Abstract
Background:
In spite of laser being the gold standard treatment for Diabetic Macular edema (DME), some patients do not respond to laser. Various treatment modalities are being tried in the management of refractory diffuse DME (DDME).
Purpose:
To compare the efficacy of intravitreal bevacizumab (IVB), intravitreal triamcinolone acetonide (IVTA), and macular grid augmentation in the management of refractory DDME.
Settings and Design:
Prospective randomized study in a tertiary eye care center.
Materials and Methods:
Sixty patients with refractory DDME were randomly assigned to three groups: Group 1 received IVB (1.25 mg/0.05 ml), Group 2 received IVTA (4 mg/0.1ml), and Group 3 underwent laser augmentation. Primary outcome measures were best corrected visual acuity (BCVA) and central macular thickness (CMT) at the end of 6 months.
Statistics:
Analysis was performed using SPSS 14.0
Results:
Group 1 and 2 showed significant improvement in mean BCVA from 20/160 at baseline to 20/80 and from 20/125 to 20/63, respectively, at 6 months (P < 0.05). These groups also showed a significant reduction in the mean CMT from 457 ± 151 μ at baseline to 316 ± 136 μ and from 394 ± 61 μ to 261 ± 85 μ, respectively, at 6 months (P < 0.05). Group 3 showed only small improvement in mean BCVA from 20/100 to 20/80 (P = 1.0) while mean CMT increased from 358 ± 89 μ at baseline to 395 ± 127 μ at 6 months (P = 0.191). Eight (40%) eyes in Group 2 had intraocular pressure (IOP) rise and 10 (50%) eyes developed cataract.
Conclusions:
Both IVB and IVTA may be effective in the treatment of refractory DDME compared with macular grid augmentation. IVTA may be associated with side effects such as IOP rise and cataract formation.
Keywords: Diabetic macular edema, Intravitreal bevacizumab, Intravitreal triamcinolone, laser photocoagulation
Introduction
Diabetic macular edema (DME) is the leading cause of vision loss in diabetic patients.[1] The Early Treatment Diabetic Retinopathy Study (ETDRS) showed that focal and grid laser photocoagulation reduced the risk of moderate visual acuity loss for all eyes with DME by as much as 50% compared with the observation group but improvement in vision was minimum, which is probably related to retinal pigment epithelium (RPE), and consequent photoreceptor damage inherent to mechanism of action of laser.[2] Thus the goal of treatment in DME is to “maintain” visual acuity. Moreover, laser photocoagulation for DME may be associated with various side effects such as increase in dark adaptation time, paracentral scotoma formation, choroidal neovascularisation, and subretinal fibrosis.[3] Moreover, about 15% patients fall into the category of refractory DME and do not respond to repeated laser treatments despite a good metabolic control. This shows that in spite of laser being the gold standard treatment of DME, some patients do not respond to laser.[4,5] Various modalities of treatment are currently being tried in the management of persistent, laser refractory DME such as supplemental laser, intravitreal steroids, and anti-vascular endothelial growth factor (VEGF) drugs such as bevacizumab, pegabtanib, and ranibizumab.[6,7] In the present study, we evaluated the efficacy of intravitreal bevacizumab (IVB), intravitreal triamcinolone acetonide (IVTA), and macular grid laser augmentation in the management of refractory DME.
Materials and Methods
The present study was a prospective, randomized, comparative case series conducted at tertiary eye care center. The study conformed to the tenets of Declaration of Helsinki and institutional review board (IRB) clearance was taken. Patients were recruited for the study over a period of 6 months and a written; informed consent was obtained from all the patients prior to enrolment in the study. Patients having diffuse DME (DDME) on fundus fluorescein angiography (FFA) refractory to at least two prior sessions of macular laser photocoagulation, with central macular thickness (CMT) greater than 250 microns on time domain-optical coherence tomography (TD-OCT) without any evidence of vitreo-retinal traction and having good metabolic control (HbA1c < 7.0%) were included in the study. Patients with a history of having received prior intraocular, peribulbar or systemic steroids or prior anti-VEGF therapy, patients having uncontrolled diabetes mellitus, diabetic nephropathy, uncontrolled hypertension, patients with a history of myocardial infarction, stroke or other thromboembolic episode, one eyed patients, and patients who were not available for a follow-up duration of at least 6 months were excluded from the study. Patients fulfilling all inclusion and exclusion criteria were evaluated with a detailed history, complete systemic examination, and a thorough ocular examination including the best corrected visual acuity (BCVA) assessment using Snellen visual acuity chart, anterior segment evaluation, slit lamp biomicroscopy, indirect ophthalmoscopy, intra ocular pressure (IOP) measurement using Goldmann applanation tonometer, stereoscopic fundus photography, FFA, and OCT (Stratus OCT, Carl Zeiss Meditec; Dublin, CA). Patients with IOP > 21 mmHg or presence of glaucomatous optic nerve head changes were labeled as having glaucoma. Lens opacity classification system (LOCS) III was used to evaluate cataract. All patients were also evaluated for their metabolic control including fasting and postprandial blood sugars, serum lipid levels, renal function tests, and glycosylated hemoglobin levels. Eyes were randomly assigned to one of the three treatment groups: Group 1 eyes received IVB (Avastin; Genetech; IL, US) 1.25 mg/0.05 ml, Group 2 eyes received IVTA (Kenalog) 4 mg/0.1 ml, and Group 3 underwent modified ETDRS macular grid laser photocoagulation augmentation.
IVB and IVTA were injected via pars plana route in the doses mentioned above by a single experienced investigator using full aseptic precautions. Postinjection, all patients were prescribed topical moxifloxacin 0.5% qid for 5 days. Macular grid laser augmentation was performed by a single experienced examiner according to the modified ETDRS protocol with a spot size of 100 μ, pulse duration of 100 ms, and a power of 50–100 mW titrated to produce mild intensity burns in areas showing diffuse leakage on the FFA in a ‘C’ shaped zone between 500 and 3000 μ from the foveal center sparing the papilla-macular bundle.[3,4]
Patients in all the three groups were examined on the first postprocedure day to look for any complications following the procedure. Thereafter, follow up visits were conducted by a single masked investigator at 1, 3, and 6 months in each group. At each follow up visit, a complete systemic and ocular examination including fundus photography, FFA, OCT, and serological investigations similar to baseline were conducted. The BCVA, presence of cataract, IOP, CMT, and the metabolic control of the patient were noted at each follow up visit. In each group, eyes with a 2 line decrease in the BCVA from the baseline, increasing leakage on FFA or a 100 μ increase in the CMT on OCT were retreated according to the treatment regimen as outlined above for the respective group.
Statistical analysis was performed using SPSS (version 14.0; IL, CA). Quantitative data were expressed as Mean ± Standard Deviation and qualitative data were expressed as percentages. Parametric data were analyzed using the paired ‘t’ test and a ‘P’ value < 0.05 was considered as statistically significant.
Results
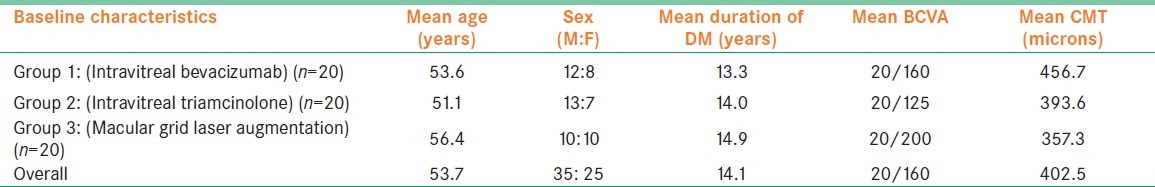
Sixty eyes of 60 patients were enrolled in the study over 6 months and all completed the study protocol with the final follow up visit at 6 months. The mean age of the study population was 53.7 ± 5.9 years. Of them, 58% were males and 42% were females. All the patients had Type 2 diabetes mellitus with 15 % requiring subcutaneous insulin injections for the control of blood sugar, whereas the rest were well controlled on oral hypoglycemic agents. None of the study eyes had preexisting glaucoma at the time of enrolment and the mean baseline IOP was 14.7 ± 2.1 mm of Hg. None of the study eyes had a visually significant cataract at the time of enrolment. Patients were consecutively randomized to one of the three treatment groups with 20 patients in each group. There were no significant differences amongst the groups for baseline characteristics [Table 1].
Table 1.
Baseline Characteristics of three treatment groups
Group 1 (IVB) eyes showed a statistically significant (P = 0.001) improvement in the mean BCVA that was well sustained over a follow up period of 6 months [Figure 1]. The mean number of IVB injections was 2.7 ± 0.4. Group 2 (IVTA) eyes also showed a statistically significant (P = 0.001) and well sustained improvement in the mean BCVA [Figure 2]. The mean number of IVTA injections received by Group 2 eyes was 1.4 ± 0.2. However, the group 3 (macular grid augmentation) eyes showed only a marginal improvement in the BCVA over a period of 6 months (P = 1.000). The mean number of laser treatment received by the Group 3 eyes was 1.8 ± 0 [Figure 3]. The mean BCVA at baseline and at each of the follow up visits in all the three study groups are summarized in Table 2.
Figure 1.
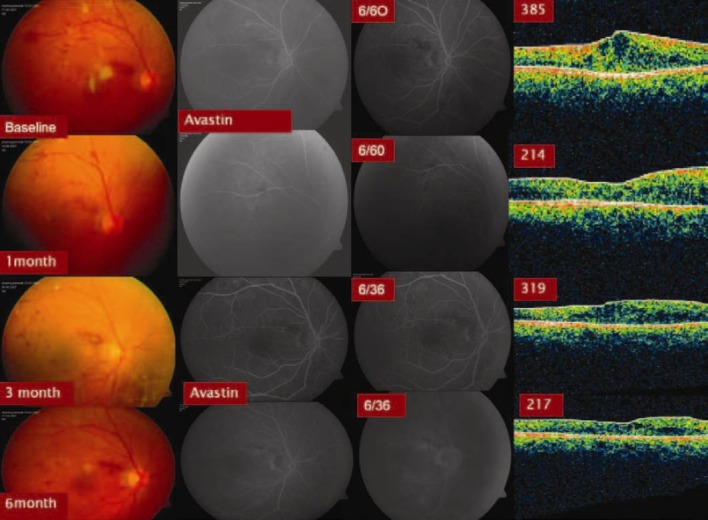
Six months results with Intravitreal Bevacizumab (Group 1) in Recalcitrant DDME
Figure 2.
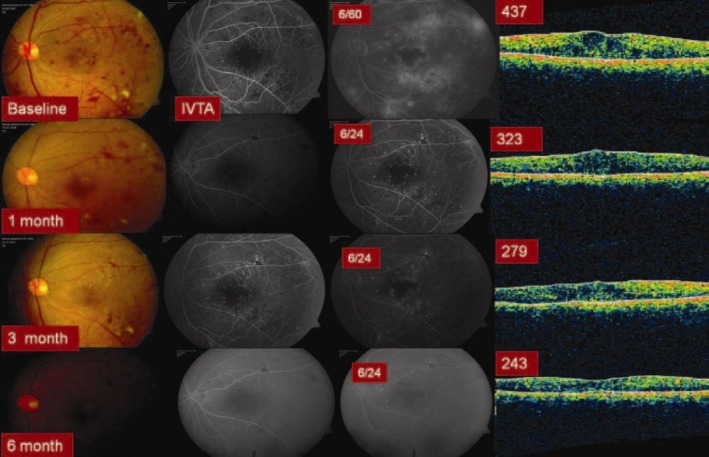
Six months results with Intravitreal Triamcinolone (Group 2) in Recalcitrant DDME
Figure 3.
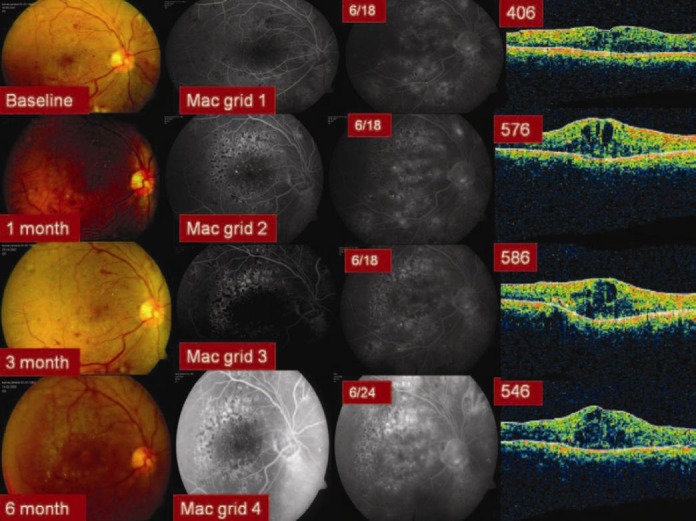
Six months results with Macular grid laser augmentation (Group 3) in Recalcitrant DDME
Table 2.
The mean Best Corrected Visual Acuity and central macular thickness (in microns) at baseline and each follow up visit in the three study groups
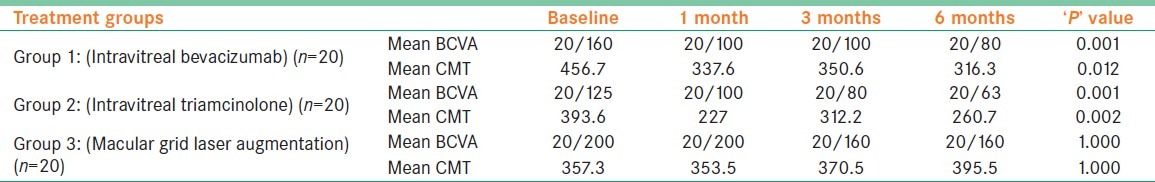
At the final follow up visit, four eyes in Group 1, two eyes in Group 2, whereas none in Group 3 showed a 3 line gain in the BCVA compared with baseline. None of the eyes in groups 1 and 2 showed a drop in the BCVA at the final follow up visit compared with baseline, whereas four eyes in group 3 showed a 1 line drop in the BCVA. The change in the BCVA at the final follow up visit compared with the baseline in each of the study groups is summarized in Table 3. Maximum vision gain in groups 1 and 2 was seen at 6th month follow-up, whereas in group 3, the same was seen at 3rd month follow-up, which persisted at 6th month follow-up [Table 2].
Table 3.
Change in the Best Corrected Visual Acuity at the final follow up visit compared with the baseline in each of the study groups

Both groups 1 and 2 showed a statistically significant decrease in the mean CMT on OCT compared with the baseline that was well sustained over a period of 6 months (P = 0.012 and P = 0.002, respectively). In contrast, group 3 eyes showed an increase in the mean CMT on OCT at the final follow up visit albeit not statistically significant. The details regarding the mean CMT at baseline and each follow up visit in the three study groups is summarized in Table 2.
Four eyes (20%) showed progression of cataract in group 1. Ten eyes (50%) in group 2 showed progression of cataract of which 6 eyes required cataract surgery within the 6 month follow up period. The mean duration of progression of cataract in IVTA group was around 3 months. None of the group 3 eyes showed progression of cataract. Similarly, no eyes in groups 1 and 3 showed a rise in the IOP that required treatment. However, 10 eyes (50%) in group 2 (IVTA) showed increase in IOP from baseline needing treatment with an average rise in IOP of 24mm of Hg. All the patients were managed successfully medically with none of the eyes needing a surgical intervention for the control of IOP. No adverse events like endophthalmitis or retinal detachment were observed in any group. No systemic side effects with IVB were observed in group 1 eyes.
Discussion
In DME blood retinal barrier is damaged as the result of loss of anchor proteins in tight junctions and trans-endothelial vesicular transport in capillary endothelial cells and/or RPE leading to passive leakage of water and electrolytes and retinal thickening.[1] Microaneurysms are almost always present in DME and contribute to retinal thickening. Thus the treatment of DDME should focus on passive permeability.[7]
Focal and/or grid laser photocoagulation is recommended and considered as the gold standard for the treatment of DME. Grid laser treatment for DME is believed to reduce permeability of leaky blood retinal barrier but several reports indicate that photocoagulation itself may induce blood retinal barrier breakdown and increased retinal thickness soon after grid laser treatment.[5] It has also been stated in previous studies that laser coagulation of macular region often does not lead to increase in vision and that macular edema especially in diffuse type may persists despite laser treatment.[3,4] Other reported adverse effects of laser photocoagulation are scotomas, field constriction decrease in dark adaptation, development of choroidal neovascularisation and subretinal scar formation.[8,9]
Despite being labeled as gold standard, nearly 15% of patients falls into the category of recalcitrant DME and do not respond to laser treatment. The latter can be classified as DME with or without vitreoretinal traction (VRT). In patients having recalcitrant DME with VRT, treatment is surgery but in case of refractory DME without VRT, repeated laser photocoagulation results in formation of scars that tends to increase with time and the likelihood of experiencing vision improvement is low in such patients.[7]
The existence of substantial group of patients with DME whose vision has failed to improve following laser photocoagulation has prompted clinicians to seek more effective treatment modalities.
IVTA has been seen to be effective treatment adjuvant in number of studies. It has been seen to both improve vision and reduce CMT in eyes with refractory DME.[10–12] IOP and cataract progression were reported as relatively common ocular side effects among others.[12–14] The exact mechanism of IVTA is unknown, but the rationale behind their usage lies in their ability to inhibit arachnoid pathway, down regulate the production of cytokines, and reduce breakdown of blood retinal barrier.[7]
The Diabetic Retinopathy Clinical Research Network (DRCR.Net) reported 2-year results of a multicenter randomized clinical trial comparing preservative free IVTA and focal/grid laser for DME. The mean visual acuity and reduction in CMT at 2 years after starting the treatment was better in the laser group compared with steroid groups. They, however, included primary cases of DME involving fovea.[15]
Nevertheless, IVTA is a promising therapy method for DME unresponsive to laser photocoagulation.[16] The therapeutic effect of the steroid is typically seen within 1 week, but in many patients, reinjections are needed every 3-6 months as the effect diminishes.[7] Repeated intravitreal injections, however, do carry their own inconvenience and risks including endophthalmitis and retinal detachment.
The advent of anti-VEGF agents marks a major advancement in treatment of various ocular diseases. Considering a key role of VEGF in pathophysiology of diabetic retinopathy, VEGF blockade is an attractive therapeutic approach.[17–19]
Bevacizumab (Avastin; Genentech Inc., San Francisco, CA) is a complete full-length humanized antibody that binds to all isoforms of VEGF-A. Safety and efficacy of off-label use of VEGF antibody IVB has been established in various studies.[20–26] Decrease in retinal thickness and improvement in visual acuity has also has been noted in patients with diffuse chronic macular edema that did not previously respond to laser photocoagulation, vitrectomy, or IVTA in the past.[25]
There is evidence to support selective blockade of VEGF-165 isoforms as a way to reduce VEGF mediated pathologic effect while preserving VEGF mediated normal physiologic function.[27,28] This Pan–VEGF blocking effect might be considered as disadvantage of IVB, however, recent popularity, more availability, and reasonable cost of this drug persuaded us to use this drug in our study.
In our study, we compare and evaluate IVB, IVTA, and standard laser treatment in nonresponsive persistent DME. Paccola et al. compared the morphological and visual outcomes associated with single IVTA versus IVB for the treatment of refractory diffuse DME and results showed that one single IVTA may offer certain advantages over IVB in short-term management of refractory DME, specifically with regard to changes in CMT.[29]
Shimura et al. compared the effect of an IVB with that of IVTA in persistent DME and results show that with generally used concentration, IVTA showed better results in reducing DME and in improvement of visual acuity than that of IVB.[30]
The effect of IVB lasts about 4-6 weeks and perhaps this would require a follow-up at second month postinjection as well for proper assessment of injection response. This alongwith a short follow-up duration of 6 months and limited sample size can be considered to be shortcomings of the study. The results of our study, however, revealed definite a benefit with both IVB and IVTA over grid laser augmentation for treatment of persistent refractory DME compared with grid laser augmentation in terms of both visual gain and reduction in CMT. No significant ocular adverse events like IOP rise were noted in eyes injected with IVB. However, significant proportion of eyes treated with IVTA showed adverse ocular events. Hence, IVB may be a better alternative in treatment of refractory DME.
Footnotes
Source of Support: None
Conflict of Interest: No.
References
- 1.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 2.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 3.Akduman L, Olk RJ. Laser photocoagulation of diabetic macular edema. Ophthalmic Surg Lasers. 1997;28:387–408. [PubMed] [Google Scholar]
- 4.Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse macular oedema: Long-term visual results. Ophthalmology. 1991;98:1594–602. doi: 10.1016/s0161-6420(91)32082-7. [DOI] [PubMed] [Google Scholar]
- 5.Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1995;113:1144–55. [PubMed] [Google Scholar]
- 6.Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003;136:122–35. doi: 10.1016/s0002-9394(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 7.Bhagat N, Ruben A, Grigorian MD, Tutela A, Zarbin MA. Diabetic Macular Edema: Pathogenesis and Treatment. Surv Ophthal. 2009;54:1–33. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Sander B, Larsen M, Moldow B, Lund-Andersen H. Diabetic macular edema: Passive and active transport of fluorescein through the blood-retina barrier. Invest Ophthalmol Vis Sci. 2001;42:433–8. [PubMed] [Google Scholar]
- 9.Chew EY, Ferris FL, 3rd, Csaky KG, Murphy RP, Agrón E, Thompson DJ, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: The early treatment diabetic retinopathy follow-up study. Ophthalmology. 2003;110:1683–9. doi: 10.1016/S0161-6420(03)00579-7. [DOI] [PubMed] [Google Scholar]
- 10.Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: Preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–24. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–7. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 12.Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 13.Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–6. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 14.Jonas JB, Kreissig I, Degenring R. Secondary chronic open-angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003;121:729–30. doi: 10.1001/archopht.121.5.729. [DOI] [PubMed] [Google Scholar]
- 15.Diabetic Retinopathy Clinical Research Network. A Randomised trial comparing intravitreal triamcinolone acetonide with focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–59. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson CA, Berkowitz BA, Sato Y, Ando N, Handa JT, de Juan E., Jr Treatment with intravitreal steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992;110:1155–9. doi: 10.1001/archopht.1992.01080200135041. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 18.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–8. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005;293:1509–13. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 20.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreat toxicity of bevacizumab Avastin. Retina. 2006;26:257–61. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–9. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ. Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol. 2006;142:162–4. doi: 10.1016/j.ajo.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–9. [PubMed] [Google Scholar]
- 24.Scott IU, Edwords AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. Diabetic retinopathy clinical research network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Sinha S. Intravitreal bevacizumab (Avastin) treatment of diffuse diabetic macular edema in an Indian population. Indian J Ophthalmol. 2007;55:451–5. doi: 10.4103/0301-4738.36481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol. 2008;86:372–6. doi: 10.1111/j.1600-0420.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 28.Kernt M, Neubauer AS, Kampik A. Intravitreal bevacizumab (Avastin) treatment is safe in terms of intraocular and blood pressure. Acta Ophthalmol Scand. 2007;85:119–20. doi: 10.1111/j.1600-0420.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 29.Paccola L, Cost RA, Folgosa MS, Barbosa JC, Scott IU, Jorge R. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME Study) Br J Ophthalmol. 2008;92:76–80. doi: 10.1136/bjo.2007.129122. [DOI] [PubMed] [Google Scholar]
- 30.Shimura M, Nakazawa T, Yasuda K, Shiono T, Iida T, Sakamoto T, et al. Comparative therapy evaluation of intravitreal bevacizumab and triomcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145:854–61. doi: 10.1016/j.ajo.2007.12.031. [DOI] [PubMed] [Google Scholar]


