Abstract
The natural transition to reproductive senescence is an important physiological process that occurs with aging, resulting in menopause in women and diminished or lost fertility in most mammalian species. This review focuses on how rodent models have informed our knowledge of age-related changes in GnRH neurosecretory function and the subsequent loss of reproductive capacity. Studies in rats and mice have shown molecular, morphological and functional changes in GnRH cells. Furthermore, during reproductive aging altered sex steroid feedback to the hypothalamus contributes to a decrease of stimulatory signaling and increase in inhibitory tone onto GnRH neurons. At the site of the GnRH terminals where the peptide is released into the portal vasculature, the cytoarchitecture of the median eminence becomes disorganized with aging, and mechanisms of glial-GnRH neuronal communication may be disrupted. These changes can result in the dysregulation of GnRH secretion with reproductive decline. Interestingly, reproductive aging effects on the GnRH circuitry are observed in middle age even prior to any obvious physiological changes in cyclicity. We speculate that the hypothalamus may play a critical role in this mid-life transition. Because there are substantial species differences in these aging processes, we also compare and contrast rodent aging to that in primates. Work discussed herein shows that in order to understand neuroendocrine mechanisms of reproductive senescence, further research needs to be conducted in ovarian-intact models.
Keywords: female reproductive aging, hypothalamus, neuroendocrine, reproductive senescence, menopause, ovarian intact
General somatic aging and reproductive aging (the latter referring to the germ cells and the reproductive axis) are distinguishable but overlapping and interacting senescent processes, leading to mortality and reproductive failure, respectively [1]. Reproductive aging occurs widely among mammalian species, ultimately resulting in diminution and loss of fertility [2]. In humans, women undergo a transition to reproductive senescence, called the perimenopausal transition. This process involves many changes to the reproductive system and results in physiological and psychological consequences due to hormonal loss. It is important to note that “menopause” is a discrete event during this process; it is clinically diagnosed after one year of amenorrhea. In order to understand the factors that underlie menopause, it is necessary to investigate the central and peripheral changes that precede it. In other words, the perimenopausal transition is an excellent period to investigate factors leading to menopause. Because of our own laboratory’s interest in the hypothalamic control of aging, and the greater feasibility to perform studies on hypothalamic genes/proteins controlling reproduction in rodents, we focus this article on rat models of the natural reproductive transition. However, we begin with some brief description of what is known about mechanisms of menopause in humans.
Menopause in women
The transition surrounding the menopause appears to be a critical period during which neurobiological and peripheral systems undergo profound changes. During the perimenopausal transition menstrual cycles first become irregular in length and timing before eventually ceasing at approximately 51 years of age [3]. Women can be classified as premenopausal (regular menstrual cycles), perimenopausal (irregular menstrual cycles), or postmenopausal (one year after amenorrhoea) to distinguish their progress in the menopausal transition. Women experience vasomotor dysfunction, insomnia, mood changes [4], and increased probability for osteoporosis [5], cardiovascular disease [6] and neurodegenerative disorders [7]. Declining oocyte number and survival contribute to the reduced reproductive potential of perimenopausal women [8]. Many women seek therapies for their perimenopausal symptoms, and research in this field has the potential to introduce novel treatments, especially when that research focuses on the perimenopausal transition.
Endocrine changes in women undergoing reproductive aging are tightly related to ovarian decline. Gonadotropin secretion from the anterior pituitary changes with aging, exhibiting increased serum follicle stimulating hormone (FSH) and later increased basal luteinizing hormone (LH) concentrations [8], primarily due to the removal of negative feedback from ovarian hormones onto the hypothalamus and pituitary. LH and FSH secretion are both regulated by gonadotropin-releasing hormone (GnRH) release from the hypothalamus; however FSH is also regulated by ovarian inhibins and activins [9]. The increase in FSH levels seen as an early marker of menopausal progression is likely due to a decrease in circulating inhibins and increases in activins [10] and may be better associated with age than with menopausal status [11]. Decreases in inhibin B and anti-Mullerian hormone (AMH) levels can also be used as endocrine predictors of menopause [12]. As ovarian decline takes place, circulating concentrations of estrogens and progestins are also dramatically altered. Perimenopausal women have similar or increased estrogen levels as compared to young women, and overall estrogen levels appear to fluctuate widely from month to month [13]. Eventually, estrogen levels begin to decline and reach their nadir postmenopause. Progesterone levels appear to decline with aging, and urinary progesterone metabolites were decreased in perimenopausal women compared to young women [14].
Despite the myriad ovarian changes that take place in humans, there is limited evidence that the pituitary and hypothalamus may undergo age-related changes. For example, the pituitary response to GnRH was decreased with aging in women [15]. Also, using GnRH antagonists to indirectly assess the relative amount of GnRH in postmenopausal women, it was shown that GnRH increases with aging [16], although GnRH pulse frequency decreases, as measured by gonadotropin free α-subunit [17]. Finally, LH surges are only found in half of the perimenopausal women who show estrogen peaks, indicating failure of the hypothalamic/pituitary system to respond to estrogen positive feedback [18]. Thus, while most research has focused on ovary, there are age-related changes at all three levels of the hypothalamic-pituitary-ovarian axis of women.
Nonhuman models of the perimenopausal transition
Menopause research has benefitted greatly from the use of animal models. Non-human models give researchers more control over their subjects to reduce potential confounding variables, such as exposure to birth control, diet, and hormonal levels. There are also more experimental manipulations and endpoints available to researchers of animal models. In particular, it is not possible to directly access GnRH neurons in the brain of human subjects, limiting the types of studies that can be done to investigate neuroendocrine contributions to menopause. In the following sections, we provide a short description of data from non-human primate models of menopause. Then, our review of the neuroendocrine control of reproductive aging will focus on the rodent model, as the majority of studies are conducted in this model, and the hypothalamic gonadotropin-releasing hormone (GnRH) circuitry is well-established.
Nonhuman Primate Models
Nonhuman primates such as macaques go through menopause at the end of their lifespan, approximately 25 years of age [19–21]. Monkey models show similar patterns of irregular menses [19, 20] and circulating hormones to women during perimenopause. Aged perimenopausal monkeys show: 1) increased FSH levels in urine [22] and plasma [21]; 2) increased plasma LH concentrations [23]; 3) declining serum estradiol [20]; 4) decreased AMH levels [21, 24]; and 5) decreased levels of inhibin B [21] (Figure 1). Also, perimenopausal non-human primate models show oocyte depletion consistent with decreased follicular reserve [25]. Thus, in both humans and nonhuman primates, ovarian decline plays a critical role in reproductive aging. As in humans, there is also some evidence that there are also important neuroendocrine changes taking place. In aged perimenopausal rhesus monkeys, pulsatile GnRH release, particularly GnRH pulse amplitude, is increased in aged monkeys, consistent with the decreased peripheral estradiol levels and the removal of negative feedback [26].
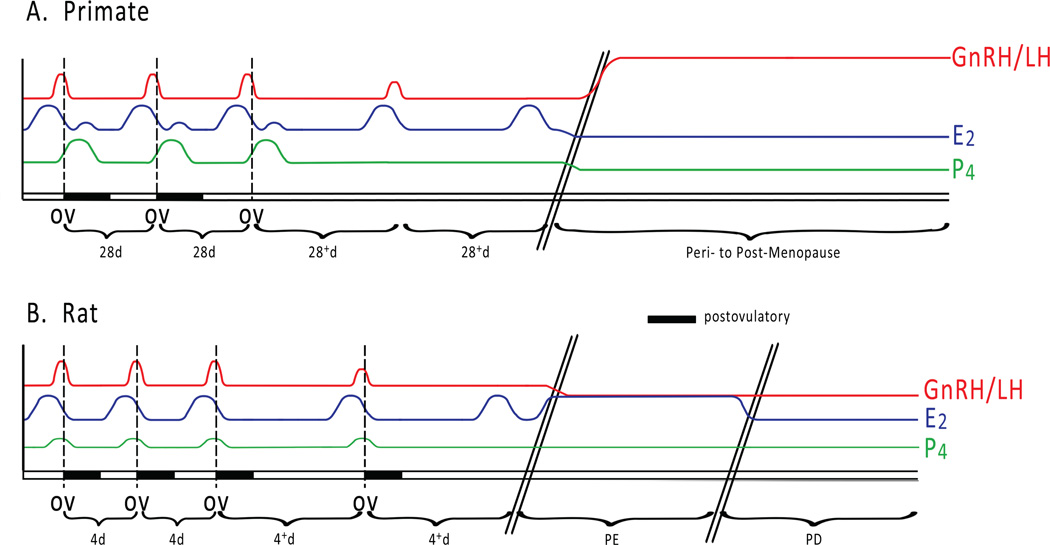
Figure 1.
The pattern of a selected subset of circulating hormone levels changes during the transition to reproductive senescence (acyclicity) for (A) the menstrual cycle of primates (human and nonhuman) and (B) the estrous cycle of rodents. A. Regularly cycling primates have approximately 28-day cycles. Positive feedback of the preovulatory estradiol (E2) peak leads to the gonadotropin-releasing hormone/luteinizing hormone (GnRH/LH) surge. The GnRH/LH surge then causes ovulation (OV), followed by the postovulatory progesterone (P4) peak and smaller E2 increase. In perimenopausal primates, the menstrual cycle becomes longer and anovulatory, and the GnRH/LH surge is attenuated. Eventually the preovulatory E2 peak no longer elicits a GnRH/LH response. During the menopausal transition, females become acyclic, and E2 and P4 levels decrease while GnRH/LH levels rise. B. Regularly cycling rodents have 4–5 day cycles. Similar to primates, there is a preovulatory E2 peak, leading to the GnRH/LH surge, which causes ovulation. Ovulation is followed by an increase in P4. At middle age the GnRH/LH surge is delayed and attenuated, the cycle length increases, and eventually the cycles become anovulatory. Next, the female transitions to persistent estrus (PE), an acyclic state characterized by elevated E2 and decreased GnRH/LH. Later, acyclic rodents move into persistent diestrus (PD), in which the circulating E2 decreases. Hash marks indicate the passing of time (years in primates, months in rodents). Dashed lines show the temporal relationship of the hormone peaks relative to ovulation. The postovulatory period is denoted by a black bar.
Rodent Models
Although rodents do not go through menopause, the rat HPG axis is highly conserved with that of other species [27], and rats undergo reproductive failure at middle-age in a manner that in many ways closely approximates the perimenopausal transition [28–30]. As they enter middle age, female rats can be grouped into regularly cycling, irregularly cycling and acyclic categories, similar to premenopausal, perimenopausal, and postmenopausal (respectively) stages in women and nonhuman primates. For purposes of this review regularly cycling rats have 4–5 day estrous cycles, irregularly cycling rats have prolonged cycles of 6 days or more, and acyclic rats are in persistent estrus (cornified epithilial cells) or persistent diestrus (leukocytic cells). This transitional process begins at approximately 10–12 months of age and can last for several months [29].
There are many differences between female rat and primate reproductive aging. In contrast to women, rats do not undergo ovarian failure at middle age. Transplantation of ovaries from old rats to young adult cycling rats did not alter cycling activity in the young animals [31]. By contrast, perimenopausal women experience decreased fertility, coinciding with diminishing ovarian follicles, declining oocyte quality and subsequent changes in ovarian hormone serum concentrations [32]. This key difference limits the aging female rodent as a model of menopause. To get around this limitation, many studies have utilized ovariectomized rodents to mimic the dramatic loss of ovarian follicles and steroid hormones. As discussed below, there is also considerable value in taking advantages of comparisons among species undergoing natural senescent changes, particularly when the endpoint is hypothalamic function.
While ovarian atrophy appears to be an important species difference between primates and rodents, the fundamental changes in the GnRH neuronal circuitry underlying the transition to reproductive senescence appears to be more highly conserved across mammalian species, possibly due to the high homology of GnRH structure, release, and neurocircuitry. For instance, kisspeptin, a neuropeptide involved in the regulation of GnRH secretion, is critical for both human [33–35] and rodent [36, 37] reproductive function. Kisspeptin upregulates GnRH secretion as measured directly in rodents [38] and nonhuman primates [39]. However, the neuroanatomy of the kisspeptin system differs between primates and rodents. Both have kisspeptin neurons in the arcuate nucleus [40], but rodents also have kisspeptin neurons in the anteroventral periventricular nucleus [41], a region that is not as clearly defined in primates. A recent study in humans observed a population of kisspeptin neurons in a rostral region of females, which may be anatomically analogous to the AVPV in the rodent [42]. In all these species, kisspeptin input onto GnRH neurons is stimulatory, so the fundamental circuitry appears to be conserved.
Ovariectomized versus ovarian-intact animal models
Although menopause can be surgically induced to take place very quickly through removal of the ovaries (oophorectomy), the natural transition to acyclicity in women can last for several years [43]. Models of senescence by ovariectomy or pharmacological treatment are extremely valuable. However, they do not allow the study of the natural transition to acyclicity, whose mechanisms are still largely undefined. This is a critical gap in knowledge since the majority of women undergo a natural, and not a surgical, menopause. We will return to the natural aging model momentarily, but we will briefly discuss the ovariectomy model. Age at ovariectomy must be taken into account in interpreting these data, as it is clear that there are age- and cycle status-related brain changes that occur independently of removal or replacement of hormone treatment. When age and previous cycle history was taken into account before ovariectomy, Scarbrough and Wise [44] found that LH pulse amplitude and frequency decreased in middle-aged rats, regardless of cycle status. In that same study, further significant decreases in LH concentrations were found in middle-aged irregularly cycling and persistant estrus groups.
Furthermore, ovariectomy as a model of the precipitous loss of hormone feedback does not necessarily reproduce results found postmenopause. For example, in older, menopausal women NPY mRNA was increased in the medial basal hypothalamus [45]. However, ovariectomy of young female rhesus monkeys did not produce the same increase [45]. These differences could be attributable to age or species differences, but they raise the point that ovariectomy in a young animal does not necessarily reflect similar changes in the aging brain. A recent study by Eghlidi et al. on female rhesus monkeys beautifully highlights differences between the intact and OVX aging models [46]. This paper showed substantial differences in hypothalamic gene expression profiles depending upon ovarian status. In intact monkeys, gene expression of Kiss1 and neurokinin B in the arcuate nucleus-median eminence were substantially increased with aging. Ovariectomy of monkeys at young and old ages up-regulated expression of these same genes in a manner that obliterated the age difference seen in the intact group. Thus, ovarian status is a key factor to take into consideration in understanding hypothalamic age-related changes. To fully understand neuroendocrine changes that take place during the transition to acyclicity, it is often necessary to utilize models of natural reproductive decline.
Reproductive Senescence and the Hypothalamic-Pituitary-Gonadal (HPG) axis
Reproductive function in females is controlled by coordinated interactions among the three levels of the HPG axis. There is feed-forward regulation from hypothalamus (GnRH network) to pituitary (gonadotropins) to gonad (steroids, proteins). There is also feedback regulation. During the period of positive feedback prior to ovulation, increasing estrogen levels cause a GnRH surge, closely followed by an LH surge, leading to ovulation. Although direct evidence for preovulatory GnRH surges in women is lacking [47], another study in humans provides indirect evidence [18]. In this latter study, although GnRH could not be measured in women, results showed the occurrence of LH surges associated with preovulatory increases in estradiol in some perimenopausal women, while other women experienced rises in estradiol but no LH surge. Monkey studies provide more direct evidence for both a spontaneous GnRH/LH surge in intact animals [48], as well as a steroid-induced GnRH surge in ovariectomized monkeys [49].
Role of gonadotropin-releasing hormone (GnRH) neurons
Although all three levels of the hypothalamic-pituitary-gonadal (HPG) axis likely undergo age-related changes, accumulating evidence suggests that modulation of GnRH neurons is an initial factor in reproductive senescence, playing a greater (rodents) or lesser (primates) role in driving this process. The release of the GnRH peptide from these neurons drives the onset of puberty and enables ovulation to occur [27], and as such has been hypothesized to contribute to the cessation of ovulation and cyclicity during reproductive decline. Evidence for a role of GnRH in aging is shown by: 1) In middle-aged rats, there are changes in pulsatile LH release [44]; 2) The preovulatory GnRH/LH surge is delayed and/or attenuated with age in rodents [50–54]; 3) The preovulatory-associated increase in GnRH gene expression is not detected in aging rats [55]; and 4) GnRH functional activity, as indicated by co-expression of GnRH neurons with the immediate early gene c-Fos, is decreased at the time of the preovulatory surge in rats [51, 56]. Together these studies suggest there is a loss of preovulatory GnRH drive at middle age. Because these effects take place in regularly cycling middle-aged rats, GnRH dysfunction at middle age likely contributes to the transition to acyclicity that occurs later in life.
Stimulatory/inhibitory inputs to GnRH neurons change with reproductive aging
Modulation of GnRH neuronal function with aging could arise due to alterations in intrinsic properties (GnRH cell numbers, morphology, gene expression and release) or extrinsic regulatory inputs (afferent inputs from neurotransmitters, neurotrophic factors and steroid feedback). GnRH neurons do not show changes in number at middle age [54, 57, 58], thus more research has focused on the myriad neurotransmitters, neurotrophic factors and steroid hormones that regulate GnRH secretion. These factors can act directly on GnRH neurons or indirectly through the action of interneurons. In rats, GnRH cell bodies reside in the preoptic area (POA) and rostral hypothalamus. In primates, most GnRH neurons are localized in the mediobasal hypothalamus. In both species, hypophysiotropic GnRH cells project their axons to the median eminence (ME) where they release GnRH into the portal capillary system leading to the anterior pituitary. Modulators of GnRH neuronal activity can act at the site of the GnRH cell bodies and/or the neuroterminals to regulate the synthesis or release of GnRH (Reviewed in [27]). There are myriad stimulatory and inhibitory inputs onto GnRH neurons. Here, we have chosen to focus the following discussion on excitatory (glutamate) and inhibitory (GABA) influences onto GnRH neurons with aging. We also provide some discussion for the potential role of the neuropeptide kisspeptin in this age-related process (Figure 2).
Figure 2.
Regulatory inputs to the gonadotropin-releasing hormone (GnRH) neuron, as well as the surrounding glial micro-environment, undergo modulation with reproductive aging. This model depicts the situation in aging rodents. Regulation of GnRH release can take place at the GnRH perikarya in the preoptic area or at the GnRH neuroterminals in the median eminence. The stimulatory influence of glutamate (GLU) and kisspeptin (KISS) on GnRH neurons decreases in middle-aged rats, particularly during the preovulatory GnRH surge. The smaller size of neurons indicates diminished influence compared to larger-sized neurons. In addition, there is an increase in inhibitory tone by GABA signaling. Glial cells may also play a role in the regulation of GnRH release during reproductive aging. In the median eminence, tanycytes (green) become larger and lose their linear organization in middle-aged rats compared to young. In addition, the pericapillary boundary (red line) becomes more convoluted. Although not shown, other neural and glial changes occur during aging, including release of transforming growth factor (TGF)α, and its effects on erb-B receptors on glial cells. Red lines (bottom) represent the portal capillary vasculature. Green features at the GnRH neuroterminals are tanycytes.
Glutamate
Glutamate is a ubiquitous neurotransmitter in the hypothalamus and the predominant excitatory neurotransmitter in the brain [59, 60]. It acts on GnRH neurons through N-methyl-D-aspartate receptors (NMDAR) [61] and non-NMDARs [62, 63] to stimulate GnRH gene expression [64] and GnRH/LH release [65]. The NMDAR is a ligand-gated ion channel, mainly composed of two obligatory NR1 subunits together with two members of the NR2 family (NR2a-d; Reviewed in [66]). During aging, the stimulatory effect of NMDAR activation on GnRH neurons decreases [53, 61, 67]. Studies indicate that a number of mechanisms at the post-translational level contribute to the decrease of NMDAR-induced activation of GnRH neurons. There is decreased phosphorylation of NMDAR subunits [68], resulting in post-translational modifications to NMDAR functional properties. Changes in NMDAR subunit composition occur that attenuate its signaling [69], and lower levels of glutamate are released from afferent projections [70
Changes in NMDAR gene expression have also been documented. A study was conducted to measure gene expression of NR1, NR2a and NR2b in the POA-anterior hypothalamus, the site of GnRH perikarya, in aging intact rats. Results showed that the NR1 subunit mRNA did not vary with age. However, middle-aged and old persistent estrous (acyclic) rats showed lower NR2a and NR2b subunit mRNA levels compared to regularly cycling middle-aged and young females [61]. A decrease in mRNA levels may indicate a decrease in the protein levels, suggesting there are fewer functional NMDARs with aging.
In the MBH, the site of GnRH terminals, all NMDAR subunit mRNA levels increased with aging, particularly in acyclic rats [61]. It is interesting to speculate whether this is a compensatory mechanism to maintain GnRH neuronal secretion at the level of the GnRH terminals or within regulatory interneurons. In a different study, middle-aged regularly cycling rats had decreased NR1 mRNA in the POA and ME/arcuate nucleus on proestrus [53]. The differences in findings from these two studies may be due to the region size sampled and RNA extraction techniques between the groups. Despite the divergent results, NMDA subunit gene expression was consistently disrupted in middle-aged rats, even before changes in estrous cyclicity were observable.
While measurements of mRNA levels are valuable indications of gene expression of the receptor subunits, these studies lack anatomical specificity of the expression of the NMDAR subunits. To determine NMDAR expression directly on GnRH neurons and whether there are changes with reproductive aging, the colocalization of NMDAR subunits and GnRH was studied using immunohistochemistry. An increase in colocalization of the NR2b subunit on GnRH perikarya occurred with aging, independent of cycle status [71]. NR2b-containing receptors open more slowly and less reliably than NR2a-containing receptors; this affects the activation of downstream signal transduction events [72, 73]. A change in the NMDAR stoichiometry to favor NR2b over NR2a levels may decrease the stimulatory effects of the NMDAR channel, contributing to the decreased excitatory drive to the GnRH neurons at middle age. This hypothesis is supported by a study that used ifenprodil, an NR2b-specific antagonist, to stimulate parameters of LH release in both young and middle-aged ovariectomized, estradiol-treated females [74]. Furthermore, NR1 subunit co-expression on GnRH cell bodies on proestrus varied with reproductive aging: 30% (young, regularly cycling), 19% (middle-aged, regularly cycling), 46% (middle-aged, persistent estrus) [61]. Because NR1 is the obligatory subunit for the functional NMDAR, a decrease in the expression of NR1 at middle-age would lead to a decrease in NMDAR excitatory signaling on GnRH neurons.
GABA
Few studies have studied the contribution of the main inhibitory neurotransmitter in the brain, GABA, during reproductive aging. However, the findings point to increased GABAergic signaling in the hypothalamus with aging. GABA neurotransmission in the POA as measured by microdialysis was elevated during the LH surge of middle-aged (regularly cycling prior to ovariectomy) versus young ovariectomized, hormone-primed female rats [75]. Also, the dynamic changes of GAD67 mRNA expression, the rate-limiting enzyme in the synthesis of GABA, in the POA were not seen in middle-aged (persistent estrous prior to ovariectomy) versus young ovariectomized, hormone-primed female rats during the LH surge. Instead, GAD67 mRNA levels were elevated in middle-aged females compared to young [76]. An increase in the enzyme that synthesizes GABA in the POA may explain the increased GABA signaling at middle-age at the site of GnRH cell bodies.
GnRH cell bodies must integrate excitatory glutamatergic inputs and inhibitory GABAergic inputs. Glutamatergic and GABAergic cells that regulate GnRH neurons were identified by their close terminal contact to GnRH cell bodies through the labeling of vesicular glutamate transporter-2 (VGlut2) and vesicular GABA transporter (VGat), respectively. The number of terminal appositions to GnRH neurons was determined. VGlut2 terminal appositions were significantly increased and VGat appositions were decreased from diestrus to proestrus in young females. However, this pattern was not seen in middle-aged regularly cycling rats. Instead both the density of VGat and VGlut2 terminals were increased in middle-aged compared to young females [77]. This study shows that the proportion of excitatory to inhibitory stimulation onto GnRH neurons is shifted to favor inhibition at middle age.
To summarize these findings, there is a disruption of stimulatory signaling on GnRH neurons with aging. Decreased excitation on GnRH neurons may underlie the attenuated preovulatory GnRH/LH surge, as well as alterations in GnRH pulsatile release, seen at middle-age. There also appears to be increased inhibitory tone from GABAergic neurons, possibly resulting from a change in overall balance from excitatory to inhibitory neurotransmission.
Kisspeptin
Kisspeptin is a neuropeptide that is crucial for preovulatory GnRH release and the initiation of puberty [37]. Kisspeptin may stimulate GnRH/LH release in part by modulating the ratio of glutamate and GABA neurotransmission in the preoptic area, and signals through its G-protein coupled receptor 54 (GPR54)[78]. The few studies on kisspeptin and aging show changes in the expression of this important peptide in concert with reproductive decline.
Postmenopausal women show an increase in the number, size and gene expression of kisspeptin-positive neurons compared to premenopausal women in the infundibular nucleus [35]. Similarly, postmenopausal rhesus macaques showed elevated kisspeptin and GPR54 gene expression compared to premenopausal monkeys in the medial basal hypothalamus [79]. A recent study by Eghlidi et al. also saw increased kisspeptin mRNA levels in perimenopausal rhesus macaques compared to premenopausal monkeys in the arcuate nucleus-median eminence [46]. In these primate studies, the increased kisspeptin expression seen with aging/menopause is likely a consequence of the loss of negative steroid hormone feedback, due to decreased circulating ovarian hormone levels.
In the rat, kisspeptin is expressed in the arcuate nucleus, as with primates, but also in the anteroventral periventricular nucleus (AVPV) in the anterior hypothalamus [80]. Kisspeptin gene expression in the anterior hypothalamus of middle-aged ovariectomized rats underwent a smaller increase during the steroid-induced LH surge than it did in young ovariectomized, hormone-primed female rats [78]. Middle-aged females also have a reduced number of kisspeptin immunoreactive neurons in the AVPV compared to young during the LH surge [81]. Furthermore, kisspeptin infusion into directly into the medial preoptic area restored the attenuated LH surge displayed in the middle-aged rats [78]. Thus, in rats there is a decrease of kisspeptin signaling and expression at middle-age, which corresponds to the attenuated GnRH surge on proestrus. The changes in kisspeptin signaling in the rat precede ovarian decline, and may play a causal role during the transition to reproductive senescence, whereas in humans, postmenopausal changes in kisspeptin appear to be secondary to ovarian decline. These species differences are important and must be borne in mind when translating data from the rodent to the human.
The evidence across species suggests that GnRH dysfunction during reproduction aging may be due, in part, to altered kisspeptin signaling. However, it is notable that these kisspeptin changes with aging appear opposite in rats and primates. These differences hearken back to age-related changes in steroid hormone feedback onto GnRH neurons (see Figure 1). In primates, loss of estradiol feedback is accompanied by an increase in GnRH and kisspeptin hypothalamic levels. In rodents, estradiol levels remain the same at middle-age until they increase in persistent estrous rats with the concomitant decline in GnRH and kisspeptin levels. It will be very important to extend this research to much more aged rats, which transition into persistent diestrus and low estradiol levels – and to relate GnRH and kisspeptin to those in the postmenopausal state in women.
Disrupted steroid hormone receptor expression during reproductive decline
The ovarian steroid hormones, estrogens and progestins, exert broad and important effects on the brain and body. The HPG axis of adult spontaneous ovulators such as rodents and primates normally operates primarily under the influence negative steroid hormone feedback. Only prior to ovulation does estrogen’s influence switch to a positive feedback mechanism leading to the GnRH/LH surge. The effects of estrogens in the brain are mediated by the classical intracellular estrogen receptors (estrogen receptor (ER) α and β) and the non-classical membrane bound ERs [82]. Classical ERs belong to the steroid hormone nuclear receptor superfamily, which act as transcription factors to modulate the expression of different genes when activated [83]. Estrogens can feedback directly onto GnRH neurons via ERβ receptors or indirectly via ERα/ERβ receptors through trans-synaptic or glial interactions (Reviewed in [84]).
ER gene expression is altered with aging in the rodent hypothalamus, as reported in the few studies conducted on ovarian-intact rats. ERβ mRNA levels were decreased in the cortex and showed disrupted rhythmicity and decreased levels in the supraoptic nucleus of middle-aged irregularly cycling and old females compared to young regularly cycling rats [85]. ERα mRNA levels were also decreased in the periventricular preoptic nucleus of old rats compared to middle-aged irregularly cycling and young females [85]. Another study showed that in the POA, ERα mRNA levels were unchanged between young and middle-aged rats, and were slightly higher in middle-aged rats in the MBH [86]. That same study found that ERβ mRNA levels were lower in young than middle-aged rats in both POA and MBH, possibly affecting direct estradiol feedback onto GnRH neurons. These changes in ER gene expression may be indicative of altered steroid hormone feedback onto GnRH neurons with reproductive aging, in a tissue-specific manner.
Progestin receptors are also ligand (hormone)-dependent transcription factors that are distributed throughout the brain [87]. Middle-aged persistent estrous rats showed decreased PR mRNA in the AVPV, a brain region crucial to the preovulatory surge, compared to regularly cycling middle-aged and young rats on proestrus [88]. Interestingly, only middle-aged females that had been in persistent estrus long-term had decreased PR mRNA in the ventromedial hypothalamus and arcuate nuclei, both areas important for reproductive function [88]. Thus, female rats show an effect of time spent in senescence, indicating this is a factor that should be incorporated in future studies.
The research described above focused on mRNA levels of ERs and PR. There are limited numbers of studies evaluating protein expression and levels with aging. Madeira et al [2000] reported no difference in ERα cell numbers and density in aging intact female rats [89]. Other studies on age-related changes in protein of these steroid receptors have focused on the OVX rat model with (or without) steroid replacement. Chakraborty et al (2003) showed relatively small changes in ERα cell numbers measured by unbiased stereological counting, with ERα cell numbers lower in middle-aged than young or aged rats in the AVPV and VMN, and no difference in MPN and arcuate nucleus [90]. A companion study on ERβ cell numbers found a significant decrease with aging in the AVPV but no difference in the bed nucleus of the stria terminalis [91]. Interestingly, both ERβ and ERα levels decreased in the AVPV with age. The decreased postive feedback during the preovulatory surge at middle-age may arise from altered steroid hormone expression in brain regions important for the initiation of the GnRH/LH surge, such as the AVPV. This work needs to be extended to the intact rat model to provide more information about natural changes in estrogen receptors with age.
Morphological and glial-GnRH neuron interactions with aging
GnRH neurons have a close physical relationship with glial cells, including astrocytes and specialized ependymoglial cells called tanycytes, allowing active cell-cell communication [92–94]. Glial ‘end-feet’ interact with GnRH terminals to regulate GnRH secretion into the portal capillary system [93]. These interactions are sensitive to gonadal steroids and show changes throughout the estrous cycle [95–97]. In ovariectomized rats, the relationship between glia and GnRH terminals becomes disorganized with aging, suggesting that ultrastructural changes in the median eminence (ME) may contribute to the altered GnRH signal with age [98, 99]. Overall, these data suggest that the ME is an important site of GnRH regulation, and its role in neuroendocrine aging needs to be clarified, especially in models of the natural reproductive aging transition.
GnRH neurosecretion can be stimulated by glial-neuronal interactions involving the production of epidermal growth factor-related peptides (Reviewed in [92]). The members of this family that modulate GnRH release are TGFα and neuregulin 1β. Both peptides elicit GnRH secretion indirectly via the activation of tyrosine kinase receptors (erbB1 and erbB4, respectively with the co-receptor erbB-2) located on astrocytes and tanycytes [93, 100, 101]. On the afternoon of proestrus, middle-aged irregular cycling rats demonstrated a delayed decrease in erbB-4 mRNA levels in the ME-arcuate nucleus, and an attenuated erbB-4/-2 peak in the POA, compared to young females [102]. Similarly, middle-aged irregularly cycling rats showed a delayed erbB-1 mRNA peak in the ME-arcuate, and did not show the same increase in the POA, compared to young rats on proestrus [103]. This attenuation likely contributes to the decrease in GnRH surge, given the role glia play in the modulation of GnRH secretion into the portal capillary vasculature of the ME.
The anatomical localization of erbB mRNAs have also been examined in the context of aging. In the organum vasculosum of lamina terminalis (OVLT), where the GnRH cell bodies reside, young females showed higher cell labeling of erbB-4/-2. In the arcuate nucleus, erbB-4 labeling was highest in middle-aged irregularly cycling females whereas erbB-2 labeling was highest in young females [102]. ErbB-1 labeling was higher in young females in both the OVLT and POA [103]. These results indicate that there is a disruption of glial-neuronal signaling with reproductive aging in the hypothalamus.
Using natural reproductive aging to model the postmenopause
In humans, gonadotropins, and presumably GnRH, increase during the menopausal transition, probably due to the decrease in steroid negative feedback as the ovarian follicles become atretic. With greater age post-menopause, this negative feedback regulation declines, resulting in some differences in the HPG axis between young and older menopausal women [16, 17, 104, 105]. In addition, the results of the Women’s Health Initiative (WHI) study on postmenopausal hormone treatments have caused a revisitation of the importance of age, time post-menstruation, and other factors (e.g. body mass index) in deciphering whether or not to take postmenopausal hormones to treat health effects resulting from diminished estrogens [106, 107].
This later timing during the lifespan also relates to female rats, which continue to display neuroendocrine changes after middle age. At around 17 months of age, female rats transition to a state of persistent diestrus characterized by predominantly leukocytic vaginal smears [29]. Serum FSH levels increase [108] and estradiol levels decrease [89] as the aged female rat population transitions from persistent estrus to a state of persistent diestrus. This appears to be much closer to the postmenopausal condition in humans. In old (about 22 months) persistent diestrus rats, GnRH gene expression was increased [61], consistent with the observation that GnRH mRNA levels were higher in postmenopausal women than premenopausal women [109]. These data show the potential opportunity to exploit the persistent diestrous rat model of natural menopause, something that has been done very little, probably due to high costs of animal care and mortality of aging rats.
Summary and Future Directions
Overall, rat work modeling the natural transition to reproductive senescence provides strong evidence that the hypothalamic neuroendocrine system that modulates GnRH function is disrupted. Excitatory inputs onto GnRH neurons, such as glutamate and kisspeptin, decrease at middle-age. In addition, inhibitory tone increases, shifting the balance to favor decreased GnRH neuronal activation on proestrus. Decreased positive feedback from estrogens also contributes to the loss of GnRH activation, as both ERβ and ERα expression in the hypothalamus is altered. At the site of the GnRH terminals in the ME, there are age-related changes in the glial-neuronal interactions. The ultrastructural relationship between glia and GnRH terminals becomes more disorganized, and glia signaling through tyrosine kinase receptors is disrupted at middle-age. However, it must be noted that these ultrastructural data came from ovariectomized rats – further work is necessary in the intact model. At least in the rodent, these changes may play a causal role in reproductive decline.
We were unable to discuss all of the potential neuroactive factors whose regulation of GnRH may play a role in reproductive aging. Of great interest to us are those factors that mediate signals between energy balance and reproduction, functions both regulated by the hypothalamus. Leptin, insulin-like growth factor-1 (IGF-1), NPY, melanocortins, and many other factors (including kisspeptin, discussed above) are future targets for such research. Considering that reproductive aging is often associated with metabolic dysregulation, this is a crucial area for future research with high translational relevance to women’s health.
Acknowledgments
We thank the NIH AG028051 for financial support and Belinda Lehmkuhle for assistance with the figures.
References
- 1.Kirkwood TBL, Shanley DP. The connections between general and reproductive senescence and the evolutionary basis of menopause. Ann N Y Acad Sci. 2010;1204:21–29. doi: 10.1111/j.1749-6632.2010.05520.x. [DOI] [PubMed] [Google Scholar]
- 2.Turbill C, Ruf T, Gursky-Doyen S. Senescence is more important in the natural lives of long-than short-lived mammals. PLoS ONE. 2010;5:e12019. doi: 10.1371/journal.pone.0012019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- 4.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 5.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporosis International. 2003;14:525–530. doi: 10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- 6.van der Schouw Y, van der Graaf Y, Steyerbery E, Eijkemans J, Banga J. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord. 2001;16:830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald C, Zimon AE, Jones EE. Aging and reproductive potential in women. Yale J Biol Med. 1998;71:367–381. [PMC free article] [PubMed] [Google Scholar]
- 9.Padmanabhan V, Sharma TP. Neuroendocrine vs. paracrine control of follicle-stimulating hormone. Arch Med Res. 2001;32:533–543. doi: 10.1016/s0188-4409(01)00318-6. [DOI] [PubMed] [Google Scholar]
- 10.Reame NE, Wyman TL, Phillips DJ, de Kretser DM, Padmanabhan V. Net increase in stimulatory input resulting from a decrease in inhibin B and an increase in activin A may contribute in part to the rise in follicular phase follicle-stimulating hormone of aging cycling women. J Clin Endocrinol Metab. 1998;83:3302–3307. doi: 10.1210/jcem.83.9.5130. [DOI] [PubMed] [Google Scholar]
- 11.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–351. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 12.Sowers M, Eyvazzadeh A, McConnell D, Yosef M, Jannausch M, Zhang D, Harlow S, Randolph J., Jr Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinology Metab Clinics North America. 2004;33:637–659. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 15.Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J Clin Endocrinol Metab. 2009;94:3259–3264. doi: 10.1210/jc.2009-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill S, Sharpless J, Rado K, Hall J. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab. 2000;85:1794–1800. doi: 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- 18.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 19.Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- 20.Walker ML. Menopause in female rhesus monkeys. Am J Primatol. 1995;35:59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 22.Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- 23.Nozaki M, Mitsunaga F, Shimizu K. Reproductive senescence in female Japanese monkeys (Macaca fuscata): Age- and season-related changes in hypothalamic-pituitary-ovarian functions and fecundity rates. Biol Reprod. 1995;52:1250–1257. doi: 10.1095/biolreprod52.6.1250. [DOI] [PubMed] [Google Scholar]
- 24.Shaw CM, Stanczyk F, Egleston BL, Kahle LL, Spittle CS, Godwin AK, Brinton L, Dorgan JF. Serum antimullerian hormone in healthy premenopausal women. Fertil Steril. 2011;95:2718–2721. doi: 10.1016/j.fertnstert.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schramm RD, Paprocki AM, Bavister BD. Features associated with reproductive ageing in female rhesus monkeys. Human Reprod. 2002;17:1597–1603. doi: 10.1093/humrep/17.6.1597. [DOI] [PubMed] [Google Scholar]
- 26.Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 27.Gore AC. GnRH: The Master Molecule of Reproduction. Norwell, MA: Kluwer Academic Publishers; 2002. [Google Scholar]
- 28.Gore AC. Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Reviews. 2001;37:235–248. doi: 10.1016/s0165-0173(01)00121-7. [DOI] [PubMed] [Google Scholar]
- 29.LeFevre J, McClintock MK. Reproductive senescence in female rats: A longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- 30.Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 31.Peng M-T, Huang H-H. Aging of hypothalamic-pituitary-ovarian function in the rat. Fertil Steril. 1972;23:535–542. doi: 10.1016/s0015-0282(16)39131-2. [DOI] [PubMed] [Google Scholar]
- 32.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 33.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianetti E, Seminara S. Kisspeptin and KISS1R: a critical pathway in the reproductive system. Reproduction. 2008;136:295–301. doi: 10.1530/REP-08-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarkson J, Han S, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 37.d'Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Anglemont De Tassigny X, Fagg L, Carlton M, Colledge W. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keen K, Wegner F, Bloom S, Ghatei M, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in LHRH-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaswamy S, Guerriero K, Gibbs R, Plant T. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- 42.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- 43.Nelson JF. Puberty, gonadal steroids and fertility: potential reproductive markers of aging. Exp Gerontol. 1988;23:359–367. doi: 10.1016/0531-5565(88)90038-1. [DOI] [PubMed] [Google Scholar]
- 44.Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–890. doi: 10.1210/endo-126-2-884. [DOI] [PubMed] [Google Scholar]
- 45.Escobar C, Krajewski S, Sandoval-Guzman T, Voytko M, Rance N. Neuropeptide Y gene expression is increased in the hypothalamus of older women. J Clin Endocrinol Metab. 2004;89:2338–2343. doi: 10.1210/jc.2003-031899. [DOI] [PubMed] [Google Scholar]
- 46.Eghlidi D, Haley G, Noriega N, Kohama S, Urbanski H. Influence of age and 17 {beta}-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF. Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA. 1994;91:6894–6898. doi: 10.1073/pnas.91.15.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pau K-YF, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology. 1993;133:1650–1656. doi: 10.1210/endo.133.4.8404606. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno M, Gearing M, Terasawa E. The role of neuropeptide Y in the progesterone-induced luteinizing hormone-releasing hormone surge in vivo in ovariectomized female rhesus monkeys. Endocrinology. 2000;141:1772–1779. doi: 10.1210/endo.141.5.7451. [DOI] [PubMed] [Google Scholar]
- 50.Matt DW, Gilson MP, Sales TE, Krieg RJ, Kerbeshian MC, Veldhuis JD, Evans WS. Characterization of attenuated proestrous luteinizing hormone surges in middle-aged rats by deconvolution analysis. Biol Reprod. 1998;59:1477–1482. doi: 10.1095/biolreprod59.6.1477. [DOI] [PubMed] [Google Scholar]
- 51.Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 1994;51:1264–1272. doi: 10.1095/biolreprod51.6.1264. [DOI] [PubMed] [Google Scholar]
- 52.Sahu A, Kalra S. Absence of increased neuropeptide Y neuronal activity before and during the luteinizing hormone (LH) surge may underlie the attenuated preovulatory LH surge in middle-aged rats. Endocrinology. 1998;139:696–702. doi: 10.1210/endo.139.2.5728. [DOI] [PubMed] [Google Scholar]
- 53.Zuo Z, Mahesh VB, Zamorano PL, Brann DW. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: A possible role in reproductive aging? Endocrinology. 1996;137:2334–2338. doi: 10.1210/endo.137.6.8641183. [DOI] [PubMed] [Google Scholar]
- 54.Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- 55.Gore AC, Oung T, Yung S, Flagg RA, Woller MJ. Neuroendocrine mechanisms for reproductive senescence in the female rat: Gonadotropin-releasing hormone neurons. Endocrine. 2000;13:315–323. doi: 10.1385/ENDO:13:3:315. [DOI] [PubMed] [Google Scholar]
- 56.Le W-W, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. Parallel declines in fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology. 2001;142:4976–4982. doi: 10.1210/endo.142.11.8470. [DOI] [PubMed] [Google Scholar]
- 57.Rubin BS, King JC, Bridges RS. Immunoreactive forms of luteinizing hormone-releasing hormone in the brains of aging rats exhibiting persistent vaginal estrus. Biol Reprod. 1984;31:343–351. doi: 10.1095/biolreprod31.2.343. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: No loss up to middle age. Neurobiol Aging. 1986;7:45–48. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 59.Hrabovszky E, Liposits Z. Novel aspects of glutamatergic signalling in the neuroendocrine system. J Neuroendocrinol. 2008;20:743–751. doi: 10.1111/j.1365-2826.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 60.Van den Pol AN, Wuarin J-P, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- 61.Gore AC, Yeung G, Morrison JH, Oung T. Neuroendocrine aging in the female rat: The changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-D-aspartate receptors. Endocrinology. 2000;141:4757–4767. doi: 10.1210/endo.141.12.7841. [DOI] [PubMed] [Google Scholar]
- 62.Bailey J, Centers A, Jennes L. Expression of AMPA receptor subunits (GluR1–GluR4) in gonadotrophin-releasing hormone neurones of young and middle-aged persistently oestrous rats during the steroid-induced luteinising hormone surge. J Neuroendocrinol. 2006;18:1–12. doi: 10.1111/j.1365-2826.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- 63.Eyigor O, Jennes L. Kainate receptor subunit-positive gonadotropin-releasing hormone neurons express c-Fos during the steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 2000;141:779–786. doi: 10.1210/endo.141.2.7299. [DOI] [PubMed] [Google Scholar]
- 64.Gore AC, Wu TJ, Rosenberg JJ, Roberts JL. Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci. 1996;16:5281–5289. doi: 10.1523/JNEUROSCI.16-17-05281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl D,L-aspartate induces the release of luteinizing hormone-releasing hormone in the prepubertal and pubertal female rhesus monkey as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000;141:219–228. doi: 10.1210/endo.141.1.7231. [DOI] [PubMed] [Google Scholar]
- 66.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Sortino MA, Aleppo G, Scapagnini U, Canonico PL. Different responses of gonadotropin-releasing hormone (GnRH) release to glutamate receptor agonists during aging. Brain Res Bull. 1996;41:359–362. doi: 10.1016/s0361-9230(96)00199-2. [DOI] [PubMed] [Google Scholar]
- 68.Adjan V, Centers A, Jennes L. Expression and activation of N-methyl-D-aspartate receptor subunit-1 receptor subunits in gonadotrophin-releasing hormone neurones of young and middle-aged mice during the luteinising hormone surge. J Neuroendocrinol. 2008;20:1147–1154. doi: 10.1111/j.1365-2826.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 69.Maffucci JA, Noel M, Gillette R, Wu D, Gore AC. Age- and hormone-regulation of N-methyl-D-Aspartate receptor subunit NR2b in the anteroventral periventricular nucleus of the female rat: Implications for reproductive senescence. J Neuroendocrinol. 2009;21:506–517. doi: 10.1111/j.1365-2826.2009.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]
- 71.Miller B, Gore A. N-Methyl-D-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology. 2002;143:3568–3574. doi: 10.1210/en.2002-220346. [DOI] [PubMed] [Google Scholar]
- 72.Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santucci DM, Raghavachari S. The effects of NR2 subunit-dependent NMDA receptor kinetics on synaptic transmission and CaMKII activation. PLoS Comput Biol. 2008;4:e1000208. doi: 10.1371/journal.pcbi.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maffucci J, Walker D, Ikegami A, Woller M, Gore A. NMDA receptor subunit NR2b: effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol. Neuroendocrinol. 2007;87:129–141. doi: 10.1159/000111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grove-Strawser D, Jimenez-Linan M, Rubin BS. Middle-aged female rats lack the dynamic changes in GAD(67) mRNA levels observed in young females on the day of a luteinising hormone surge. J Neuroendocrinol. 2007;19:708–716. doi: 10.1111/j.1365-2826.2007.01579.x. [DOI] [PubMed] [Google Scholar]
- 77.Khan M, De Sevilla L, Mahesh V, Brann D, Tena-Sempere M. Enhanced glutamatergic and decreased gabaergic synaptic appositions to GnRH neurons on proestrus in the rat: Modulatory effect of aging. PLoS ONE. 2010;5:e10172. doi: 10.1371/journal.pone.0010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk G, Etgen A. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–3708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mikkelsen J, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacol. 2010;58:314–320. doi: 10.1016/j.neuropharm.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spary EJ, Maqbool A, Batten TFC. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat. 2009;38:185–196. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 83.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 84.Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- 85.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 86.Böttner M, Leonhardt S, Wuttke W, Wedel T, Jarry H. Expression of estrogen receptors in the hypothalamo-pituitary-ovarian axis in middle-aged rats after re-instatement of estrus cyclicity. Biogerontology. 2010;11:75–85. doi: 10.1007/s10522-009-9230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mani S. Progestin receptor subtypes in the brain: the known and the unknown. Endocrinology. 2008;149:2750–2756. doi: 10.1210/en.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mills RH, Romeo HE, Lu JKH, Micevych PE. Site-specific decrease of progesterone receptor mRNA expression in the hypothalamus of middle-aged persistently estrus rats. Brain Res. 2002;955:200–206. doi: 10.1016/s0006-8993(02)03440-6. [DOI] [PubMed] [Google Scholar]
- 89.Madeira MD, Andrade JP, Paula-Barbosa MM. Hypertrophy of the ageing rat medial preoptic nucleus. J Neurocytol. 2000;29:173–197. doi: 10.1023/a:1026598906739. [DOI] [PubMed] [Google Scholar]
- 90.Chakraborty T, Hof P, Ng L, Gore A. Stereologic analysis of estrogen receptor alpha (ERα) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- 91.Chakraborty TR, Ng L, Gore AC. Age-related changes in estrogen receptor beta in rat hypothalamus: A quantitative analysis. Endocrinology. 2003;144:4164–4171. doi: 10.1210/en.2003-0052. [DOI] [PubMed] [Google Scholar]
- 92.Prevot V, Dehouck B, Poulain P, Beauvillain J-C, Buée-Scherrer V, Bouret S. Neuronal-glial-endothelial interactions and cell plasticity in the postnatal hypothalamus: implications for the neuroendocrine control of reproduction. Psychoneuroendocrinol. 2007;32(Suppl 1):S46–S51. doi: 10.1016/j.psyneuen.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 93.Ojeda S, Lomniczi A, Sandau U. Glial–gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- 94.Yin W, Gore AC. The hypothalamic median eminence and its role in reproductive aging. Ann NY Acad Sci. 2010;1204:113–122. doi: 10.1111/j.1749-6632.2010.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King JC, Rubin BS. Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm Behav. 1994;28:349–356. doi: 10.1006/hbeh.1994.1031. [DOI] [PubMed] [Google Scholar]
- 96.Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, Beauvillain JC. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94:809–819. doi: 10.1016/s0306-4522(99)00383-8. [DOI] [PubMed] [Google Scholar]
- 97.de Seranno S, d'Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain J-C, Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology. 2010;151:1760–1772. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yin W, Mendenhall JM, Monita M, Gore AC. Three-dimensional properties of GnRH neuroterminals in the median eminence of young and old rats. J Comp Neurol. 2009;517:284–295. doi: 10.1002/cne.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yin W, Wu D, Noel M, Gore A. Gonadotropin-releasing hormone neuroterminals and their microenvironment in the median eminence: effects of aging and estradiol treatment. Endocrinology. 2009;150:5498–5508. doi: 10.1210/en.2009-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma Y, Berg-von der Emde K, Rage F, Wetsel W, Ojeda S. Hypothalamic astrocytes respond to transforming growth factor-{alpha} with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- 101.Voigt P, Ma YJ, Gonzalez D, Fahrenbach WH, Wetsel WC, Berg-Von der Emde K, Hill DF, Taylor KG, Costa ME, Seidah NG, Ojeda SR. Neural and glial-mediated effects of growth factors acting via tyrosine kinase receptors on luteinizing hormone releasing hormone neurons. Endocrinology. 1996;137:2593–2605. doi: 10.1210/endo.137.6.8641214. [DOI] [PubMed] [Google Scholar]
- 102.Hou J, Li B, Yang Z, Fager N, Ma M. Functional integrity of ErbB-4/-2 tyrosine kinase receptor complex in the hypothalamus is required for maintaining normal reproduction in young adult female rats. Endocrinology. 2002;143:1901–1912. doi: 10.1210/endo.143.5.8801. [DOI] [PubMed] [Google Scholar]
- 103.Hou J, Li B, Yang Z, Fager N, Ma M. Altered gene activity of epidermal growth factor receptor (ErbB-1) in the hypothalamus of aging female rat is linked to abnormal estrous cycles. Endocrinology. 2002;143:577–586. doi: 10.1210/endo.143.2.8632. [DOI] [PubMed] [Google Scholar]
- 104.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 105.Rossmanith WG. Gonadotropin secretion during aging in women: Review article. Exp Gerontol. 1995;30:369–381. doi: 10.1016/0531-5565(94)00030-7. [DOI] [PubMed] [Google Scholar]
- 106.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gass M. Highlights from the latest WHI publications and the latest North American Menopause Society position statement on use of menopausal hormone therapy. Cleve Clin J Med. 2008;75(Suppl 4):S13–S16. doi: 10.3949/ccjm.75.suppl_4.s13. [DOI] [PubMed] [Google Scholar]
- 108.Bestetti GE, Reymond MJ, Blanc F, Boujon CE, Furrer B, Rossi GL. Functional and morphological changes in the hypothalamo-pituitary-gonadal axis of aged female rats. Biol Reprod. 1991;45:221–228. doi: 10.1095/biolreprod45.2.221. [DOI] [PubMed] [Google Scholar]
- 109.Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab. 1996;81:3540–3546. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]