Abstract
Background
We carried out a prospective clinical study to evaluate the impact of the Recurrence Score (RS) on treatment decisions in early breast cancer (EBC).
Patients and methods
A total of 379 eligible women with estrogen receptor positive (ER+), HER2-negative EBC and 0–3 positive lymph nodes were enrolled. Treatment recommendations, patients' decisional conflict, physicians' confidence before and after knowledge of the RS and actual treatment data were recorded.
Results
Of the 366 assessable patients 244 were node negative (N0) and 122 node positive (N+). Treatment recommendations changed in 33% of all patients (N0 30%, N+ 39%). In 38% of all patients (N0 39%, N+ 37%) with an initial recommendation for chemoendocrine therapy, the post-RS recommendation changed to endocrine therapy, in 25% (N0 22%, N+ 39%) with an initial recommendation for endocrine therapy only to combined chemoendocrine therapy, respectively. A patients' decisional conflict score improved by 6% (P = 0.028) and physicians' confidence increased in 45% (P < 0.001) of all cases. Overall, 33% (N0 29%, N+ 38%) of fewer patients actually received chemotherapy as compared with patients recommended chemotherapy pre-test. Using the test was cost-saving versus current clinical practice.
Conclusion
RS-guided chemotherapy decision-making resulted in a substantial modification of adjuvant chemotherapy usage in node-negative and node-positive ER+ EBC.
Keywords: adjuvant, breast cancer, chemotherapy, node negative, node positive, recurrence score
introduction
For hormone receptor-positive early-stage breast cancer (EBC), the risk of recurrence and the potential benefit of chemotherapy have to be balanced with the potential risks of adverse events and the possibility of overtreatment with no additional benefit from chemotherapy. It is also accepted that the presence of ipsilateral positive lymph nodes does not automatically mandate the administration of chemotherapy [1]. The Oncotype DX® assay has been shown to provide prognostic and predictive information beyond traditional parameters in node-negative (N0) and node-positive (N+) hormone receptor-positive disease [2–6].
The use of the Recurrence Score® (RS) to guide clinical treatment decisions has been incorporated within clinical guidelines such as ASCO [7], NCCN [8], ESMO [9] and the St Gallen Consensus Guidelines [1, 10].
Meanwhile, several prospective clinical utility studies demonstrated that the knowledge of RS affects management of patients [11–15]. A recent meta-analysis [16] showed that the results are very consistent for N0 ER-positive disease in countries with different therapeutic traditions with a consistent change in treatment recommendations in ∼35% of cases. Importantly, the studies reveal that every second patient originally recommended adjuvant chemoendocrine treatment is recommended endocrine treatment alone after knowledge of the RS. The use of the assay has been found to be cost-effective or even cost-saving in different national health care systems [17–21].
The data regarding the impact of using the assay in N+ disease are still evolving [14, 15, 22, 23].
We sought to prospectively evaluate the impact of Oncotype DX on adjuvant decision-making in German clinical practice for patients with ER-positive EBC with both N0 and N+ disease.
patients and methods
This multi-center study was carried out in 15 German centers. The Oncotype DX RS assay was provided free of charge by Genomic Health, Inc. The study was approved by a central and all local ethics committees. All patients provided their signed informed consent.
study objectives
The primary study objective was to evaluate the impact of the RS on physicians' adjuvant therapy decision-making in a cohort of consecutive patients with ER+, HER2-negative breast cancer. Secondary study objectives were to assess the impact of the RS on patients' decisional conflict, on physicians' confidence in their treatment recommendations, to evaluate the rate of therapy actually administered in relation to recommended therapies and to assess the pharmaco-economic impact of RS-guided adjuvant decision-making for German clinical practice.
patients
Enrolment was offered consecutively to eligible women presenting with operable EBC, ER positive, HER2 negative by IHC or FISH, tumor size of ≥1 cm (T1, 2, 3 excluding those with dermal involvement) or <1 cm if at least one histological unfavorable characteristic (high histological grade, angiolymphatic invasion and p-53 positive), node negative or histologically verified lymph node metastases in up to three lymph nodes. Further inclusion criteria were age ≥18 years, good performance status (ECOG 0–1, Karnofsky Index ≥70) and no contraindication for receiving systemic chemoendocrine therapy. Also, the treating physician and the enrolled patient had to confirm that she/he was principally willing to consider her/his initial recommendation and decision.
treatment recommendations
The protocol did not provide guidance regarding preferred treatment options for specific RS groups nor a specific algorithm how to derive treatment recommendations. Each patient's case was discussed twice within the respective institution's multidisciplinary tumor board. In the first meeting, adjuvant treatment was recommended based on available clinical and histopathological data according to the center's common treatment algorithms. In a second meeting, the case was rediscussed and treatment recommended with the RS result as an additional piece of information.
questionnaires
Physicians completed a pre-test questionnaire recording the initial treatment recommendation and their confidence in this recommendation before knowing the patient's RS and a post-test follow-up questionnaire after knowledge of RS results stating their final treatment recommendation and their confidence in it. Confidence could be rated on a semi-quantitative scale as minimum, low, intermediate, high or absolute. In the end-of-study questionnaire the treatment actually administered was recorded. Treatment always needed to be detailed in terms of agents, dosing, number of cycles and treatment duration.
Patients completed a questionnaire assessing decisional conflict (decisional conflict scale DCS) [24, 25] after their treating physician had discussed his/her initial treatment recommendation and a second time after their treating physician had discussed their final recommendation when the RS was known.
statistical analyses
The total sample size was planned to be at least 360 patients. The goal was to enable sufficiently precise estimates of the change rate of treatment recommendations (from an initial recommendation for chemoendocrine to endocrine treatment and vice versa) in patients with N+ ER+ EBC which was assumed to be one-third of the patients. At the time the study was designed, no data were available for change rates in N+ disease. Based on change rates in the range 21% and 44% published for N0 patients, we assumed the rate to be ∼25%, requiring a sample size of at least 100 patients to estimate it with a 95% confidence interval of width <18%.
Statistical analysis was exploratory. Evaluation of end points was carried out for all assessable patients and by predefined subgroups (N0, N+, low, intermediate and high RS risk group) and included comparisons before and after the Oncotype DX assay. Change rates are reported with 95% confidence intervals calculated using the Clopper–Pearson method. A logistic regression on the change rate with RS as an explanatory variable was carried out to evaluate the influence of the RS on treatment recommendations separately for patients initially recommended hormonal therapy or chemotherapy.
Data on physicians' confidence pre- and post-test were tabulated and compared the McNemar's test on dichotomized values (absolute and high confidence into high, low and minimum into low confidence, intermediate did not occur). A paired-sample t-test was used to determine the significance of the change from pre- to post-test in the means for the total DCS. Linear regression on the change of the total DCS with the RS as an explanatory variable was carried out to determine the impact of the RS on the decisional conflict of patients regarding treatment recommendation for patients initially recommended hormonal therapy or chemotherapy.
pharmacoeconomic analysis
A Markov model was developed to make long-term projections of distant recurrence, survival, quality-adjusted life expectancy and direct costs. Scenarios using conventional diagnostic procedures or the 21-gene assay to inform treatment recommendations for adjuvant chemotherapy were modeled based on the pre- and post-test data of our study. Transition probabilities and risk adjustment were based on published landmark trials. Costs (2011 Euros (€)) were estimated from a sick fund perspective based on the resource use in patients receiving chemotherapy. Future costs and clinical benefits were discounted at 3% annually [26].
results
Between June 2010 and April 2011 a total of 379 patients were enrolled. Two patients dropped out and Oncotype DX could not be carried out in another 11 individuals, leaving 366 assessable patients.
patient and tumor characteristics
Complete patient and tumor characteristics are listed in Table 1. The distribution of patients by RS risk group was similar for all assessable patients and in the N0 and N+ subsets.
Table 1.
Patient characteristics
| Characteristic | All | N0 | N+ (1–3 positive nodes) |
|---|---|---|---|
| N = 366 (100%) | N = 244 (67%) | N = 122 (33%) | |
| Mean age | |||
| Years | 56 | 56 | 57 |
| Tumor size, n (%) | |||
| ≤2 cm | 201 (55) | 137 (56) | 64 (53) |
| >2 cm | 165 (45) | 107 (44) | 58 (48) |
| Tumor grade, n (%) | |||
| Well | 52 (14) | 33 (14) | 19 (16) |
| Moderate | 281 (75) | 188 (77) | 93 (76) |
| Poor | 33 (9) | 23 (9) | 10 (8) |
| Progesterone receptor, n (%) | |||
| Positive | 326 (89) | 216 (89) | 110 (90) |
| Negative | 40 (11) | 28 (12) | 12 (10) |
| Recurrence Score, n (%) | |||
| Mean | 18 | 18 | 18 |
| Low | 198 (54) | 131 (54) | 67 (55) |
| Intermediate | 139 (38) | 95 (39) | 44 (36) |
| High | 29 (8) | 18 (7) | 11 (9) |
treatment recommendations before and after knowledge of RS
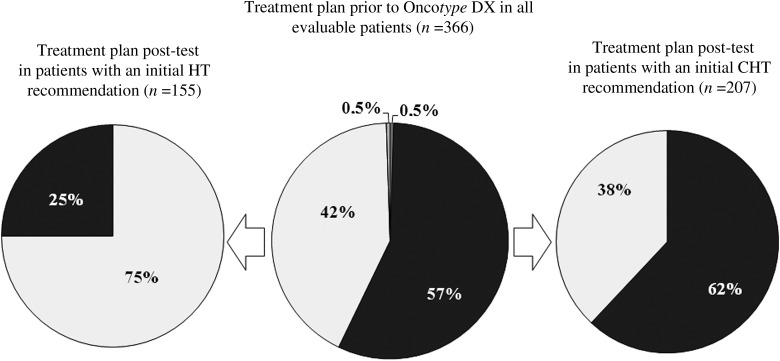
Changes in treatment recommendations from pre- to post-knowledge of Oncotype DX results are summarized in Table 2. Initial treatment recommendation was revised in 33% of all assessable patients, 30% in N0 and 39% in N+ subgroups after knowledge of the RS. The shift was predominantly from adjuvant combined chemoendocrine (CHT) to endocrine therapy alone (HT). Before the test adjuvant CHT would have been recommended to 209 of the 366 women (57%) whereas after Oncotype DX testing recommendation excluded adjuvant chemotherapy in 54% of patients (Figure 1). In 38% of all assessable patients, 39% of N0 and 37% of N+ patients with an initial recommendation for CHT the post-RS recommendation was revised to HT. In 25% of all assessable patients, 22% of N0 and 39% of N+ patients who had been initially recommended HT the post-RS recommendation changed to CHT.
Table 2.
Changes in physicians' treatment recommendations pre- to post-Oncotype DX
| Patients | N | Overall change rate | CHT to HT, n (%) | HT to CHT, n (%) | Other, n (%) |
|---|---|---|---|---|---|
| All assessable | 366 | 121 (33%; 95% CI 28.3–38.1) | 79 (22) | 39 (11) | 3 (1) |
| Low RS | 198 | 72 (36%; 95% CI 29.7–43.5) | 68 (34) | 2 (1) | 2a,b(1) |
| Intermediate RS | 139 | 43 (31%; 95% CI 23.4–39.3) | 11 (8) | 31 (22) | 1c (1) |
| High RS | 29 | 6 (21%; 95% CI 8.0–39.7) | 0 (0) | 6 (21) | 0 (0) |
| Node negative | 244 | 74 (30%; 95% CI 24.6–36.5) | 45 (18) | 28 (12) | 1c (0) |
| Low RS | 131 | 39 (30%; 95% CI 22.1–38.4) | 38 (29) | 1 (1) | 0 (0) |
| Intermediate RS | 95 | 31 (33%; 95% CI 23.4–43.0) | 7 (7) | 23 (24) | 1c (1) |
| High RS | 18 | 4 (22%; 95% CI 6.4–47.6) | 0 (0) | 4 (22) | 0 (0) |
| Node positive | 122 | 47 (39%; 95% CI 29.9–47.8) | 34 (28) | 11 (9) | 2a,b (2) |
| Low RS | 67 | 33 (49%; 95% CI 36.8–61.8) | 30 (45) | 1 (2) | 2a,b (3) |
| Intermediate RS | 44 | 12 (27%; 95% CI 15.0–42.8) | 4 (9.1) | 8 (18) | 0 (0) |
| High RS | 11 | 2 (18%; 95% CI 2.3–51.8) | 0 (0) | 2 (18) | 0 (0) |
RS, Recurrence Score; CHT, chemoendocrine therapy; HT, endocrine therapy; CT, chemotherapy; 95% confidence intervals calculated using the Clopper–Pearson method.
aObservation to CHT.
bObservation to HT.
cCT to CHT.
Figure 1.
Changes in treatment recommendations: black squares, chemoendocrine therapy (CHT); dark gray squares, chemotherapy; very light gray squares, endocrine therapy only (HT); light gray squares, observation.
Recommendations changed in 13 of 57 (23%) N0 patients with Ki-67 <15% and a grading G1 or G2 (4 HT to CHT, 9 CHT to HT). Of the 244 N0 patients there were 23 with a grading G3 and 45 with G1 or G2 but Ki-67 ≥15%. In 14 of these 68 patients (21%) recommendations changed (7 CHT to HT, 7 HT to CHT).
Influence of the RS on the change rate was highly substantial if patients with initial recommendation for chemotherapy or hormonal therapy were considered separately (P < 0.001 for both).
physicians’ level of confidence in their adjuvant treatment recommendation
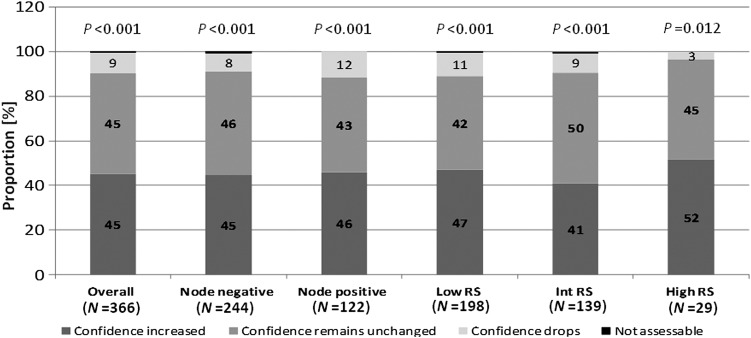
After knowledge of the test results, physicians classified their confidence in their treatment recommendation as absolute or high in 82% of cases when compared with a baseline of 55%. Overall, physicians' confidence increased in 45% and in 45% of N0 and 46% of N+ cases (P < 0.001) (Figure 2).
Figure 2.
Changes in physicians' confidence in treatment recommendations. The change in physicians' confidence from pre- to post-test assessment. Physicians had the choice between absolute, high, intermediate, low and minimal to classify the confidence in their treatment recommendations. RS, Recurrence Score, P-values of McNemar's test.
patients’ decisional conflict
Completed questionnaires pre- and post-test were available for 325 patients. The mean value for the DCS was 1.72 pre-test, indicating a low level of decisional conflict at baseline and decreased moderately to 1.61 post-test. The difference from the pre- to post-test DCS was statistically significant for all patients (6%, P = 0.028) and for patients with low RS values (11%, P = 0.003). Linear regression showed a significant influence if those with an initial HT recommendation (P = 0.048) but not if patients with initial CHT recommendation (P = 0.275) were analyzed.
actual treatment administered
In 45 (12%) cases treatment actually administered differed from the post-RS recommendation. Twenty-one (9%) N0 patients did not follow their physician's post-test recommendation: 6 patients in the low (2 HT to CHT, 4 CHT to HT) and 15 women in the intermediate RS group (15 CHT to HT). Twenty-four (20%) N+ patients decided against their physician's post-test recommendation: 13 individuals with low (3 HT to CHT, 9 CHT to HT, 1 HT to observation) and 11 patients with intermediate RS values (2 HT to CHT, 9 CHT to HT). This included three women (N0 1, N+ 2) with low RS and CHT post-test treatment recommendation after a pre-test recommendation against chemotherapy, who decided to have endocrine therapy as actual adjuvant treatment.
In absolute numbers 69 (N0 34, N+ 35, 73 low RS group, 2 intermediate RS group) patients were spared chemotherapy when treatment actually administered was compared with the initial treatment recommendation. This constituted an overall 19% net reduction in adjuvant chemotherapy usage when the actual number of chemotherapies given was compared with the initial treatment recommendation regardless of the nature of the pre-test recommendation (Table 3). If only those patients with an initial chemotherapy recommendation were considered as the denominator, the relative reduction in chemotherapy use was 33% (N0 29%, N+ 38%, low RS group 73%, intermediate RS group 2%) In patients with high RS values, there was a 27% relative increase of actual chemotherapy use.
Table 3.
Adjuvant chemotherapy use
| Patients | Pre-Oncotype DX recommendation | Therapy actually administered | Reduction in chemotherapy use |
||
|---|---|---|---|---|---|
| n (%) | n (%) | n | Net change (%)a | Relative change (%)b | |
| All, N = 366 | |||||
| No CT | 157 (43) | 226 (62) | 69 | 19 | 33 |
| CT | 209 (57) | 140 (38) | |||
| N0, N = 244 | |||||
| No CT | 127 (52) | 161 (66) | 34 | 14 | 29 |
| CT | 117 (48) | 83 (34) | |||
| N+, N = 122 | |||||
| No CT | 30 (25) | 65 (53) | 35 | 29 | 38 |
| CT | 92 (75) | 57 (47) | |||
N0, node negative; N+, node positive; CT, chemotherapy.
aTotal change in the number of chemotherapies applied regardless of pre-test recommendation.
bRelative change in the number of chemotherapies applied in relation to the number of patients with initial chemotherapy recommendation.
cost-effectiveness analysis
The 21-gene assay was projected to increase the mean life expectancy by 0.06 years and the quality-adjusted life expectancy by 0.06 quality-adjusted life years (QALYs) compared with current clinical practice over a 30-year time horizon. Costs from a health care payer perspective were lower with the assay by ∼€561 versus standard of care. Probabilistic sensitivity analysis indicated that there was an 87% probability that the use of the 21-gene assay would be dominant (cost- and life-saving) to standard of care.
discussion
This is the largest prospective study on the impact of the Oncotype DX assay on adjuvant decision-making as of today and, in particular, the first that reported prospective data in women with node-positive EBC [23].
Overall, treatment recommendations changed in 30% of node-negative cases after RS results were known. This is quiet consistent with findings from the prospective studies by Lo et al. [11] (32%) and Albanell et al. [12] (32%).
The predominant change in our study was from a recommendation of CHT to HT alone in 18% of N0 cases, which compares well with the findings from all reported prospective decision impact studies. The lower overall shift in treatment recommendations of 23% reported for N0 patients from the Australian study recently [15] can be explained by the much lower proportion of patients of 30% with an initial recommendation for CHT, whereas 47% of all N0 patients in our study were recommended CHT before Oncotype DX based on traditional criteria.
The change rate of 39% from pre- to post-test recommendation in patients with N+ disease in our study was higher than the rate of 30% in the N0 subset. But the difference was not statistically significant (P = 0.116, chi-square). The predominant change was from CHT to HT in 28% of all N+ cases and 37% of the 92 N+ patients with an initial recommendation for CHT. This is remarkable if one implies that the decision to omit chemotherapy in patients with 1–3 positive lymph nodes is considered more deeply and eventually hesitantly. Even if data indicate that patients with 1–3 positive nodes have a moderately elevated risk of recurrence, especially if they have low RS values [1, 5], some will regard chemotherapy as mandatory regardless of the number of positive nodes [8].
Four other studies reported about the clinical use of Oncotype DX in N+ disease. A UK study including patients with nodal micrometastases did not report results of this particular subgroup [13]. A web-based physicians' survey [22] reported a higher change rate of 51% in a probably highly selected population of 138 N+ ER-positive patients—92.6% had 1–3 positive nodes—with a change from CHT to HT in 33%. A prospective Japanese study reported on 90 patients including a subset of 17 patients with 1–3 positive nodes [14]. Recommendations changed in 12 (71%) women, all from CHT to HT. In the 50 patients with 1–3 positive lymph nodes from the Australian study [15], the overall change rate was 26%: 12 patients to HT and 1 to CHT.
In our study physicians seemed to be hesitant omitting chemotherapy in patients with intermediate RS: HT was recommended in 13% of patients initially recommended CHT but 60% of patients with initial recommendation for HT were advised CHT post-test. Also, 32% of patients with initial CHT recommendation were not advised to omit chemotherapy despite a low RS result and initial HT recommendation was revised to CHT post-test in three women with low RS values.
This may be explained by a large adjuvant study that had been ongoing in Germany defining an RS of 11 as the threshold for omitting chemotherapy in hormone receptor-positive EBC. It may also reflect that the use of Oncotype DX is not as widespread in German clinical practice as it is, e.g. in the United States [27]. This goes along with a lower reported increased confidence of physicians' after the test when compared with respective numbers from the United States (76%) [11] and the Spanish study (60%) [12]. A score combining pathological and clinical factors with the RS has been shown to improve the prognostic value compared with the RS alone and to substantially reduce the proportion of patients with intermediate risk which may help treatment decisions. However, RS alone is a better predictor of the benefit of chemotherapy [28].
Importantly, 45 patients—all with low or intermediate RS values—did not follow their physician's post-test recommendation, the majority (82%) of them deciding against chemotherapy regardless of the nodal status. This may have been the result of patients pondering their actual numeric relative risk of recurrence with HT alone and the potential benefit to be gained by CHT. Interestingly, Lo et al. [11] could demonstrate that patients perceived their actual risk of recurrence to be lower after knowing the RS result.
When comparing the initial treatment recommendation and treatment actually given, the use of adjuvant chemotherapy decreased with the limitation that it is assumed that without Oncotype DX all patients would have accepted their physician's initial treatment recommendation. This was even more pronounced for the subgroup of patients with 1–3 positive nodes. A reduction in chemotherapy usage may translate into actual pharmacoeconomic savings from the sick funds' perspective as indicated by results of our group reported here and in more detail in a separate publication [26]. While the outcome data of using Oncotype DX in clinical routine practice are pending, assumptions have to be based on consistent data from multiple prospectively designed studies of archival specimens from randomized clinical trials with a long-term follow-up [2, 3, 5, 6]. Findings from various health care systems [21] show that using the Oncotype DX assay as a decision aid in ER-positive EBC is, at minimum, cost-effective.
Grade and Ki-67 are frequently used as key markers to guide adjuvant treatment decisions and the St Gallen consensus recommends Ki-67 to discriminate luminal A and B tumors, although the relevant cut-off is debated. We carried out a retrospective analysis to assess the potential value of the RS in assumed low-risk patients with G1-2 with Ki-67 <15% and in assumed high-risk patients with G3 with Ki67 ≥15% for the patients where data were available. There were relevant shifts in treatment recommendations in both directions post-test. These results may be explained by a limited concordance among pathologists regarding grade and a high interobserver variability in Ki-67 assessment [29]. The adjuvant PlanB study recently reported a 68% concordance between local and central grading [30]. The correlation between the RS and Ki-67 by central assessment in PlanB was weak (r = 0.34) [31] and the current data indicate that Ki-67 is not predictive of the benefit of adjuvant chemotherapy in node-negative EBC [32].
The RS has been demonstrated to provide additional prognostic and predictive information beyond classical and histopathological criteria in N0 and N+ hormone receptor-positive disease [2–6] Recently, a score derived from immunohistochemical staining of ER, PgR, HER2 and Ki-67 (IHC4) was reported to deliver similar prognostic information as the RS if obtained by an optimized and centralized testing method [33]. Correlation between the IHC score and the RS was modest (r = 0.7). Moreover, this still has to prove the test of applicability in broad routine clinical practice. Lack of reproducibility of IHC assays is an issue of major concern as are the ∼20% of immunohistochemically false HR-negative patients [34] and the ∼20% of potentially inaccurate HER2 tests [35]. Also, the IHC4 is only a prognostic score while the RS also has predictive importance quantifying the benefit of adjuvant chemotherapy.
In conclusion, our results confirm that the RS had an impact on physicians' and patients' adjuvant decision-making in both node-negative and node-positive ER-positive disease. This resulted in a substantial reduction of adjuvant chemotherapy usage and should thus support efforts to improve the access for patients with ER-positive EBC to the test.
funding
This study was sponsored by Genomic Health Inc. Oncotype DX was supported free of charge. Authors had full access to the data. The paper reflects the views of the authors. No restrictions were set by the sponsor.
disclosure
WE and KF are members of the speakers' bureau for Genomic Health, Inc., SK, CJ and JB received speakers' honoraria from Genomic Health, Inc., SH is a medical consultant to Genomic Health, Inc. MR, TK, MW, AS, SM, HE, JH, IW, HE and AB have declared no conflicts of interest.
acknowledgements
We would like to acknowledge Anja Liebert and Rolf Vogel from ClinAssess for their valuable support in study coordination and data management. We would also like to thank Dr Kerstin Reuter for her continuous support and advice.
references
- 1.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–1329. doi: 10.1093/annonc/mdp322. doi:10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. doi:10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. doi:10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 4.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. doi:10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 6.Albain K, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncology. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. doi:10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. doi:10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Practice Guidelines in Oncology. Breast Cancer (version v2.2011). http://www.NCCN.org .
- 9.Aebi S, Davidson T, Gruber G, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v9–v14. doi: 10.1093/annonc/mdq159. doi:10.1093/annonc/mdq159. [DOI] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Wood C, Coates AS. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. doi:10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo S, Mumby P, Norton J, et al. Prospective Multicenter Study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. doi:10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 12.Albanell J, Gonzales A, Ruiz-Borrego M, et al. Prospective transGEICAM Study of Oncotype DX® in clinical decision making in women with estrogen receptor-positive, node-negative breast cancer. Ann Oncol. 2012;23:625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 13.Holt SDH, Durrani S, Pudney D, et al. Results from a prospective clinical study on the impact of Oncotype DX® on adjuvant treatment decision in a cohort of 142 UK patients. SABCS. 2011 Abstract #P5-14-26. [Google Scholar]
- 14.Yamauchi H, Nakagawa C, Yamashige S, et al. Decision Impact and Economic Evaluation of the 21-Gene Recurrence Score® Assay for Physicians and Patients in Japan. Stockholm, Sweden: Poster presented at the annual European Society for Medical Oncology Congress; 2011. [Google Scholar]
- 15.De Boer RH, Baker C, Speakman D. Australian Decision Impact Study: the impact of Oncotype DX Recurrence Score (RS) on adjuvant treatment decisions in hormone receptor positive (HR+), node negative and node positive (N+9 early breast cancer (ESBC) in the multidisciplinary clinic. SABCS. 2011 Abstract #P4-09-18. [Google Scholar]
- 16.Hornberger J, Chien R. Meta-analysis of the decision impact of the 21-gene breast cancer Recurrence Score in clinical practice. Poster Presented at the St Gallen International Breast Cancer Conference; St Gallen, Switzerland: 2011. Abstract P201. [Google Scholar]
- 17.Klang SH, Hammerman A, Liebermann N, et al. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health. 2010;13:381–387. doi: 10.1111/j.1524-4733.2010.00724.x. doi:10.1111/j.1524-4733.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsoi DT, Inoue M, Kelly CM, et al. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist. 2010;15:457–465. doi: 10.1634/theoncologist.2009-0275. doi:10.1634/theoncologist.2009-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M, Hoshi S, Yamanaka T, et al. Economic evaluation of the 21-gene signature (Oncotype DX) in lymph node negative/positive hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03) Breast Cancer Res Treat. 2011;127:739–749. doi: 10.1007/s10549-010-1243-y. doi:10.1007/s10549-010-1243-y. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Cosler LE, NM Kuderer NM, et al. Impact of a 21-Gene RT-PCR assay on treatment decisions in early-stage breast cancer, an economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. doi:10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 21.Pronzato P, Plun-Favreau J. Is the 21-gene breast cancer test (Oncotype DX) cost-effective. SABCS 2012, P1-10-05.
- 22.Oratz R, Kim B, Chao C, et al. Physician survey of the effect of the 21-gene recurrence score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer. J Oncol Pract. 2011;7(2):94–99. doi: 10.1200/JOP.2010.000046. doi:10.1200/JOP.2010.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blohmer J, Kühn T, Rezai M, et al. German multicentre decision impact study of Oncotype DX recurrence score (RS) on adjuvant treatment in estrogen receptor positive (ER+) node negative (N0) and node positive (N+) early breast cancer. Presented as a Poster at the 12th International St. Gallen Breast Cancer Conference; 2011. P206. [Google Scholar]
- 24.O'Connor A. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. doi:10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 25.Höldtke B. Evidence based medicine für Laien. Modellhafte Entwicklung eines Konzeptes zur Vermittlung von wissenschaftlichen Informationen zum Thema, Früherkennung von Brustkrebs mit Mammographie, Hamburg 2002.
- 26.Blohmer JU, Rezai M, Kümmel S, et al. Using Oncotype DX® to guide adjuvant chemotherapy decision making in early-stage breast cancer: a cost-effectiveness evaluation in the German setting. J Med Econ. 2012 doi: 10.3111/13696998.2012.722572. in press. [DOI] [PubMed] [Google Scholar]
- 27.Hassett M, Niland JC, Hughes ME, et al. Gene expression profile testing for breast cancer: Patterns and predictors of use and impact on chemotherapy. J Clin Oncol. 2010;28:15s. (Suppl; abstr 566) [Google Scholar]
- 28.Tang G, Cuzick J, Costantino JP, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011;29(33):4365–4372. doi: 10.1200/JCO.2011.35.3714. doi:10.1200/JCO.2011.35.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga Z, Diebold J, Dommann-Scherrer C, et al. How reliable is Ki-67 immunohistochemistry in Grade 2 breast carcinomas? A QA study of the Swiss working group of breast- and gynecopathologists. PLoS ONE. 2012;7(5):e37379. doi: 10.1371/journal.pone.0037379. doi:10.1371/journal.pone.0037379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gluz O, Kreipe HH, Christgen M. Prospective comparison of recurrence score and independent central pathology assessment of prognostic tools in early breast cancer (BC): Focus on HER2, ER, PR, Ki-67 results from the phase III WSG-Plan B trial. J Clin Oncol. 2012;30 (Suppl; abstr 552) [Google Scholar]
- 31.Gluz O, Kreipe HH, Degenhardt T, et al. Prospective comparison of risk assessment in early breast cancer: correlation analysís from the Phase III WSG-Plan B Trial. Ann Oncol. 2012;23(suppl 2):ii17–ii24. doi:10.1093/annonc/mds039. [Google Scholar]
- 32.Viale G, Regan MM, Mastropasqua MG, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008;100:207–212. doi: 10.1093/jnci/djm289. doi:10.1093/jnci/djm289. [DOI] [PubMed] [Google Scholar]
- 33.Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, porgesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the genomic health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. doi:10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 34.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. doi:10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. doi:10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]