enteric fever whenever possible, especially when high resistance to Nalidixic acid is found in that region. Chloramphenicol may be re-considered as the first choice of therapy and third generation cephalosporins should be used judiciously and preserved as a reserve drug. With the emergence of susceptibility to older antibiotics, it may be warranted to recycle antibiotics against enteric fever periodically by analyzing Salmonella antibiogram and formulate the best possible treatment strategies at that point of time.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Drug-resistant Salmonella (WHO website) 2005. Apr, [Last Accessed on 2012 Mar 04]. Available from: http://www.who.int/mediacentre/factsheets/fs139/en/
- 2.Harish BN, Menezes GA. Antimicrobial resistance in typhoidal salmonellae. Indian J Med Microbiol. 2011;29:223–9. doi: 10.4103/0255-0857.83904. [DOI] [PubMed] [Google Scholar]
- 3.Ananthnarayan R, Paniker KJ. Ananthnarayan and Paniker's Textbook of Microbiology. 8th ed. Hyderabad: Universities Press (India) Private Limited; 2009. Enterobacteriaceae III: Salmonella; pp. 288–301. [Google Scholar]
- 4.Kumar Y, Sharma A, Mani KR. Re-emergence of susceptibility to conventionally used drugs among strains of Salmonella of Salmonella Typhi in central west India. J Infect Dev Ctries. 2011;5:227–30. doi: 10.3855/jidc.1310. [DOI] [PubMed] [Google Scholar]
- 5.Capoor MR, Nair D. Quinolone and cephalosporin resistance in enteric fever. J Glob Infect Dis. 2010;2:258–62. doi: 10.4103/0974-777X.68529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson JE, Gay K, Barrett TJ, Medalla F, Chiller TM, Angulo FJ. Increase in Nalidixic acid resistance among Non-Typhi Salmonella enterica isolates in the United States from 1996 to 2003. Antimicrob Agents Chemother. 2007;51:195–7. doi: 10.1128/AAC.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. WaynePA: Clinical and Laboratory Standards Institute; 2009. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Infromational Supplement.CLSI Document M100-S19. [Google Scholar]
- 8.Gorman R, Adley CC. Nalidixic acid-resistant strains of Salmonella showing decreased susceptibility to fluorquinolones in the mid-west region of the republic of Ireland. J Antimicrob Chemother. 2003;51:1047–9. doi: 10.1093/jac/dkg159. [DOI] [PubMed] [Google Scholar]
- 9.Kumar Y, Sharma A, Mani KR. High level of resistance to Nalidixic acid in Salmonella enteric serovar Typhi in Central India. J infect Dev Ctries. 2009;3:467–9. doi: 10.3855/jidc.419. [DOI] [PubMed] [Google Scholar]
- 10.Lakshmi V, Ashok R, Susmita J, Shailaja VV. Changing trends in the antibiogram of Salmonella isolates at a tertiary care hospital in Hyderabad. Indian J Med Microbiol. 2006;24:45–8. doi: 10.4103/0255-0857.19894. [DOI] [PubMed] [Google Scholar]
- 11.Koneman EW, Allen SD, Janda WM, Schreckberger PC, Winn CW., Jr . Colour Atlas and textbook of diagnostic Microbiology. 5th ed. PA: LippinTMP. SMZt Williams and Wilkins Company; 1997. The Enterobacteriaceae; pp. 171–252. [Google Scholar]
- 12.Miles RS, Amyes SG. Laboratory Control of Antimicrobial Therapy. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. NY: Churchill Livingstone; 1996. pp. 151–78. [Google Scholar]
- 13.Barrow GI, Feltham RK, editors. Cowan and Steel's Manual for Identification of Medical Bacteria. 3rd ed. Cambridge University Press: Cambridge; 2003. Characters of Gram negative bacteria; pp. 94–164. [Google Scholar]
- 14.Tankhiwale SS, Agrawal G, Jalgaonkar SV. An unusually high occurrence of Salmonella enteric serotype Paratyphi A in patients with enteric fever. Indian J Med Res. 2003;117:10–12. [PubMed] [Google Scholar]
- 15.Palit A, Ghosh S, Dutta S, Sur D, Bhattacharya MK, Bhattacharya SK. Increasing prevalence of Salmonella enterica serotype Paratyphi-A in patients with enteric fever in a periurban slum setting of Kolkata India. Int J Environ Health Res. 2006;16:455–9. doi: 10.1080/09603120601093188. [DOI] [PubMed] [Google Scholar]
- 16.Khanal B, Sharma SK, Bhattacharya SK, Bhattarai NR, Deb M, Kanungo R. Antimicrobial susceptibility patterns of Salmonella enteric Serotype Typhi in Eastern Nepal. J Health Popul Nutr. 2007;25:82–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Mengo DM, Kariuki S, Muigai A, Revathi G. Trends in Salmonella enteric serovar Typhi in Nairobi, Kenya from 2004 to 2006. J infect Dev Ctries. 2010;4:393–6. [PubMed] [Google Scholar]
- 18.Chitnis V, Chitnis D, Verma S, Hemvani N. Multidrug resistant Salmonella typhi in India. The Lancet. 1999;354:514–5. doi: 10.1016/S0140-6736(05)75549-5. [DOI] [PubMed] [Google Scholar]
- 19.Angulo FJ, Nargund VN, Chiller TC. Evidence of an association between use of anti-microbial agents in food animals and anti-microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. J Vet Med B Infect Dis Vet Public Health. 2004;51:374–9. doi: 10.1111/j.1439-0450.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay C, Rao PS, Shetty AK, Vidyasagar S, Varma M. Haemorrhagic pleural effusion in an HIV patient with Salmonella Typhimurium. J Clin Diagn Res. 2007;4:299–302. [Google Scholar]


