Abstract
Background
We evaluated the risk of sampling errors in specimens of biopsy size, which may be caused by heterogeneous overexpression of Her2/neu in gastric cancer (GC).
Patients and methods
The study cohort comprised 454 gastrectomy patients with adenocarcinoma of the stomach or esophago-gastric junction. Tissue micro-arrays (TMAs) served as ‘biopsy procedure’ and were generated from formalin-fixed and paraffin-embedded tissue: five tissue cylinders were collected randomly from each tumor, rendering 2230 core cylinders. These were compared with 454 whole tissue sections obtained from the same paraffin blocks. Her2/neu expression and gene amplification were analyzed by immunohistochemistry and in situ hybridization. The Her2/neu status was determined according to GC scoring system by two independent observers.
Results
In whole tissue sections, 37 (8.1%; observer 1) and 38 (8.4%; observer 2) of the GCs, and in the corresponding TMAs, 28 (6.3%; observer 1) and 28 (6.3%; observer 2) of the GCs were classified as Her2/neu-positive (kappa value 98.5% and 96.2%; P < 0001). Comparison of whole tissue sections with corresponding TMAs showed a false-negative rate of 24% and a false-positive rate of 3% for TMAs.
Conclusion
Assessment of the Her2/neu status in tissue biopsies carries a significant risk of sampling errors, thereby rendering patients unsuitable for treatment with trastuzumab.
Keywords: biopsy, gastric cancer, Her2/neu, sampling error
introduction
For many years, only the anatomical location of the primary tumor, its histological phenotype and the tumor stage tailored chemotherapy. However, in clinical practice, many patients with a seemingly identical tumor responded differently to the same therapy. Research on cancer biology provided ample explanations [1]. Various genetic alterations and distinct molecular phenotypes were unraveled, which influence patient prognosis and response to chemotherapy. Meanwhile, the choice of treatment increasingly depends on the identification of these particular molecular phenotypes. As a consequence, predictive biomarkers were sought, which prognosticate treatment responses before therapy. This approach bears hope to make treatment more efficient in responders and to avoid unnecessary side-effects in non-responders. Despite these advancements, new problems emerged. The value of a predictive biomarker test depends on the robustness, sensitivity and specificity of the assay. These factors can be negatively influenced by tumor heterogeneity and tissue sampling errors.
Recently, Her2/neu was introduced as a predictive biomarker for the treatment of gastric cancer (GC) with trastuzumab. Trastuzumab is an antibody targeting Her2/neu and is applied in combination with chemotherapy for the treatment of Her2/neu-positive advanced GC [2]. The Her2/neu status is assessed in tumor biopsies or resection specimens by a combination of immunohistochemistry and in situ hybridization. Based on the breast cancer scoring system, a modified scoring system was developed for GC [3]. An almost overwhelming number of studies recently demonstrated the robustness of the Her2/neu testing (for a review, see also [4]). However, evidence is accumulating that Her2/neu overexpression is heterogeneous in GC, carrying the risk of a sampling error [5–14]. Particularly in patients with advanced, unresectable GC eligible for trastuzumab, only biopsies may be available for Her2/neu testing. In this study, we assessed the reliability of Her2/neu testing in tissue samples of biopsy size by comparing the Her2/neu status of whole tissue sections with corresponding tissue micro-arrays (TMAs).
materials and methods
ethics statement
This project was approved by the local ethics committee of the University Hospital in Kiel, Germany (reference number D 453/10). All patient data were pseudonymized before study inclusion.
study population
We retrieved from the archive of the Institute of Pathology, University Hospital Kiel, all Caucasian patients who had undergone either total or partial gastrectomy for adenocarcinoma of the stomach or esophago-gastric junction between 1997 and 2009. The following patient characteristics were retrieved: type of surgery, age at diagnosis, gender, tumor localization, tumor type, tumor grade, depth of invasion, number of lymph nodes resected and number of lymph nodes with metastases. Each resection specimen underwent gross sectioning and histological examination by trained histopathologists. Date of patient death was obtained from the Epidemiological Cancer Registry of the state of Schleswig-Holstein, Germany. Follow-up data of patients still alive were retrieved from hospital records and general practitioners.
study inclusion and exclusion criteria
Inclusion and exclusion criteria were defined as follows: patients were included when histology confirmed an adenocarcinoma of the stomach or esophago-gastric junction, and the date of death or survival data were available. Patients were excluded when histology identified a tumor type other than adenocarcinoma, histopathological data were incomplete, patients had previously undergone a partial stomach resection (Billroth-II) and now had locally recurrent GC and date of patient death or survival data had not been recorded. Patients who received perioperative chemotherapy were also excluded.
histology and TNM classification
Tissue specimens were fixed in formalin and embedded in paraffin. Deparaffinized sections were stained with hematoxylin and eosin. Tumors were classified according to the Laurén classification [15]. pTNM stage of all study patients was determined according to seventh edition of the UICC guidelines [16] and our recent proposal (‘Kiel-stage’) [17] and was based solely on surgical pathological examination including classification of distant metastases (pM-category). In the seventh edition, all tumors of the esophago-gastric junction as well as tumors of the proximal 5 cm of the stomach with extension into the esophagus are classified as esophageal tumors [16]. Patients were re-categorized accordingly.
TMA construction
Formalin-fixed and paraffin-embedded tissue samples were used to generate TMAs as described previously [18]. Briefly, five morphologically representative regions of the paraffin ‘donor’ blocks were chosen. Tissue cylinders of 1.5 mm diameter were punched from these areas and precisely arrayed into a new ‘recipient’ paraffin block using a custom-built instrument (Beecher Instruments, Silver Spring, MD). After completing block construction, 4 µm sections of the resulting tumor TMA block were cut for further analysis.
Her2/neu status
The Her2/neu status was assessed using the monoclonal anti-Her2/neu antibody (clone 4B5), the HER2-SISH double-labeling in situ-hybridization system and the Ventana BenchMark XT automated slide staining system (all Roche Diagnostics GmbH, Mannheim, Germany). The Her/2neu status was assessed by two independent observers (VSW and CB) according to the GC scoring system separately for resection specimens (whole tissue sections) and for biopsies (TMAs) [3]. The intensity of Her2/neu immunostaining varied from negative (0) to strong (3+). All GCs with an Her2/neu immunostaining of 2+ were forwarded to silver-enhanced in situ hybridization. Both observers were blinded with regard to the clinicopathological patient characteristics. After independent evaluation of the whole tissue sections and the TMAs by both observers, a final consensus evaluation was carried out with three observers (VSW, CB and CR).
statistics
Statistical analyses were carried out using IBM SPSS Version 20 (IBM Corp., Armonk, NY). Median overall survival was determined using the Kaplan–Meier method, and the log-rank test was used to determine significance. For comparison purposes, the median survival time, its standard deviation and 95% confidence interval were calculated. Prognostic relevance was investigated by multivariate Cox regression analysis, using the backward LR method with inclusion/exclusion significances of 0.100, respectively. The significance of correlation between clinicopathological parameters and the Her2/neu status was tested using Fisher's exact test. For parameters of ordinal scale (T, N, stage), we applied Kendall's tau test instead. For measuring the interrater agreement of Her2/neu scores between two observers, Cohen's kappa coefficient was calculated. A kappa value of 0.20 was considered to be poor, of 0.21–0.40 to be fair, of 0.41–0.60 to be moderate, of 0.61–0.80 to be good, and of 0.81–1.00 to be very good. A P-value ≤0.05 was considered statistically significant. No adjustments were made.
results
In this study, 454 patients fulfilled all study criteria (Table 1). According to Laurén, an intestinal type GC was found in 232 (51.1%), a diffuse type in 140 (30.8%), an unclassified type in 53 (11.7%) and a mixed type in 29 (6.4%) patients.
Table 1.
Clinicopathological patient characteristics
| Patient characteristics | |
|---|---|
| Patients (n) | 454 |
| Age (years) | |
| Mean ± SD | 67.3 ± 11.1 |
| Median | 68 |
| Gender, n (%) | |
| Men | 283 (62.3) |
| Women | 171 (37.7) |
| Follow-up data, n (%) | |
| Alive | 120 (27.1) |
| Dead | 322 (72.9) |
| Laurén phenotype, n (%) | |
| Intestinal | 232 (51.1) |
| Diffuse | 140 (30.8) |
| Unclassified | 53 (11.7) |
| Mixed | 29 (6.4) |
| Localization, n (%) | |
| Proximal | 144 (31.7) |
| Distal | 310 (68.3) |
| pT-category, n (%) | |
| pT1a | 10 (2.2) |
| pT1b | 37 (8.1) |
| pT2 | 55 (12.1) |
| pT3 | 185 (40.7) |
| pT4a | 128 (28.2) |
| pT4b | 39 (8.6) |
| pN-category, n (%) | |
| pN0 | 124 (27.5) |
| pN1 | 65 (14.4) |
| pN2 | 81 (18.0) |
| pN3/a/b | 181 (40.1) |
| Resection margin, n (%) | |
| pR0 | 380 (88.0) |
| pR1 | 49 (11.3) |
| pR2 | 3 (0.7) |
| Lymphatic invasion, n (%) | |
| pL0 | 212 (48.3) |
| pL1 | 227 (51.7) |
| Venous invasion, n (%) | |
| pV0 | 386 (88.3) |
| pV1 | 51 (11.7) |
| UICC stage (7th edition), n (%) | |
| IA | 36 (8.1) |
| IB | 30 (6.7) |
| IIA | 57 (12.8) |
| IIB | 46 (10.3) |
| IIIA | 54 (12.1) |
| IIIB | 80 (18.0) |
| IIIC | 64 (14.4) |
| IV | 78 (17.5) |
| Stage according to Kiel proposal, n (%) | |
| I | 36 (8.0) |
| II | 83 (18.4) |
| IIIA | 47 (10.4) |
| IIIB | 148 (32.7) |
| IV | 138 (30.5) |
| Resected lymph nodes | |
| Mean ± SD | 19.1 ± 8.2 |
| Median, n | 18 |
| Positive lymph nodes | |
| Mean ± SD | 6.5 ± 7.4 |
| Median, n | 3.5 |
| Lymph node ratio | |
| Median, n | 0.22 |
| Tumor grade, n (%) | |
| G1/G2 | 99 (22.4) |
| G3/G4 | 343 (77.6) |
Her2/neu status in whole tissue sections
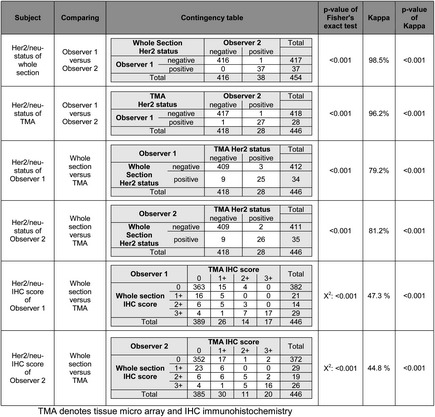
The Her2/neu status was assessed according to the GC scoring system for resection specimens, using whole tissue sections [3]: GCs were classified as Her2/neu-positive when ≥10% of the tumor area showed strong immunostaining (3+) for Her2/neu, or moderate immunostaining (2+) in conjunction with HER2 gene amplification (ratio ≥2.0). Observer 1 classified 37 (8.1%) and observer 2 classified 38 (8.4%) of the GCs as Her2/neu-positive. The interobserver agreement was very good (kappa 98.5%, P < 0.001; Table 2).
Table 2.
Interrater agreement on the assessment of the Her2/neu status (kappa)
 |
Her2/neu status in TMAs
Next we assessed the Her2/neu status in TMAs obtained from the same tumor-bearing paraffin blocks used for the assessment of whole tissue sections. Five core cylinders were obtained from each paraffin ‘donor’ block, finally rendering 2230 tumor-bearing core cylinders (in eight cases, the amount of material was too low for the generation of a TMA). The Her2/neu status was assessed according to the GC scoring system for biopsy specimens [3]: GCs were classified as Her2/neu-positive when five or more tumor cells showed strong immunostaining (3+) for Her2/neu, or moderate immunostaining (2+) in conjunction with HER2 gene amplification (ratio ≥2.0) in at least one of the five core cylinders obtained from a given tumor. Observer 1 classified 28 (6.3%) and observer 2 classified 28 (6.3%) of the GCs as Her2/neu-positive. Again, the interobserver agreement was very good (kappa 96.2%, P < 0.001; Table 2).
comparison of the Her2/neu status between whole tissue sections and TMAs
In the next step, we compared the Her2/neu status between whole tissue sections and TMAs. Observer 1 classified nine (2.0%) GCs as Her2/neu-positive in whole tissue sections and negative in the corresponding TMAs. To the contrary, three (0.7%) cases were classified as Her2/neu-positive in TMAs and negative in whole tissue sections. Observer 2 classified nine (2.0%) GCs as Her2/neu-positive in whole tissue sections and negative in the corresponding TMAs. To the contrary, two (0.4%) cases were classified as Her2/neu-positive in the TMAs and negative in the whole tissue sections. The interassay agreement was good to very good for both observers (kappa 79.2% and 81.2%, respectively; P < 0.001; Table 2).
comparison of Her2/neu immunostaining between whole tissue sections and TMAs
According to the GC scoring system, Her2/neu immunostaining is the first diagnostic procedure for the assessment of the Her2/neu status. An Her2/neu immunoreactivity score of ≤1+ and of 3+ does not necessitate in situ hybridization. Thus, a heterogeneous Her2/neu immunostaining has a major impact on the Her2/neu status classified in a biopsy specimen. Therefore, we next compared the results of immunostaining between whole tissue sections and TMAs. As shown in Table 2, the interassay agreement further declined for both observers, when only the Her2/neu immunostaining results were compared between whole tissue sections and TMAs (kappa 47.3% and 44.8%, respectively). However, Her2/neu immunostaining results correlated still highly significantly between whole tissue sections and TMAs for both observers (P < 0.001).
case-by-case analysis of discrepant Her2/neu status
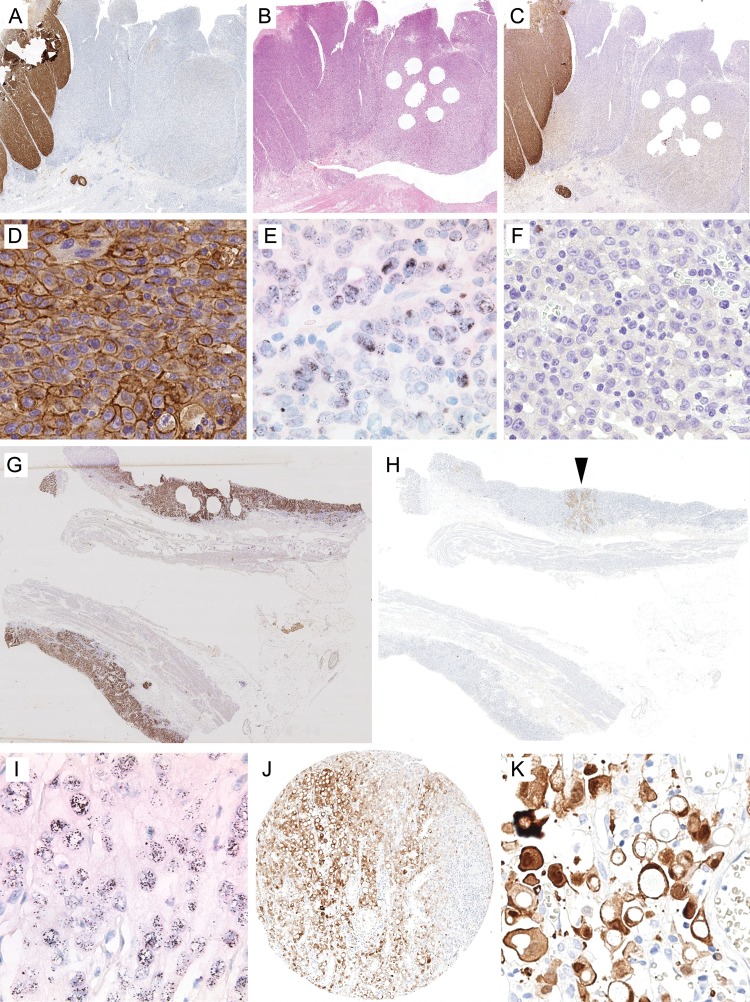
Following the comparison of the immunostaining results, we compared case-by-case the whole tissue sections with the TMAs. Interestingly, the discrepancies between whole tissue sections and TMAs were mainly related to sampling errors (Table 3). In six cases, the TMA was false negative since the GC enclosed an Her2/neu-positive tumor cell clone (IHC 3+ or IHC2+/ISH-amplified) comprising only 10%–20% (four cases) or 40% (one case) of the entire tumor area. The remaining tumor area was completely devoid of Her2/neu expression (‘black-and-white’ expression pattern; Figure 1). In three cases, the intensity of immunostaining for Her2/neu was uneven. The GCs enclosed areas with weak (1+), moderate (2+) and strong (3+) immunostaining (‘gray-scale’ expression pattern). The intensity of Her2/neu-immunostained tumor areas enclosed in the TMAs was not representative for the entire tumor.
Table 3.
Case-by-case analysis of discrepant Her2/neu status
| No. | Observer 1 |
Observer 2 |
Comment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue micro-array |
Whole tissue section |
Tissue micro-array |
Whole tissue section |
|||||||||||
| IHC | ISH | Her2 | IHC | ISH | Her2 | IHC | ISH | Her2 | IHC | ISH | Her2 | |||
| False negative | 1 | 2+ | NA | − | 3+ | A | + | 2+ | NA | − | 3+ | A | + | Tumor cell clone with IHC 3+ comprising 40% of the tumor area; clone reaching the mucosal surface; available for endoscopic biopsy procedure |
| 2 | 0 | − | 3+ | A | + | 0 | − | 3+ | A | + | Mucinous adenocarcinoma; tumor cell clone with IHC 3+ comprising 15% of the tumor area; clone localized at the invasion front unavailable for endoscopic biopsy procedure | |||
| 3 | 0 | − | 3+ | A | + | 0 | − | 3+ | A | + | Tumor cell clone IHC 3+ comprising 10%–20% of the tumor area; clone reaching the mucosal surface; available for endoscopic biopsy procedure | |||
| 4 | 0 | − | 3+ | A | + | 0 | − | 3+ | A | + | Tumor cell clone IHC 3+ comprising 10%–20% of the tumor area; clone reaching from mucosal surface to the level of the subserosa; available for endoscopic biopsy procedure | |||
| 5 | 0 | − | 3+ | + | 0 | − | 3+ | + | Tumor cell clone IHC 3+ comprising 10%–20% of the tumor area; clone reaching from mucosal surface down to the level of the muscularis propria; available for endoscopic biopsy procedure | |||||
| 6 | 0 | − | 2+ | A | + | 0 | − | 2+ | A | + | Tumor cell clone IHC 2+ comprising 20% of the tumor area; clone reaching mucosal surface; available for endoscopic biopsy procedure | |||
| 7 | 1+ | − | 2+ | A | + | 1+ | − | 2+ | A | + | Variable Her2/neu expression (IHC 0, 1+, 2+, 3+); tumor areas with IHC 3+ localized at the mucosal surface; available for endoscopic biopsy procedure | |||
| 8 | 1+ | − | 3+ | A | + | 1+ | − | 3+ | A | + | Variable Her2/neu expression (IHC 0, 1+, 2+, 3+); tumor areas with IHC 3+ localized at the mucosal surface; available for endoscopic biopsy procedure | |||
| 9 | 1+ | − | 2+ | A | + | 1+ | − | 2+ | A | + | Variable Her2/neu expression (IHC 0, 1+, 2+, 3+); gene amplification was even across entire tumor area; possibly fixation artifact. | |||
| False positive | 10 | 2+ | A | + | 0 | − | 2+ | A | + | 2+ | A | + | Tumor cell clone IHC 3+ comprising <10% of the tumor area; in the TMA, more than five cells with IHC 3+ were present in one of five core cylinders; in the remaining four core cylinders tumor cells were completely Her2/neu negative | |
| 11 | 2+ | A | + | 0 | − | 2+ | NA | − | 0 | − | Tumor cell clone IHC 2+ comprising <10% of the tumor area in whole tissue section; clone localized at the mucosal surface; available for endoscopic biopsy procedure | |||
| 12 | 2+ | NA | − | 0 | − | 3+ | + | 0 | − | Staining artifact: signet ring cells showed strong cytoplasmic immunostaining, which was misinterpreted as membranous staining in TMA | ||||
A, amplified; NA, not amplified; IHC, immunohistochemistry; Her2, Her2/neu status; ‘−’ denotes negative; ‘+’ denotes positive.
Figure 1.
Her2/neu expression of gastric cancer assessed in whole tissue sections and tissue micro-arrays (TMAs). A case-by-case analysis illustrated the causes of discrepant Her2/neu test results between whole tissue sections and TMAs: (A–F) Case no. 5 (see Table 3) encloses a tumor cell clone comprising 10%–20% of the entire tumor area, which showed strong (IHC 3+) expression of Her2/neu (A, C and D) and HER2 gene amplification (E). The remaining tumor area was completely negative for Her2/neu (IHC 0; F). A tissue section cut after the TMA was generated shows that, unintentionally, only the negative tumor area was ‘biopsied’. (G and H) Case no. 10 (see Table 3) illustrates the reverse situation of a false-positive test result. An Her2/neu-overexpressing tumor cell (H, arrowhead) clone comprised <10% of the entire tumor area, as illustrated by cytoketarin immunolabeling (G). This clone also showed HER2 gene amplification (I). (J and K) Case no. 12 illustrates a unique but well-recognized staining artifact of the monoclonal antibody we used (clone 4B5). Strong cytoplasmic immunostaining of a signet ring cell carcinoma was erroneously interpreted as membrane staining in TMAs. However, both observers also classified the same case as negative in the whole tissue sections, where the staining artifact was more apparent. Her2/neu immunostaining (A, B, D, F, H, J and K); HER2-SISH double-labeling in situ hybridization (E and I); hematoxylin and eosin (B); pancytokeratin (the brown stain is virtually identical with the tumor area (G). No magnification (A–C, G, H, J); original magnifications ×400 (D, F and K) and ×600 (E and I). The digitalized original slides of (C) and (H) can be viewed at http://www.uni-kiel.de/path/vm/her2-manuscript.
Two cases were false positive in the TMA for similar reasons (‘black-and-white’ expression pattern): five or more cells showed strong (3+) or moderate immunostaining (2+) in conjunction with HER2 gene amplification (ratio ≥2.0) in the TMAs samples, while the whole tissue section showed that these clones comprised <10% of the entire tumor area (Figure 1). In these cases, we also noticed that the interobserver variability was due to observer-dependent differences in the estimation of the cut-off value, i.e. <10% versus ≥10% of the tumor area (case no. 10; Table 3).
Finally, one case (no. 12) was categorized as false positive due to a staining artifact. The monoclonal antibody used in this study is known to occasionally generate a strong cytoplasmic and nuclear immunoreaction [19]. The signet ring cell carcinoma of patient no. 12 showed strong cytoplasmic staining, which was misinterpreted by both observers as membranous staining and became evident for both observers in the whole tissue section (Figure 1).
Statistical analyses
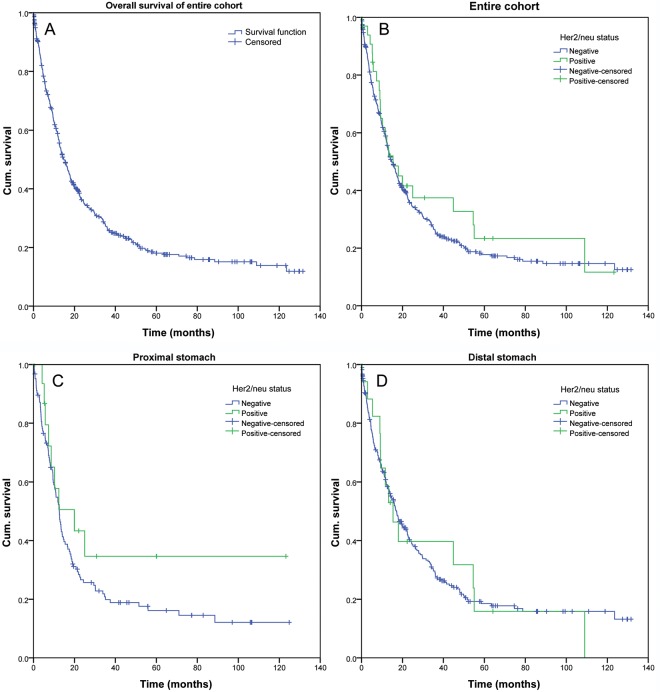
Finally, a consensus score was reached by three observers (VSW, CB, CR) and a total of 37 (8.1%) GCs were classified as Her2/neu-positive (supplementary Table S1, available at Annals of Oncology online). The Her2/neu status correlated highly significantly with tumor type (P = 0.002) and tumor grade (P = 0.003). Proximal tumors were more commonly Her2/neu-positive (11.8%), compared with distal tumors (6.5%). However, this difference did not reach statistical significance (P = 0.065). We next separated proximal tumors from distal tumors. This showed that the Her2/neu status correlated significantly with nodal spread (P = 0.034; proximal tumors) and tumor grade (P = 0.009; distal tumors). Patient survival did not correlate with the Her2/neu status (Figure 2). However, patient prognosis highly significantly depended on patient age, Laurén phenotype, tumor grade, T-category, N-category, lymph node ratio, R-status, as well as UICC stage and ‘Kiel stage’ (data not shown).
Figure 2.
Patient survival according to the Her2/neu status. Kaplan–Meier curves depicting patient survival of the entire patient cohort (A), according to the Her2/neu status in all cases (B, P = 0.452), the Her2/neu status in proximal (C; P = 0.203) and distal cancers (D; P = 0.984).
discussion
The phenotypic and genotypic characterization of cancer is increasingly used to tailor patient management. The introduction of trastuzumab for distant metastatic Her2/neu-positive GC in the first-line treatment added GC to a growing list of cancers to which targeted therapy can be applied [2]. Trastuzumab can only be used when the cancer has been shown to overexpress Her2/neu, i.e. when ≥10% of the tumor area of a GC resection specimen or when a tumor cell cluster (five or more tumor cells) of a biopsy specimen shows strong immunostaining (3+) for Her2/neu, or moderate immunostaining (2+) in conjunction with Her2/neu gene amplification (ratio ≥2.0) (http://www.ema.europa.eu). Various protocols have been tested and found suitable to test the Her2/neu status in GC. This includes different primary antibodies and in situ-hybridization protocols [3, 20]. We used the monoclonal anti-Her2/neu antibody (clone 4B5), the HER2-SISH double-labeling in situ-hybridization system and the Ventana BenchMark XT automated slide staining system, which is a highly standardized protocol and was validated independently in the past [3].
Using whole tissue sections, we found an Her2/neu overexpression in 8.1% of our patients. It was more common in intestinal-type GCs compared with diffuse-type GCs (12.9% versus 3.6%), and was more common in proximal GCs compared with distal GCs (11.8% versus 6.5%). These findings are in line with previous observations. A recent systematic review reported a prevalence of Her2/neu overexpression ranging from 4%−53% (median 18%) [4, 21]. A correlation with the anatomical localization was also found by Yu et al. [22]. Thus, neither methodological issues (fixation and staining protocols) nor the selection of the study cohort explain the discrepancy between whole tissue sections and TMAs.
The NCCN Guidelines recommend that multiple (8–10) biopsies should be carried out to provide an adequate-sized material for histological interpretation [23]. Similarly, the German S3-guideline for the diagnosis and treatment of esophago-gastric cancer recommends that at least eight biopsies should be taken from tumor suspicious lesions [24]. Ten biopsies should be obtained from large lesions. Particularly, diffuse-type GC can easily be missed by biopsies and these numbers were chosen to reach a safe diagnosis of GC by avoiding false-negative results. They were not based on the assumption that GC can only be diagnosed when a minimum number of eight biopsies enclose cancer. In our experience, the number of cancer-bearing biopsies is usually less than eight, and five core cylinders obtained from the formalin-fixed and paraffin-embedded tumor may be considered to be representative for the evaluation of endoscopic biopsy sampling of GC.
We believe that we are the first to systematically study the effect of tissue sampling on the Her2/neu status in a single patient cohort of 454 Caucasian patients, using the GC scoring system. Yano et al. [25] compared whole tissue sections and biopsies in an Asian study population. In their cohort, the biopsy was positive in 76.7% of the cases with overexpression in subsequent resection specimens. However, Yano et al. [25] applied the breast cancer scoring system to both biopsy and resection specimens. Yan et al. [14] compared 15 resection specimens with 12 matched biopsy specimens obtained from the same patients before gastrectomy, using the GC scoring system. They did not find a significant correlation of the Her2/neu status between biopsy and surgical resection specimens. Neither Yano et al. [25] nor Yan et al. [14] specified the number of tumor-bearing biopsies studied. Kim et al. [26] recently studied the Her2/neu status, by immunohistochemistry only, in two large separate Asian cohorts. In the first cohort of 1414 patients, whole tissue sections were analyzed. In the second, independent cohort TMAs were generated from 595 resection specimens (one core cylinder per tumor and patient). The frequency of Her2/neu-positive cases was 12.3% in whole tissue sections and 17% in TMAs [26]. However, based on the study design, no conclusions can be drawn from this study regarding the positive or negative predictive value of biopsy sampling. Finally, Lee et al. [11] compared biopsies (mean number of biopsy fragments was 4.9; range 1–11) and resection specimens obtained from 54 patients. The concordance for the Her2/neu status between biopsy and gastrectomy was 74.1% [11]. However, the time interval between biopsy procedure and gastrectomy was not reported, and confounding factors, such as perioperative chemotherapy, were not specifically mentioned and cannot be completely ruled out [11].
To the contrary, our study investigated whole tissue sections and TMAs obtained from the same paraffin blocks, addressing specifically the issue of a sampling error. We provide strong evidence that sampling procedures carry a significant risk to generate a false-negative Her2/neu status. Twenty-five percent of our GC patients with Her2/neu overexpression would have been missed in a biopsy procedure harvesting five tumor-bearing biopsies. This sampling error is related to the well-recognized heterogeneity of Her2/neu expression in GC. This has been reported independently now by many authors [5–8, 10, 12–14]. However, biopsy sampling carries also a minor risk of a false-positive rate. Six cases showed a ‘black-and-white’ expression pattern (Figure 1 and Table 3). Small Her2/neu-overexpressing tumor cell clones (<10% of the entire tumor volume) were present in an overall Her2/neu-negative GC. Sampling of these tumor cell clones carries the risk of a false-positive test result, according to the GC scoring system. The prognostic impact of these cell clones is unknown. At least we were unable to show any prognostic significance of Her2/neu overexpression in our cohort.
Patient-individualized treatment aims to avoid unnecessary medication in patients who are unlikely to respond to therapy. To the contrary, targeted therapy should reach every patient eligible for the treatment. In this respect, unresectable GC patients may be withheld a life-prolonging medication due to a sampling error. Since the overall number of GC patients eligible for trastuzumab is already small (<20%), we recommend that, whenever possible, the Her2/neu status should be assessed on whole tissue sections of gastrectomy specimens. Furthermore, in case of metastatic disease, it should be considered to re-evaluate the Her2/neu status from metastatic sites, when Her2/neu results are negative from primary tumor biopsies, in order to increase the probability of finding Her2/neu-positivity, which allows for treatment with trastuzumab in stage-IV GC. Additionally, more in-depth investigations on the correlation of the percentage of Her2/neu-immunopositive GCs with clinical outcomes may help to improve predictive diagnostics. Changing the cut-off value to above or below 10% positivity influences the risk of a sampling error, i.e. it may also alter the discrepancies between whole tissue sections obtained from a GC resection specimen and tumor biopsies. Alternatively, a predictive biomarker should be sought, which is less sensitive to sampling procedures.
funding
This work was supported by grant no. F344605 of Roche Diagnostics Deutschland GmbH and Roche Pharma AG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We wish to thank Sandra Krüger for her excellent technical assistance.
references
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. doi:10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Van CE, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. doi:10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 3.Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299–307. doi: 10.1007/s00428-010-0952-2. doi:10.1007/s00428-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-Positive gastric cancer and its impact on survival outcomes – a systematic review. Int J Cancer. 2012;130(12):2845–2856. doi: 10.1002/ijc.26292. [DOI] [PubMed] [Google Scholar]
- 5.Bilous M, Osamura RY, Ruschoff J, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2010;41:304–305. doi: 10.1016/j.humpath.2009.10.006. doi:10.1016/j.humpath.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372–1376. doi: 10.1038/bjc.2011.121. doi:10.1038/bjc.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura M, Tsuda H, Morita D, et al. Usefulness and limitation of multiple endoscopic biopsy sampling for epidermal growth factor receptor and c-erbB-2 testing in patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2005;35:324–331. doi: 10.1093/jjco/hyi089. doi:10.1093/jjco/hyi089. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Rauser S, Feith M, et al. Assessment of ErbB2 (Her2) in oesophageal adenocarcinomas: summary of a revised immunohistochemical evaluation system, bright field double in situ hybridisation and fluorescence in situ hybridisation. Mod Pathol. 2011;24:908–916. doi: 10.1038/modpathol.2011.52. doi:10.1038/modpathol.2011.52. [DOI] [PubMed] [Google Scholar]
- 9.Abraham SC, Park SJ, Lee JH, et al. Genetic alterations in gastric adenomas of intestinal and foveolar phenotypes. Mod Pathol. 2003;16:786–795. doi: 10.1097/01.MP.0000080349.37658.5E. doi:10.1097/01.MP.0000080349.37658.5E. [DOI] [PubMed] [Google Scholar]
- 10.Kim MA, Lee HJ, Yang HK, et al. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59:822–831. doi: 10.1111/j.1365-2559.2011.04012.x. doi:10.1111/j.1365-2559.2011.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, de Boer WB, Fermoyle S, et al. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–840. doi: 10.1111/j.1365-2559.2011.04017.x. doi:10.1111/j.1365-2559.2011.04017.x. [DOI] [PubMed] [Google Scholar]
- 12.Tafe LJ, Janjigian YY, Zaidinski M, et al. Human epidermal growth factor receptor 2 testing in gastroesophageal cancer: correlation between immunohistochemistry and fluorescence in situ hybridization. Arch Pathol Lab Med. 2011;135:1460–1465. doi: 10.5858/arpa.2010-0541-OA. doi:10.5858/arpa.2010-0541-OA. [DOI] [PubMed] [Google Scholar]
- 13.Yan B, Yau EX, Bte Omar SS, et al. A study of HER2 gene amplification and protein expression in gastric cancer. J Clin Pathol. 2010;63:839–842. doi: 10.1136/jcp.2010.076570. doi:10.1136/jcp.2010.076570. [DOI] [PubMed] [Google Scholar]
- 14.Yan B, Yau EX, Choo SN, et al. Dual-colour HER2/chromosome 17 chromogenic in situ hybridisation assay enables accurate assessment of HER2 genomic status in gastric cancer and has potential utility in HER2 testing of biopsy samples. J Clin Pathol. 2011;64:880–883. doi: 10.1136/jclinpath-2011-200009. doi:10.1136/jclinpath-2011-200009. [DOI] [PubMed] [Google Scholar]
- 15.Lauren T. The two histologic main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th edition. Wiley-Blackwell: Oxford; 2009. [Google Scholar]
- 17.Warneke VS, Behrens HM, Hartmann JT, et al. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol. 2011;29:2364–2371. doi: 10.1200/JCO.2010.34.4358. doi:10.1200/JCO.2010.34.4358. [DOI] [PubMed] [Google Scholar]
- 18.Weichert W, Röske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9:139–148. doi: 10.1016/S1470-2045(08)70004-4. doi:10.1016/S1470-2045(08)70004-4. [DOI] [PubMed] [Google Scholar]
- 19.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 20.Fox SB, Kumarasinghe MP, Armes JE, et al. Gastric HER2 Testing Study (GaTHER): an evaluation of gastric/gastroesophageal junction cancer testing accuracy in Australia. Am J Surg Pathol. 2012;36:577–582. doi: 10.1097/PAS.0b013e318244adbb. doi:10.1097/PAS.0b013e318244adbb. [DOI] [PubMed] [Google Scholar]
- 21.Park YS, Hwang HS, Park HJ, et al. Comprehensive analysis of HER2 expression and gene amplification in gastric cancers using immunohistochemistry and in situ hybridization: which scoring system should we use. Hum Pathol. 2012;43:413–422. doi: 10.1016/j.humpath.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135:1331–1339. doi: 10.1007/s00432-009-0574-8. doi:10.1007/s00432-009-0574-8. [DOI] [PubMed] [Google Scholar]
- 23.Ajani JA, Barthel JS, Bekaii-Saab T, et al. Gastric cancer. J Natl Compr Canc Netw. 2010;8:378–409. doi: 10.6004/jnccn.2010.0030. [DOI] [PubMed] [Google Scholar]
- 24.Moehler M, Al-Batran SE, Andus T, et al. German S3-guideline ‘Diagnosis and treatment of esophagogastric cancer. Z Gastroenterol. 2011;49:461–531. doi: 10.1055/s-0031-1273201. doi:10.1055/s-0031-1273201. [DOI] [PubMed] [Google Scholar]
- 25.Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 26.Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. doi:10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.