Abstract
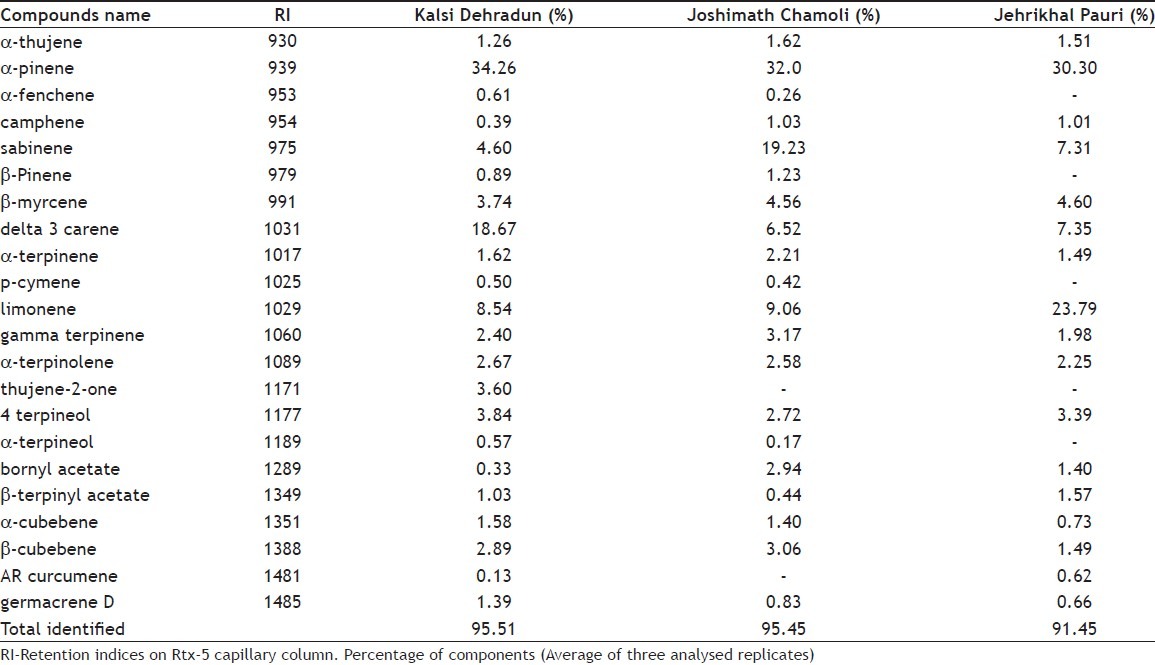
The aim of the present study was to investigate the various chemical components present in the volatile oil of the leaf of Cupressus torulosa and to find variation of essential oil components among the populations. Twenty-two, 17 and 20 compounds were identified with 95.45, 95.45 and 91.45% in Kalsi, Joshimath and Jeharikhal, respectively were identified by gas chromatography-mass spectrometry and quantify by gas chromatography and flame ionization detector (GC-FID). The major compound identified was α-pinene in all the populations and it varied between 30.30 and 34.26%. Results of the study stated that α-pinene, δ- 3-carene, limonene and sabinene components were detected in high concentration, thus competent for use in related industries and as a favourite ornamental aromatic tree.
Keywords: α-pinene gas chromatography, Cupressus torulosa, gas chromatography-mass spectrometry, variation, volatile oil
The word Cupressus is taken from the Greek Kuparissos, the ancient name of Cypress. This genus consists of approximately a dozen species[1]. The common names for Cupressus torulosa are Bhutan cypress and Himalayan cypress. It is an evergreen tree that grows up to 30-40 m tall. Its branches are horizontal and branchlets are 2–3 pinnate, curved and whip like. Leaves are four to many ranks. The stamens are numerous, each with 2–6 globose anther cells. Volatile oil obtained from the essential oil of the leaves is used to treat rheumatism and whooping cough, and as an astringent[2]. Biflavones, viz. amentoflavone, cupressuflavone, hinokiflavone, and apigenin, are present in the leaves of C. torulosa[3]. Chemical analysis of the essential oil present in the foliage of C. torulosa contains mono-, sesqui- and di-terpenes[4]. Keeping this in view, the present study was designed to access variation in volatile constituents of the leaves from three distinct locations. Fresh leaves of C. torulosa were collected from Kalsi district Dehradun, Joshimath district Chamoli and Jeharikhal district Pauri Garhwal during the month of April, 2010. The specimens were kept in Centre Herbarium and identified at our centre. The essential oil from 500 g of the sample was extracted by hydro-distillation for 6 h using Clevenger apparatus[5]. The essential oil content was determined as percentage (v/w %) on fresh weight basis as an average of three independent extractions of each population to minimise error. The oil obtained was dehydrated over anhydrous sodium sulphate and kept in refrigerator at 4° before analysis. The gas chromatography (GC) analyses of the oil samples were carried out using Agilent (model 6890) gas chromatograph equipped with a Flame Ionization detector (FID) and a HP-5 fused silica column (30 m×032 mm, 0.2 μm film thickness). Nitrogen was used as a carrier gas during analysis. The injector and detector temperature were maintained at 210° and 230°, respectively. The column oven temperature was programmed from 60° to 220° with an increase in rate of 3°/min. The injection volume was 0.2 μl. The gas chromatography-mass spectrometry (GC-MS) analysis of the oil was performed on a Perkin Elmer mass spectrometer (Model Claurus 500) coupled to a Perkin Elmer Claurus 500 gas chromatograph with a 60 m×0.32 mm, 0.2 μm film thickness column (RtX5). Helium was used as the carrier gas (flow rate 1 ml/min). The oven temperature was programmed range from 60° to 220° at 3°/min. Other conditions were the same as described under GC. The mass spectrum was taken with a mass range of 40-600 Daltons. The identification of constituents was performed on the basis of retention index (RI), determined with reference to the homologous series of n-alkanes, C9-C32 under experimental conditions, co-injection with standards (Sigma Aldrich USA), MS library search (NIST/Pfleger/Wiley), and by comparing with the MS literature data[6,7]. The relative amounts of the individual components were calculated based on the GC peak area without correction factors. Present study revealed that the essential oil content varied among the populations. Maximum yield was obtained in the sample collected from Joshimath population (1.3%). Maximum amount of α-pinene was found in the sample collected from Kalsi region as compared to Joshimath and Jeharikhal populations (Table 1). α-pinene (34.25%), δ-3 carene (18.67%), limonene (8.54%) and sabinene (4.60%) were the major components in the sample collected from Kalsi, a total of 22 components detected including other minor components like β-myrcene, 4-terpineol, β-cubnene, α-terpinolene found greater than 2% of peak area of FID response, whereas sample from population Jehrikhal district Pauri Garhwal showed α-pinene (30.30%), limonene (23.79%), sabinene (7.30%), β-myrcene (5.49%), 4-terpinolene (3.38%), and α-terpinene (2.25%) as the major components. Joshimath populations also found α-pinene (31.99%), as a major component followed by sabinene (19.23%), limonene (9.06%), δ-3-carene (6.51%), β-cubnene (3.0%), bornyl acetate (2.9%), 4-terpinolene (2.7%) and terpinene (2.7%). Study from Argentina showed α-pinene (25.8%), sabinene (22.3%), terpinen-4-ol (9.3%), α-thujene (4.2%) and myrcene (4.7%) as their major components and it is the same with our result sample collected from Joshimath populations situated in Chamoli district whereas other species C. arizonica oil from Argentina showed α-pinene (22.9%), limonene (8.5%), umbellulone (16.5%), terpinen-4-ol (5.5%) and cis-muurola-4 (14), 5-diene (9.0%); and C. macrocarpa oil possessed α-pinene (20.2%), sabinene (12.0%), p-cymene (7.0%) and terpinen-4-ol (29.6%)[8]. The major compound α-pinene is widely used in perfumery industry due to the pleasant aroma[9]. Limonene is used in the preparation of commercially available flea shampoos, mosquito repellents and different agrochemicals[10]. The bicyclic monoterpenes cis-sabinene hydrate and cis-sabinene hydrate acetate are considered to be responsible for the special flavour of marjoram (Origanum majorana L.)[11]. The study concluded that the worthwhile application of C. torulosa volatile constituents can be harvest coupled with other minor components. On the basis of the above results, it was concluded that C. torulosa samples collected from three distinct populations showed three types of group. Kalsi population possessed α-pinene, δ-3-carene type, Jeharikhal population possessed α-pinene, limonene type and Joshimath population possessed α-pinene, sabinene type components. This variation is due to difference genetic makeup, microclimatic and environmental conditions of the places where the species are grown. The study also recommended that further careful investigations of essential oil composition along with habitats characteristics features should be done.
TABLE 1.
ESSENTIAL OIL COMPOSITION OF CUPRESSUS TORULOSA D. DON LEAVES FROM UTTARAKHAND
Footnotes
Lohani, et al.: α-Pinene Rich Volatile Constituents
REFERENCES
- 1.Ambasta SP. The Useful Plants of India. New Delhi: CSIR Publications; 1986. p. 705. [Google Scholar]
- 2.Dhanabal SP, Manimaran S, Subburaj T, Elango K, Kumar EP, Dhanaraj SA. Evaluation of antimicrobial and antiinflammatory activity of volatile oil from Cupressus. Drug Lines. 2000;3:9–12. [Google Scholar]
- 3.Natarajan S, Murti VV, Seshadri TR. Bioflavones of some Cupressaceae plants. Phytochem. 1970;9:575–9. [Google Scholar]
- 4.Cool LG, Hu ZL, Eugene Z. Foliage terpenoids of Chinese Cupressus. Biochem Syst Ecol. 1998;26:899–913. [Google Scholar]
- 5.Clevenger JF. Apparatus for the Determination of Volatile Oil. J Am Pharm Assoc. 1928;17:345–9. [Google Scholar]
- 6.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Illinois: Allured Publishing; 2007. [Google Scholar]
- 7.Davies NW. Gas chromatographic retention indices of monoterpences and sesquiterpenes on methyl silicone and Carbowax 20M phases. J Chromatogr A. 1990;503:1–24. [Google Scholar]
- 8.Malizia RA, Cardell DA, Molli JS, Gonzalez S, Guerra PE, Grau RJ, et al. Volatile constituents of leaf oils from the Cupressacea family. I. Cupressus macrocarpa Hartw. C. arizonica Greene and C. torulosa Don species growing in Argentina. J Essent Oil Res. 2000;12:59–63. [Google Scholar]
- 9. [Last accessed on 2012 January 23]. Available from: http://www.exportersindia.com/uttaranchalterpeneproducts/producst.htm .
- 10.Dubey NK, Shukla R, Kumar A, Singh P, Prakash B. Prospects of botanical pesticides in sustainable agriculture. Curr Sci. 2010;98:479–80. [Google Scholar]
- 11.Novak J, Bitsch C, Pank F, Langbehn J, Franz CM. Distribution of the cis-sabinene hydrates acetate-chemotype in accessions of marjoram (Origanum majorana L.) Euphytica. 2002;127:69–74. [Google Scholar]


