Abstract
Background
Type 1 insulin-like growth factor receptor (IGF-1R) mediates resistance to chemotherapy and targeted agents. This study assessed the safety, pharmacokinetics (PK), and tolerability of humanized IGF-1R antibody AVE1642 with other cancer treatments.
Patients
Patients with advanced solid tumors received three weekly AVE1642 dosed at 6 mg/kg, chosen following previous study, with 75 (cohort A) or 100 mg/m2 (B) docetaxel, 1250 mg/m2 gemcitabine/100 mg erlotinib (C1), or 60 mg/m2 doxorubicin (D1). Blood samples were assayed for PK, IGFs, and IGF-BP3.
Results
Fifty-eight patients received 317 AVE1642 infusions. The commonest adverse events were diarrhea (37/58 patients), asthenia (34/58), nausea (30/58), and stomatitis (21/58). Dose-limiting toxic effects in cohorts C1 (diarrhea) and D1 (neutropenia) prompted addition of cohorts C2 (1000 mg/m2 gemcitabine/75 mg erlotinib) and D2 (50 mg/m2 doxorubicin). Grade 3–4 hyperglycemia (three cases) accompanied steroid premedication for docetaxel administration. No PK interactions were detected. There were three partial responses in cohorts B (melanoma) and C (leiomyosarcoma, two cases) and 22 stabilizations ≥12 weeks, giving a control rate of 25/57 (44%). On treatment IGF-II rose by 68 ± 25 ng/ml in patients discontinuing treatment <12 weeks, and fell by 55.5 ± 21 ng/ml with disease control (P < 0.001).
Conclusion
AVE1642 was tolerable with 75–100 mg/m2 docetaxel and 1000 mg/m2 gemcitabine/75 mg erlotinib, achieving durable disease control in 44%, with an association between IGF-II and response.
Keywords: AVE1642, chemotherapy, erlotinib, type 1 IGF receptor, monoclonal antibody
introduction
Type 1 insulin-like growth factor receptor (IGF-1R) regulates cell cycle progression, cell survival, and invasion and, in preclinical studies, mediates resistance to chemotherapy and targeted biological agents [1]. Clinical trials testing IGF-1R antibodies with chemotherapy showed activity in nonsmall-cell lung cancer (NSCLC), prostate, and pancreatic cancer [2–5]. However, a phase II study in colorectal cancer suggested antagonism between IGF-1R antibody and irinotecan [6], and phase III figitumumab trials were discontinued after interim analysis indicated no benefit in unselected NSCLC patients [7]. To enable effective use of these new drugs, it will be essential to identify factors predicting response, and to understand how IGF-1R inhibition impacts on response to chemotherapy.
AVE1642 is a humanized version of murine monoclonal IGF-1R antibody EM164; both antibodies have been shown to induce regression of human tumor xenografts, inhibit metastasis, and enhance chemosensitivity [8–11]. AVE1642 also enhances bortezomib-induced apoptosis in CD45 negative multiple myeloma cells [12]. In a phase I myeloma trial, AVE1642 was well tolerated but had insufficient activity to warrant further investigation, although myeloma cells were not screened for IGF-1R or CD45 [13]. The primary objective of this current study was to determine the safety of AVE1642 in patients with solid tumors. The first part of this phase I study had assessed escalating doses of AVE1642 alone or with 75 mg/m2 docetaxel. The maximal tolerated dose of AVE1642 was 12 mg/kg, and 6 mg/kg was selected for further study, based on AVE1642 pharmacokinetics (PK) and pharmacodynamic (PD) measures of IGF-I response. Tolerance of 6 mg/kg AVE1642 with 75 mg/m2 docetaxel suggested the feasibility of further docetaxel escalation [14; Massard et al. submitted]. This second part of the phase I study tested the safety and feasibility of the selected dose of AVE1642 (6 mg/kg) in combination with four different anticancer regimens.
patients and methods
trial design and objectives
This was an uncontrolled, four-arm multicenter study of IGF-1R antibody AVE1642 administered by an intravenous infusion in three weekly cycles with other anticancer therapies to patients with advanced solid tumors. On completion of chemotherapy, patients remained on AVE1642 until disease progression. The aim was to assess the feasibility of the treatment combinations, based on the incidence and severity of adverse events (AEs) and serious adverse events (SAEs) during the first cycle of treatment.
eligibility
To be eligible, patients were required to have pathologically confirmed advanced stage of solid tumor, one of the combination therapies should be a reasonable option given tumor characteristics and prior therapy, and provided no grade ≥3 toxic effects occurred during previous treatment with compounds in the same class. The specific inclusion criteria were age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, at least one measurable or evaluable lesion, and adequate hematologic, hepatic, and renal function (Hb ≥9 g/dl, neutrophils ≥1.5 × 109/l, platelets ≥100 × 109/l, creatinine <1.5× upper limit of normal (ULN) or calculated creatinine clearance ≥60 ml/min, bilirubin ≤ULN, AST/SGOT and ALT/SGPT ≤2.5× ULN (≤5× ULN if liver metastases), and alkaline phosphatase ≤2.5× ULN (≤5× ULN if bone or liver metastases). Patients in the doxorubicin cohort were required to have left ventricular ejection fraction (LVEF) ≥50% within 1 month before the trial entry. Patients were excluded for brain metastases, peripheral neuropathy grade ≥2, pregnancy/lactation, HbA1c >8% within 2 months of inclusion, >2 prior lines of cytotoxic chemotherapy or >4 prior lines of therapy in total (including targeted agents) for advanced disease, known severe hypersensitivity to docetaxel or other drugs formulated in polysorbate 80, or any other contraindication to the selected combination therapy. The study was approved by the Research Ethics Committees and Institutional Review Boards of the participating centers. All participants gave written informed consent.
drug administration
All patients received 6 mg/kg AVE1642 by a slow intravenous infusion on day 1 of each 21-day cycle (q21), followed 30 min later by day 1 chemotherapy. The additional anticancer regimens are shown by cohort in Table 1. Patients were enrolled in groups of three, with a 1-week interval between recruitment of the first three patients, and observation of the first group for 3 weeks before further enrollment. The intention was to recruit 15–20 patients to each cohort. However, a decision was made to discontinue the study in May 2009, for reasons unrelated to safety or efficacy, limiting recruitment to cohorts C and D.
Table 1.
Patient demographics by treatment cohort
| Cohort: | A | B | C1 | C2 | D1 | D2 |
|---|---|---|---|---|---|---|
| Doc 75 | Doc 100 | G 1250, E100 | G 1000, E 75 | Dox 60 | Dox 50 | |
| No. of patients | 20 | 20 | 4 | 6 | 4 | 4 |
| Demographics | ||||||
| Male : female | 8 : 12 | 8 : 12 | 1 : 3 | 2 : 4 | 0 : 4 | 1 : 3 |
| Age (years) | 54 (29–74) | 55 (27–72) | 50.5 (35–64) | 43 (25–57) | 60.5 (47–68) | 55.5 (40–63) |
| ECOG 0/1/2 | 11/8/1 | 14/6/0 | 3/1/0 | 2/4/0 | 1/3/0 | 0/4/0 |
| Prior surgery | 12 | 18 | 3 | 5 | 4 | |
| Prior radiotherapy | 9 | 9 | 3 | 4 | 1 | |
| Prior drug therapy | 20 | 15 | 4 | 4 | 4 | |
| Prior chemotherapy | 20 | 15 | 4 | 4 | 4 | |
| Prior taxanes | 9 | 4 | 2 | 1 | 4 | 1 |
| Prior endocrine therapy | 3 | 3 | 0 | 1 | 0 | |
| Prior targeted agents | 4 | 2 | 2 | 2 | 1 | |
| No. of prior regimens | 2 (1–7) | 2 (0–5) | 3 (2–4) | 1 (0–4) | 2 (1–3) | 2 (1–3) |
| Last therapy—first dose | 10.4 (0–90) | 21 (5–418) | 7.6 (5–155) | 11.3 (2–91) | 31.9 (6–55) | 21.1 (5–41) |
| Diagnosis | ||||||
| Ovarian | 6 | 1 | 0 | 0 | 4 | 1 |
| Melanomaa | 1 | 5 | 0 | 0 | 0 | 0 |
| Adrenocortical | 0 | 1 | 0 | 1 | 0 | 2 |
| Breast | 1 | 2 | 0 | 1 | 0 | 0 |
| Sarcomab | 3 | 0 | 2 | 0 | 0 | 0 |
| Head and neck | 3 | 1 | 0 | 0 | 0 | 0 |
| Thyroid adenoca | 0 | 1 | 0 | 0 | 0 | 0 |
| Nonsmall-cell lung | 2 | 1 | 0 | 0 | 0 | 0 |
| Gastroesophageal | 2 | 1 | 0 | 0 | 0 | 0 |
| Colorectal | 0 | 2 | 1 | 0 | 0 | 0 |
| Appendix | 0 | 1 | 0 | 0 | 0 | 0 |
| Pancreas | 2 | 0 | 0 | 1 | 0 | 0 |
| Adenocystic | 0 | 1 | 0 | 1 | 0 | 0 |
| Hepatocellular | 0 | 0 | 1 | 0 | 0 | 0 |
| Gallbladder | 0 | 0 | 0 | 1 | 0 | 0 |
| Pheochromocytoma | 0 | 0 | 0 | 0 | 0 | 1 |
| Prostate | 0 | 1 | 0 | 0 | 0 | 0 |
| Cervical | 0 | 1 | 0 | 1 | 0 | 0 |
| ACUP | 0 | 1 | 0 | 0 | 0 | 0 |
Patient characteristics, tumor types, and treatments are shown for each cohort. Age: median (range) years. ACUP, adenocarcinoma of unknown primary site.
aOf six metastatic melanomas, four were cutaneous and two were ocular, one each in cohorts A and B.
bSarcomas were all of soft tissue including four cases of leiomyosarcoma and one desmoplastic small round cell tumor. Number of prior regimens is shown as median (range), time from last prior treatment to first dose of study medication as median (range) in weeks. All patients received 6-mg/kg AVE1642 by a slow intravenous infusion on day 1 of each 21-day cycle, followed by day 1 chemotherapy: Doc, docetaxel; G, gemcitabine; E, erlotinib; Dox, doxorubicin. Chemotherapy was administered 30 min after completion of AVE1642 infusion: docetaxel by a 1-h intravenous infusion, gemcitabine by 30-min infusion, doxorubicin by intravenous injection, and erlotinib orally daily on an empty stomach. Prophylactic hematopoietic growth factors were not allowed during the first cycle of treatment. Thereafter, patients could receive G-CSF (Pegfilgrastim) for prophylaxis or treatment of neutropenia at the investigators' discretion.
pretreatment investigations and on study monitoring
Before treatment and before each treatment cycle, patients underwent physical examination and blood tests for hematology, renal and liver function, coagulation screen, and fasting glucose. For patients on doxorubicin, LVEF was reassessed on completing treatment. Tumor response was assessed by computed tomography scan or magnetic resonance imaging at baseline, on completion of alternate treatment cycles, for clinical suspicion of disease progression, and on completing treatment. Activity was evaluated according to Response Evaluation Criteria in Solid Tumors and toxic effects by National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Patients were assessed for toxicity after the first cycle and for response after two cycles. Dose-limiting toxicity (DLT) was defined as occurring during cycle 1 and included grade 3–4 neutropenia complicated by fever ≥38.5°C or documented infection, grade 4 neutropenia >7 days, grade 3–4 thrombocytopenia or anemia >7 days, grade 3–4 thrombocytopenia complicated by hemorrhage, or grade 3–4 nonhematologic toxic effects.
pharmacokinetic and pharmacodynamic monitoring
Patients underwent blood sampling for AVE1642 predose, during cycle 1 at intervals during the first 24 h, on days 3, 8, and 15, for subsequent cycles at day 22 before the next infusion, and 50–70 days after final study drug administration. Blood samples were collected for docetaxel, gemcitabine, erlotinib, and doxorubicin levels at cycle 1 predose, and at intervals during the first 48 h after the start of administration, with additional sampling for docetaxel PK for 6 h after administration of cycle 2. Blood for PD assessment was collected during cycle 1 at baseline; days 1, 2, 7, 14, and 21; at the end of each cycle for circulating IGF-I, IGF-II, and IGF-BP3; and human anti-humanized antibodies (HAHA). PK and PD parameters and AVE1642 HAHA were determined in serum or plasma using validated bioanalytical methods. AVE1642 was measured by enzyme immunoassay (EIA; lower limit of quantification, LLOQ: 2 µg/ml), and additional therapies by liquid chromatography/mass spectrometry (LC/MS-MS), with LLOQs of 1 ng/ml docetaxel, 0.1 ng/ml doxorubicin, 2 ng/ml erlotinib, 50 ng/ml gemcitabine. IGFs were measured by radioimmunoassay, with LLOQ for IGF-I of 16.7 ng/ml, and IGF-II 99.9 ng/ml. IGFBP3 was quantified by EIA (LLOQ 77.4 ng/ml) and HAHA by enzyme-linked immunosorbent assay. IGF levels were analyzed using GraphPad Prism version 5 (GraphPad, San Diego, CA).
results
patient characteristics
The characteristics of the 58 enrolled patients are shown in Table 1. In total, 317 cycles of study treatment were administered, divided by cohort as shown in Table 2. All patients were assessable for toxicity and PK/PD, and all but one for efficacy.
Table 2.
Summary of treatments and toxicity by cohort
| Cohort: | A | B | C1 | C2 | D1 | D2 |
|---|---|---|---|---|---|---|
| Doc 75 | Doc 100 | G 1250, E100 | G 1000, E 75 | Dox 60 | Dox 50 | |
| No. patients | 20 | 20 | 4 | 6 | 4 | 4 |
| Treatments | ||||||
| Total cycles | 106 | 124 | 33 | 37 | 9 | 8 |
| Median | 4 | 4 | 7 | 2 | 2 | 2 |
| Range | 1–16 | 2–16 | 1–18 | 2–24 | 2–3 | 1–3 |
| Dose delay | 8 | 9 | 2 | 1 | 3 | 0 |
| G3/4 TEAEs: | ||||||
| Neutropenia | 8 | 2 | 2 | 0 | 3 | 1 |
| Thrombocytopenia | 1 | 0 | 0 | 2 | 0 | 1 |
| Anemia | 0 | 0 | 1 | 0 | 2 | 0 |
| Renal impairment | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic impairment | 1 | 0 | 2 | 0 | 0 | 0 |
| Hyperglycemia | 1 | 2 | 0 | 0 | 0 | 0 |
| Oral mucositis | 0 | 0 | 1 | 0 | 0 | 0 |
| Diarrhea | 0 | 1 | 2 | 0 | 0 | 0 |
| DLTs: number (grade) | ||||||
| Neutropenia | 3 (3–4) | 0 | 1 (3) | 0 | 3 (3–4) | 1 (4) |
| Hyperglycemia | 1 (4) | 1 (3) | 0 | 0 | 0 | |
| Diarrhea | 0 | 0 | 2 (3) | 0 | 0 | 0 |
| Stomatitis | 0 | 0 | 0 | 1 (3) | 0 | 0 |
Doc, docetaxel; G, gemcitabine; E, erlotinib; Dox, doxorubicin. Doses in mg/m2 for cytotoxic agents, total daily dose for erlotinib. TEAE, treatment emergent adverse event. DLT, dose limiting toxicity, shown as number (grade).
safety
All 58 patients had at least one AE attributed to trial treatment, the most common being diarrhea in 37/58 (64%), asthenia (34/58, 59%), nausea (30/58, 52%), and stomatitis (21/58, 36%). After documentation of grade 3 diarrhea in two patients in cohort C1, and febrile neutropenia in three patients in cohort D1, the protocol was amended, adding dose-reduced cohorts C2 and D2 (see Table 1). Grade 3 or 4 AEs were experienced by 36/58 patients (62%), including neutropenia in 16/58 (see Table 2), requiring treatment delay and/or dose-reduction in six patients. Grade 3 or 4 hyperglycemia occurred in 3/58 patients (5%), including two cases of grade 3 hyperglycemia in cohort B, and one grade 4 hyperglycemia in a cohort A patient with known type II diabetes, responding to insulin with normalization of blood glucose within 24 h.
Dose delays were experienced by 23/58 (40%) patients, and 13/58 (22%) experienced DLTs of neutropenia, hyperglycemia, diarrhea, or stomatitis (Table 2). These results indicated that 6 mg/kg AVE1642 was tolerable in combination with 75 or 100 mg/m2 docetaxel in cohorts A and B, but not with 1250 mg/m2 gemcitabine/100 mg erlotinib (cohort C1), or 60 mg/m2 doxorubicin (cohort D1). The reduced doses introduced in cohort C2 (1000 mg/m2 gemcitabine/75 mg erlotinib) were tolerable, as was treatment in cohort D2 (50 mg/m2 doxorubicin), and one patient tolerated doxorubicin escalation to 60 mg/m2. Premature cessation of this study prevented further recruitment to cohorts C2 and D2. There was grade 3 impairment of hepatic function in three patients (one in cohort A, two in C1) but no grade 3–4 deterioration in renal function or reduction in LVEF. One patient in cohort B experienced deteriorating cognitive function possibly related to study medication after 13 cycles of treatment. Forty SAEs occurred in 19 patients, of which 13 events were possibly related to AVE1642, and 3 patients experienced a SAE leading to death. These were the grade 4 hyperglycemia described above (cohort A), grade 3 oral stomatitis (cohort C2), and grade 3 febrile neutropenia (cohort D1). Fourteen additional patients died on study, all from disease progression.
pharmacokinetic analysis
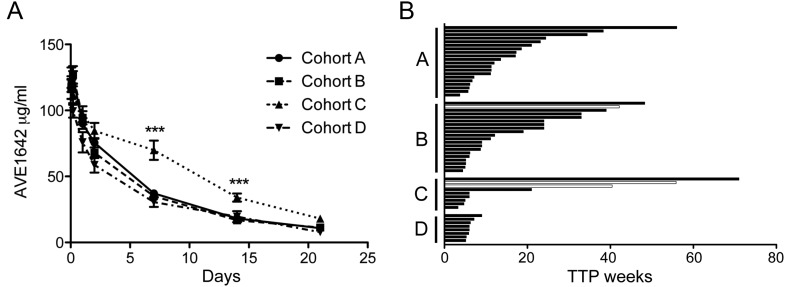
In the previous dose-escalation study, AVE1642 exposure had been approximately dose-proportional between 3 and 24 mg/kg, and clearance was similar (mean 363–477 ml/day) at all dose levels, suggesting attainment of a plateau of clearance from the lowest dose of 3 mg/kg [14; Massard et al. submitted]. In the current study, all patients received AVE1642 at 6 mg/kg. AVE1642 levels during cycle 1 showed a similar pattern across all cohorts, with the exception that levels were higher on days 7 and 14 in cohort C (Figure 1A), and AVE1642 clearance appeared greater in cohort D2 (Table 3), for reasons that are currently unclear. Otherwise, there were no differences between cohorts in AVE1642 Cmax values, achieved 30–90 min after the start of AVE1642 infusion, final measured concentration (Clast), AVE1642 AUC, plasma terminal half-life (t½), and clearance (Table 3). These data are comparable with PK parameters recorded for the 6 mg/kg cohort in the AVE1642 dose-escalation phase (Massard et al. submitted). Comparison of PK data for the cytotoxic drugs (Table 3) with historical and in-house data (not shown) indicated that there was no significant drug–drug interaction between AVE1642 and the cytotoxic drugs used in this study.
Figure 1.
AVE1642 PK and clinical responses. (A) Plasma concentrations of AVE1642 (mean ± SEM in µg/ml) are shown during treatment cycle 1. ***P < 0.001 for comparison of AVE1642 concentrations in cohort C with levels in other cohorts. (B) Graph shows TTP (weeks) by cohort, in 57 patients assessable for response. White bars represent patients achieving PR.
Table 3.
Summary of PK parameters
| Cohort | A | B | C1 | C2 | D1 | D2 |
|---|---|---|---|---|---|---|
| Doc 75 | Doc 100 | G 1250, E100 | G 1000, E 75 | Dox 60 | Dox 50 | |
| AVE1642 PK parameters | ||||||
| Cmax (μg/ml) | 129 ± 22.0 (17) | 129 ± 28.6 (22) [126] | 135 ± 23.8 (18) | 118 ± 24.8 (21) | 118 ± 13.4 (11) | 115 ± 29.5 (26) |
| Clast (μg/ml) | 11.8 ± 4.87 (41) | 11.3 ± 4.78 (42) | 13.2 ± 3.22 (24) | 11.1 ± 3.03 (27) | 8.06 ± 4.61 (57) | 17.6 ± 17.9 (102) |
| AUC (day µg/ml) | 899 ± 224 (25) | 858 ± 225 (26) | 918 ± 341 (37) | 819 ± 192 (23) | 805 ± 260 (32) | 629 ± 386 (61) |
| t½ (day) | 7.97 ± 2.34 (29) | 9.10 ± 2.73 (30) | 8.87 ± 2.02 (23) | 7.51 ± 2.42 (32) | 10.3 ± 2.66 (26) | 11.2 ± 8.73 (78) |
| Cl (ml/day) | 472 ± 189 (40) | 566 ± 206 (36) | 476 ± 153 (32) | 520 ± 180 (35) | 439 ± 207 (47) | 876 ± 189 (22) |
| Chemotherapy PK parameters | ||||||
| Cmax (ng/ml) | N/A | N/A | 8950 ± 5360 (60) | 8040 ± 3710 (46) | 1900 ± 529 (28) | 3210 ± 1750 (55) |
| Clast (ng/ml) | N/A | N/A | 466 ± 112 (24) | 208 ± 115 (56) | 12.6 ± 8.57 (68) | 6.81 ± 1.83 (27) |
| AUC | 4.59 ± 4.2a (91.5) | 4.00 ± 1.23a (52.8) | 3060 ± 1360 (44) | 3050 ± 851 (28) | 5310 ± 1490 (28) | 5690 ± 2560 (46) |
| t½ (h) | N/A | N/A | 0.281 ± 0.069 (25) | 0.242 ± 0.039 (16) | 28.0 ± 8.53 (30) | 27.2 ± 8.85 (33) |
| Cl (L/h/m2) | 22.9 ± 8.9a (39.0) | 26.3 ± 7.8a (29.5) | 867 ± 444 (51.2) | 634 ± 230 (36.3) | 11.9 ± 3.15 (26) | 9.57 ± 4.14 (43) |
Doc, docetaxel; G, gemcitabine; E, erlotinib; Dox, doxorubicin. PK data are provided as mean ± SD (CV%). Cmax, maximum observed concentration; Clast, last measured concentration; AUC, area under the curve (for docetaxel, μg h/ml; for gemcitabine and doxorubicin, ng h/ml); t½, terminal half-life; Cl, clearance.
aTabulated data are for docetaxel PK at cycle 1. N/A, not available. Analysis of cycle 2 data following 1-h infusion of docetaxel at 75 mg/m2 showed mean ± SD docetaxel clearance of 26.4 ± 14.6 l/h/m² with AUC 3.67 ± 1.94 µg h/ml. Equivalent data for cycle 2 administration at 100 mg/m2 showed docetaxel clearance of 28.9 ± 10.3 l/h/m² with AUC 3.87 ± 1.59 µg h/ml. There were no statistically significant differences in AVE1642 PK parameters between cohorts, or from PK parameters at 6 mg/kg in the dose-escalation phase (AUC 1030 ± 348 day µg/ml, plasma t½ 9.02 ± 1.89 days). Erlotinib PK parameters could not be determined due to erratic plasma profiles.
efficacy
Response data are summarized in Table 4. Three patients achieved durable partial responses (PR), including one with metastatic melanoma who received 14 cycles of treatment in cohort B, with time to progression (TTP) of 42 weeks. The other responses were in two patients with leiomyosarcoma, both treated in cohort C1 with 1250 mg/m2 gemcitabine and 100 mg erlotinib. Both patients required dose reduction after experiencing DLT (grade 3 diarrhea) during cycle 1, continuing treatment for 18 and 12 cycles, respectively, achieving TTP of 56 and 40.5 weeks. Stable disease was documented in 36 patients, including 14/20 in cohort A, 14/20 in cohort B, 4 in cohort C, and 4 in cohort D; information on TTP is shown in Table 4 and Figure 1B. Durable stabilizations (≥12 weeks) were achieved by 11/20 of patients in cohort A, 10/20 in cohort B, and 2/10 in cohort C. In patients experiencing PR or durable (≥12 weeks) stabilization, median TTP was 21 weeks (range 12–56 weeks) in cohort A, 28.5 weeks (range 12–48.3 weeks) in cohort B, and 48 weeks (range 21–71) in cohort C. Patients ultimately discontinuing treatment for progressive disease (PD) included 15/20 in cohort A, 10/20 in cohort B, 3/4 in C1, 2/6 in C2, 3/4 in D1, and 2/4 patients in D2. Six patients discontinued treatment because of toxicity, attributed to asthenia, paresthesia, general physical deterioration, deep vein thrombosis, hypocalcemia, and neutropenia. At the study cutoff date (August 2009, 60 days after completion of the first cycle of the final enrolled patient), nine patients remained on study (one patient in cohort A, five in B, two in C2, and one in D2).
Table 4.
Responses and reasons for discontinuing treatment
| Cohort | A | B | C1 | C2 | D1 | D2 |
|---|---|---|---|---|---|---|
| Doc 75 | Doc 100 | G 1250, E100 | G 1000, E 75 | Dox 60 | Dox 50 | |
| No. patients | 20 | 20 | 4 | 6 | 4 | 4 |
| Total cycles | 106 | 124 | 33 | 37 | 9 | 8 |
| Median | 4 | 4 | 7 | 2 | 2 | 2 |
| Range | 1–16 | 2–16 | 1–18 | 2–24 | 2–3 | 1–3 |
| PR | 0 | 1 | 2 | 0 | 0 | 0 |
| SD | 14 | 14 | 0 | 4 | 2 | 2 |
| PD | 6 | 5 | 2 | 1 | 2 | 2 |
| TTP (weeks) | 18 (11–56) | 24 (6–48) | 48 (40.5–56) | 6 (6–71) | 6 (6–6.1) | 7.5 (6–9) |
| Reasons for discontinuationa | ||||||
| Toxicity | 4 | 1 | 0 | 0 | 1 | 0 |
| PD | 15 | 10 | 3 | 2 | 3 | 2 |
| Other | 3 | 4 | 1 | 3 | 0 | 1 |
PR, partial response; SD, stable disease; PD, progressive disease; TTP, time to progression for patients with PR or SD, shown as median (range). One patient in cohort C2 was not assessable for response.
aSome patients discontinued treatment for a combination of PD, toxicity, and other reasons including patient choice. At the data cutoff date, nine patients remained on treatment, including one patient in cohort A, five in B, two in C2, and one in D2.
pharmacodynamic monitoring
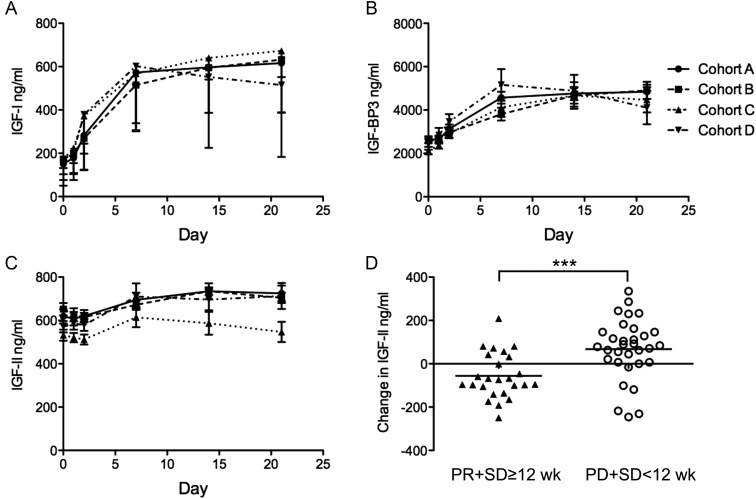
AVE1642 HAHAs were detected in one patient, only at the end of treatment. Plasma IGF-I, IGF-II, and IGF-BP3 levels are shown in Figure 2. Circulating IGF-I and IGF-BP3 levels are regulated by the hypothalamic–pituitary axis [15] and are sensitive to blockade of pituitary IGF-1R. Pretreatment circulating IGF-I levels (34–374 ng/ml) were within the normal range, with no difference between patients achieving TTP ≥12 weeks (mean IGF-I 157.5 ng/ml, range 57–289 ng/nl), and those with TTP <12 weeks (169.6 ng/ml, range 33.8–374.0 ng/ml). Circulating IGF-I rose approximately fourfold within 7 days of initiating treatment, with a subsequent plateau (Figure 2A). Thus, in cohort A, baseline values were 151 ± 20 ng/ml, rising to 616 ± 65 ng/ml by day 21. Very similar increases were observed in cohorts B, C, and D (P < 0.001 for comparison of baseline versus peak cycle 1 value in each cohort), with no difference between cohorts. Circulating levels of the principal IGF-binding protein, IGF-BP3, showed approximately twofold treatment-related elevation, again without difference between cohorts (Figure 2B). These data are consistent with sustained IGF-1R blockade at least in the pituitary, compatible with effects of other IGF-1R antibodies [16–18].
Figure 2.
Treatment-related changes in circulating IGFs and IGF-BP3. Circulating levels during cycle 1 are shown as mean ± SEM in ng/ml of (A) IGF-I; (B) IGF-BP3; (C) IGF-II; legend as Figure 1. (D) Comparison of differences between baseline IGF-II concentration and final IGF-II concentration measured during definitive response. Circulating IGF-II fell by 55.5 ± 21 ng/ml (mean ± SEM) in patients achieving disease control (PR or stable disease ≥12 weeks; n = 25), and rose by 68 ± 25 ng/ml in non/brief-responders (n = 32; ***P < 0.001 by t-test).
Circulating IGF-II levels are higher than IGF-I and are only weakly regulated by the pituitary [15]. Study subjects had baseline IGF-II ranging from 323–945 ng/ml, with no difference between cohorts. We noted treatment-related IGF-II rise (Figure 2C): in cohort A, baseline IGF-II levels were 619.0 ± 33.32 ng/ml, rising by day 7–21 to 735.0 ± 36.67, with equivalent changes in cohorts B and D. The fold change was less marked (∼1.2-fold) than changes in IGF-I and IGF-BP3, but the differences between pretreatment and day 21 IGF-II levels were significant (P < 0.05) in all cohorts but C. By day 21 of cycle 1, IGF-II levels were significantly lower in cohort C than in A, B, and D (P < 0.05 by ANOVA; Figure 2C). Given that cohort C included two of the three patients achieving PR, and two achieving prolonged stabilization, we examined the relationship between IGF-II and response. Patients were divided into those achieving disease control (PR or stable disease of ≥12 weeks, 25 patients), and those on treatment for <12 weeks (32 patients). There was no difference in baseline IGF-II (596 ± 24 ng/ml in non/brief-responders versus 635 ± 22 ng/ml in the disease control group). On treatment IGF-II increased in patients discontinuing treatment <12 weeks, but not in those achieving prolonged disease control (mean values, respectively, 664 ± 25 versus 579 ± 24 ng/ml, P < 0.05). Comparing on treatment changes in these two groups, circulating IGF-II rose by 68 ± 25 ng/ml in non/brief-responders and fell by 55.5 ± 21 ng/ml in the disease control group (P < 0.001; Figure 2D).
discussion
The combination of AVE1642 with docetaxel caused no greater toxicity than was previously reported using 75 or 100 mg/m2 docetaxel alone [19, 20]. AVE1642 was also tolerable with gemcitabine/erlotinib at the dose-reduced schedule administered in cohort C2. AVE1642 with 1250 mg/m2 gemcitabine and 100 mg erlotinib caused dose-limiting diarrhea in 2/4 patients, compared with <10% reported previously on 100–150 mg erlotinib with gemcitabine [21, 22]. AVE1642 with 60 mg/m2 doxorubicin induced neutropenia in 3/4 cases, compared with historical rates of ∼50% in patients treated with single-agent 60 mg/m2 doxorubicin [23]. These data suggest that IGF-1R inhibition may exacerbate chemotherapy-induced toxicity, although numbers are small. In the absence of PK interaction, toxicity is unlikely be due to increased drug exposure, raising the prospect of pharmocodynamic interaction. Indeed, IGF-1R antibodies can induce myelotoxicity, more often thrombocytopenia than neutropenia [16, 17, 24].
Hyperglycemia is a known class effect of IGF-1R inhibitors [1]. AVE1642 does not bind to the insulin receptor (IR), but EM164 was shown to downregulate IRs by binding to IGF-1R:IR hybrid receptors [25], potentially impairing the function of variant IRs (IR-A) that contribute to tumor growth, and classical IRs (IR-B) that mediate glucose uptake [26]. In this study, significant hyperglycemia occurred only after steroid premedication for docetaxel dosing. Otherwise, the lack of serious hyperglycemia is consistent with data from trials of similar IGF-1R antibodies [17, 27, 28], and with the dose-escalation phase of this study, where grade 3 hyperglycemia occurred in 2/8 patients receiving 75 mg/m2 docetaxel with AVE1642 at 18 mg/kg but not 6 mg/kg (Massard et al. submitted).
Responses occurred in three patients and durable stabilizations in 22, giving a disease control rate of 25/57 (44%). Responding patients included one with metastatic melanoma treated with docetaxel and two patients with leiomyosarcoma on the erlotinib/gemcitabine arm. Both cytotoxic drugs have reported single-agent activity in these tumor types: docetaxel of 17% (5/30) in melanoma [29], and gemcitabine of 7% (1/14) in previously treated leiomyosarcoma [30]. Prior studies have noted the activity of IGF-1R antibody in sarcomas [17, 24, 31], and the utility of IGF-1R antibody with gemcitabine in pancreatic cancer [5]. IGF-1R inhibition may enhance chemosensitivity via regulation of apoptosis and DNA repair [32–35], but there is potential for antagonism if cell cycle arrest protects from phase-specific cytotoxic drugs, possibly explaining the adverse outcome when IGF-1R antibody was added to irinotecan in patients with colorectal cancer [6]. Therefore, scheduling is an important issue when combining targeted agents with chemotherapy, with evidence of more effective chemosensitization when administering IGF-1R therapeutics after the cytotoxic drug [36], rather than before, as in this study.
Dramatic and durable responses have been noted in this and other studies of IGF-1R therapeutics [2, 5, 17], but as yet there is no clear biomarker for sensitivity [7]. High pretreatment levels of free or total IGF-I appear to correlate with response to IGF-1R antibodies figitumumab and R1507 [24, 37–40]. In the current study, free IGF-I was not measured, and there was no association between baseline total IGF-I and response. There was, however, evidence that on treatment IGF-II increased in non/brief-responders, and fell in patients achieving durable disease control. At present, it is not clear whether these differences are biologically significant; it is plausible that rising IGF-II could mediate AVE1642 resistance by activating IR-A, as was recently shown for IGF-1R antibody SCH717454 in sarcoma models [41].
In summary, 6 mg/kg AVE1642 was tolerable in combination with 75–100 mg/m2 docetaxel and with 1000 mg/m2 gemicibine and 75 mg erlotinib, but not with 1250 mg/m2 gemicibine and 100 mg erlotinib due to gastrointestinal toxicity, or 60 mg/m2 doxorubicin due to myelotoxicity. Of 57 patients assessable for response, 25 (44%) achieved durable disease control, and we identified an association between on treatment IGF-II and disease response. Issues of scheduling and identification of predictive biomarkers remain to be resolved.
funding
This study was supported by sanofi-aventis and by funding from the Oxford National Institute for Health Research (NIHR) Biomedical Research Centre (VMM and MRM), Medical Research Council (G0601061 to VM), and Higher Education Funding Council for England (HEFCE) Clinical Senior Lectureship (VM). JCS and RB are supported by the French Institut National du Cancer labeling of phase I centers. JYB is funded by the French Institut National du Cancer labeling of phase I centers (CLIP2) and the French NCI SIRIC (Integrated Cancer Research Site) program LYRIC (Lyon SIRIC).
disclosure
DMM is an employee of Sanofi-aventis. All remaining authors have declared no conflicts of interest.
acknowledgement
We are grateful to Nikki Hayward, I. Ray-Coquard, Ph. Cassier, Antoine Deslandes and to the patients and families who took part in this study.
references
- 1.Chitnis MM, Yuen JS, Protheroe AS, et al. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 2.Karp DD, Paz-Ares LG, Novello S, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 3.Collins IM, Beddy P, O'Byrne KJ. Radiological response in an incidental meningioma in a patient treated with chemotherapy combined with CP-751,871, an IGF-1R inhibitor. Acta Oncol. 2010;49:872–874. doi: 10.3109/02841861003752408. [DOI] [PubMed] [Google Scholar]
- 4.Molife LR, Fong PC, Paccagnella L, et al. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332–339. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javle MM, Varadhachary GR, Fogelman DR, et al. Randomized phase II study of gemcitabine (G) plus anti-IGF-1R antibody MK-0646, G plus erlotinib (E) plus MK-0646 and G plus E for advanced pancreatic cancer. J Clin Oncol. 2011;29(Suppl) doi: 10.1186/s13045-018-0616-2. abstr 4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins DJ, Tabernero J, Schmoll H, et al. A randomized phase II/III study of the anti-IGF-1R antibody MK-0646 (dalotuzumab) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory metastatic colorectal cancer (mCRC) with wild-type (wt) KRAS status. J Clin Oncol. 2011;29(Suppl) abstr 3501. [Google Scholar]
- 7.Basu B, Olmos D, de Bono JS. Targeting IGF-1R: throwing out the baby with the bathwater? Br J Cancer. 2011;104:1–3. doi: 10.1038/sj.bjc.6606023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073–5083. [PubMed] [Google Scholar]
- 9.Sachdev D, Zhang X, Matise I, et al. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2010;29:251–262. doi: 10.1038/onc.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiliotaki M, Markomanolaki H, Mela M, et al. Targeting the insulin-like growth factor I receptor inhibits proliferation and VEGF production of non-small cell lung cancer cells and enhances paclitaxel-mediated anti-tumor effect. Lung Cancer. 2011;73:158–165. doi: 10.1016/j.lungcan.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Geoerger B, Brasme JF, Daudigeos-Dubus E, et al. Anti-insulin-like growth factor 1 receptor antibody EM164 (murine AVE1642) exhibits anti-tumour activity alone and in combination with temozolomide against neuroblastoma. Eur J Cancer. 2011;46:3251–3262. doi: 10.1016/j.ejca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Descamps G, Gomez-Bougie P, Venot C, et al. A humanised anti-IGF-1R monoclonal antibody (AVE1642) enhances Bortezomib-induced apoptosis in myeloma cells lacking CD45. Br J Cancer. 2009;100:366–369. doi: 10.1038/sj.bjc.6604839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau P, Cavallo F, Leleu X, et al. Phase I study of the anti insulin-like growth factor 1 receptor (IGF-1R) monoclonal antibody, AVE1642, as single agent and in combination with bortezomib in patients with relapsed multiple myeloma. Leukemia. 2011;25:872–874. doi: 10.1038/leu.2011.4. [DOI] [PubMed] [Google Scholar]
- 14.Tolcher AW, Patnaik A, Till E, et al. A phase I study of AVE1642, a humanized monoclonal antibody IGF-1R (insulin like growth factor1 receptor) antagonist, in patients(pts) with advanced solid tumor (ST) J Clin Oncol. 2008;26(Suppl) abstr 3582. [Google Scholar]
- 15.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 16.Lacy MQ, Alsina M, Fonseca R, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 17.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 18.Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 19.Cerny T, Kaplan S, Pavlidis N, et al. Docetaxel (Taxotere) is active in non-small-cell lung cancer: a phase II trial of the EORTC Early Clinical Trials Group (ECTG) Br J Cancer. 1994;70:384–387. doi: 10.1038/bjc.1994.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller VA, Rigas JR, Francis PA, et al. Phase II trial of a 75-mg/m2 dose of docetaxel with prednisone premedication for patients with advanced non-small cell lung cancer. Cancer. 1995;75:968–972. doi: 10.1002/1097-0142(19950215)75:4<968::aid-cncr2820750411>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Boeck S, Vehling-Kaiser U, Waldschmidt D, et al. Erlotinib 150 mg daily plus chemotherapy in advanced pancreatic cancer: an interim safety analysis of a multicenter, randomized, cross-over phase III trial of the ‘Arbeitsgemeinschaft Internistische Onkologie. Anticancer Drugs. 2010;21:94–100. doi: 10.1097/CAD.0b013e32833123ed. [DOI] [PubMed] [Google Scholar]
- 22.Okusaka T, Furuse J, Funakoshi A, et al. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci. 2011;102:425–431. doi: 10.1111/j.1349-7006.2010.01810.x. [DOI] [PubMed] [Google Scholar]
- 23.Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193) J Clin Oncol. 2003;21:588–592. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. 2011;29:4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachdev D, Singh R, Fujita-Yamaguchi Y, et al. Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: implications for anti-insulin-like growth factor therapy in breast cancer. Cancer Res. 2006;66:2391–2402. doi: 10.1158/0008-5472.CAN-05-3126. [DOI] [PubMed] [Google Scholar]
- 26.Belfiore A, Frasca F, Pandini G, et al. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 27.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 28.Atzori F, Tabernero J, Cervantes A, et al. A phase I, pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-IGF-1R monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 29.Aamdal S, Wolff I, Kaplan S, et al. Docetaxel (Taxotere) in advanced malignant melanoma: a phase II study of the EORTC Early Clinical Trials Group. Eur J Cancer. 1994;30A:1061–1064. doi: 10.1016/0959-8049(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 30.Ferraresi V, Ciccarese M, Cercato MC, et al. Gemcitabine at fixed dose-rate in patients with advanced soft-tissue sarcomas: a mono-institutional phase II study. Cancer Chemother Pharmacol. 2008;63:149–155. doi: 10.1007/s00280-008-0723-9. [DOI] [PubMed] [Google Scholar]
- 31.Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warshamana-Greene GS, Litz J, Buchdunger E, et al. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–1571. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 33.Haluska P, Carboni JM, Loegering DA, et al. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- 34.Flanigan SA, Pitts TM, Eckhardt SG, et al. The insulin-like growth factor I receptor/insulin receptor tyrosine kinase inhibitor PQIP exhibits enhanced antitumor effects in combination with chemotherapy against colorectal cancer models. Clin Cancer Res. 2010;16:5436–5446. doi: 10.1158/1078-0432.CCR-10-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turney BW, Kerr M, Chitnis MM, et al. Depletion of the type 1 IGF receptor delays repair of radiation-induced DNA double strand breaks. Radiother Oncol. 2012;103:402–409. doi: 10.1016/j.radonc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Zeng X, Zhang H, Oh A, et al. Enhancement of doxorubicin cytotoxicity of human cancer cells by tyrosine kinase inhibition of insulin receptor and type I IGF receptor. Breast Cancer Res Treat. 2011;133:117–126. doi: 10.1007/s10549-011-1713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gualberto A, Hixon ML, Karp DD, et al. Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab. Br J Cancer. 2010;104:68–74. doi: 10.1038/sj.bjc.6605972. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Habben K, Delmar P, Brownstein CM, et al. Investigation of predictive biomarkers for R1507, an anti-IGF1R antibody, in patients with advanced non-small cell lung cancer with progression after first-line chemotherapy. J Clin Oncol. 2011;29:7584. [Google Scholar]
- 39.Ramalingam SS, Spigel DR, Steins M, et al. Randomized, double-blind, phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor receptor-1 (IGF-1R), for advanced-stage non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(Suppl 20):7527. doi: 10.1200/JCO.2011.36.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goto Y, Sekine I, Tanioka M, et al. Figitumumab combined with carboplatin and paclitaxel in treatment-naive Japanese patients with advanced non-small cell lung cancer. Invest New Drugs. 2011;30:1548–1556. doi: 10.1007/s10637-011-9715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bid HK, Zhan J, Phelps DA, et al. Potent Inhibition of Angiogenesis by the IGF-1 Receptor-Targeting Antibody SCH717454 Is Reversed by IGF-2. Mol Cancer Ther. 2011;11:649–659. doi: 10.1158/1535-7163.MCT-11-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]