Abstract
Preparations of Agelanthus dodoneifolius have been used in the traditional Nigerian medicine to treat malaria and this practice has remained till date without scientific validation. The antiplasmodial property of the water extract of Agelanthus dodoneifolius was evaluated in vivo and in vitro against Plasmodium berghei and clinical isolates of Plasmodium falciparum, respectively. There was a dose-dependent inhibition of parasitaemia in the in vivo antiplasmodial tests likewise, the in vitro screening demonstrated a strong and concentration-dependent activity (21.54 μg/ml < IC50 < 50 μg/ml) of the extract against the clinical isolates of Plasmodium falciparum. The phytochemical analysis revealed the presence of tannins, saponins, sterols, glycosides, phenols, anthraquinones, terpenes, reducing sugars and resins. It also showed a strong free-radical scavenging activity on 2, 2-diphenyl-2-picrylhydrazyl. The oral median lethal dose (LD50) in mice was estimated to be greater than 5000 mg/kg. Our results evidence that Agelanthus dodoneifolius may contain biologically active principles those are relevant in the treatment of malaria, thus supporting further studies of its active components.
Keywords: Acute toxicity, Agelanthus dodoneifolius, antioxidant, antiplasmodial, phytochemical screening
Agelanthus dodoneifolius, (synonyms – Tapinanthus dodoneifolius, DC Danser (Loranthaceae) is a ubiquist plant, especially parasitizing Mimosaceae which largely grow in West Africa[1]. African mistletoe (Agelanthus dodoneifolius [DC]) called ‘Kauchi’ in Hausa is a hemi-plant parasite used ethno medicinally by the Hausa and the Fulani tribes of Northern Nigeria as a remedy for several human and animal ailments that include stomach ache, diarrhoea, dysentery, wound and cancer[2]. The leaves and young twigs of the plants have been used in folklore medicine to treat different diseases such as circulatory and respiratory diseases, malaria, diabetes, hypertension and sterility[3]. The cardiovascular, spasmolytic and antiinflammatory activities of water extract of A. dodoneifolius have been reported[4]. Cepleanu et al.[5] also reported the larvicidal and molluscicidal activities of this plant.
The Loranthaceae constitutes the largest group of parasitic plants with about 950 plants distributed in 77 genera[6]. Loranthacean mistletoe, including A. dodoneifolius (DC) and other species are widely distributed in Nigeria and the plants are found on many host trees such as Mangifera indica, Phyllanthus niruri, Parkia biglobosa, Ziziphus spina-christi and Azadirachta indica trees[2].
The present study was undertaken to evaluate the antiplasmodial activity of the water extract of the twigs of A. dodoneifolius parasitic on Parkia biglobosa against Plasmodium berghei-infected Swiss albino mice and clinical isolate of Plasmodium falciparum to validate its potential values in the Nigerian traditional medicine for the treatment of malaria.
MATERIALS AND METHODS
The twigs of A. dodoneifolius were collected from host plant P. biglobosa in the month of February, 2009 from Chaza village in Niger state of Nigeria. The plant was identified and authenticated and a voucher specimen (NIPRD/H/6543) was deposited at NIPRD Herbarium for future reference.
Extraction of plant materials:
The plant material was cleaned, air dried under shade and pounded into fine powder using a mortar and pestle. A 100 g quantity of the powder was boiled with 1 l of distilled water for 30 min. The decoction was decanted, centrifuged at 4500 rpm (Hamburg-Eppendorf, Germany) for 30 min and freeze-dried. The total yield of dark brown extract was 11.33% w/w of crude starting material. The freeze-dried powder was stored in an airtight container and used for the study.
Test systems:
Swiss albino mice (20-25 g) of either gender maintained at the Animal Facility Centre (AFC) of the Department of Pharmacology and Toxicology, NIPRD, Abuja, Nigeria, were used for the study. The animals were fed ad libitum with the standard feed (Ladokun feeds, Ibadan, Nigeria) and had free access to water. They were also maintained under standard conditions of humidity, temperature and 12 h light/ darkness cycle. The animals were acclimatised for 2 weeks before the commencement of the study. A standard protocol was drawn up in accordance with the Good Laboratory Practice (GLP) regulations of the ENV/MC/CHEM,1998)[7]. The principle of laboratory animal care[8] was also followed in this study.
The chloroquine-sensitive P. berghei (NK-65) obtained from the National Institute for Medical Research (NIMR), Lagos, Nigeria and kept at the Department of Pharmacology and Toxicology, NIPRD, Idu, Abuja, Nigeria were used for this study. The parasites were kept alive by continuous re-infestation (i.p.) in mice every 4 days. Parasitised erythrocytes were obtained from a donor-infected mouse by cardiac puncture in heparin and made up to 20 ml with normal saline. Animals were inoculated intraperitoneally with infected blood suspension (0.2 ml) containing 1×107 parasitised erythrocytes on day 0. Infected mice with parasitaemia of 5-7% were allocated to eight groups of six mice each[9].
Phytochemical tests:
The phytochemical screening of the water extract of A. dodoneifolius twig was carried out to determine the presence of the following compounds alkaloids, flavonoids, tannins, anthraquinones, saponins, glycosides, sterols, resins, volatile oil, terpenes and phenols using the standard procedures[10].
Antioxidant potential:
Ascorbic acid was used as the antioxidant standard and the methanol-soluble portion of the freeze-dried water extract of A. dodoneifolius was extracted, concentrated and used for the antioxidant assessment. A. dodoneifolius and ascorbic acid at equal concentrations of 0.0675, 0.125, 0.25 and 0.5 mg/ml in methanol were prepared. The radical-scavenging activities of the extract against 2,2-diphenyl-1-picryl hydrazyl radical (DPPH) were determined by UV spectrophotometric methods[11]. One millilitre quantities of the extract and ascorbic acid were placed in a test tube, and 3 ml of methanol was added followed by 0.5 ml of 1 mM DPPH in methanol. These were allowed to stand for 30 min before the absorbance were taken at 517 nm. The blank solution consists of the same amount of methanol and DPPH. The radical scavenging activity presented as percentage inhibition was calculated using Eqn. 1. % Inhibition=[(Ab-Aa)/Ab]×100…(1), Where Ab is the absorbance of the blank sample and Aa is the absorbance of the drug (A. dodoneifolius or ascorbic acid).
Acute toxicity study:
The acute toxicity of the extract was determined using modifications of Lorkes method[12]. The test was carried out in two phases. Phase 1: Nine mice were divided into three groups of three mice per group. The three groups were administered orally with graded doses (10, 100 and 1000 mg/kg, respectively) of the extract. Phase 2: Another nine mice were divided into three groups of three mice per group, which received graded doses (1600, 2900 and 5000 mg/kg) of the extract, respectively. The number of deaths in each group within 24 h was recorded and the final LD50 values were calculated as the geometric mean of the highest nonlethal dose (with no deaths) and the lowest lethal dose (where deaths occurred).
In vivo studies; suppressive test:
A total of 54 mice were used for this study. Each mouse was given standard intraperitoneal inoculums of P. berghei parasites. The animals were randomly divided into nine groups of six mice each. The animals were administered graded doses (50, 100, 200, 300, 400, 500 and 600 mg/kg/day) doses of the extract, chloroquine diphosphate 25 mg/ kg/ day (positive control) and 0.2 ml of normal saline (negative control) for 4 consecutive days (D0 to D3). On the 5th day (D4), thick blood smears were prepared and blood films were fixed with methanol. The blood films were stained with Giemsa, and examined with ×100 magnification microscope. The percentage suppression of parasitaemia was calculated for each dose level by comparing the parasitaemia in infected controls with those of treated mice[13].
In vivo studies; curative test:
On the 1st day (D0), standard inoculums of (1×107) P. berghei-infected erythrocytes were injected intraperitoneally. Seventy two hours later, the mice were randomly divided into nine groups of six mice each. Seven groups received graded doses of the extract (50, 100, 200, 300, 400, 500 and 600 mg/kg/ day) for 5 days. The remaining two groups received Chloroquine diphosphate (25 mg/ kg/ day) and 0.2 ml of normal saline, respectively for 5 days. Thick blood smears were prepared from tail of each mouse for 5 days, to monitor the parasitaemia level. The mean survival time for each group was determined arithmetically by finding the average survival time (days) of the mice (postinoculation) in each group over a period of 28 days (D0–D27)[14].
In vivo studies; prophylactic test:
Mice of either gender were randomly selected and divided into nine groups of six mice per group. Seven groups received graded (50, 100, 200, 300, 400, 500 and 600 mg/kg/day) doses of the extract orally for 4 consecutive days. The remaining two groups received 25 mg/kg/day of chloroquine diphosphate (positive control) and 0.2 ml/mouse/day normal saline (negative control) for 4 consecutive days (D0-D3), respectively. On the 5th day (D4), the mice were inoculated with P. berghei-infected red blood cells. Seventy-two hours later, the parasitaemia level was assessed by studying the Giemsa-stained blood smears[15].
In vitro studies:
Three fresh blood specimens were collected from three patients suffering from fever and other malaria symptoms with confirmed infection by P. falciparum. Already prepared, dried-in-acridine orange-stained thin smears were examined for Plasmodium species identification. The parasite density was determined by counting the number of infected erythrocytes amongst 20,000 erythrocytes. From each patient, 4 ml of venous blood was collected in an EDTA anticoagulant–coated tube. Samples with monoinfection due to P. falciparum and a parasite density between 1 and 2% were used for the in vitro antimalarial tests[16].
The assay was performed in duplicate in a 96-well microtiter plate, according to WHO method in vitro micro test[16]. RPMI 1640 (Sigma-Aldrich, USA) was the culture medium used for cultivation of P. falciparum. Dilutions were prepared from the crude plant extract and the final concentrations prepared by dilution were (100, 50, 25, 12.50, 6.25 and 3.125 μg/ml). Negative controls (treated with solvent) and positive controls (chloroquine phosphate) were added to each set of experiments. Fifty microliters of blood mixture media was added to each well in the plate and incubated in a candle jar (with gas environment of about 3% O2, 6% CO2 and 91% N2)[17] at 37.0° for 24-30 h. After incubation, contents of the wells were harvested and stained for 5 min in an already prepared dried-in-acridine orange reagent. The parasites were counted in five fields of vision (>200 total cells) using a fluorescence microscope (Partec cyscope malaria fluorescence microscope, Partec GmbH, Germany) at a magnification of ×40.
Statistical analysis:
Results were expressed as the mean±standard error of mean (SEM). Statistical analysis of data was carried out using one-way analysis of variance (ANOVA). The IC50 values were determined graphically on a log dose-response curve (log concentration versus percent inhibition curves) by interpolation whereas those values for the antioxidant activities were calculated from the linear regression of plots of concentration of the test compounds (mg/ml) against the percentage of inhibition of DPPH.
RESULTS AND DISCUSSION
Phytochemical screening of the water extract of twig of A. dodoneifolius revealed the presence of saponins, tannins, anthraquinones, terpenes, phenols, sterols and reducing sugars. Alkaloids, flavonoids and volatile oil were not detected (Table 1). The active principles of many drugs found in plants are secondary metabolites. Therefore, basic phytochemical investigation of these extracts for their major phytoconstituents is also vital, hence standard chemical tests were carried out to determine the presence of alkaloids, terpenes, steroids, flavonoids, tannins, anthraquinones and cardiac glycosides, phenols, reducing sugars, resins, volatile oil and saponins[18]. The presence of saponins, anthraquinones, tannins, terpenes, phenols, reducing sugars and sterols in A. dodoneifolius may explain some of the antiplasmodial actions of the extract, since the antiplasmodial actions of most of these phytochemical substances were found in some of the host plants and had been documented[19–23].
TABLE 1.
PHYTOCHEMICAL ANALYSIS OF WATER EXTRACT OF A. DODONEIFOLIUS

Fig. 1 shows the comparative antioxidant effect of the crude extract of A. dodoneifolius and the ascorbic acid. The antioxidant activities of A. dodoneifolius and ascorbic acid increased with increase in concentration. The water extract of A. dodoneifolius and ascorbic acid showed maximum DPPH radical-scavenging activity of 95.7 and 98.1%. Although the mechanism of action of the antiplasmodial activities of the extract has not been evaluated, the presence of a number of bioactive secondary metabolites with antiplasmodial potential may mean that the antiplasmodial action of the extract may be by interplay of different mechanisms. Ayoola et al.[11] had reported high level of antioxidants in antiplasmodial activity, thus, the presence of such metabolites as tannins, flavonoids, phenols, saponins and terpenes with antioxidant activities could be related to the antiplasmodial action of A. dodoneifolius.
Fig. 1.
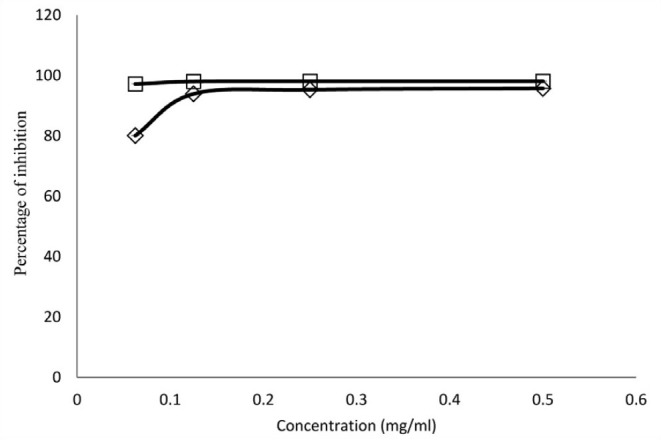
Comparative radical scavenging effect of A. dodoneifolius and ascorbic acid
-◊- Ascorbic acid and -□- A. dodoneifolius
In the acute toxicity test, A. dodoneifolius up to the dose level of 5000 mg/kg of body weight did not exhibit any lethality or toxic symptoms. The absence of death at all doses up to 5000 mg extract/ kg showed that the LD50 of the water extract of the twig of A. dodoneifolius is greater than 5000 mg/ kg p.o. The acute toxicity of A. dodoneifolius has been investigated to determine any adverse effect that may arise as a result of a short-time animal exposure to the water extract within a 24 h period. Although A. dodoneifolius has been used by traditional medicine practitioners without report of any mortality due to toxicity, this claim has been authenticated by the lack of death at oral treatment of over 5000 mg/kg body weight of the extract. The results thus suggest that the water extract of the twigs of A. dodoneifolius is acutely nontoxic[24].
The water extract of A. dodoneifolius indicated a dose-dependent inhibition of parasitaemia at the different doses employed. The extract at 600 mg/ kg weight of the mice showed maximum inhibition (78.7±1.6, 80.1±1.0 and 69.8±1.2%) of parasitaemia (fig. 2). The values were found to be significant from dose of 200 mg extract/kg; chloroquine also exhibited a significant reduction in parasitaemia (Table 2) The water extract of A. dodoneifolius also displayed dose-dependent chemosuppressive activities similar to chloroquine (fig. 3). There was a dose-dependent mean survival time in mice treated with graded doses of the extract (fig. 4), with significant values from 18.9±1.6 to 28.0±1.3 days (Table 3). Plasmodium species that cause human disease are essentially unable to infect nonprimate animal models. So, in vivo evaluation of antimalarial compounds begins with the use of rodent malaria parasite, P. berghei, this parasite is sensitive to chloroquine. P. berghei 4 day suppressive test was used to assess the efficacy of our extract by comparison of blood parasitaemia and mouse survival time in treated and untreated mice[19]. Chloroquine, which has been used for curative, suppressive and prophylactic treatment of malaria, was used as the standard antimalarial in this study[25]. A. dodoneifolius extract demonstrated similar antiplasmodial activities to chloroquine. The extract exhibited a significant dose-dependent suppressive and curative activity against P. berghei in mice and enhanced the mean survival time period of the treated mice.
Fig. 2.
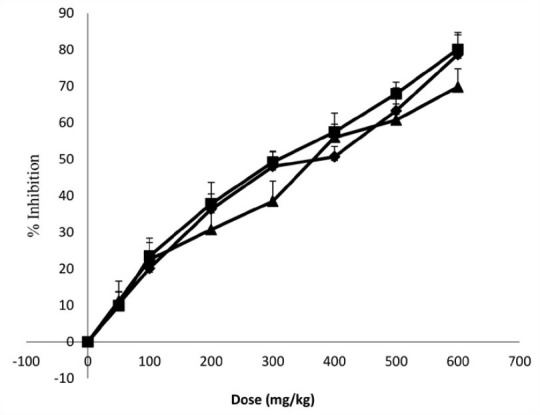
In vivo antiplasmodial activity of water extract of Agelanthus dodoneifolius, twig
-♦- Suppressive activity A.D; -■- Curative activity A.D; -▲- Prophylactic activity A.D
TABLE 2.
EFFECT OF A. DODONEIFOLIUS ON PLASMODIUM BERGHEI INFECTION IN MICE
Fig. 3.
In vivo antiplasmodial activity of water extract of Agelanthus dodoneifolius, twig versus chloroquine
-■- CQ (25mg/ml)  A. D (600mg/ml)
A. D (600mg/ml)
Fig. 4.
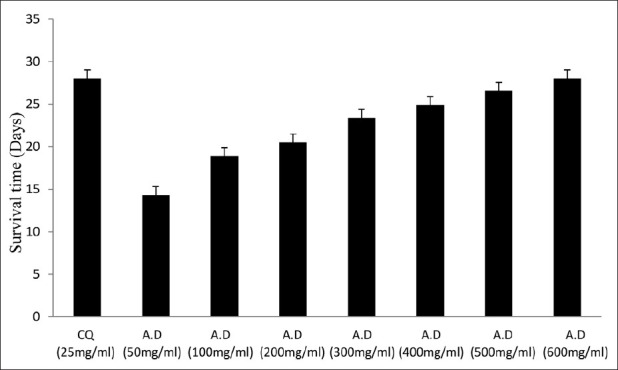
Mean survival time of treated mice.
Survival time of mice treated with water extract of Agelanthus dodoneifolius in comparision to that of mice treated with chloroquine
TABLE 3.
MEAN SURVIVAL PERIOD OF MICE TREATED WITH A. DODONEIFOLIUS
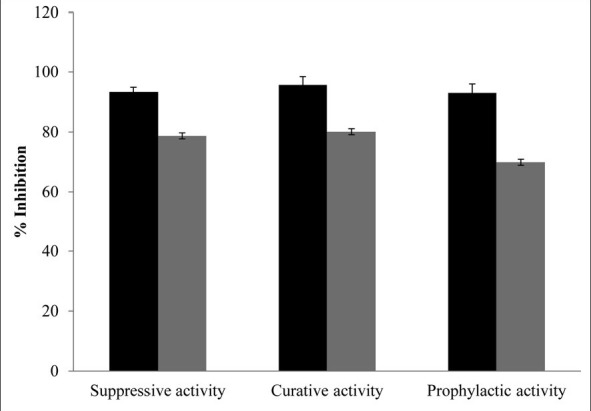
The photomicrographs of the in vitro antiplasmodial activity of the water extract of A. dodoneifolius are presented in fig. 5. The water extract of A. dodoneifolius twigs showed a concentration-dependent inhibition of P. falciparum. Maximum plasmodia inhibition of 75.0±1.2% at a concentration of 100 μg/ml for the water extract of A. dodoneifolius and 100±1.0% for chloroquine phosphate at a concentration of 0.2 μg/ml (fig. 6). The IC50 of the water extract of A. dodoneifolius was determined as 21.54 μg/ml (fig. 7). This screening also showed that the extract has a significant antiplasmodial activity. With the rapid spread of antimalarial drug resistance over the last few decades, the need for monitoring has increased. The most commonly used method for the antimalarial in vitro testing for resistance is Micro-test (Mark III)[16]. It provides information on the quantitative drug response of P. falciparum irrespective of the patient's immune system. Quantitative assessment of in vitro malarial activity was determined by means of the fluorescence microscopy, particularly with acridine orange based on use of Plasmodium nucleic acid-specific fluorescent dyes to facilitate detection of the parasites[27]. The IC50 A. dodoneifolius is 21.54 μg/ml suggesting that the plant has a moderate antiplasmodial activity[28]. These workers proposed that a plant with an IC50 of less than 50 μg/ml has a moderate antimalarial activity. Although A. dodoneifolius (21.54 μg/ml) has a moderate antiplasmodial activity compared to the standard chloroquine phosphate (0.050 μg/ml), this result is similar to those obtained from other medicinal plants with high antiplasmodial potential[29]. The relatively higher values of IC50 obtained from the extract may be due to crude nature of the bioactive materials. The decrease in parasitaemia with increasing concentration of the extract reflects an inhibitory activity on parasite replication, thus supporting the isolation and development of the biologically active substances of this medicinal plant as antimalarial agents.
Fig. 5.
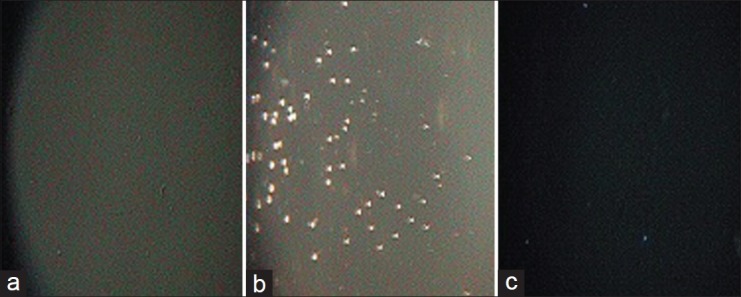
Photomicrographs of in vitro antiplasmodial activity of the water extract of Agelanthus dodoneifolius.
Magnification: ×5 (a) Complete RPM1 medium; (b) Untreated RPM1 medium with P. falciparium; (c) RPM1 medium treated with Agelanthus dodoneifolius extract
Fig. 6.
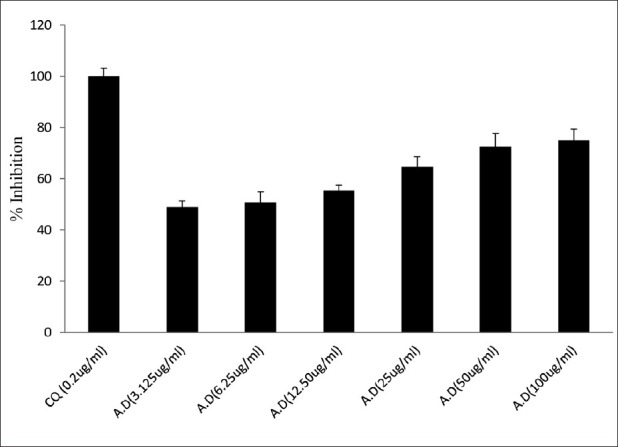
In vitro antiplasmodial activity.
Antiplasmodic activity of water extract of Agelanthus dodoneifolius in comparison to that of chloroquine against P. falciparum
Fig. 7.
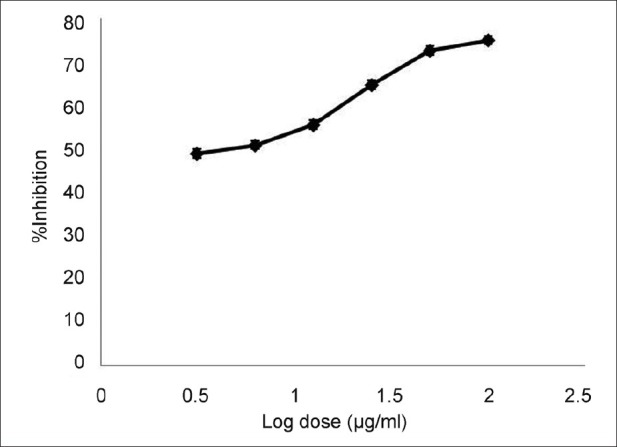
Dose-dependent in vitro antiplasmodial activity.
In vitro antiplasmodial activity of water extract of Agelanthus dodoneifolius against P. falciparum
A. dodoneifolius harvested from P. biglobosa is a parasite of the xylem tissue of P. biglobosa and depends on its host for water, nutrients and some carbon compounds[30], by a series of chemical exchange. This dependence may be responsible for its antiplasmodial activity since P. biglobosa has known antiplasmodial activity[19]. This study has also shown possible similarities in the phytochemical constituent[2] as well as the pharmacological activities between a parasitic plant and its host due to the intimate and complex chemical exchange between the parasite and the host plant.
The antimalarial activity shown by the water extract of A. dodoneifolius could have been as a result of the active antiplasmodial components contained in the extract, mainly saponins, tannins, terpenes, anthraquinones, phenols, sterols and reducing sugars. Antioxidant activity may represent another mechanism that contributes to its antimalarial activity. Although the bioactive components and mechanism are yet to be identified, the results of this study provide the basis for further studies. This study also confirms the rational usage of this plant in traditional Nigerian medicine.
ACKNOWLEDGEMENTS
This work was carried out in laboratories of Department of Medicinal Plant Research and Traditional Medicine, Department of Pharmacology and Toxicology and Department of Microbiology and Biotechnology (NIPRD), Idu Industrial area, Abuja, Nigeria. Authors are thankful to Dr. Jemilat Ibrahim, Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development (NIPRD), Abuja for plant authentication. The authors are grateful to Prof. U. A. Osunkwo and Mrs. G. A. Ajoku for providing support and encouragement.
Footnotes
Builders, et al.: Antiplasmodial Potential of the Agelanthus dodoneifolius
REFERENCES
- 1.Boussim IJ, Guinko S, Tuquet C, Salle G. Mistletoes of the agroforestry parklands of Burkina Faso. Agroforestry Syst. 2004;60:39–49. [Google Scholar]
- 2.Deeni YY, Sadiq NM. Antimicrobial properties and phytochemical constituents of the leaves of African mistletoe (Tapinanthus dodoneifolius (DC) Danser) (Loranthaceae): An ethnomedicinal plant of Hausaland, Northern Nigeria. J Ethnopharmacol. 2002;83:235–40. doi: 10.1016/s0378-8741(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 3.Efuntoye MO, Ayodele AE, Thomas BT, Ajayi TO. Does host plant affect the antibacterial activity of Tapinanthus bangwensis (Engl. and K. Krause) Danser (Loranthaceae)? J Med Plant Res. 2010;4:1281–4. [Google Scholar]
- 4.Ouédraogo S, Traoré A, Somé N, Lompo M, Guissou PI, Schott C, et al. Cardiovascular properties of aqueous extract from Tapinanthus dodoneifolius DC DANSER. Afr J Tradit Complement Altern Med. 2005;1:25–30. [Google Scholar]
- 5.Cepleanu F, Hamburger MO, Sordat B, Msonthi JD, Gupta MP, Saadou M, et al. Screening of tropical medicinal plants for molluscicidal, larvicidal, fungicidal and cytotoxic activities and brine shrimp toxicity. Int J Pharmacol. 1994;323:294–307. [Google Scholar]
- 6.Engone Obiang NL, Sallé G. Is there any point to eradicate Phragmanthera capitata parasitizing African rubber trees? C R Biol. 2006;329:185–95. doi: 10.1016/j.crvi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.ENV/MC/CHEM. OECD series on principles of good laboratory practice and compliance monitoring number 1. OECD Principles on good laboratory practice (as revised in 1997). Environment Directorate Organization for Economic Co.operation and Development Paris. 1998. [Last accessed 2012 May 24]. Available from: http://www.iris.pharma.com/download/Principles.on.GLP.pdf .
- 8.NIH Publication No. 85-23, (1985). Respect for life. National Institute of Environmental Health Sciences-NIEHS. [Last accessed on 2012 May 24]. Available from: http://www.niehs.nih.gov/oc/factsheets/wrl/studybgn.htm .
- 9.Hilou A, Nacoulma OG, Guiguemde TR. In vivo antimalarial activities of extracts from Amaranthus spinosus L and Boerhaavia erecta L. in mice. J Ethnopharmacol. 2006;103:236–40. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Builders MI, Wannang NN, Ajoku GA, Builders PF, Orisadipe A, Aguiyi JC. Evaluation of the antimalarial potential of Vernonia ambigua Kotschy and Peyr (Asteraceae) Int J Pharmacol. 2011;7:238–47. [Google Scholar]
- 11.Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, et al. Phytochemical screening and antioxidant activities of some selected medicinal plant used for malaria therapy in South western Nigeria. Trop J Pharm Res. 2008;7:1019–24. [Google Scholar]
- 12.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 13.Knight DJ, Peters W. The antimalarial activity of N-benzyloxydihydrotriazines. I. The activity of clociguanil (BRL 50216) against rodent malaria, and studies on its mode of action. Ann Trop Med Parasitol. 1980;74:393–404. [PubMed] [Google Scholar]
- 14.Chandel S, Bagai U. Antiplasmodial activity of Ajuga bracteosa against Plasmodium berghei infected BALB/c mice. Indian J Med Res. 2010;131:440–4. [PubMed] [Google Scholar]
- 15.Okokon JE, Ofodum KC, Ajibesin KK, Danladi B, Gamaniel KS. Pharmacological screening and evaluation of antiplasmodial activity of Croton zambesicus against Plasmodium berghei berghei infection in mice. Indian J Pharmacol. 2005;37:243–6. [Google Scholar]
- 16.WHO. In vitro micro test (Mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine and artemisinin. 2001 CTD/MAL/97, 20 Rev. [Google Scholar]
- 17.Mohd Ridzuan MA, Noor Rain A, Zhari I, Zakiah I. Effect of Eurycoma longifolia extract on Gluthathione level in Plasmodium falciparum infected erythrocytes in vitro. Trop Biomed. 2005;22:155–63. [PubMed] [Google Scholar]
- 18.Inge ND. Magic and Medicine of Plants. 2nd ed. Pleasantville (NY): The Readers Digest; 1993. [Google Scholar]
- 19.Builders M, Wannang N, Aguiyi J. Antiplasmodial activities of Parkia biglobosa: In vivo and in vitro studies. Ann Biol Res. 2011;2:8–20. [Google Scholar]
- 20.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (mango) Pharmacogn Rev. 2010;4:42–8. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chianese G, Yerbanga SR, Lucantoni L, Habluetzel A, Basilico N, Taramelli D, et al. Antiplasmodial triterpenoids from the fruits of neem, Azadirachta indica. J Nat Prod. 2010;73:1448–52. doi: 10.1021/np100325q. [DOI] [PubMed] [Google Scholar]
- 22.Singh B, Agrawal PK, Thakur RS. Isolation of trans-Phytol from Phyllanthus niruri. Planta Med. 1991;57:98. doi: 10.1055/s-2006-960039. [DOI] [PubMed] [Google Scholar]
- 23.Adzu B, Haruna AK, Salawu OA, Katsayal UA, Njan A. In vivo antiplasmodial activity of ZS-2A: A fraction from chloroform extract of Zizyphus spina-christi rootbark against Plasmodium berghei berghei in mice. Int J Biol Chem Sci. 2007;1:281–6. [Google Scholar]
- 24.Cobett JR, Wight K, Baille AC, editors. The Biochemical Mode of Action of Pesticides. 2nd ed. London and New York: Academic Press; 1984. [Google Scholar]
- 25.Ajaiyeoba E, Falade M, Ogbole O, Okpako L, Akinboye D. In vivo antimalarial and cytotoxic properties of Annona senegalensis extract. Afr J Tradit Complement Altern Med. 2006;3:137–41. [Google Scholar]
- 26.Kalra BS, Chawla S, Gupta P, Valecha N. Screening of antimalarial drugs: An overview. Indian J Pharmacol. 2006;38:5–12. [Google Scholar]
- 27.Hassan SD, Okoued SI, Mudathir MA, Malik EM. Testing the sensitivity and specificity of the fluorescence microscope (Cyscope) for malaria diagnosis. [Last accessed on 2012 May 24];Malar J. 2010 9:88. doi: 10.1186/1475-2875-9-88. Available from: http://www.malariajournal.com/content/9/1/88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasoanaivo P, Petitean A, Ratsimamanga-Urverg S, Rakoti RA. Medicinal plants used to treat malaria in Madagascar. J Ethnopharmacol. 1992;37:117–27. doi: 10.1016/0378-8741(92)90070-8. [DOI] [PubMed] [Google Scholar]
- 29.Palaniswamy M, Pradaep RV, Sathya R, Angayarkanni J. In vitro antiplasmodial activity of Trigonella foenum graecum L. Evid Based Complement Alternat Med. 2008;10:1–5. doi: 10.1093/ecam/nen030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Didier DS, Ndongo D, Jules PR, Desire TV, Henri F, Georges S, et al. Parasitism of host trees by the Loranthaceae in the region of Douala (Cameroon) Afr J Env Sci Tech. 2008;11:371–8. [Google Scholar]


