Abstract
Background
Understanding socio-demographic inequalities in stage at diagnosis can inform priorities for cancer control.
Patients and methods
We analysed data on the stage at diagnosis of East of England patients diagnosed with any of 10 common cancers, 2006–2010. Stage information was available on 88 657 of 98 942 tumours (89.6%).
Results
Substantial socio-demographic inequalities in advanced stage at diagnosis (i.e. stage III/IV) existed for seven cancers, but their magnitude and direction varied greatly by cancer: advanced stage at diagnosis was more likely for older patients with melanoma but less likely for older patients with lung cancer [odds ratios for 75–79 versus 65–69 1.60 (1.38–1.86) and 0.83 (0.77–0.89), respectively]. Deprived patients were more likely to be diagnosed in advanced stage for melanoma, prostate, endometrial and (female) breast cancer: odds ratios (most versus least deprived quintile) from 2.24 (1.66–3.03) for melanoma to 1.31 (1.15–1.49) for breast cancer. In England, elimination of socio-demographic inequalities in stage at diagnosis could decrease the number of patients with cancer diagnosed in advanced stage by ∼5600 annually.
Conclusions
There are substantial socio-demographic inequalities in stage at diagnosis for most cancers. Earlier detection interventions and policies can be targeted on patients at higher risk of advanced stage diagnosis.
Keywords: cancer, demographic, diagnosis, inequalities, socio-economic, stage
introduction
Eliminating population exposure to known lifestyle and environmental risk factors would only prevent up to 45% of all new diagnoses of cancer, and this potential may not be achievable for many years [1]. Therefore, in addition to primary prevention efforts to decrease cancer incidence, current cancer control policies aim to reduce the proportion of patients diagnosed at advanced disease stage. Early-stage detection renders currently available treatments more effective and can therefore amplify the impact of primary prevention initiatives to help decrease cancer-related deaths [2, 3].
However, the evidence about how to achieve earlier stage detection is still developing [3]. The great majority of cancer patients are diagnosed after first presenting to a general practitioner with symptoms relating to their cancer [4]. In those patients, interventions to improve awareness and appraisal of cancer symptoms could help to decrease the time interval between symptom onset and presentation to a doctor [5]. Such interventions could be further targeted and tailored on population groups at higher risk of advanced stage diagnosis, for example older women (who are at higher risk of late-stage diagnosis of breast cancer compared with younger women) [6, 7] or men (who are at higher risk of late-stage diagnosis of melanoma compared with women) [8]. For cancers with large socio-demographic inequalities in stage at diagnosis, such targeting may be judged particularly appropriate. However, for most common cancers, up-to-date UK evidence about whether socio-demographic inequalities in stage at diagnosis exist and their magnitude and direction is limited.
Against this background, we set out to examine socio-demographic inequalities in stage at diagnosis for 10 common cancers. Our aim was to explore and characterise variation in advanced stage at diagnosis for different cancers and patient groups to help inform priorities for cancer control.
methods
data
We analysed information on the stage at diagnosis of East of England patients with cancer aged 30 years or over with a new diagnosis of (International Classification of Diseases–10 code): colon (C18), rectal (C19–20), lung (C34), melanoma (C43), female breast (C50), endometrial (C54), ovarian (C56), prostate (C61), renal (C64) and bladder (C67) cancer during 2006–2010. This represents the longest most recent period for which stage information was available with high completeness for all 10 cancers. Together, the respective cancer sites represent 67% of new cancer diagnoses in England (2009) and 57% of all cancer deaths. Anonymous data were extracted from the Eastern Cancer Registration and Information Centre (ECRIC), a cancer registry covering a population of ∼5.7 m. This registry has an excellent record of registration quality as indicated by measures, such as death-certificate only registrations of 0.02% in 2010 compared with an average national rate of 1.6% [9]. Uniquely among English cancer registries, currently it holds stage at diagnosis information for a high proportion of tumours [10]. As part of the registration process, stage at diagnosis was assigned by CHB and BAR, based on clinical, imaging and pathological information according to the TNM classification [11]. Information on the income domain of the Index of Multiple Deprivation (IMD) 2004 score of the Lower Super Output Area of patients' residence at diagnosis was available as part of the registration record and was subsequently used to define socio-economic status quintiles (1 = least deprived, or ‘affluent’; 5 = most deprived) [12]. The use of this index (i.e. of the income domain of IMD) is standard practice in UK cancer statistics and research because it avoids the potential for endogeneity between health outcomes (e.g. cancer stage at diagnosis) and composite (multidimensional) deprivation measures already incorporating measures of health deprivation.
analysis
The main analysis was confined to patients with known stage (complete case analysis). We defined advanced stage at diagnosis as diagnosis in stages III/IV. We used a two-step modelling approach, which allows us to examine overall socio-demographic differences before investigating how they vary by cancer [13].
Initially, a logistic regression model was used to predict advanced stage diagnosis, adjusting for age group (65–69-year olds used as reference), gender (men as reference), deprivation quintile (least deprived group as reference), cancer (rectal cancer as reference); tumour type (morphology); and for breast, colon and rectal cancers, whether the cancer was detected symptomatically or by screening (diagnosis of cancer within 90 days from a screening episode defined screening-detected cancers). We used this initial model which only included main effect variables to summarise overall associations between age, gender and deprivation with advanced stage at diagnosis across all studied cancers. Because of interactions between socio-demographic variables and cancer (P < 0.001 for all), in subsequent analysis we used a series of separate logistic regression models for each cancer.
We assessed the robustness of the findings using different sensitivity analyses. We re-ran the initial model using different definitions of advanced stage categories, i.e. diagnosis in stages II–IV versus stage I; or in stage IV versus stages I–III. We also explored potential confounding of gender, deprivation and age inequalities by ethnicity by repeating the analysis adjusting for ethnic group in the two-thirds of all patients for whom ethnicity information was available. We explored potential bias arising from missing stage information using multiple imputation of missing stage repeating the analysis in a complete dataset including patients with imputed stage [7, 14, 15]. All exposure variables included in the analysis models were complete, and data were only missing for the outcome variable (stage). In this case, the analysis model will provide unbiased estimates of associations under the missing at random (MAR) assumption (that outcome data are MAR given the exposure variables). However, the MAR assumption can be made more reasonable if stage is imputed on additional variables to those used in the analysis. We therefore imputed stage using an imputation model including information on survival, tumour histological grade, basis of diagnosis (i.e. whether the diagnosis was verified with histology or not), and oestrogen receptor status (for patients with breast cancer only) in addition to all the variables used in the analysis models. Imputation variables were complete, except for grade and oestrogen receptor status (used in imputation models only). Survival was censored at 365 days so that a reasonably consistent approach was applied to patients of any age regarding competing mortality risk. For the imputation model to be congenial with the analysis, model imputation was stratified by cancer. Multiple imputation was conducted using chained equations which created 20 imputed datasets.
We illustrate the potential population health impact of the findings by estimating the number of cancers diagnosed in advanced stage in England annually that could be prevented by eliminating gender, deprivation and old age inequalities. Potential improvements were estimated only where there was significant variation. For the prediction of potential proportions, the odds ratio values for patients belonging to patient groups with higher probability of advanced stage diagnosis were set to that of the relevant group with the lowest odds ratios. As more deprived patients with cancer are under-represented in the East of England compared with the England average, we used inverse probability weights calculated using the national deprivation group-specific incidence of each cancer. We focused on the impact of eliminating age inequalities on patients aged 65 or over because this age group includes about two-thirds of all patients with cancer and because of concerns about poorer relative survival and avoidable excess cancer deaths in older patients [16, 17]. Confidence intervals for these impact values were estimated using a bootstrap with 1 000 replications, accounting for both the uncertainty in stage inequalities and the uncertainty in the distribution of different patient groups.
In order to account for the fact that patients may attend the same health care organisation and that some tumours occur in the same patient, we use a sandwich estimator throughout. All analysis was conducted in STATA 11 (StataCorp. 2009, College Station, TX), including using the ice and mim commands used for multiple imputation [18]. See also supplementary file S1, for additional details on methods, available at Annals of Oncology online.
results
In total, there were 98 942 patients diagnosed with a relevant cancer during the study period. Information on stage at diagnosis was available for 88 657 (89.6%) of all patients, and among those patients the proportion of those diagnosed in stages III/IV varied substantially by cancer (Table 1 and supplementary file S2, available at Annals of Oncology online).
Table 1.
Distribution of stage, gender, age and deprivation categories by cancer (n = 98 942)a
| Bladder | Breast | Colon | Endometrial | Renal | Lung | Melanoma | Ovarian | Prostate | Rectal | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 4924 | n = 22 447 | n = 12 019 | n = 3644 | n = 3476 | n = 16 714 | n = 5693 | n = 2744 | n = 20 372 | n = 6909 | n = 98 942 | |
| Stage I | 2219 45% |
8595 38% |
1489 12% |
2612 72% |
1208 35% |
1949 12% |
3536 62% |
524 20% |
2111 10% |
1460 21% |
25 703 26% |
| Stage II | 1403 28% |
9124 41% |
3661 30% |
315 9% |
395 11% |
901 5% |
1142 20% |
136 5% |
11 815 58% |
1451 21% |
30 343 30% |
| Stage III | 277 6% |
1999 9% |
2917 24% |
321 9% |
552 16% |
4339 26% |
629 11% |
1323 48% |
2323 11% |
1807 26% |
16 487 17% |
| Stage IV | 551 11% |
1030 5% |
2374 20% |
167 5% |
977 28% |
6360 38% |
77 1% |
461 17% |
2958 15% |
1169 17% |
16 124 16% |
| Unknown stage | 474 10% |
1699 8% |
1578 13% |
229 6% |
344 10% |
3165 19% |
309 5% |
300 11% |
1165 6% |
1022 15% |
10 285 10% |
| Stage I/II | 3622 81% |
17 719 85% |
5150 49% |
2927 86% |
1603 51% |
2850 21% |
4678 87% |
660 27% |
13 926 73% |
2911 49% |
56 046 63% |
| Stages III/IV | 828 19% |
3029 15% |
5291 51% |
488 14% |
1529 49% |
10 699 79% |
706 13% |
1784 73% |
5281 27% |
2976 51% |
32 611 37% |
| All known stage | 4450 | 20 748 | 10 441 | 3415 | 3132 | 13 549 | 5384 | 2444 | 19 207 | 5887 | 88 657 |
| Men | 3625 74% |
6069 51% |
2207 63% |
9627 58% |
2855 50% |
20 372 100% |
4207 61% |
48 962 49% |
|||
| Women | 1299 26% |
22 447 100% |
5950 49% |
3644 100% |
1269 37% |
7087 42% |
2838 50% |
2744 100% |
2702 39% |
49 980 51% |
|
| 30–49 | 111 2% |
4245 19% |
446 4% |
178 5% |
293 8% |
447 3% |
1261 22% |
291 11% |
171 1% |
316 5% |
7759 8% |
| 50–54 | 119 2% |
2574 11% |
342 3% |
244 7% |
224 6% |
566 3% |
461 8% |
189 7% |
441 2% |
285 4% |
5445 6% |
| 55–59 | 247 5% |
2412 11% |
638 5% |
492 14% |
355 10% |
1117 7% |
563 10% |
264 10% |
1345 7% |
523 8% |
7956 8% |
| 60–64 | 418 8% |
3119 14% |
1270 11% |
650 18% |
451 13% |
1950 12% |
748 13% |
387 14% |
2760 14% |
901 13% |
12 654 13% |
| 65–69 | 601 12% |
2724 12% |
1602 13% |
546 15% |
432 12% |
2197 13% |
635 11% |
379 14% |
3686 18% |
1020 15% |
13 822 14% |
| 70–74 | 766 16% |
1873 8% |
1859 15% |
547 15% |
467 13% |
2722 16% |
636 11% |
377 14% |
3900 19% |
1136 16% |
14 283 14% |
| 75–79 | 919 19% |
1963 9% |
2113 18% |
428 12% |
536 15% |
2970 18% |
571 10% |
319 12% |
3746 18% |
1068 15% |
14 633 15% |
| 80–84 | 896 18% |
1652 7% |
1968 16% |
323 9% |
394 11% |
2641 16% |
442 8% |
262 10% |
2506 12% |
916 13% |
12 000 12% |
| 85+ | 847 17% |
1885 8% |
1781 15% |
236 6% |
324 9% |
2104 13% |
376 7% |
276 10% |
1817 9% |
744 11% |
10 390 11% |
| Affluent | 1143 23% |
6031 27% |
2902 24% |
809 22% |
712 20% |
3093 19% |
1696 30% |
686 25% |
5601 27% |
1709 25% |
24 382 25% |
| Deprivation group 2 | 1154 23% |
5837 26% |
3164 26% |
943 26% |
920 26% |
3943 24% |
1552 27% |
703 26% |
5629 28% |
1756 25% |
25 601 26% |
| Deprivation group 3 | 1273 26% |
5453 24% |
3036 25% |
958 26% |
949 27% |
4313 26% |
1417 25% |
682 25% |
4935 24% |
1747 25% |
24 763 25% |
| Deprivation group 4 | 1003 20% |
3881 17% |
2211 18% |
700 19% |
677 19% |
3834 23% |
800 14% |
493 18% |
3175 16% |
1282 19% |
18 056 18% |
| Deprived | 351 7% |
1245 6% |
706 6% |
234 6% |
218 6% |
1531 9% |
228 4% |
180 7% |
1032 5% |
415 6% |
6140 6% |
aFor each cancer, column percentages for patient groups in each category are presented in italics.
Considering socio-demographic inequalities for all cancers together, there was strong evidence that advanced stage at diagnosis was less likely in women and more likely with increasing level of deprivation (P = 0.003 for gender and P < 0.001 for deprivation, Table 2). Advanced stage also varied by age (P < 0.001), being more likely in older age. There was strong evidence that the associations between advanced stage at diagnosis and gender, age and deprivation varied between cancers (P < 0.001 for all).
Table 2.
Independent associations of gender, deprivation and age with advanced stage at diagnosis (stage III/IV versus stage I/II)* (n = 88 657)
| Adjusted odds ratio* (95% confidence intervals) | P | |
|---|---|---|
| Men | Reference | 0.003 |
| Women | 0.93 (0.89, 0.98) | |
| Affluent | Reference | <0.001 |
| 2 | 1.10 (1.05, 1.15) | |
| 3 | 1.11 (1.06, 1.16) | |
| 4 | 1.15 (1.09, 1.21) | |
| Deprived | 1.21 (1.12, 1.30) | <0.001 |
| 30–49 | 0.90 (0.83, 0.98) | |
| 50–54 | 1.03 (0.94, 1.12) | |
| 55–59 | 1.09 (1.01, 1.17) | |
| 60–64 | 1.01 (0.95, 1.08) | |
| 65–69 | Reference | |
| 70–74 | 1.00 (0.94, 1.06) | |
| 75–79 | 1.01 (0.96, 1.08) | |
| 80–84 | 1.08 (1.01, 1.15) | |
| 85+ | 1.28 (1.19, 1.37) |
*From a model which also adjusted for breast, colon and rectal screening detection status, cancer and tumour type (all cancers).
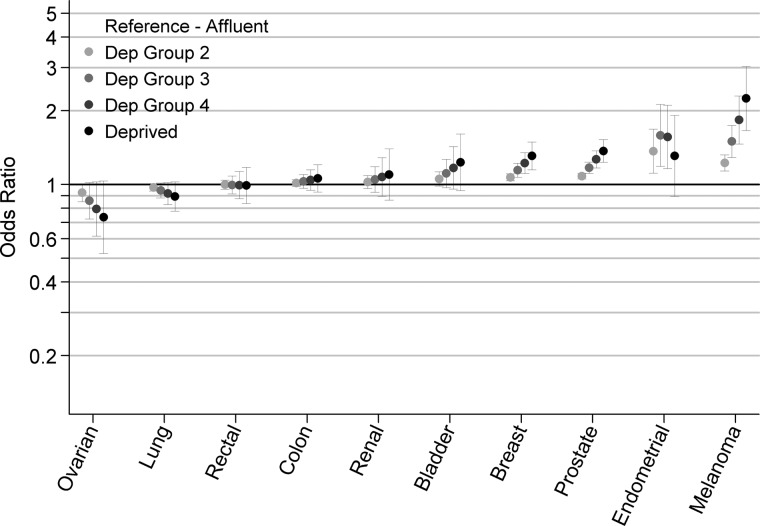
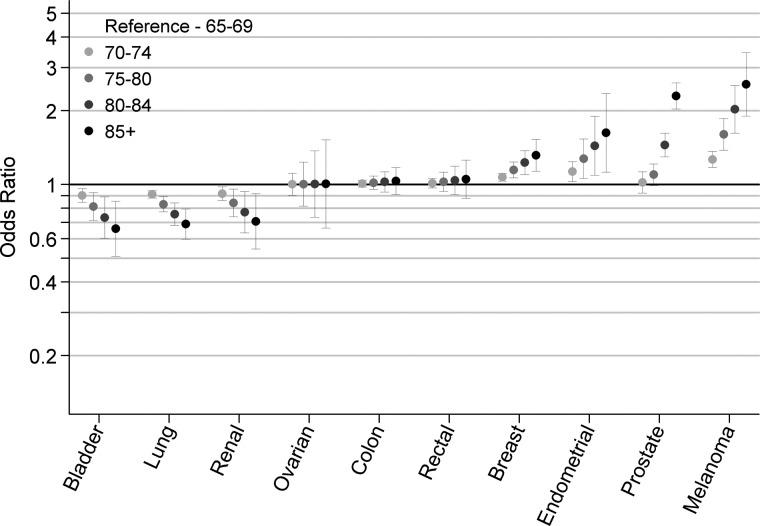
In separate models (by cancer), women were less likely to be diagnosed in advanced stage compared with men for melanoma and lung cancer [odds ratios (OR) for women versus men 0.68 (0.57–0.81) P < 0.001 and 0.88 (0.81–0.96) P = 0.003, respectively] (Table 3). There was evidence (P ≤ 0.007 for all) for deprivation gradients in patients with 4 of the 10 cancers (i.e. for melanoma, breast, endometrial and prostate cancer), with most deprived patients having a higher probability of advanced stage diagnosis (Figure 1). Among patients aged 65 or over, the strength and direction of associations between age and stage at diagnosis varied greatly between cancers (Figure 2). Older patients with melanoma, prostate, endometrial and breast cancer were more likely to be diagnosed in advanced stage compared with patients aged 65–69 (P = 0.01 for endometrial and P < 0.001 for others). Conversely, older patients with bladder, lung and renal cancer were less likely to be diagnosed in advanced stage compared with 65–69-year-old patients (P = 0.002, P < 0.001, and P = 0.009, respectively).
Table 3.
Association between gender, deprivation and age and advanced stage at diagnosis by cancer (using stratified models for each cancer)
| Bladder* |
Breast* |
Colon* |
Rectal* |
Endometrial† |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | |
| Men | Reference | 0.065 | n/a | Reference | 0.372 | Reference | 0.362 | n/a | ||
| Women | 1.19 (0.99, 1.43) | 1.04 (0.96, 1.12) | 0.95 (0.86, 1.06) | |||||||
| Affluent | Reference | 0.126 | Reference | <0.001 | Reference | 0.391 | Reference | 0.915 | Reference | 0.007 |
| 2 | 1.05 (0.99, 1.13) | 1.07 (1.04, 1.10) | 1.01 (0.98, 1.05) | 1.00 (0.96, 1.04) | 1.37 (1.11, 1.68) | |||||
| 3 | 1.11 (0.97, 1.27) | 1.14 (1.07, 1.22) | 1.03 (0.96, 1.10) | 1.00 (0.91, 1.08) | 1.59 (1.19, 2.12) | |||||
| 4 | 1.17 (0.96, 1.43) | 1.22 (1.11, 1.35) | 1.04 (0.95, 1.15) | 0.99 (0.87, 1.13) | 1.56 (1.16, 2.10) | |||||
| Deprived | 1.23 (0.94, 1.61) | 1.31 (1.15, 1.49) | 1.06 (0.93, 1.20) | 0.99 (0.84, 1.17) | 1.31 (0.89, 1.92) | |||||
| 30–49 | 1.50 (1.00, 2.24) | <0.001b | 0.79 (0.69, 0.90) | <0.001b | 1.78 (1.48, 2.14) | <0.001b | 1.78 (1.43, 2.22) | <0.001b | 1.21 (0.78, 1.87) | 0.032b |
| 50–54 | 1.35 (1.00, 1.83) | 0.84 (0.76, 0.92) | 1.54 (1.34, 1.77) | 1.54 (1.31, 1.82) | 1.15 (0.83, 1.60) | |||||
| 55–59 | 1.22 (1.00, 1.50) | 0.89 (0.83, 0.95) | 1.33 (1.22, 1.46) | 1.33 (1.19, 1.49) | 1.10 (0.89, 1.37) | |||||
| 60–64 | 1.11 (1.00, 1.22) | 0.94 (0.91, 0.97) | 1.16 (1.10, 1.21) | 1.16 (1.09, 1.22) | 1.05 (0.94, 1.17) | |||||
| 65–69 | Reference | Reference | Reference | Reference | Reference | |||||
| 70–74 | 0.90 (0.84, 0.96) | 0.002c | 1.07 (1.03, 1.11) | <0.001c | 1.01 (0.98, 1.04) | 0.618c | 1.01 (0.97, 1.06) | 0.585c | 1.13 (1.03, 1.24) | 0.010c |
| 75–79 | 0.81 (0.71, 0.92) | 1.15 (1.06, 1.24) | 1.02 (0.95, 1.08) | 1.03 (0.94, 1.12) | 1.27 (1.06, 1.53) | |||||
| 80–84 | 0.73 (0.60, 0.89) | 1.23 (1.10, 1.37) | 1.02 (0.93,1.13) | 1.04 (0.91, 1.19) | 1.44 (1.09, 1.90) | |||||
| 85+ | 0.66 (0.51, 0.86) | 1.32 (1.13, 1.53) | 1.03 (0.91, 1.17) | 1.05 (0.88, 1.26) | 1.62 (1.12, 2.35) | |||||
| Renal* |
Lung* |
Melanoma* |
Ovarian* |
Prostate‡ |
||||||
| Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | Odds ratio (95% CI) | Pa | |
| Men | Reference | 0.118 | Reference | 0.003 | Reference | <0.001 | n/a | n/a | ||
| Women | 0.89 (0.77, 1.03) | 0.88 (0.81, 0.96) | 0.68 (0.57, 0.81) | |||||||
| Affluent | Reference | 0.448 | Reference | Reference | Reference | Reference | ||||
| 2 | 1.02 (0.96, 1.09) | 0.97 (0.94, 1.01) | 0.109 | 1.22 (1.14, 1.32) | <0.001 | 0.93 (0.85, 1.01) | 0.077 | 1.08 (1.05, 1.11) | <0.001 | |
| 3 | 1.05 (0.93, 1.18) | 0.95 (0.88, 1.01) | 1.50 (1.29, 1.74) | 0.86 (0.72, 1.02) | 1.17 (1.11, 1.23) | |||||
| 4 | 1.07 (0.90, 1.29) | 0.92 (0.83, 1.02) | 1.83 (1.46, 2.30) | 0.79 (0.61, 1.03) | 1.27 (1.17, 1.37) | |||||
| Deprived | 1.10 (0.86, 1.40) | 0.89 (0.78, 1.03) | 2.24 (1.66, 3.03) | 0.74 (0.52, 1.03) | 1.37 (1.23, 1.52) | |||||
| 30–49 | 0.68 (0.52, 0.88) | <0.001b | 1.50 (1.20, 1.87) | <0.001b | 0.46 (0.34, 0.63) | <0.001b | 0.51 (0.36, 0.71) | <0.001b | 0.93 (0.76, 1.15) | <0.001b |
| 50–54 | 0.75 (0.61, 0.91) | 1.35 (1.15, 1.60) | 0.56 (0.45, 0.70) | 0.60 (0.46, 0.78) | 0.95 (0.81, 1.11) | |||||
| 55–59 | 0.82 (0.72, 0.94) | 1.22 (1.09, 1.37) | 0.68 (0.58, 0.79) | 0.71 (0.60, 0.84) | 0.97 (0.87, 1.07) | |||||
| 60–64 | 0.91 (0.85, 0.97) | 1.11 (1.05, 1.17) | 0.82 (0.76, 0.89) | 0.84 (0.77, 0.92) | 0.98 (0.93, 1.04) | |||||
| 65–69 | Reference | Reference | Reference | Reference | Reference | |||||
| 70–74 | 0.92 (0.86, 0.98) | 0.009c | 0.91 (0.88, 0.94) | <0.001c | 1.27 (1.17, 1.36) | <0.001c | 1.00 (0.90, 1.11) | 0.980c | 1.02 (0.92, 1.13) | <0.001c |
| 75–79 | 0.84 (0.74, 0.96) | 0.83 (0.77, 0.89) | 1.60 (1.38, 1.86) | 1.00 (0.81, 1.23) | 1.10 (0.99, 1.21) | |||||
| 80–84 | 0.77 (0.63, 0.94) | 0.76 (0.68, 0.84) | 2.03 (1.62, 2.54) | 1.00 (0.74, 1.37) | 1.45 (1.30, 1.62) | |||||
| 85+ | 0.71 (0.54, 0.92) | 0.69 (0.60, 0.79) | 2.57 (1.90, 3.46) | 1.01 (0.66, 1.52) | 2.30 (2.03, 2.60) | |||||
aAll testing is based on Wald tests with joint tests used where applicable.
bTest for overall variation between different age groups.
cTest for variation between age groups for patients 65 years or older.
*The models for these eight cancers (i.e. colon, rectal, lung, melanoma, female breast, ovarian, renal and bladder cancer) were adjusted for tumour type, gender and screening detection status (as applicable) and age and deprivation. Deprivation was treated as a continuous variable. Age was treated as two continuous variables (above and below the reference group) included together in the model (see also supplementary file S1, available at Annals of Oncology online).
†The model for endometrial cancer was adjusted for tumour type and linear terms for young and old age groups (see footnote * above). For deprivation, both a linear and a squared term were included, given evidence that such parameterisation improved model fit (P = 0.023). The presented odds ratio values for each deprivation group are derived by the combination of the linear and the squared terms [i.e. odds ratio = 1.48 (95% CI 1.12–1.95) and odds ratio = 0.92 (95% CI 0.86–0.99), respectively].
‡The model for prostate cancer was adjusted for tumour type and linear terms for deprivation category and young age group (see footnote * above). Old age group was treated as a categorical variable, because of strong evidence that such parameterisation improved model fit (<0.001). CI, Confidence Interval.
Figure 1.
Deprivation inequalities in advanced stage at diagnosis by cancer (odds ratios and 95% confidence intervals for diagnosis in stage III/ IV versus I/II).
Figure 2.
Age inequalities in advanced stage diagnosis in patients ≥65 by cancer (odds ratios and 95% confidence intervals for diagnosis in stage III/ IV versus I/II).
The sensitivity analysis (carried out using the initial model) produced overall similar findings (supplementary files S3–S6, available at Annals of Oncology online). Multiple imputation produced similar findings to those obtained by complete case analysis for gender, deprivation and age inequalities, although it indicated a slight underestimation of associations with advanced stage diagnosis in older patients.
In England, there are ∼186 000 new diagnoses for the 10 studied cancers every year (2009), and we estimated (see Methods) that ∼73 000 (39%) of those cancers are diagnosed in advanced stage currently. For the seven cancers with evidence of socio-demographic variation in stage at diagnosis there are ∼146 000 new diagnoses, and we estimated that ∼52 000 (36%) of those cancers are diagnosed in advanced stage currently. If it were possible to eliminate all gender, deprivation and old age inequalities in stage at diagnosis, there would be ∼5600 fewer cases diagnosed in advanced stage in England annually (Table 4) representing ∼3% of all diagnoses of the 10 studied cancers. Alternatively, this figure represents an ∼8% reduction in the advanced stage cases of all 10 studied cancers, or an ∼11% reduction in the advanced stage cases of the seven cancers for which there was evidence of potential gains in early stage diagnosis. Prostate, melanoma and endometrial cancer have the largest proportional early diagnosis gains. About 5000 of those tumours (∼90%) comprise reductions in advanced stage at diagnosis for prostate (∼2000 cancers), breast (∼1000 cancers), lung (∼1300 cancers) and melanoma (∼700 cancers), with colon, rectal and ovarian cancer contributing no cases.
Table 4.
Estimated annual reduction in cancers diagnosed in stage III/IV as opposed to stage I/II in England that could be achievable if gender, deprivation and old age inequalities (2006–2010) were eliminated*
| Gender | Deprivation | Old age | Gender, deprivation and old age combined | |
|---|---|---|---|---|
| Potential reduction in cancers diagnosed in advanced stage as a percentage of all new cancer diagnoses | ||||
| Melanoma | 2.00 (1.10, 2.89) | 3.06 (1.91, 4.22) | 2.67 (1.86, 3.48) | 6.78 (5.48, 8.09) |
| Prostate | n/a | 2.62 (1.73, 3.51) | 2.99 (2.07, 3.92) | 5.45 (4.29, 6.62) |
| Endometrial | n/a | 3.10 (1.25, 4.95) | 1.51 (0.46, 2.57) | 4.39 (2.52, 6.27) |
| Lung | 1.21 (0.41, 2.01) | 2.45 (1.45, 3.44) | 3.75 (2.42, 5.08) | |
| Renal | 2.75 (0.66, 4.83) | 2.75 (0.66, 4.83) | ||
| Breast (women) | n/a | 1.45 (0.78, 2.13) | 0.93 (0.47, 1.38) | 2.32 (1.54, 3.11) |
| Bladder | 1.87 (0.71, 3.03) | 1.87 (0.71, 3.03) | ||
| Potential reduction in the number of cancers diagnosed in advanced stage | ||||
| Prostate | n/a | 952 (628, 1277) | 1089 (753, 1425) | 1984 (1561, 2407) |
| Lung | 423 (143, 703) | 854 (507, 1202) | 1310 (846, 1774) | |
| Breast (women) | n/a | 611 (328, 894) | 390 (199, 580) | 976 (646, 1306) |
| Melanoma | 209 (115, 302) | 320 (200, 440) | 279 (194, 363) | 708 (572, 844) |
| Endometrial | n/a | 202 (82, 323) | 99 (30, 167) | 287 (164, 409) |
| Renal | 186 (45, 328) | 186 (45, 328) | ||
| Bladder | 170 (64, 275) | 170 (64, 275) | ||
*Details are provided in Methods section. Note that the estimated potential combined impact of eliminating inequalities in stage at diagnosis by gender, deprivation and old age is not equal to the sum of the individual impacts because certain individuals may be subject to more than one inequality. No data shown for colon, rectal and ovarian cancer, because of no evidence of socio-demographic variation by gender (as applicable), deprivation or old age group (see Table 3).
discussion
We found substantial and potentially avoidable gender, deprivation and age inequalities in advanced stage diagnosis of seven common cancers and relatively limited socio-demographic variation in stage at diagnosis of three other cancers. Among cancers with substantial inequalities, the size and direction of differences in advanced stage diagnosis varied greatly by cancer. The results were robust to various assumptions investigated with sensitivity analyses, including adjustment for ethnic group. Multiple imputation of missing stage indicated that the effect of age in older patients may have been slightly underestimated. Potential elimination of socio-demographic inequalities could help diagnose a substantial number of patients with cancer in earlier stage. Although, in absolute terms, most of these earlier detection gains relate to four common cancers, there are also notable relative gains for endometrial cancer.
The findings should be interpreted taking into account evidence about the socio-demographic differences in awareness and appraisal of cancer symptoms; and on the promptness by which cancer is suspected in symptomatic patients with different cancers. For melanoma, breast and endometrial cancers the observed socio-demographic inequalities in advanced stage at diagnosis are unlikely to reflect delays in suspecting and investigating cancer after symptomatic presentation to a doctor: these cancers are among the ‘easiest to suspect and diagnose’ and delays after presentation are limited [13, 19]. Therefore, inequalities in stage at diagnosis for those three cancers are likely to reflect socio-demographic differences in awareness and interpretation of cancer symptoms which have been previously described [20–22].
For lung, renal and bladder cancer, we observed ‘negative’ age gradients in advanced stage at diagnosis among patients aged 65 or over (older patients having lower probability of diagnosis at advanced stage). Similar associations between older age and stage at diagnosis of lung cancer have been reported previously in both related and other patient populations [7, 19, 23]. These findings are unlikely to reflect improved awareness and appraisal of cancer symptoms for lung, renal and bladder cancer in older compared with younger patients. Healthcare factors however may be implicated. Older patients tend to have more co-morbidities, which may lead to incidental identification of lung or renal cancers through imaging investigations (e.g. by chest X-ray or abdominal ultrasound, respectively, for symptoms unrelated to cancer). This may reflect a paradoxical apparent benefit of increasing level of co-morbidity, similar to other improvements in care reported for patients with multiple conditions [24]. An alternative explanation may be that these patterns reflect cognitive or psychological barriers by the doctors in suspecting cancer in middle-aged patients. Strong inverse age patterns in the number of consultations with a general practitioner with cancer symptoms before specialist referral exist (with younger patients more likely to be referred to hospital less promptly)—such patterns also exist for patients with lung, renal and bladder cancer [13]. Another hypothesis is that these three cancers have more (and perhaps more specific) symptoms in older age. Given that there is no robust evidence to explain the inverse age inequalities in the stage at diagnosis of lung, renal and bladder cancer, research to establish the causes of these patterns should be considered a priority. If the mechanisms responsible for these inequalities are better understood, interventions to reduce advanced stage diagnosis in middle-aged patients with lung, renal and bladder cancer may be feasible.
The findings substantially amplify previous research [7, 23, 25–33]. Particular strengths of the present study are that it encompasses several common cancers; it examines old age alongside other socio-demographic inequalities; it takes into account potential confounding by tumour type, ethnicity and (for breast, colon and rectal cancer) screening detection status; it uses highly complete stage information and explores potential bias arising from missing information in sensitivity analysis; and it illustrates the population health impact of eliminating socio-demographic inequalities in stage at diagnosis. Adjustment for tumour type minimises the potential for confounding of socio-demographic differences in stage at diagnosis by socio-demographic differences in tumour morphology because of differential prior long-term exposure to risk factors.
An important limitation is that (unlike adjusting for breast and colorectal screening status), it was not possible to adjust for whether patients with prostate cancer were diagnosed with or without symptoms [e.g. because of prostate-specific antigen (PSA) testing in asymptomatic men]. There is ongoing uncertainty about the potential benefits of asymptomatic detection of prostate cancer [34]. Although the uptake of PSA testing in the UK is overall low, it is higher among older and least deprived patients [35]. Consequently, among symptomatic patients, deprivation gradients in advanced stage of prostate cancer may be lower than those observed in our study; conversely, old age gradients may indeed be larger than those estimated by our study. Therefore, we urge caution against the potential for the prostate cancer findings to be dismissed as clinically unimportant. Melanoma, breast and prostate cancer in England have higher incidence among patients with higher socio-economic status [36], and it is likely that the observed higher frequency of advanced stage at diagnosis among more deprived patients partly reflects over-diagnosis of non-advanced stage disease among the more affluent patients.
A further limitation is the lack of availability of national data with similarly high complete information on stage for the studied cancers and period: this prevented expanding the analysis to a wider population. The relatively small number of patients for some rarer cancers may have prevented the detection of moderate or small inequalities in stage at diagnosis (e.g. this may apply to gender and deprivation differences in stage at diagnosis of bladder cancer, Table 3). Incidence patterns by age and gender between the study population and England are very similar. However, there are relative fewer patients with cancer in the most deprived group in the study population. For this reason, weights accounting for this difference were used in the estimation of the population health impact of eliminating socio-demographic inequalities in stage at diagnosis.
Among the seven cancers with substantial socio-demographic inequalities in stage at diagnosis, the size and direction of these associations varied widely. This complexity may signal a substantial potential for achieving earlier detection by targeting interventions to improve awareness and appraisal of cancer signs and symptoms on specific population groups [6, 8]. Policymakers could consider targeted approaches focusing either on a cancer or a specific population group. For example, given strong deprivation, old age and gender inequalities for this cancer, melanoma awareness interventions could particularly target more deprived old men. Among older people, interventions may specifically focus on improving awareness and appraisal of symptoms and signs of cancers associated with age inequality as a ‘bundle’ (e.g. ‘breast and endometrial cancer’ awareness interventions for older women, or ‘melanoma and prostate’ cancer awareness interventions for older men). National strategies can combine both cancer and population group approaches. Development and validation of new, tailored and more effective awareness interventions is likely to be required [6], given the persistence over time of some of these inequalities [21].
The absence of socio-demographic variation in stage at diagnosis of three cancers (colon, rectal and ovarian cancer) should not be interpreted as an indication that patient awareness interventions for those three cancers are not justified. Such interventions are currently being implemented for colorectal cancer [37], while evidence increasingly supports their consideration for ovarian cancer [38]. The findings need to be interpreted in relation to their temporal context (2006–2010). Evidence from breast cancer and melanoma which both benefited from long-standing awareness campaigns indicates that when such interventions are effective [39], they tend to also generate health inequalities (younger and more affluent people typically being able to benefit more so than older and less affluent people) [40]. Avoiding potential inequality that can be generated by future effective patient awareness campaigns about colorectal and ovarian cancer presents a challenge.
In conclusion, for most cancers, there are appreciable socio-demographic inequalities in stage at diagnosis, and this realisation can help motivate and support targeting of interventions on patients at higher risk.
funding
This work was supported by a post-doctoral fellowship award to GL by the National Institute for Health Research.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank all staff at the Eastern Cancer Registration and Information Centre who helped collect and code data used in this study. This manuscript is independent research arising from a Post-Doctoral Fellowship award to GL supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. As was the case in this study, cancer registries in the UK are entitled to hold data on cancer cases under Section 251 of the NHS Act 2006 and have policies that permit sharing of anonymised (non-identifiable) data for purposes of public health research, with the aim of generating evidence that can be of use to improving patient care. More information about the regulatory environment underpinning the function of UK Cancer Registries can be found at the UK Association of Cancer Registries website http://www.ukacr.org/.
references
- 1.Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S77–S81. doi: 10.1038/bjc.2011.489. doi:10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable. Br J Cancer. 2009;101(Suppl 2):S115–S124. doi: 10.1038/sj.bjc.6605401. doi:10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards MA. The size of the prize for earlier diagnosis of cancer in England. Br J Cancer. 2009;101(Suppl 2):S125–S129. doi: 10.1038/sj.bjc.6605402. doi:10.1038/sj.bjc.6605402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Intelligence Network. Routes to diagnosis—NCIN data briefing. http://www.ncin.org.uk/publications/data_briefings/routes_to_diagnosis.aspx. 4 September 2012, date last accessed. [Google Scholar]
- 5.Simon AE, Waller J, Robb K, et al. Patient delay in presentation of possible cancer symptoms: the contribution of knowledge and attitudes in a population sample from the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2272–2277. doi: 10.1158/1055-9965.EPI-10-0219. doi:10.1158/1055-9965.EPI-10-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes LJ, Linsell L, Atkins L, et al. A promoting early presentation intervention increases breast cancer awareness in older women after 2 years: a randomised controlled trial. Br J Cancer. 2011;105(1):18–21. doi: 10.1038/bjc.2011.205. doi:10.1038/bjc.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyratzopoulos G, Abel GA, Barbiere JM, et al. Variation in advanced stage at diagnosis of lung and female breast cancer in an English region 2006–2009. Br J Cancer. 2012;106(6):1068–1075. doi: 10.1038/bjc.2012.30. doi:10.1038/bjc.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merseyside and Cheshire Cancer Network. Targeting Men Aged 50+ to Increase Early Detection of Melanoma Local Campaign Planning Guide. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@hea/documents/generalcontent/cr_045568.pdf. 4 September 2012, date last accessed. [Google Scholar]
- 9.UK Association of Cancer Registries (UKACR) Trends in Death Certificate Only (DCO). Table 7. http://www.ukacr.org/content/data-quality. 4 September 2012, date last accessed. [Google Scholar]
- 10.National Audit Office. Department of Health. Delivering the Cancer Reform Strategy. Report by the Comptroller and Auditor General. 2010. HC 568. Session 2010–2011. http://www.nao.org.uk/publications/1011/cancer_reform_strategy.aspx. 4 September 2012, date last accessed. [Google Scholar]
- 11.Sobin LH, Wittekind CH, editors. International Union Against Cancer (UICC) (1997) TNM Classification of Malignant Tumors. 5th edition. New York: John Wiley & Sons Inc; [Google Scholar]
- 12.Office of the Deputy Prime Minister. The English Indices of Deprivation 2004: Summary (revised) 2004. http://www.communities.gov.uk/documents/communities/pdf/131206.pdf. 4 September 2012, date last accessed. [Google Scholar]
- 13.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. doi:10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Yucel R, Zaslavsky AM. Misreporting, Missing Data, and Multiple Imputation: Improving Accuracy of Cancer Registry Databases. Chance (N Y). 2008;21(3):55–58. doi: 10.1007/s144-008-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nur U, Shack LG, Rachet B, et al. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiol. 2010;39(1):118–128. doi: 10.1093/ije/dyp309. doi:10.1093/ije/dyp309. [DOI] [PubMed] [Google Scholar]
- 16.Quaglia A, Tavilla A, Shack L, et al. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer. 2009;45(6):1006–1016. doi: 10.1016/j.ejca.2008.11.028. doi:10.1016/j.ejca.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Moller H, Flatt G, Moran A. High cancer mortality rates in the elderly in the UK. Cancer Epidemiol. 2011;35(5):407–412. doi: 10.1016/j.canep.2011.05.015. doi:10.1016/j.canep.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on interval censoring. Stata J. 2007;7:445–464. [Google Scholar]
- 19.Royal College of General Practitioners. National Audit of Cancer Diagnosis in Primary Care. http://www.rcgp.org.uk/pdf/National_Audit_of_Cancer_Diagnosis_in_Primary-Care.pdf. 4 September 2012, date last accessed. [Google Scholar]
- 20.Linsell L, Burgess CC, Ramirez AJ. Breast cancer awareness among older women. Br J Cancer. 2008;99(8):1221–1225. doi: 10.1038/sj.bjc.6604668. doi:10.1038/sj.bjc.6604668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melia J, Ellman R, Chamberlain J. Investigating changes in awareness about cutaneous malignant melanoma in Britain using the Omnibus Survey. Clin Exp Dermatol. 1994;19(5):375–379. doi: 10.1111/j.1365-2230.1994.tb02685.x. doi:10.1111/j.1365-2230.1994.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 22.Robb K, Stubbings S, Ramirez A, et al. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009;101(Suppl 2):S18–S23. doi: 10.1038/sj.bjc.6605386. doi:10.1038/sj.bjc.6605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalton SO, Frederiksen BL, Jacobsen E, et al. Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001–2008. Br J Cancer. 2011;105(7):1042–1048. doi: 10.1038/bjc.2011.342. doi:10.1038/bjc.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356(24):2496–2504. doi: 10.1056/NEJMsa066253. doi:10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 25.Booth CM, Li G, Zhang-Salomons J, et al. The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer. 2010;116(17):4160–4167. doi: 10.1002/cncr.25427. doi:10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 26.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0. doi:10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. doi: 10.1016/S1470-2045(08)70032-9. doi:10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 28.Brewster DH, Thomson CS, Hole DJ, et al. Relation between socioeconomic status and tumour stage in patients with breast, colorectal, ovarian, and lung cancer: results from four national, population based studies. BMJ. 2001;322(7290):830–831. doi: 10.1136/bmj.322.7290.830. doi:10.1136/bmj.322.7290.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baade PD, Dasgupta P, Aitken J, et al. Geographic remoteness and risk of advanced colorectal cancer at diagnosis in Queensland: a multilevel study. Br J Cancer. 2011;105(7):1039–1041. doi: 10.1038/bjc.2011.356. doi:10.1038/bjc.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuthbertson SA, Goyder EC, Poole J. Inequalities in breast cancer stage at diagnosis in the Trent region, and implications for the NHS Breast Screening Programme. J Public Health (Oxf). 2009;31(3):398–405. doi: 10.1093/pubmed/fdp042. doi:10.1093/pubmed/fdp042. [DOI] [PubMed] [Google Scholar]
- 31.Adams J, White M, Forman D. Are there socioeconomic gradients in stage and grade of breast cancer at diagnosis? Cross sectional analysis of UK cancer registry data. BMJ. 2004;329(7458):142. doi: 10.1136/bmj.38114.679387.AE. doi:10.1136/bmj.38114.679387.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macleod U, Ross S, Gillis C, et al. Socio-economic deprivation and stage of disease at presentation in women with breast cancer. Ann Oncol. 2000;11(1):105–107. doi: 10.1023/a:1008385321476. doi:10.1023/A:1008385321476. [DOI] [PubMed] [Google Scholar]
- 33.Barbiere JM, Greenberg DC, Wright KA, et al. The association of diagnosis in the private or NHS sector on prostate cancer stage and treatment. J Public Health (Oxf) 2012;34(1):108–114. doi: 10.1093/pubmed/fdr051. doi:10.1093/pubmed/fdr051. [DOI] [PubMed] [Google Scholar]
- 34.Neal DE, Donovan JL, Martin RM, et al. Screening for prostate cancer remains controversial. Lancet. 2009;374(9700):1482–1483. doi: 10.1016/S0140-6736(09)61085-0. doi:10.1016/S0140-6736(09)61085-0. [DOI] [PubMed] [Google Scholar]
- 35.Williams N, Hughes LJ, Turner EL, et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int. 2011;108(9):1402–1408. doi: 10.1111/j.1464-410X.2011.10163.x. doi:10.1111/j.1464-410X.2011.10163.x. [DOI] [PubMed] [Google Scholar]
- 36.National Cancer Intelligence Network. Cancer Incidence and Survival By Major Ethnic Group, England, 2002–2006. London. 2009. http://www.ncin.org.uk/view.aspx?rid=75. 4 September 2012, date last accessed. [Google Scholar]
- 37.National Awareness and Early Diagnosis Initiative. ‘Be clear on cancer’ National Bowel Cancer Campaign. http://info.cancerresearchuk.org/spotcancerearly/naedi/beclearoncancer/ 4 September 2012, date last accessed. [Google Scholar]
- 38.Hamilton W, Peters TJ, Bankhead C, et al. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339:b2998. doi: 10.1136/bmj.b2998. : [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austoker J, Bankhead C, Forbes LJ, et al. Interventions to promote cancer awareness and early presentation: systematic review. Br J Cancer. 2009;101(Suppl 2):S31–S39. doi: 10.1038/sj.bjc.6605388. doi:10.1038/sj.bjc.6605388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victora CG, Vaughan JP, Barros FC, et al. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet. 2000;356(9235):1093–1098. doi: 10.1016/S0140-6736(00)02741-0. doi:10.1016/S0140-6736(00)02741-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.