Abstract
Pompe disease is a neuromuscular disease caused by an inherited deficiency of the lysosomal enzyme acid α-glucosidase (GAA). The resulting accumulation of glycogen causes muscle weakness with the severe form of the disease resulting in death by cardiorespiratory failure in the first year of life. The only available treatment, enzyme replacement therapy (ERT) with recombinant GAA (rhGAA), is severely hampered by antibody responses that reduce efficacy and cause immunotoxicities. Currently, Pompe mice represent the only pre-clinical model for development of new treatments and for immunological studies. While antibody formation following ERT in this model has been described, the underlying T cell response has not been studied. In order to define the T helper response to rhGAA in Pompe mice, immunodominant CD4+ T cell epitopes were mapped in GAA−/− 129SVE mice using ELISpot. Additionally, cytokine responses and antibody formation against rhGAA during ERT were measured. Among the three CD4+ T cell epitopes identified, only epitope IFLGPEPKSVVQ, predicted to be the strongest MHC II binder, consistently contributed to IL-4 production. Frequencies of IL-4 producing T cells were considerably higher than those of IL-17 or IFN-γ producing cells, suggesting a predominantly Th2 cell mediated response. This is further supported by IgG1 being the prevalent antibody subclass against rhGAA during ERT and consistent with prior reports on IgE formation and anaphylaxis in this model. These results will facilitate mechanistic studies of the immune response to rhGAA in Pompe mice during development of new therapies and tolerance protocols.
Keywords: alpha-Glucosidase, Pompe, Immune response, T cell, Antibody
1. Introduction
Pompe disease is a debilitating neuromuscular disorder, caused by partial or complete deficiency of the lysosomal enzyme acid α-glucosidase (OMIM # 232300) (GAA). The disease is a continuum of clinical spectrums including progressive skeletal muscle weakness, cardiorespiratory failure. The recessive inheritance has been detected with varying frequencies in several ethnic groups. Severity of Pompe disease varies with the amount of residual endogenous enzyme production thereby influencing the age of and rate of progression of muscle weakness [1]. The early-onset phenotype, diagnosed within the first 6 months of life, is considered to be the most severe form of the disorder. All early onset patients are found to have severe cardiac hypertrophy, profound muscle weakness, hypotonia, and die due to cardiorespiratory failure within a year of age [2–5].
Diagnostic challenges are often encountered since as a rare disease there will be only 20–30 early onset children born in the US each year, yet more rapid diagnostic methods have shortened the time from symptom onset to definitive diagnosis [3]. The diagnosis is established by measurement of GAA activity in a blood spot assay, whole blood sample, or in skin fibroblasts. Although detection of GAA from skin fibroblasts was considered to be the gold standard, preference is being given to assays run on dried blood spots for their accuracy and rapid mode of diagnosis [3].
Therapeutic approaches, including gene and enzyme replacement therapies have been developed for replacing the deficient/dysfunctional enzyme. In vivo and in vitro studies using viral vectors, cell lines, and recombinant proteins have shown that the defect can be corrected by gene transfer to the affected cell (from autonomous expression) or from cross-correction [2,6]. In the latter case, extracellular GAA is taken up by cells via the mannose-6-phosphate receptor and traffics to the lysosome, resulting in clearance of glycogen. This mechanism has been the basis for enzyme replacement therapies (ERT) and some novel gene therapy approaches. Currently, the recombinant human enzyme, Myozyme® has been approved by the US and EU for use in infantile Pompe disease. The pivotal study of ERT in infants showed significant improvement in survival as a secondary outcome measure, however limitations in ventilator free survival, the primary outcome of the study, was observed over time.
Notably, very high doses of enzyme are required for a therapeutic effect and ERT for Pompe disease faces a major hurdle in the form of antibody formation against the intravenously infused recombinant GAA (rhGAA). The incidence of antibody formation is very high in early onset disease (>90%), negatively effects efficacy of treatment, and immunotoxicities such as allergic/anaphylactic reactions and tissue toxicities can occur [2,7–9]. Therefore, a better understanding of the immune response to rhGAA is imperative, and the development of immune tolerance protocols is desirable. Pre-clinical and translational studies in animal models will aid in these efforts.
Although there are several naturally occurring animal models for Pompe disease including the Lapland dog, cats, sheep and Brahman and Shorthorn cattle and a particular strain of Japanese quail, only the GAA−/− mouse model serves as a more phenotypically and genotypically similar model for the human disease. To this end, knockout mice had been generated by several groups by targeting exons 6, 13, or 14. Generalized and progressive muscle weakness was observed in these models, along with elevated levels of lysosomal glycogen in the heart, liver and striated muscles. Therefore, Pompe mice are currently the animal model of choice for basic and translational studies toward improved therapies for the disease [10].
Pompe mice form high-titer antibodies to human GAA during ERT, including IgG and IgE, and develop fatal anaphylactic reaction after subsequent exposure [11]. Experiments in this model have clearly shown that the antibodies interfere with efficacy of treatment. CD4+ T cells play a critical role in B cell activation and antibody production in protein replacement therapies. However, the underlying T helper cell response that drives antibody formation in the Pompe mouse has not yet been studied. Using a Pompe mouse strain which is congenic with the 129SVE line, we mapped three CD4+ T cell epitopes in this study and found a response dominated by Th2.
2. Materials and methods
2.1. Animals
Pompe mice (8–12 weeks old) were generated by targeted disruption of exon 6 with the neomycin resistance gene (6neo/6neo) were used in this study [10]. With assistance by Taconic, these mice had been repeatedly backcrossed to 129SVE for over 10 generations to obtain a pure strain background. The mice were housed under specific pathogen free conditions in the Animal Facility at the University of Florida.
2.2. Peptide library
A peptide library spanning the mature rhGAA sequence of 952 amino acids was generated in the form of 12-mers with an overlap of 2 amino acids on both ends (Anaspec, San Jose, CA). The peptide stocks were dissolved using dimethylsulfoxide (DMSO) to a concentration of 2 mg/ml. Two-dimensional arrays of the peptides were designed to obtain 20 pools of peptides [12,13]. Each pool was composed of 8–10 peptides, and each peptide was represented in two pools. The final concentration of each peptide in the pool was 4 µg/ml. The reconstituted peptides were stored at −70 °C. Alternatively, to avoid the high concentration of DMSO during cell culture, peptides were diluted using 5-MLC media, and used to construct smaller pools consisting of only 3 peptides. Thus, 32 pools of 3 peptides each were created with a final concentration of each peptide in the pool at 10 µg/ ml.
2.3. Immunizations and ERT
Pompe mice [GAA−/− (129SVE)] were immunized subcutaneously with 200 µl of rhGAA protein (Myozyme, 50 µg/mouse) and Complete Freund's Adjuvant (cFA; GIBCO/BRL, Rockville, MD) and sacrificed 10 days later for the peptide mapping experiments. To mimic ERT, Pompe mice were treated intravenously (tail vein) with 20 mg/kg of rhGAA protein once per week for 3 weeks and sacrificed 10 days later for T cell assays. Myozyme was kindly provided by Genzyme (Framingham, MA).
2.4. Antibody assays
GAA-specific antibody titers were determined from plasma samples of the immunized mice using subclass-specific ELISA for IgG1, IgG2a and IgG2b anti-rhGAA as published [7]. Briefly, plates were coated overnight with 1 µg/ml of rhGAA protein (in 1× PBS) and treated with mouse plasma samples (1:20 dilution) and detected using horseradish peroxidase conjugated secondary IgG antibodies (at 1:2000 dilution) (Roche Molecular Biochemicals, Indianapolis, IN). Standard curves were plotted using two fold dilutions of mouse immunoglobulin standards (Sigma-Aldrich, St Louis, MO) starting at 2000 ng/ml for IgG1 and IgG2a and at 500 ng/ml for the IgG2b ELISA's. The plates were developed using OPD (o-phenylen diamine substrate, Louis, MO) substrates and the antibody titers were determined by reading the optical density measurements at 450 nm. Anti-rhGAA IgE titers were also measured by ELISA using plasma samples depleted for IgG with sepharose beads as published [14]. Plasma samples containing high-titer IgG2a anti-rhGAA were assayed for ability of antibody–antigen complex to fix complement using an ELISA sandwich assay as described by others [15]. Briefly, wells that were coated overnight with rhGAA were incubated with plasma samples for 2 h, washed and further incubated with fresh mouse complement (1:20 dilution) (Innovative Research, Peary Court Novi, MI) for an hour at 37 °C. Anti-mouse C3 conjugated with horse radish peroxidase (1:2000) (ICL, Inc, Portland, OR) was added for detection as published [14,15].
2.5. ELISpot assays
ELISpots were performed for rhGAA-specific IL-4, IL-17, and IFN-γ responses. Sterile 96 well multiscreen filter ELISpot plates (Millipore, Billerica, MA) were pre-wetted using 15 µl of 35% ethyl alcohol to overcome the hydrophobicity of the membrane and coated overnight at 4 °C with anti IFN-γ/IL-4/IL-17 capture antibodies (BD Biosciences, San Jose, CA). Splenocytes were isolated from 129SVE mice 10–12 days after their immunizations and cultured using 5-MLC media (DMEM, sodium-pyruvate, HEPES, non-essential amino acids, 2-beta-mercaptoethanol, 1% penicillin/streptomycin and 5% mouse serum/FBS). The splenocytes were also positively selected for CD4+ and CD8+ T cell populations by magnetic cell separation (Miltenyi Biotec, CA, USA) and used for mapping the CD4+ T cell dominant epitopes. Plates were blocked using PBS with 5% sucrose and 1% BSA for 2 h at room temperature after the overnight Ab incubation and then used for cell culture, with 200 µl of 106 splenocytes or peripheral blood mononuclear cells. The cells were stimulated using 10 µg/ml of the peptide pools or 1 µg/ml individual peptides or 10 µg/ml of the whole rhGAA protein for 14–17 h at 37 °C in 5% CO2 for the mapping experiments (using IFN-γ ELISpots). Staphylococcal Enterotoxin B (1 µg/100 µl; Sigma Aldrich, St. Louis, MO), PMA-Phorbol 12-myristate 13-acetate (0.05 g/ml)/Ionomycin (1 µg/ml; Sigma Aldrich), and recombinant mouse IFN-γ (R&D systems Minneapolis, MN) at 1 µg/ml were also used as positive controls. DMSO or an irrelevant peptide was added to media at identical concentration for mock-stimulation (negative controls). For responses to ERT, splenocytes were stimulated with 10 µg/ml of a specific peptide or of GAA protein. The cells were stimulated with peptides and controls at 37 °C in 5% CO2 for 48 h for the IL-4 ELISpots and for 24 h for the IL-17 ELISpot's. The plates were then washed and plated with anti IFN-γ/IL-4/IL-17 secondary antibodies (BD Biosciences, San Jose, CA). The color development of the spots was carried out using HRP-streptavidin (BD Biosciences, San Jose, CA) and BCIP/NBT chromogen (BD Biosciences). IFN-γ/IL-4/IL-17 secreting cells were analyzed and counted with the CTL-ImmunoSpotH S5 UV analyzer (Cellular Technology, Shaker Heights, OH) [16].
2.6. In silico analysis
The immune epitope database analysis resource (IEDB, sponsored by National Institute of Allergy and Infectious Diseases) was utilized for the prediction of I-Ab MHC class II epitopes in the GAA protein sequence. The NET MHC II pan epitope prediction tool was preferred due to its performance score and its ability to predicting murine specific MHC class II epitopes. The algorithms were based on neural networks and binding affinities of the peptides with MHC class II molecules as described previously [17].
2.7. Statistics
The mean ± SD results of all the experiments were plotted using GraphPad Prism 5.0 software. Un-paired Student t tests was used for determining statistical significant values, with p values<0.05 considered to be significant.
3. Results
3.1. Identification of a dominant CD4+ T cell epitope
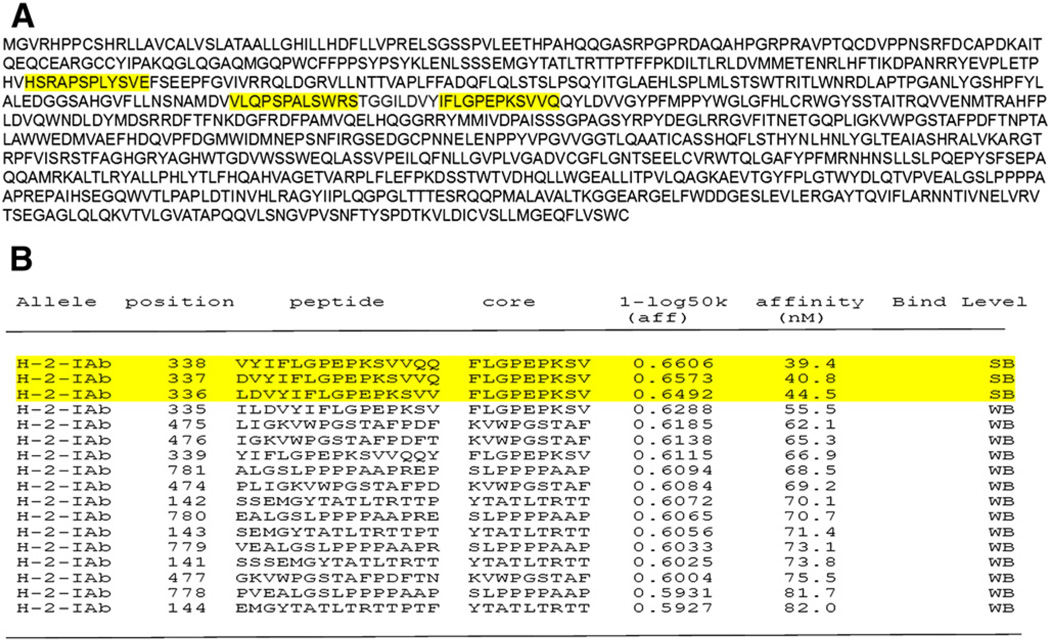
Efficient immunoglobulin class switching, strong antibody responses and development of memory B cells are typically dependent on T help by MHC II-restricted CD4+ T cells. This is well documented for treatment of other protein deficiencies such as hemophilia [18]. Consistent with this notion, repeated administration of rhGAA resulted in antibody formation in wild-type but not in CD4−/− C57BL/6 mice (data not shown). In order to generate appropriate tools for T cell studies, we sought to identify CD4+ T cell epitopes. The strain of mouse used here (GAA−/− 129SVE, MHC haplotype H-2bc) expresses the MHC II molecule I-Ab, which by in silico analyses is predicted to tightly bind GAA-derived peptides containing the core sequence IFLGPEPKSVQQ (Fig. 1B). We decided to experimentally identify the immunodominant T cell epitope against the rhGAA protein in Pompe mice using peptide libraries [13,19].
Fig. 1.
GAA primary amino acid sequence and MHC II epitope prediction. A. Primary amino acid sequence of the lysosomal protein, acid α-glucosidase. Yellow highlights represent sequences, which were subsequently identified to contain CD4+ T cell epitopes: peptide 19 (HSRAPSPLYSVE), peptide 29 (VLQPSPALSWRS), and peptide 83 (IFLGPEPKSVVQ). B. Prediction of the immunodminant I-Ab-restricted epitope by NET MHC II, a T cell epitope prediction server of the immune epitope database analysis source. Protein was loaded in the Fasta format and sequence IFLGPEPKSVVQ highlighted in yellow was identified as the strongest binder based on its threshold values (strong binder threshold 50.00, weak binder threshold 500.00).
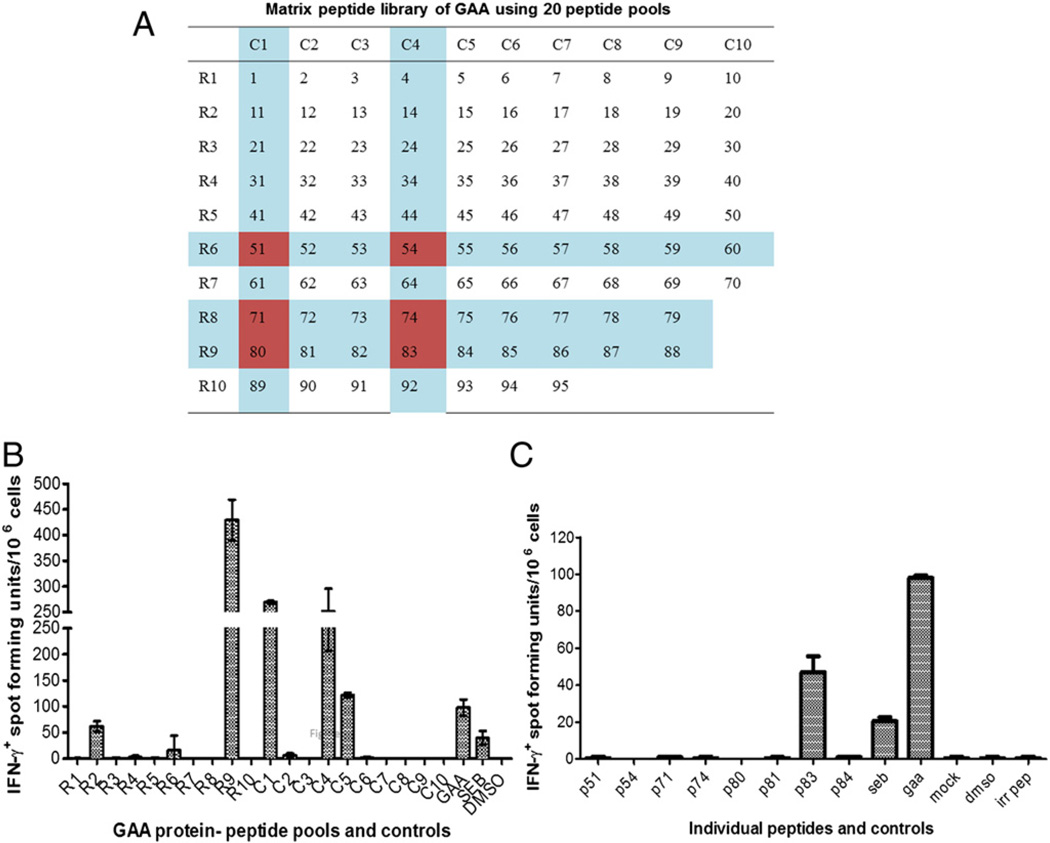
A library spanning the entire mature GAA sequence of 952 amino acids was generated in the form of 12-mers with an overlap of 2 amino acids on both ends. The library was initially split into 20 pools with 10 peptides per pool and arranged in the form of a matrix, with each peptide being represented by two pools as shown in (Fig. 2A). Pompe mice were immunized with rhGAA protein in cFA. After 10 days, their splenocytes were extracted and stimulated in vitro with peptide pools for analysis by IFN-γ ELISpot. Initial experiments done with the 20 peptide-pools narrowed down the response to 8 potential dominant epitopes (Fig. 2B). The experiment was repeated, and splenocytes were stimulated with the single candidate peptides. Here, only peptide # 83 yielded a response (Fig. 2C). These results are in agreement with in silico analyses, since peptide 83 contains the predicted high-affinity core sequence (Figs. 1A and B).
Fig. 2.
Initial identification of a dominant T cell epitope using a peptide library. Pompe mice (GAA−/− 129SVE) were immunized with rhGAA/CFA followed by in vitro stimulation of splenocytes with peptides and IFN-γ ELISpot. A. Matrix of 20 peptide pools, each containing 10 peptides with each peptide being represented in 2 pools. Candidate peptides that emerged from the assay are indicated. B. Average frequencies of IFN-γ spot forming units (± SD) obtained for each pool. C. Average frequencies of IFN-γ spot forming units (± SD) obtained for stimulation with individual candidate peptides. All stimulations were performed in triplicate (using splenocytes pooled from 3 animals). Control stimulations included mock (adding an irrlevant peptide or an identical amount of DMSO solvent to the media), SEB superantigen, and rhGAA protein.
3.2. Identification of two additional CD4+ T cell epitopes
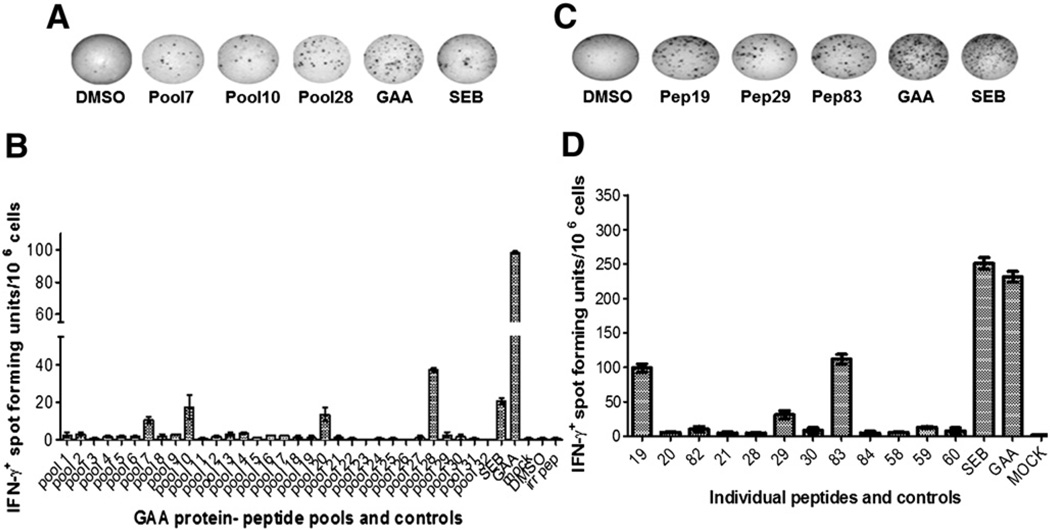
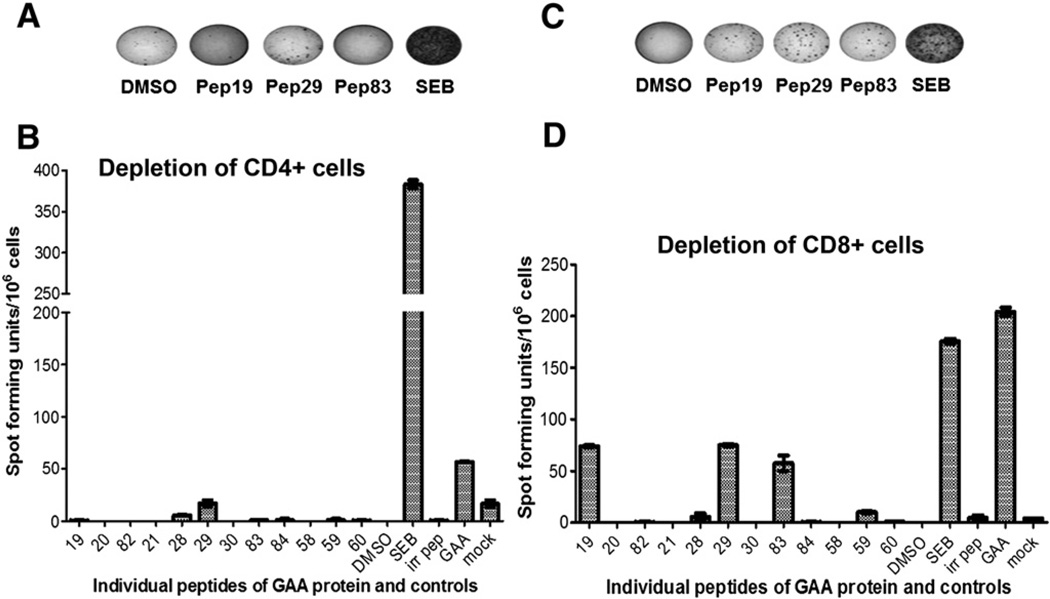
As the initial matrix arrangement of peptide pools were large and possessed high concentrations of DMSO, the peptides were rearranged into 32 smaller pools, consisting of 3 peptides per pool, to identify epitopes which could have been missed due to competitive effects in the previous assays. Among the 32 pools of peptides, 4 emerged positive for IFN-γ secretion, including the pool that contained peptide 83 (Figs. 3A and B). In the next experiment, stimulation with single peptides derived from these pools resulted in positive responses for peptides 19, 29, and 83 (Figs. 3C and D). Identical results were obtained when peripheral blood cells instead of splenocytes were used for the assay (data not shown). ELISpot results remained positive for peptides 19, 29, and 83, when the splenocytes were depleted for CD8+ cells, but were negative upon on depletion of CD4+ cells, confirming that the identified epitopes were recognized by CD4+ T cells (Figs. 4A–D). Interestingly, all 3 identified epitopes were clustered around in the same region of the GAA protein (amino acids 200–350, Fig. 1A).
Fig. 3.
Identification of additional dominant T cell epitopes for rhGAA in Pompe mice. A. Representative examples of well images from IFN-γ ELISpot assays after stimulation of splenocytes with small peptide pools, individual peptides, and controls. GAA−/− 129Sv mice had been immunized with rhGAA/CFA. B. Average frequencies of IFN-γ spot forming units (± SD, triplicate) after stimulation with small peptide pools or C. Individual peptides derived from pools that had tested positive.
Fig. 4.
Confirmation that identified peptides contain CD4+ T cell epitopes. A. Representative examples of well images from IFN-γ ELISpot assays after stimulation of CD4+ cell-depleted splenocytes with peptides 19, 29, 83, rhGAA protein, SEB or solvent control. GAA−/− 129SVE had been immunized with rhGAA/CFA. B. Average frequencies of IFN-γ spot forming units (± SD, triplicate) after CD4+ depletion and stimulation with indicated peptides or controls. C. Representative examples of well images from IFN-γ ELISpot assays after stimulation of CD8+ cell-depleted splenocytes. D. Average frequencies of IFN-γ spot forming units (± SD, triplicate) after CD8+ depletion and stimulation with indicated peptides or controls.
3.3. Relative contribution of the epitopes to Th2 responses in ERT
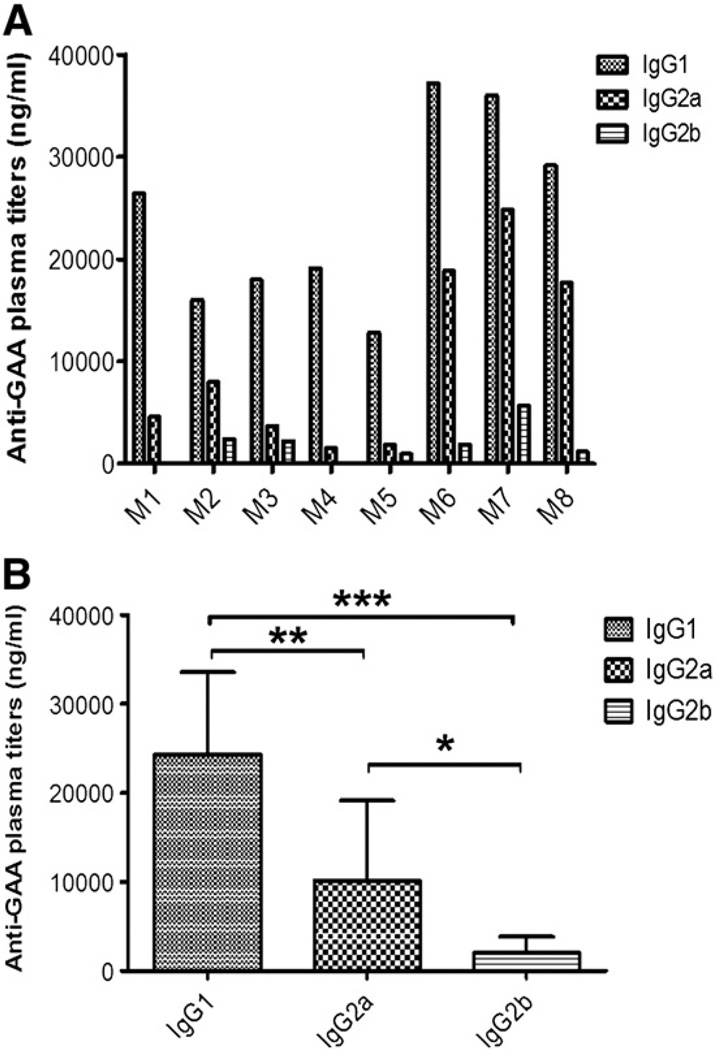
In order to study the contribution of the identified epitopes in the immune response to ERT, Pompe mice received 3 weekly therapeutic doses of rhGAA (20 mg/kg) by intravenous (tail vein) injection, which consistently resulted in high-titer IgG1 anti-rhGAA formation (Figs. 5A and B). Less consistently and at lower titers, IgG2a was also observed as well as low-titer IgG2b (Figs. 5A and B). No evidence for the ability of antibody–rhGAA complexes to activate complement was obtained (data not shown).
Fig. 5.
Antibody formation against rhGAA during ERT in Pompe mice. GAA 129SVE mice were treated with 3 weekly intravenous doses of rhGAA (20 mg/kg). One week, later, plasma samples were collecte, and rhGAA-specific IgG titers were measured. A. GAA-specific IgG1, IgG2a, and IgG2b titers for 8 individual animals. B. Average titers (± SD) for the 3 subclasses. Statistically significant differences are indicated as * for P<0.05, ** for P<0.01, and *** for P<0.001.
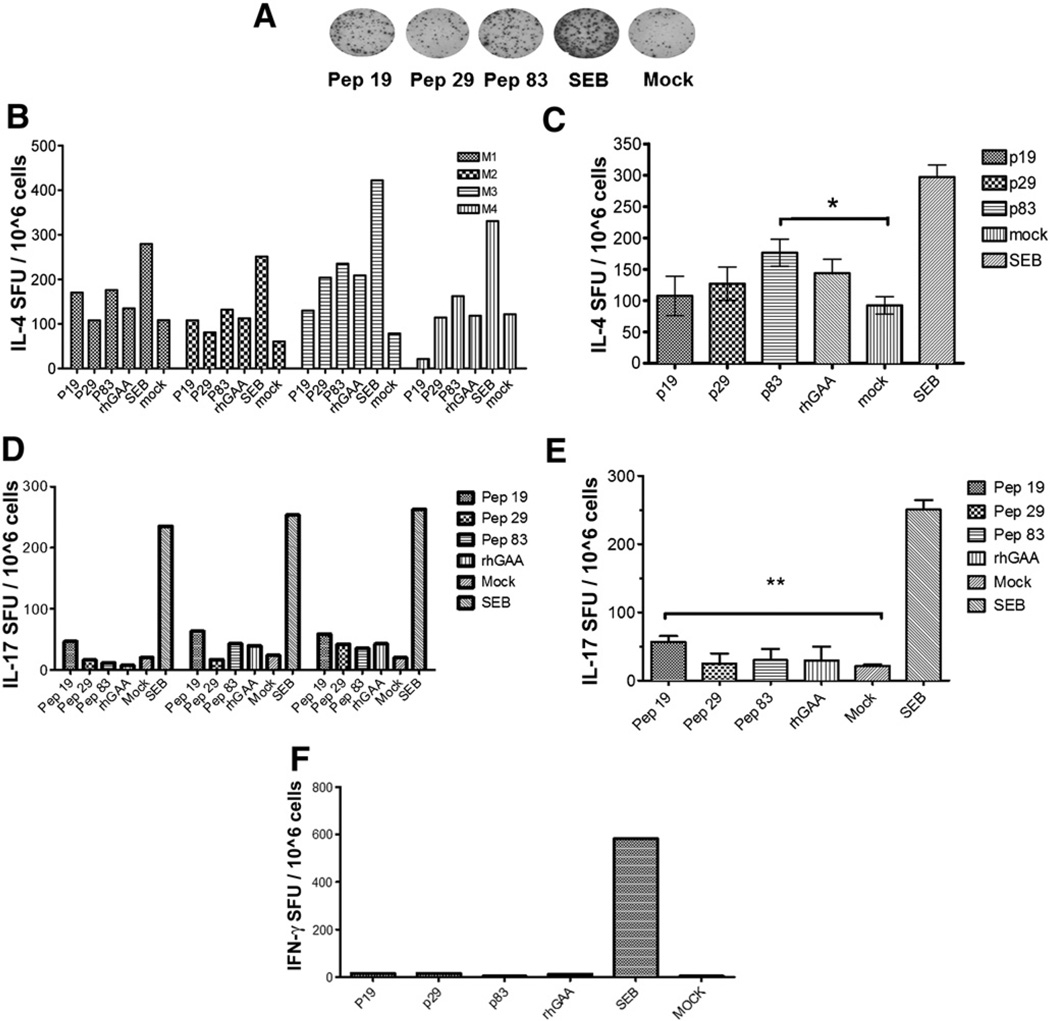
Given that IgG1 formation is driven by Th2, and that others reported IgE formation (another Th2-dependent antibody), we performed ELISpots for IL-4 production to assess Th2 immunity. Stimulation with peptide 83 consistently resulted an IL-4 response, while responses to peptides 19 and 29 were also observed, albeit less consistently and typically at lower frequency (Figs. 6A–C). Three of 4 mice also showed an IL-4 response upon stimulation with GAA protein, which was not as high as when stimulated with peptide 83 (This was somewhat expected, since the protein has to be processed prior to peptide presentation by MHC). In other assays, we also found a low-frequency IL-17 response, suggesting some level of Th17 activation (Figs. 6D–E). These responses were primarily to peptide 19. In contrast to immunization with cFA, ERT did not result in a detectable IFN-γ response (Fig. 6F). In all assays performed for Fig. 6, ELISpot data from GAA-stimulated cells tracked very well with peptide responses for each individual mouse and cytokine.
Fig. 6.
Cytokine responses to the immunodominant epitopes upon ERT in Pompe mice. GAA−/− 129SVE mice received 3 weekly intravenous doses of rhGAA (20 mg/kg) followed by splenocyte assays. A. Representative examples of well images IL-4 ELISpot assay after stimulation with peptide 19, 29, or 83, or GAA protein, or SEB, or mock controls. B. Average frequencies of IL-4 spot forming units for 4 mice assayed individually (duplicate stimulations). C. Average values (± SD) for the group of 4 animals. Peptide 83 showed statistically significant responses compared to mock stimulation. D. Average frequencies of IL-17 spot forming units for 3 mice assayed individually (duplicate stimulations). E. Average values (± SD) for the group of 3 animals. Peptide 19 showed statistically significant responses compared to mock stimulation. F. Average values (± SD) for 4 animals assayed by IFN-γ ELISpot.
4. Discussion
Immunodominant epitopes are essential for mounting effective immune responses in vivo. In the past decade, more attention has been focused on mapping and characterization of T cell epitopes from protein antigens. CD4+ T cells have been garnering additional importance due to their significant roles in the development and functioning of both the cell mediated and humoral responses of the adaptive immune system [19]. T cell assays following immunization or ERT and in silico analyses were all in agreement and identified peptide IFLGPEPKSVVQ to contain the dominant epitope that derived CD4+ T cell responses to human rhGAA in GAA−/− 129SVE Pompe mice. Two additional epitopes that contribute to the response were also identified. This knowledge should facilitate future studies on T cell responses to rhGAA in gene and protein therapies in the murine model and provide important tools for T cell assays. Furthermore, peptides containing CD4+ T cell epitopes can be useful in the development of immune tolerance protocols, as we and others have shown in form of nasal tolerance and co-administration of peptides with immune modulatory drugs [11,18,20–24]. For example, in the right context, peptides may stimulate immune regulatory CD4+ T cells that actively suppress responses. Translation of this approach will depend on the ability to effectively map T cell epitopes in patients.
Continued ERT in the Pompe mice beyond three rhGAA administrations performed here leads to fatal anaphylactic reactions. Others found that Pompe mice form IgE formation in addition to IgG, suggesting involvement of the classical anaphylaxis pathway in this severe systemic allergic response [7,11]. Anaphylactoid reactions mediated by an alternative IgG-PAF (platelet activating factor) pathway cannot be ruled out as another contribution to the pathogenic antibody response, which requires further study [25]. While we did not detect circulating IgE anti-rhGAA here (data not shown), we found that prolonged administration of a lower dose of rhGAA (5 mg/kg, which did not result in anaphylaxis as rapidly) resulted in high-titer IgE formation (unpublished observations). It should also be pointed out that IgE is primarily associated with cell surfaces, e.g. of mast cells to promote histamine release, and thus is difficult to detect in circulation. Consistent with IgG1 and IgE formation, T help against rhGAA was primarily of the Th2 subset during the first month of ERT. In addition to the IL-4 response revealed by ELISpot, quantitative RT-PCR analyses identified induction of IL-13, another Th2 cytokine that promotes class-switch to IgG1 and to IgE (data not shown). ELISpot for IL-17 results and IgG2a formation (Th1-dependent antibody) suggest that additional subsets of T helper cells such as pro-inflammatory Th17 or Th1 cells are also activated by ERT, but to substantially lesser extent than Th2. In contrast, introduction of rhGAA antigen in the context of strong inflammation, as provided by cFA, can result in strong Th1 responses specific to the same three epitopes.
In conclusion, multiple epitopes contribute to the CD4+ T cell response to rhGAA during ERT in Pompe mice. This results in Th2 immunity that drives the formation of pathogenic antibodies, which are known to reduce efficacy, cause toxicities and potentially life-threatening anaphylactic reactions. Knowledge of dominant CD4+ T cell epitopes should be useful for measurement and mechanistic studies on the immune response to rhGAA and to develop immune tolerance protocols.
Acknowledgments
This study was supported by NIH grants P01 DK058327 and P01 HL59412 to BJB and R01 HL106687 to HD and RWH and by a Scientist Development Grant from the American Heart Association to OC
Footnotes
Author contributions
SN, RS, and OC performed experiments. SN, RS, OC, HD, BJB, and RWH designed experiments. OC, BJB, and RWH supervised the study. RS, SN, HD, BJB and RWH wrote the manuscript.
Contributor Information
Barry J. Byrne, Email: bbyrne@ufl.edu.
Roland W. Herzog, Email: rherzog@ufl.edu.
References
- 1.Tinkle BT, Leslie N. Glycogen storage disease type II (Pompe disease) In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. Seattle (WA): University of Washington; 1993. [Google Scholar]
- 2.Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME, Collins SW, Conlon TJ, Clement N, Cleaver BD, Cloutier DA, Porvasnik SL, Islam S, Elmallah MK, Martin A, Smith BK, Fuller DD, Lawson LA, Mah CS. Pompe disease gene therapy. Hum. Mol. Genet. 2011;20:R61–R68. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne BJ, Kishnani PS, Case LE, Merlini L, Muller-Felber W, Prasad S, der Ploeg AV. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol. Genet. Metab. 2011;103:1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kishnani PS, Steiner RD, Bali D, Berger K, Byrne BJ, Case LE, Crowley JF, Downs S, Howell RR, Kravitz RM, Mackey J, Marsden D, Martins AM, Millington DS, Nicolino M, O'Grady G, Patterson MC, Rapoport DM, Slonim A, Spencer CT, Tifft CJ, Watson MS. Pompe disease diagnosis and management guideline. Genet. Med. 2006;8:267–288. doi: 10.1097/01.gim.0000218152.87434.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raben N, Plotz P, Byrne BJ. Acid alpha-glucosidase deficiency (glycogenosis type II, Pompe disease) Curr. Mol. Med. 2002;2:145–166. doi: 10.2174/1566524024605789. [DOI] [PubMed] [Google Scholar]
- 6.Schoser B, Hill V, Raben N. Therapeutic approaches in glycogen storage disease type II/Pompe Disease. Neurotherapeutics. 2008;5:569–578. doi: 10.1016/j.nurt.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cresawn KO, Fraites TJ, Wasserfall C, Atkinson M, Lewis M, Porvasnik S, Liu C, Mah C, Byrne BJ. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum. Gene Ther. 2005;16:68–80. doi: 10.1089/hum.2005.16.68. [DOI] [PubMed] [Google Scholar]
- 8.de Vries JM, van der Beek NA, Kroos MA, Ozkan L, van Doorn PA, Richards SM, Sung CC, Brugma JD, Zandbergen AA, van der Ploeg AT, Reuser AJ. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol. Genet. Metab. 2010;101:338–345. doi: 10.1016/j.ymgme.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Sun B, Bird A, Young SP, Kishnani PS, Chen YT, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am. J. Hum. Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B, Plotz P. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J. Biol. Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 11.Sun B, Kulis MD, Young SP, Hobeika AC, Li S, Bird A, Zhang H, Li Y, Clay TM, Burks W, Kishnani PS, Koeberl DD. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe disease. Mol. Ther. 2010;18:353–360. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthony DD, Lehmann PV. T-cell epitope mapping using the ELISPOT approach. Methods. 2003;29:260–269. doi: 10.1016/s1046-2023(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 13.Tobery TW, Caulfield MJ. Identification of T-cell epitopes using ELISpot and peptide pool arrays. Methods Mol. Med. 2004;94:121–132. doi: 10.1385/1-59259-679-7:121. [DOI] [PubMed] [Google Scholar]
- 14.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Zenzo G, Calabresi V, Olasz EB, Zambruno G, Yancey KB. Sequential intramolecular epitope spreading of humoral responses to human BPAG2 in a transgenic model. J. Invest. Dermatol. 2010;130:1040–1047. doi: 10.1038/jid.2009.309. [DOI] [PubMed] [Google Scholar]
- 16.Kalyuzhny AE. Handbook of ELISPOT [electronic resource]: Methods and Protocols. Totowa, N.J.: Humana Press; 2005. [Google Scholar]
- 17.Nielsen M, Lundegaard C, Blicher T, Peters B, Sette A, Justesen S, Buus S, Lund O. Quantitative predictions of peptide binding to any HLA-DR molecule of known sequence: NetMHCIIpan. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000107. e1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao O, Armstrong E, Schlachterman A, Wang L, Okita DK, Conti-Fine B, High KA, Herzog RW. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed RK, Maeurer MJ. T-cell epitope mapping. Methods Mol. Biol. 2009;524:427–438. doi: 10.1007/978-1-59745-450-6_31. [DOI] [PubMed] [Google Scholar]
- 20.Nayak S, Sarkar D, Perrin GQ, Moghimi B, Hoffman BE, Zhou S, Byrne BJ, Herzog RW. Prevention and reversal of antibody responses against factor IX in gene therapy for hemophilia B. Front. Microbiol. 2011;2:244. doi: 10.3389/fmicb.2011.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J. Thromb. Haemost. 2009;7:1523–1532. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staines NA, Harper N, Ward FJ, Malmström V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184–198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin. Exp. Immunol. 1996;103:368–375. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JB, Czerkinsky C, Holmgren J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand. J. Immunol. 2010;71:1–11. doi: 10.1111/j.1365-3083.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yang J, Jin L, Feng J, Lu Y, Sun Y, Li T, Cao R, Wu J, Fan H, Liu J. Immunotherapy of autoimmune diabetes by nasal administration of tandem glutamic acid decarboxylase 65 peptides. Immunol. Invest. 2009;38:690–703. doi: 10.3109/08820130903124770. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD. Anaphylaxis: lessons from mouse models. J. Allergy Clin. Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]