Abstract
Panic disorder (PD) is a severe anxiety disorder characterized by susceptibility to induction of panic attacks by subthreshold interoceptive stimuli such as sodium lactate infusions or hypercapnia induction. Here we review a model of panic vulnerability in rats involving chronic inhibition of GABAergic tone in the dorsomedial/ perifornical hypothalamic (DMH/PeF) region that produces enhanced anxiety and freezing responses in fearful situations, as well as a vulnerability to displaying acute panic-like increases in cardioexcitation, respiration activity and “flight” associated behavior following subthreshold interoceptive stimuli that do not elicit panic responses in control rats. This model of panic vulnerability was developed over 15 years ago and has provided an excellent preclinical model with robust face, predictive and construct validity. The model recapitulates many of the phenotypics features of panic attacks associated with human panic disorder (face validity) including greater sensitivity to panicogenic stimuli demonstrated by sudden onset of anxiety and autonomic activation following an administration of a sub-threshold (i.e., do not usually induce panic in healthy subjects) stimulus such as sodium lactate, CO2, or yohimbine. The construct validity is supported by several key findings; DMH/PeF neurons regulate behavioral and autonomic components of a normal adaptive panic response, as well as being implicated in eliciting panic-like responses in humans. Additionally, Patients with PD have deficits in central GABA activity and pharmacological restoration of central GABA activity prevents panic attacks, consistent with this model. The model’s predictive validity is demonstrated by not only showing panic responses to several panic-inducing agents that elicit panic in patients with PD, but also by the positive therapeutic responses to clinically used agents such as alprazolam and antidepressants that attenuate panic attacks in patients. More importantly, this model has been utilized to discover novel drugs such as group II metabotropic glutamate agonists and a new class of translocator protein enhancers of GABA, both of which subsequently showed anti-panic properties in clinical trials. All of these data suggest that this preparation provides a strong preclinical model of some forms of human panic disorders.
Keywords: hypothalamus, perifornical, orexin, GABA, glutamate, panic, anxiety, fear, serotonin, norepinephrine, locus coeruleus, raphe, animal model, l-allylglycine, dorsomedial hypothalamus, DMH, PeF, sodium lactate, hypercapnia, yohimbine, cholecystokinin, CCK, CRF, BNST, amygdala, lateral septum
1. Panic attacks and panic disorder
Anxiety disorders are the most prevalent psychiatric disorders [1], with a life time prevalence of about 20% in the general population [2]. Currently, there is clear evidence that anxiety disorders are a heterogeneous group. Several distinct syndromes have been delineated, based primarily on clinical presentations. One severe anxiety syndrome is panic disorder where recurrent ‘spontaneous’ panic attacks occur that are discrete periods of intense fear or discomfort with at least 4 characteristic symptoms such as tachycardia, hyperventilation or dyspnea, locomotor agitation, etc [1]. Current estimates are that about 7-10% of the population experience occasional panic attacks and about 2-5% of the population have panic disorder (i.e., frequent and/or disabling panic attacks)[3].
Although the cause of panic disorder and associated panic attacks is largely unknown there are predisposing factor that increase the likelihood of the development of panic attacks. The onset of panic attacks usually occurs in late adolescence or early adulthood, and women are twice as likely as men to develop recurrent panic attacks. Sexual maturation in adolescence [see review [4]], and fluccuating sex hormones in women [see review [5]] appear to play a significant role in the vulnerability to panic attacks, but other factors such as early life stress or higher incidence of trauma such as rape in women could also account for this vulnerability. Genetic factors also appear to play a significant role since it has been estimated that 30-40% of monozygotic twins of persons diagnosed with a panic disorder will experience recurrent panic attacks [6, 7].
Normally an adaptive ‘panic’ response is a survival reflex that occurs in response to an imminent threat [8] that can be associated with either external or internal sensory stimuli (exteroceptive- or interoceptive-cues, respectively) [9, 10]. For instance, normal panic is an adaptive response to imminent threats that are exteroceptive (e.g., predator attacks) or interoceptive (e.g., severe hypercapnia that leads to a suffocation sensation). However, in patients with panic disorder, the panic attacks (i.e., aberrant panic responses) often initially occur “spontaneously” in the absence of any obvious external threatening stimuli. Although panic attacks are considered “spontaneous”, they can be consistently triggered in patients with panic disorder by normal interoceptive cues. For instance, patients with panic disorder are hyper-responsive to normal interoceptive cues [11, 12], and are also susceptible to induction of panic attacks by subthreshold interoceptive stimuli such as 0.5 M sodium lactate (NaLac) infusions and 7.5% CO2 inhalations, which are agents that normally do not elicit panic attacks in healthy controls [13-15]. Patients with panic disorder are also susceptible to precipitation of panic attacks by variety of other agents such as yohimbine, cholecystokinin, caffeine etc. [16], all at subthreshold doses that normally do not elicit panic attacks in most healthy controls (i.e., by subthreshold interoceptive cues). Thus, the initial pathology in these patients appears to be an alteration somewhere in the central neural pathways regulating normal panic response, thus rendering them susceptible to ‘spontaneous’ panic attacks [17]. These initial spontaneous attacks can eventually become associated with contextual cues where previous panic attacks have occurred such as bridges, crowded spaces, or situations where rapid exit is difficult [18]. This development of cognitive bias towards threat perception [19] and conditioning of fear responses to panic cues [20] can lead to phobias which are the likely mechanisms underlying recurrent and situational panic attacks in later stages of the illness. Within the anxiety disorder spectrum, recurrent panic attacks are the hallmark of diagnosis for panic disorder, but can also occur in other severe anxiety disorders such as post traumatic stress disorder [21], but not other anxiety disorders such as generalized anxiety and obsessive compulsive disorders [22, 23], or pure depressive disorders [24].
2. Neurochemical and neuroanatomical systems implicated in panic disorder
Currently, the neuroanatomical circuits and associated neurochemicals underlying the initial vulnerability to spontaneous panic attacks in human are poorly understood. An ongoing hypothesis of ours is that an alteration in the central neural pathways regulating normal panic response underlies ‘spontaneous’ panic attacks and the development of panic disorder. If these putative brain areas that are postulated to be regulatory sites for a normal panic response fail to properly function, this could trigger an episodic “alarm” and activate the brain “panic circuit” resulting in a panic attack. Very little is known about such a circuit except that structures such as the periaqueductal grey (PAG), hypothalamus, amygdala and frontal cortex are frequently implicated [25-27]. While much is known about conditioned fear and induction of fear responses by contextual cues, little is known about the mechanisms underlying the vulnerability to the initial ‘spontaneous’ panic attacks, the true etiological basis of panic disorder [28]. A number of neurochemical hypothesis are also proposed for the etiology of panic disorder, primarily based on the therapies that work in treating panic attacks.
For example, both spontaneous and laboratory-induced panic attacks can be blocked by successful treatment of panic disorder with: 1) benzodiazepines like alprazolam [29-31]; 2) tricyclic antidepressants [32] or monoamine oxidase (MAO) inhibitors [33] that target monoaminergic systems in general (i.e., serotonin, norepinephrine, epinephrine, dopamine and histamine); 3) serotonergic (SSRI) or norephinephrine (NRI) reuptake inhibitors [see review [34]]. There is also evidence of reduced inhibitory GABAergic tone in patients with panic disorder. For instance patients with panic disorder have reduced GABAAr binding in the frontal cortex [35], genetic polymorphisms in the GABA synthesizing genes (glutamic acid decarboxylase: GAD) are associated with vulnerability to panic disorder [36] and panic patients demonstrate deficits in central GABA concentrations [37]. This most likely explains why benzodiazepines are so effective at treating panic symptoms [34, 38-40]. Here, we will review both the putative neuroanatomical substrates implicated in regulating a panic response and the potential neurochemical mechanisms engaged in these circuits in the context of this particular animal model.
3. Hypothalamus - one of the putative sites for generating panic-like responses
Since the early studies by Bard and Hess, the hypothalamus is hypothesized to be a critical site for generating a coordinated “defense reaction” i.e., emotional arousal appropriate for a “fight or flight” response [41, 42], In the 1940s Hess and colleagues electrically stimulated the hypothalamus of awake and freely moving cats and discovered that stimulation of some parts of posterior hypothalamic regions evoked strong autonomic and somatic responses resembling adaptive panic/defense reactions (e.g., increased blood pressure, piloerection, arching of back) [43]. Further studies, conducted in rodents, used microelectrodes to stimulate discrete regions of the hypothalamus and found that stimulation of DMH/PeF elicits cardiovascular and behavioral components of the “fight or flight” response including hypertension, tachycardia and hyperventilation [44, 45], decreases in visceral blood flow and increases in hindlimb blood flow (McCabe et al., 1994) and “agitated” running proportional to stimulus intensity [46]. However, a major confound of all of these electrical stimulation studies is that in addition to local cells, fibers of passage (e.g., fornix and nearby mammillothalamic tracts and median forebrain bundle) also travel through these regions. More sophisticated pharmacological studies using discrete microinjections into the hypothalamic nuclei of rats have confirmed that stimulating or disinhibiting the DMH region (with excitatory amino acids or GABAA receptor antagonist bicuculline methiodide, respectively) elicits coordinated emotional arousal [47-49]. escape- [50] and anxiety- [51] associated behavior as well as ‘panic’ associated cardiorespiratory responses (e.g., hypertension, tachycardia [50, 52-55].
The More specifically, blockade of inhibitory GABA neurotransmission in the DMH/PeF elicits escape- [50] and anxiety- [51] associated behavior as well as increases in cardioexcitation (i.e., tachycardia and pressor response), tachypnea and increases in release of stress hormones into the plasma [e.g., catecholamines [56, 57], adrenocorticotrophic hormone (ACTH) and corticosterone [58, 59] [see hypothetical illustration in Figure 1a]. This pattern of autonomic and respiratory responses is similar to responses observed during panic attacks in humans [60]. These responses to site-specific stimulation of adjacent structures such as the lateral hypothalamus (LH) or regions dorsal to the DMH/PeF do not result in any cardiovascular response [61]. This region of the hypothalamus has since been referred to as the hypothalamic “defense area” in light of these findings. Deep brain stimulation of the posterior hypothalamus (that contains the DMH/PeF) of humans also leads to tachycardia and self-reported ‘panic’ [62, 63].
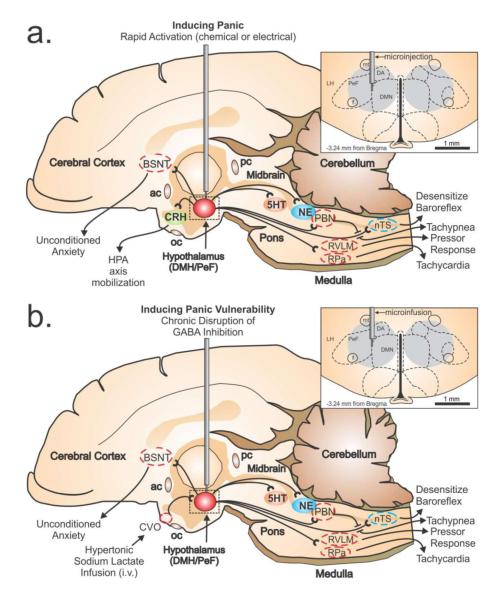
Figure 1.
Illustrates a mid-sagittal image of the rat brain showing the neural circuits that mobilize panic-associated behavior and cardiorespiratory responses following either: a) rapid loss of GABA inhibition with a GABAA receptor antagonist (i.e., bicuculline methiodide), or b) chronic infusion of a GABA synthesis inhibitor (i.e., l-allyglycine) followed by an intravenous sodium lactate infusion. The top right panel in fig. 1a-b illustrates a coronal brain section with subnuclei of the hypothalamus delineated by dashed lines with reference to a standard stereotaxic atlas of the rat brain [208]. The perifornical hypothalamus (PeF) is just dorsal to the fornix (f), and the dorsomedial hypothalamus (DMH) collectively contains the dorsal hypothalamic area (DA), and the dorsomedial hypothalamic nucleus (DMN). The gray circle in the panel indicates the target sites for injections/infusions and estimated diffusion distances [209]. Additional abbreviations: ac, anterior commissure; BNST, bed nucleus of the stria terminalus; LH, lateral hypothalamus; mt, mammillothalamic tract; nTS, nucleus of the solitary tract; pc, posterior commissure; PBN, parabrachial nucleus; RVLM, rostroventrolateral medulla.
4. Panic generating circuit is tonically suppressed at rest
As noted above, tonic GABA release in the DMH/PeF plays an important role in suppressing defensive ‘panic’ responses. This section discusses neural circuits that play an important role in regulating the tonic GABA release in the DMH/PeF. In the 1920s Cannon and Britton found that decorticating cats produced a variety of sympathetic nervous system responses (e.g., increases in blood pressure and plasma concentrations of stress hormones) and defensive/panic behavior (e.g., hissing, arching of back and attempts to bite) to fairly non-threatening stimuli [64]. Bard and colleagues discovered that transections of the cat forebrain produced “sham rage”, but if the transection were caudal to posterior regions of the hypothalamus, then the “sham rage” responses disappeared [65]. This led Bard to propose that the forebrain suppresses emotional responses to inconsequential or trivial stimuli by actions in the posterior hypothalamus, but when the organism is exposed to an imminent threat (e.g., a conspecific male or predator) then tonic inhibition of nuclei within posterior regions of the hypothalamus is removed and this produces a defensive ‘panic’ response. This hypothesis is supported by retrograde tracing in the DMH/PeF leading to robust labeling in the infralimbic region of the medial prefrontal cortex [66-68]. Since most of the projection neurons in the medial prefrontal cortex (mPFC) are glutamatergic it is hypothesized that these neurons project to local GABAergic interneurons in the DMH/PeF to tonically inhibit their activity, which has been determined to be the case for mPFC projections to the amygdala. There are some compelling findings from human studies supporting the hypotheses that panic vulnerability may result from a loss of normal regulatory mechanisms of the limbic structures that elicit a panic response. For example, during anticipatory anxiety, regional cerebral blood flow is reduced in the mPFC [69]. In addition, panic patients have decreased regional cerebral blood flow [70] in the mPFC and metabolism in the mPFC of panic disorder patients is reduced during panic attacks [71]. Furthermore, during an acute laboratory induced panic in human subjects, the first area to be activated is the posterior regions of the hypothalamus with a corresponding reduction in the activation of prefrontal cortex [72]. Supporting a disruption of GABA function in panic disorder are data showing that humans with genetic polymorphisms in the glutamic acid decarboxylase genes are vulnerable to panic disorder [73], GABA enhancers such as benzodiazepine agonists attenuate sodium lactate induced panic-related responses in panic patients [74]. Panic disorder patients demonstrate deficits in cortical GABA concentrations [37]. Furthermore, in a functional in vivo imaging study patients with panic disorder have reduced GABAA receptor binding in the frontocortical brain regions [35].
5. Development of panic-vulnerable rats
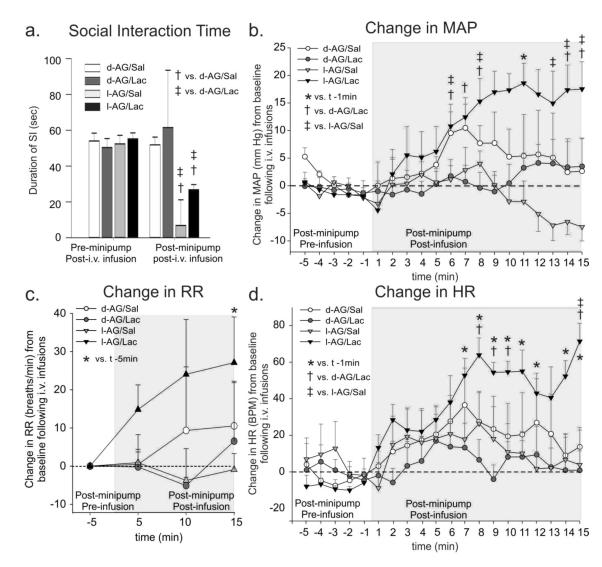
If DMH/PeF is indeed one of the sites regulating a panic-like response, then it could be predicted that rats that have a dysfunction of GABA in this region would show a susceptibility to ‘panic-like’ responses. They would also be predicted to exhibit physiological arousal by sodium lactate (NaLac) infusions similar to patients with panic disorder. To induce GABA dysfunction in the DMH/PeF, a GABA synthesis inhibitor (l-allylglycine:l-AG) is unilaterally infused into the DMH/PeF via an osmotic minipump filled with l-AG. Allylglycine is the precursor to 2-keto-4-pentanoic acid, which is a nonspecific inhibitor of isoforms of glutamic acid decarboxylase (i.e., GAD65 and GAD67). In order to inhibit the synthesis of GABA, allylglycine must first be transformed into its active form, 2-keto-4-pentenoic acid by amino acid oxidase enzymes [75-77]. For example, In vivo systemic administration of l-AG into rats reduces levels of GABA throughout the brain [78], but in vitro l-AG does not affect GAD activity [77, 79]. Although the amino acid oxidases are expressed throughout the brain, there are two isoforms that are selective in their substrates, such that one of them only oxidizes the l-form of an amino acid while the second is selective to the d-form the amino acids. In the forebrain and diencephalon, only the L-form selective amino acid oxidase is predominant. Thus, only l-AG significantly blocks the synthesis of GABA in the hypothalamus and d-AG serves as an inactive pharmacological control [80, 81]. Previous studies have determined that the dose of l-AG utilized here is subthreshold to the dose that acutely reduces local GABA concentrations [based on microdialysis study [52] but chronically reduces GABA levels to by approximately 60% following unilateral infusions as determined by enzyme assays [50, 82-84] and supported by immunohistochemistry [85]. In most cases, the l-AG infusion leads to increases anxiety-like behavior [i.e., as measured by the social interaction (SI) test and elevated plus-maze (EPM)] without altering baseline cardiorespiratory activity [50, 82-84]. However, following exposure to subthreshold cues such as 0.5 M sodium lactate [68, 82-88] or subthreshold doses of yohimbine [82] that are known to provoke panic attacks in humans suffering from panic disorder, the rats have increased anxiety-like behavior (greater than already present from l-AG infusion) and marked and rapid increases in cardioexcitation (evidenced by either tachycardia and/or a pressor response), tachypnea and an increase in general locomotor activity [see Figure 2 reproduced with permission from [68]].
Figure 2.
The effect of GABA synthesis inhibition in the DMH/PeF region with l-allylglycine (l-AG: or inactive isomer d-AG) and i.v. sodium lactate (or isotonic saline control) infusions on anxiety-like behavioral and cardiorespiratory responses. a) Social interaction (SI) time pre and post l-AG (GABA synthesis inhibitor)- or d-AG (control isomer) treatment in rats also receiving i.v. infusions of saline (Sal) or lactate (Lac). Change in b) mean arterial blood pressure (MAP), c) respiration rate (RR), d) and heart rate (HR) from 5 min baseline post-i.v. infusion. Bars or lines represent the mean + S.E.M. Figure 4-c adapted by permission from [68].
This animal model of panic disorder has now been established for over 15 years and has robust face, predictive, and construct validity. As noted above, the model recapitulates many of the phenotypics features of human panic disorder. The model’s predictive validity is demonstrated by responses, similar to those observed in subjects with panic disorder, to both panic-inducing agents (e.g. sodium lactate, yohimbine, and inhalations of CO2) and anti-panic effects of therapeutic agents such as alprazolam [86, 89] and group II metabotropic glutamate agonists [88]. Further predictive validity comes from a study that recently used this hypothalamic animal model and determined that a novel GABA enhancer drug showed anti-panic properties preclinically and in later clinical trials [90]. The construct validity of this model is supported by the fact that neural circuits of the DMH/PeF regulate behavioral and autonomic components of an adaptive panic/defense response in rats [91], and are implicated in eliciting panic-like responses in humans [92] and animals [93]. Furthermore, panic disorder subjects have reported deficits in central GABA activity [37] and pharmacological restoration of central GABA activity prevents panic attacks [67], which is consistent with GABA reductions in key brain areas resulting in panic vulnerability as demonstrated with this animal model.
6. Afferent pathways for the classic panic-inducing interoceptive stimuli
6. 1 Sodium lactate sensory pathways
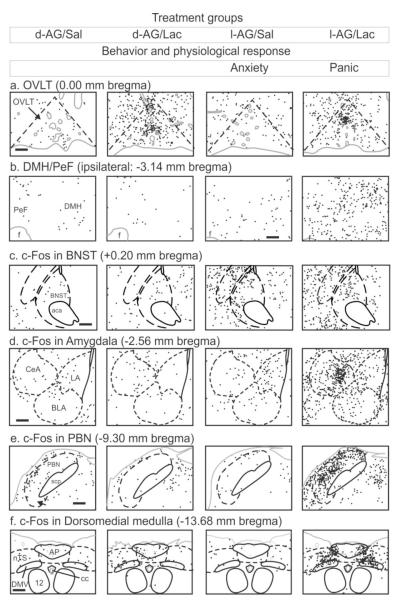
The DMH/PeF has extensive projections to and from several circumventricular organs (CVOs)[94, 95], which are areas in the CNS that lack a blood-brain barrier and express “sensors” to detect changes in osmolarity, sodium concentrations and other plasma parameters [96]. These anatomical connections suggest a potential mechanism by which information about many peripheral plasma parameters can be directly relayed to the DMH/PeF. Among the three major CVOs that are connected to the DMH/PeF, the region of the antero 3rd ventricle [A3Vr; containing the organum vasculosum lamina terminalis (OVLT), the median preoptic nucleus (MnPO), and anteroventral periventricular nucleus (AVP)] has particularly dense connections with the panic-generating region of the DMH/PeF [87]. The A3Vr cells are also highly responsive to intravenous infusions of sodium lactate at doses used to provoke panic in the panic model [68]. We have previously mapped out functional cellular responses in brain regions implicated in the sensing of sodium lactate (i.e., A3Vr) and in the regulation of components of the panic response in the panic model [see Figure 3 reproduced with permission from [68]]. The importance of the A3Vr in sensing changes in plasma sodium lactate in the panic model was confirmed when injecting tetrodotoxin into the A3Vr but not in another CVO (i.e., subfornical organ) blocked sodium lactate induced panic responses in the panic model [97]. Furthermore, directly injecting a small amount (100 nl) of NaLac into the A3Vr of panic vulnerable rats elicited panic-associated cardiorespiratory and behavioral responses [83].
Figure 3.
Drawings illustrating cellular responses [i.e. numbers of c-Fos-immunoreactive (ir) cells with each black dot = 1 c-Fos-ir cell] from each of four treatment groups from figure 2 [columns respectively represent d-allylglycine (d-AG) inactive isomer into DMH/PeF+ i.v. isotonic saline infusion (Sal), d-AG+i.v. sodium lactate infusion (Lac), GABA synthesis inhibitor l-allyglycine (l-AG) into DMH/PeF+Lac, and l-AG+Lac] in key subregions involved in: a) sensing plasma perameters such as sodium lactate (i.e., organum vasculosum lamina terminalis, OVLT); b) panic generating site (i.e., DMH/PeF); c) unconditioned anxiety (i.e., bed nucleus of the stria terminalis, BNST); d) conditioned fear and sympathoexcitation (i.e., central, CeA; basolateral, BLA; and lateral, LA; amygdala); e) respiratory regulation (i.e., parabrachial nucleus, PBN); f) baroreceptor desensitization (i.e., nucleus of the solitary tract, nTS; dorsal motor nucleus of the vagus, DMV). Gray solid lines indicate the outline of the brain section and blood vessels. Black solid lines indicate white matter tracts or cranial nerves. Black dashed lines indicate the brain nuclei. A standard rat stereotaxic atlas of the brain was used to determine, bregma, and delineated areas [210]Columns 3-4 has increased anxiety, and column 4 also had cardiorespiratory responses (see Figure 2 for details). Figure 3a-f adapted by permission from [68]. See also [68] article for additional information.
Previous clinical studies conducted on panic disorder patients suggest that increased sodium, not lactate or osmotic stress, may be the critical change for provoking panic attacks. For instance, i.v. infusions of hypertonic (0.5M) NaLac, sodium chloride (NaCl) [98] or sodium bicarbonate [99] provoke equivalent panic associated responses in panic disorder patients. Furthermore, panic disorder patients are significantly more likely to have a panic attack following i.v. infusions of hypertonic NaCl rather than lactate dissolved in dextrose [100]. Consistent with this, we recently determined that sodium was the specific sensory component of sodium lactate challenge that is critical for provoking panic in our panic model [87]. Utilizing whole-cell patch clamp preparations, we also showed that bath applications of NaLac (positive control), but not lactic acid (lactate stimulus) or D-mannitol (osmolar stimulus) increased firing rates of DMH/PeF projecting neurons in the A3Vr that were retrogradely labeled from the DMH/PeF. These neurons are also most likely glutamatergic cells [87]. The cellular mechanism by which neurons in the A3Vr detect changes in sodium concentrations may be through specialized sodium X channel (NaX) that are expressed in the A3Vr and have been shown to be critical for drinking behavior associated with salt intake [101, 102]. Recently, Grob and colleagues have shown that neurons in the MnPO region express NaX channels that are excited by local application of hypertonic NaCl, but not changes in osmolarity [103] which fits the phenotype of the neurons that project to the DMH/PeF. Overall, these data are consistent with the hypothesis that Na+ is exciting glutamatergic neurons in the A3Vr through Nax receptors that project to the DMH/PeF region to elicit panic-responses in panic-prone rats.
6.2 Carbon dioxide sensory pathways
Mild elevations of CO2 in the blood (i.e., hypercapnia) can occur from hypoventilation. Mild changes in hypercapnia initially lead to an increase in respiration activity to help “blow off” excess CO2 without much conscious awareness of the change [see review [104]]. However, if CO2 levels continue to increase there is a sense of “suffocation” and additional physiologic responses are initiated, including adaptive behavioral, autonomic, and neuroendocrine responses. For instance, exposing rats to mild hypercarbic gas [e.g., 7% CO2 [105]] increases respiration rate that reduces hypercapnia without mobilizing other components of a “panic-like” response. However, exposing rats to higher concentrations of hypercarbic gas (e.g., ≤10% CO2) elicits additional components of a panic-associated responses as evidenced by increases in sympathetic activity [106], blood pressure [107], anxiety-like behaviors [108, 109] and mobilization of the hypothalamic-pituitary-adrenal (HPA) axis [110-112]. Mild changes in hypercapnia from exposure to air containing 5-7% CO2 that increases respiratory activity in healthy humans provokes panic attacks in the majority of patients with panic disorder [113-115]], which suggests they are hyper-responsive to CO2. This hyper-reactivity to hypercapnia could be a relevant trigger of panic attacks in environmental settings where ambient CO2 levels are increasing and where panic attacks are known to occur such as people with asthma or obstructive lung diseases, and even physically healthy panic patients trapped in closed spaces such as an elevator with groups of people exhaling high levels of end tidal CO2 (approximately 5-6%, compared to atmospheric concentrations of CO2 that are less that 0.05%).
Overall, this hyper-sensitivity to hypercapnia has led to a hypothesis that a respiratory center abnormality in the brain leads to false suffocation alarms [116]. CO2 readily crosses the blood-brain barrier to directly interact with specialized central chemoreceptive neurons [117, 118] that are critical for regulating breathing in response to mild hypercapnia [104]. Orexin producing neurons found only in the dorsomedial/perifornical (DMH/PeF) and adjacent lateral hypothalamus (LH) [119] also display CO2/H+-sensitive properties, but with lesser chemosensitivity [120], suggesting that they may respond to only panic threshold hypercapnia. As mentioned previously, a loss of inhibitory GABAergic tone in the DMH/PeF leads to a hyperactive ORX system in our panic model [86], which could lead to hyperactive responses to mild hypercania. Further evidence of orexin involvement is that prepro-ORX knockout mice have blunted respiratory responses to 5-10% hypercarbic gas exposure, and injecting wild type mice with an ORX1 receptor antagonist attenuates hypercapnic-induced respiratory responses [121]. In a recent publication, we have also determined that pretreating rats with an ORX1 receptor antagonist attenuates panic-associated anxiety behavior and pressor responses following a panic threshold exposure to hypercapnic gas (20%CO2)[122]. Finally, humans with episodes of severe hypercapnia (such as patients with COPD, bronchitis or asthma) have significant comorbidity with severe anxiety and sympathetic arousal, both of which can make management of these patients difficult. In a recent preclinical study, COPD was modeled in rats by exposing them to chronic cigarette smoke [123] which induced COPD-associated lung pathology (i.e., coalesced alveoli and thickened bronchiolar walls). Ex vivo analyses revealed that there was a 100% increase in hypothalamic ORXA protein expression; and heightened medullary responses phrenic nerve responses to ORXA. In a recent clinical study, plasma orexin A levels [orexin A crosses the blood brain barrier with ease [124]] were over 100% higher in COPD patients with hypercapnic respiratory failure compared to control subjects [125]. Overall, orexin neurons in the DMH/PeF could be a critical substrate in the hyper-reactivity to hypercapnia seen in in patients with panic disorder, but also in severe hypercapnic episodes see in conditions such as COPD.
7. Efferent DMH/PeF pathway for induced panic
We have extensively characterized the afferent pathways that activate the DMH/PeF region following sodium lactate infusions [83, 87], some of the mechanisms within the DMH/PeF that regulate the panic-like response [82, 85, 88, 89, 126], as well as identifying the efferent pathways from the DMH/PeF implicated in the panic-like response using c-Fos immunohistochemistry [see Figure 3 reproduced with permission from [68]].
The DMH/PeF region has extensive efferent projections to many sites that are critical for behavioral and physiological responses associated with stress, anxiety and fear. The putative efferent pathways utilized for generating the different components of the panic responses following sodium lactate infusions are hypothesized to be the following structures: 1) Paraventricular nucleus (PVN) for stress hormone release and sympathetic activation [58, 127-129]; 2) BNST [130-132] for anxiety-like responses; 3) Lateral septum (LS) [131] which may be critical for anxiety and enhancement of aversively conditioned responses by DMH/PeF activation [133]; 4) DPAG for locomotor and flight responses [131, 134]; 5) The NE systems of the locus ceruleus (LC) and 5-HT systems of the dorsal raphe nucleus (DRN) to regulate arousal; and 6) Brainstem regions such as the lateral parabracial nucleus [LPBN: [135, 136]] for regulating respiratory responses, 7) rostroventrolateral medulla for pressor responses [RVLM: [137]], 8) raphe pallidus for tachycardia [RPa: [138]] and also 9) sympathetic mobilization through regions of the spinal cord [139] for mobilizing sympathetic activity and 8) nucleus of the solitary tract (nTS) for desensitizing parasympathetic autonomic responses [see review [140]]. In addition, there are poorly understood pathways that we have not listed here. For example, nausea is a common symptom associated with acute panic attacks and the robust activation of area postrema [see Figure 3 and [68]] and the emetic pathways may be involved in generating the symptoms of nausea during a panic attack. There are also many redundant pathways to elicit many of these responses within this panic circuit. For example, ‘anxiety’ responses can be elicited from the BNST, amygdala, hippocampus or lateral septum. Similarly, sympathetic mobilization can be induced via several brain stem centers. However, in this animal model of panic a unique subset of DMH/PeF efferent pathways appears to be activated during the panic-like response induced by panic inducing stimuli such as sodium lactate [68].
7.1 Anxiety and fear mobilization during panic
Following sodium lactate infusions, several limbic structures (i.e., the BNST, LSV and several subnuclei of the amygdala) are selectively activated during panic-like responses [68]. Although other studies have demonstrated that the BNST regulates autonomic activity [141], the BNST’s role may depend on the context since we previously determined that pharmacological inactivation (GABAA receptor agonist) of the rostro-caudal BNST region blocked the anxiety-associated behavior in the panic model but did not alter sodium lactate provoked cardiorespiratory activation, providing strong support for the hypothesis that the BNST selectively regulates anxiety behavior, but not panic-like cardiorespiratory responses in the panic model presented here [68]. Consistent with this finding, chronic disinhibition of the BNST with l-allyglycine or chronic excitation with CRF agonist urocortin 1 produced rats with high anxiety , but unlike the rats with l-AG in the DMH/PeF region, these animals were not vulnerable panic-like responses following sodium lactate infusions [142].
Although fear and phobia development has not been fully studied yet in this panic model, robust cellular responses occur in the central amygdala (CeA) in rats that display panic-associated cardiorespiratory responses following sodium lactate [68]. This strong CeA response could indicate the onset of conditioned fear memories [see review [143]] that and the formation of secondary phobia following initial panic attacks in panic disorder patients [144, 145]. We do however see that there is significant contextual fear that develops in the panic prone animals in the defensive burying test (Shekhar et al., unpublished results), suggesting that they are more susceptible to induction of conditioned fears and show delayed extinction.
7.2 Sympathetic mobilization during panic
Identification of the efferent pathways from the DMH/PeF implicated in the sympathetic mobilization were mapped using c-Fos expression in l-AG and d-AG infused into the DMH/PeF region and then challenged with either normal saline or hypertonic sodium lactate infusions (Johnson, 2008). We identified activation of multiple autonomic regulatory regions including the dorsal cap of the PVN (i.e. PaDC), the RVLM (spefically C1 adrenergic neurons) and the RPa. Some of the other autonomic regulatory sites responded to sodium lactate infusion (i.e. RPa, LC noradrenergic and C1 and C2 adrenergic neurons), while yet other brainstem regions associated with sympathetic mobilization failed to respond to sodium lactate or l-AG (e.g., subdivisions of the PAG, etc.). There are several putative ways the DMH could potentially induce cardioexcitatory responses following sodium lactate infusions in l-AG-treated rats (see Fig. 1b, 2 for a hypothetical model for DMH-mediated cardioexcitatory responses). The DMH/PeF could drive hypertensive responses at efferent targets such as the PaDC, SON, RVLM or sympathetic regions of the spinal cord. The DMH/PeF directly innervates the PaDC, SON [95] and also sympathetic regions of the spinal cord [139]. Neurons in the PaDC also directly innervate sympathetic regions of the spinal cord [146]. Yet another explanation is that C1 adrenergic neurons were firing at a higher rate in sodium lactate+l-AG-treated rats, relative to sodium lactate+d-AG-treated rats, even though sodium lactate induced a similar level of c-Fos expression in C1 adrenergic neurons in the RVLM similarly in both d-AG and l-AG treated rats challenged with lactate. Consistent with a role for the RVLM/C1 adrenergic cell groups in DMH/PeF-mediated cardiovascular responses, pressor responses, elicited from disinhibition of the DMH, can be severely attenuated by microinjecting muscimol into the RVLM [137]. Tachycardia responses following l-AG+sodium lactate infusions are most likely being mobilized by a combination of direct and indirect projections from the DMH/PeF to sympathetic preganglionic neurons.
7.3 Parasympathetic coordination during panic
In order for the sympathetic limb of the autonomic nervous system to simultaneously induce hypertension and tachycardia, the baroreflex mechanism which stimulates vagal parasympathetic output and inhibits the sympathetic output needs to be desensitized [see review [140]]. The nTS region is critical for the baroreflex [147] and contains parasympathetic motor neurons. The DMH/PeF directly innervates the nTS [137], and electrical or chemical [see review [140]] stimulation of the DMH/PeF decreases the sensitivity of the baroreflex through a GABAergic mechanism in the nTS [148]. This circuit represents an adaptive means of inhibiting the baroreflex during “fight or flight” responses and exercise [149]. The nTS also contains numerous GABAergic neurons [150] which could be dampening parasympathetic activity by inhibiting acetylcholinergic vagal preganglionic neurons. This notion is supported by recent evidence that stimulation of nTS neurons in vitro inhibits DMV neurons [151, 152].
The finding that panic-related conditions exhibit parasympathetic and sympathetic imbalance, and baroreflex disruption is not surprising [see review [153]]. Panic disorder patients have higher baseline HR than controls [154, 155] and electrocardiograms in panic disorder patients reveals sympathetic dominance and parasympathetic withdrawal [156, 157]. Patients with panic disorder also show disrupted baroreceptor function that persists even when they are in remission [158].
8. Neurochemical mechanisms within the DMH/PeF that regulate panic as putative therapeutic targets
8.1 Glutamate receptor activation is necessary for panic response generation
The current evidence suggests that there is a balance between tonic GABAergic inhibition and glutamate mediated excitation within the DMH/PeF that makes rats vulnerable to NaLac-induced panic responses [85, 88]. Under basal conditions, the activity of DMH/PeF neurons is regulated by a tonic GABAergic inhibition of excitatory glutamatergic drive [159]. However, when the GABAergic inhibitory tone is reduced with l-AG infusions, the glutamate-mediated excitation appears to become prominent, leading to chronic activation and a panic-prone state [85]. In light of this, there is increasing recognition that glutamatergic mechanisms may be a potential avenue to develop new treatment for anxiety problems such as panic disorder [160].
Group II metabotropic glutamate receptors (mGluR 2 and 3) are expressed on presynaptic terminals of glutamatergic neurons [161] which reduce synaptic release of glutamate when glutamate binds to them [see review [162]], thus negatively modulating presynaptic glutamate neurotransmission. This suggests that mGluR 2/3 potentiators could potentially reduce excessive synaptic glutamate release in panic-prone rats to reduce panic vulnerability without altering basal glutamate release [163]. Using our panic model, we determined that pretreating panic-vulnerable rats with either a specific mGLUR2/3 allosteric agonist [88] or a more selective mGluR2 agonist (i.e., CBiPES or THIIC) (unpublished data) attenuates panic responses (see Figure 4]. This is consistent with a previous data showing that mGluR2/3 allosteric agonists reduce anxiety-associated behaviors in rodents [88, 164-166] and also has anxiolytic and antipanic properties in humans [167, 168] without benzodiazepine associated side effects such as sedation [168].
Figure 4.
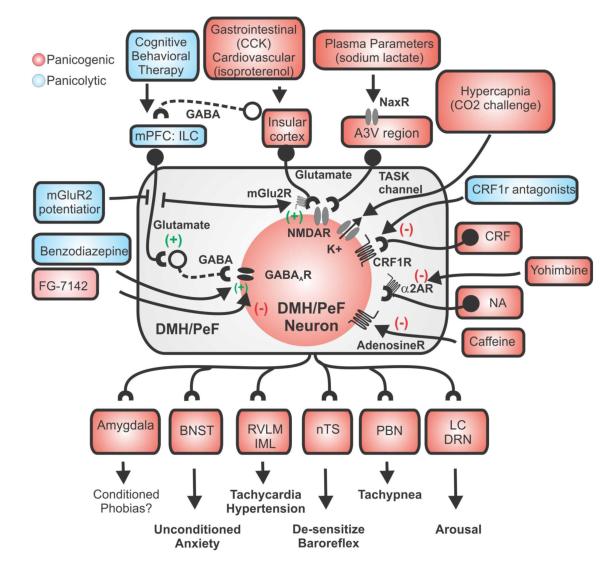
Hypothetical schema of the sensory and neurochemical mechanisms by which panicogenic stimuli triggers panic-like responses in the hypothalamic panic model. Sources of GABA in the DMH/PeF that are inhibited by l-allyglycine include local interneurons, which are tonically driven by glutamatergic input from medial prefrontal cortex (mPFC) [41, 65]. Elevated plasma levels of Na+ from sodium lactate infusion are “sensed” by specialized NaX ion channels [101, 102] that are highly expressed in the anterior 3rd ventricle region (A3Vr) [103]. The AV3r contains many DMH/PeF projecting glutamatergic (Glu) neurons [87] which (following a hypertonic Na+ Challenge) excites the DMH/PeF region to mobilize panic-like responses. This figure also illustrates how other panic provoking stimuli such as hypercapnia [via CO2 sensitive K+ channels (TASK) [120]], cortocotrophin releasing factor (CRF) [89], isoproterenol, [211], cholecystokinin (CCK)[212], and yohimbine, caffeine and FG-7142 [213]] increase neuronal responses in the DMH/PeF. Afferent targets of the DMH/PeF which are implicated in the regulation of panic-like responses are also listed [see [68] CNS responses in panic model and also (Chamberlin and Saper, 1994;Chen et al., 2004;Fontes et al., 2001;Thompson and Swanson, 1998)]. GABAergic and glutamatergic neurons are represented by circles attached to dashed or solid lines, respectively. Abbreviations: BNST, bed nucleus of the stria terminalis; CBT, cognitive behavioral therapy; IML, intermediolateral cell column of spinal cord; LC, locus coeruleus; nTS, nucleus of solitary tract; PBN, parabrachial nucleus; RVLM, rostroventrolateral medulla.
8.2 Benzodiazepines agonists and inverse agonists modulate DMH/PeF activity and panic responses
As shown previously, acute blockage of tonic GABAergic inhibion in the DMH/PeF leads to panic responses, whereas a subtler chronic inhibition of GABA tone leads to panic vulnerability in our model. We have also shown that restoring GABAergic tone in panic vulnerable rats just prior to a sodium lactate challenge blocks panic responses [68]. The concept of loss of GABAergic tone resulting in a panic-prone state implied in the preclinical animal model is also implicated in humans with panic disorder where reduced GABA levels are seen in the brain [35, 37]. Also, supporting disruption of GABA function in panic disorder are data showing that genetic polymorphisms in the GAD genes are associated with vulnerability to panic disorder [36].
This most likely may also explains why GABA enhancers such as benzodiazepines are so effective in rapidly treating panic symptoms [74]. Benzodiazepines such as alprazolam also have rapid anti-panic properties in the DMH/PeF model of panic vulnerability [86, 88], which could be partially attributed to restoring GABAergic tone in the DMH/PeF (see Figure 4].
8.3 The NE arousal system input into the DMH/PeF is a critical component of panic responses
Activation of norepinephrine (NE) locus ceruleus (LC) neurons is postulated to mobilize anxiety and panic responses in humans [see recent review [169]]. There is also evidence that the NE system is hyperactive in patients with panic disorder [170-172]. For instance, patients with panic disorder (compared to controls) have exacerbated anxiety and cardiovascular responses following pharmacological enhancement of NE release with an oral 20mg dose of yohimbine (an alpha 2 adrenoreceptor antagonist). Yohimbine (intravenous) also provokes panic attacks in 70% (40% experienced flashbacks) of combat veterans with post-traumatic stress disorder (PTSD), while having minimal effects on control subjects [173].
The DMH/PeF may be an important relay site for LC induced panic responses. The DMH/PeF is densely innervated with noradrenergic terminals which most likely largely represent LC input [see [174]]. Both stress-related stimuli and panicogenic drugs such as beta carbolines (e.g., FG-7142) or yohimbine cause increases in NE release in the DMH/PeF [see review [175]] and fear-potentiated startle tests in rats also leads to increases in NE levels in the DMH [176]. Futhermore, acute disinhibition of the DMH/PeF with bicuculline methiodide (BMI, a GABAA receptor antagonist) causes panic responses that coincide with an increase in local extracellular NE levels [126]. This NE release in the DMH/PeF following GABAA receptor inhibition appears to play an important role in DMH/PeF mediated panic responses, since lesioning NE terminals in the DMH/PeF (using 6-hydroxydopamine neurotoxin) results in a significant blunting of the panic response to local disinhibition with BMI [126]. Furthermore, panic associated autonomic responses elicited by LC stimulation are attenuated in rats with lesions of the hypothalamus that contain the DMH/PeF [177]. Finally, yohimbine hypersensitivity is also present in the hypothalamic panic model, where panic prone rats, but not controls, display panic associated behavior and cardiovascular responses following intravenous yohimbine challenges [82] and noradrenergic systems such as the LC are highly sensitive to interoceptive stimuli such as NaLac in the hypothalamic panic model [68].
8.4 CRF inputs to the DMH/PeF may be an important signal in the panic response
In addition to endocrine functions [178], the neuropeptide corticotropin releasing factor (CRF) is synthesized (and released) in other extra-hypothalamic brain regions [179, 180]], where it acts as a neurotransmitter/neuromodulator to coordinate behavioral and autonomic responses to stress. Central injections of CRF mobilizes “panic/defense” responses in rodents [181, 182], and clinical evidence suggests that polymorphisms in CRF1 receptor gene may be associated with panic vulnerability [183]. Overall this suggests that the CRF system could be an important therapeutic target for panic disorder [184]. We recently determined that systemically pretreating panic vulnerable rats with a centrally active highly specific and selective CRF1 receptor antagonist attenuated sodium lactate induced panic responses [[89] see Figure 4]. Our finding is consistent with other reports where this same CRF1 receptor antagonist had anxiolytic-like effects in rodents [185-188], which suggests that the CRF1 receptor may be a novel target for the treatment of acute panic and/or anxiety symptoms. However in a recent clinical trial, patients with generalized anxiety disorder did not show improvement of primary outcome measures following treatment with the CRF1 antagonist pexacerfont, compared to placebo group [189]. There are significant differences in symptoms and underlying pathological causes between panic disorder and GAD [1] and CRF1 receptor antagonists may be efficacious in the treatment of panic disorder symptoms. For instance, profound cardioexcitatory responses occur during panic attacks which are more specific for panic disorder compared to other anxiety disorders such as GAD, and in our preclinical study the highest dose of the CRF1 receptor antagonist was as effective as a benzodiazepine in blocking panic-associated cardioexcitatory responses [89].
9. The phenotype of neurons in the DMH/PeF that mobilize panic
9.1 Orexin synthesizing neurons
An important question is what neurons are impacted by removal of local GABA inhibition in the DMH/PeF region that makes rats display increases in anxiety-like behavior and panic-like physiology. An enticing candidate is a group of ORX producing neurons found only in the DMH/PeF region and adjacent lateral hypothalamus (LH)] [119, 190] These ORX producing neurons are critical for maintaining wakefulness and vigilance [191], contain GABAA receptor subunits [192] are inhibited by GABAA receptor agonist muscimol [193]] and excited by bicuculline methiodide [194]]. Regions of the brain which are critical for anxiety-related (unconditioned) stress responses such as the BNST [143, 195, 196] contain extensive orexinergic fibers [119] and ORX1 (ORX1R) [197] and 2 receptors (ORX2R)[198]. Central ORX release also appears to mobilize centrally mediated sympathetic responses. Intracerebroventricular (icv) injections of ORX produces tachycardia, hypertension and increases in renal sympathetic activity and plasma concentrations of NE and epinephrine [199, 200]. Furthermore, mice lacking prepro ORX have attenuated cardioexcitatory responses following disinhibition of the DMH/PeF [201], and specific lesions of ORX neurons (ulilizing a ORX-saporin technique) reduces conditioned fear-induced tachycardia and hypertention [202]. Other line of evidence comes from studies assessing the role of ORX in parasympathetic regions such as the RVLM, and NTS/DMV complex that are innervated with ORX containing fibers and express ORX 1 and 2 receptors [190]. The pressor responses elicited from disinhibition of the DMH/PeF appears to be mediated via the RVLM [137]. Injecting ORX into the RVLM elicits tachycardia [203, 204] and hypertension [203-205]. Furthermore, ORX dose-dependently increases firing rates of RVLM neurons in vitro [206] and ORX containing fibers are in close apposition to RVLM neurons expressing the ORX1R [204] and tyrosine hydroxylase (i.e. C1 adrenergic neurons)[205]. The DMH also directly innervates the nTS [137], and electrical [207] or chemical [140] stimulation of the DMH/PeF lowers the sensitivity of the baroreflex presumably by regulating activity in the nTS region. Recent studies have shown that ORX may also modulate the baroreflex through actions in the nTS complex. ORX perfusions onto nTS slices excites and inhibits the majority of nTS neurons [190], respectively. Furthermore, ORX can enhance inhibitory input to the dorsal motor nucleus of the vagus arising from the nTS and elicit tachycardia and pressor responses [190].
9.2 Angiotensin synthesizing neurons
We previously demonstrated that injecting an angiotensin II (A-II) type 1 receptor (AT1r) antagonist losartan or the nonspecific A-II receptor antagonist saralasin into the DMH/PeF of “panic-prone” rats prior to sodium lactate challenge blocked the entire panic responses [82]. In addition, direct injections of A-II into the DMH/PeF of these “panic-prone” rats also elicited panic-like responses, which were blocked by pretreatment with saralasin. Microinjections of saralasin into the DMH did not block the panic-like responses elicited by i.v. infusions of the noradrenergic agent yohimbine or by direct injections of NMDA into the DMH/PeF, suggesting that angiotensin release in the DMH/PeF may be associated with the initial sodium lactate signal to the DMH/PeF.
10. Conclusions
Acute activation of DMH/PeF leads to panic-like behavior and increased cardiorespiratory responses in rats. After chronically inhibiting GABA synthesis in the PeF/DMH of rats with l-AG, a GABA synthesis inhibitor, sodium lactate challenges produce anxiety as measured by social interaction, elevated plus maze, open field test and freezing in defensive probe burying test, as well as acute panic-like responses such as increased “flight”-like locomotion, increased respiration, increased heart rate, and increased mean arterial pressure responses following intravenous sodium lactate infusions. This animal model of panic vulnerability (see Figures 2 and 4] has provided an excellent preclinical system with robust face, predictive and construct validity. The model recapitulates several of the key phenotypic characteristics of PD (face validity), including greater sensitivity to panicogenic stimuli demonstrated by sudden onset of anxiety and autonomic activation following a administration of a sub-threshold (i.e., do not usually induce panic in healthy subjects) stimulus such as sodium lactate, CO2, or yohimbine. The construct validity is supported by several key findings; DMH/PeF neurons regulate behavioral and autonomic components of the “fight or flight” response, as well as being implicated in eliciting panic-like responses in humans. Additionally, Patients with PD have deficits in central GABA activity and pharmacological restoration of central GABA activity prevents panic attacks, consistent with this model. The model’s predictive validity is demonstrated by not only showing panic responses to several panic-inducing agents that elicit panic in patients with PD, but also by the positive therapeutic responses to clinically used agents such as alprazolam and antidepressants that attenuate panic attacks in patients. More importantly, discovery of novel drugs to treat panic have been identified by this model such as group II metabotropic glutamate agonists and a new class of translocator protein enhancers of GABA, both of which subsequently showed anti-panic properties in clinical trials [90]. All of these data suggest that this preparation provides a strong preclinical model of some forms of human panic disorders.
Highlights.
This article reviews a rat hypothalamic model of panic disorder
The face, predictive and construct validity of the panic disorder model
The role of the dorsomedial/perifornical hypothalamus in adaptive and pathological panic
Neurochemical systems implicated in adaptive and pathological panic
How existing and novel panicolytic drugs may treat panic disorder symptoms
Collectively, the sections mentioned previously led to the hypothesis that chronic disruption of GABA inhibition in the DMH/PeF that is below the threshold of inducing panic could model a inhibition of the DMH/PeF, which could produce rats that are vulnerable to displaying panic-like behavioral and physiological responses to ordinarily mild interceptive stimuli.
Acknowledgements
This work was supported by Indiana CTSI Project Development Team Pilot Grant (RR025761to PLJ), NIH Student LRP and NARSAD Young Investigator Award to PLJ; R01 MH52619 and MH065702 to AS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].DSM-IV . Diagnostic and Statistical Manual. Fourth Edn. (DSM - IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- [2].Weissman MM, Merikangas KR. The epidemiology of anxiety and panic disorders: an update. J.Clin.Psychiatry. 1986;47(Suppl):11–7. [PubMed] [Google Scholar]
- [3].Goodwin RD, Faravelli C, Rosi S, Cosci F, Truglia E, de Graaf R, et al. The epidemiology of panic disorder and agoraphobia in Europe. Eur Neuropsychopharmacol. 2005;15:435–43. doi: 10.1016/j.euroneuro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [4].Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Current topics in behavioral neurosciences. 2010;2:21–35. [PubMed] [Google Scholar]
- [5].Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clinical psychology review. 2011;31:1183–91. doi: 10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crowe RR. The genetics of panic disorder and agoraphobia. Psychiatr.Dev. 1985;3:171–85. [PubMed] [Google Scholar]
- [7].Sheikh SN, Martin SB, Martin DL. Regional distribution and relative amounts of glutamate decarboxylase isoforms in rat and mouse brain. Neurochem.Int. 1999;35:73–80. doi: 10.1016/s0197-0186(99)00063-7. [DOI] [PubMed] [Google Scholar]
- [8].Stein DJ, Bouwer C. A neuro-evolutionary approach to the anxiety disorders. J.Anxiety.Disord. 1997;11:409–29. doi: 10.1016/s0887-6185(97)00019-4. [DOI] [PubMed] [Google Scholar]
- [9].Ehlers A, Breuer P. How good are patients with panic disorder at perceiving their heartbeats? Biol Psychol. 1996;42:165–82. doi: 10.1016/0301-0511(95)05153-8. [DOI] [PubMed] [Google Scholar]
- [10].Street LL, Craske MG, Barlow DH. Sensations, cognitions and the perception of cues associated with expected and unexpected panic attacks. Behav Res Ther. 1989;27:189–98. doi: 10.1016/0005-7967(89)90078-8. [DOI] [PubMed] [Google Scholar]
- [11].Hoehn-Saric R, McLeod DR, Funderburk F, Kowalski P. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Arch Gen Psychiatry. 2004;61:913–21. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- [12].Pollock RA, Carter AS, Amir N, Marks LE. Anxiety sensitivity and auditory perception of heartbeat. Behav Res Ther. 2006 doi: 10.1016/j.brat.2005.12.013. [DOI] [PubMed] [Google Scholar]
- [13].Gorman JM, Papp LA, Coplan JD, Martinez JM, Lennon S, Goetz RR, et al. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am.J.Psychiatry. 1994;151:547–53. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- [14].Keck ME, Strohle A. Challenge studies in anxiety disorders. Handb Exp Pharmacol. 2005:449–68. doi: 10.1007/3-540-28082-0_16. [DOI] [PubMed] [Google Scholar]
- [15].Liebowitz MR, Gorman J, Fyer A, Levitt M, Levy G, Dillon D, et al. Biological accompaniments of lactate-induced panic. Psychopharmacol.Bull. 1984;20:43–4. [PubMed] [Google Scholar]
- [16].Price LH, Goddard AW, Barr LC, Goodman WK, Bloom FE, Kupfers DJ. Psychopharmacology: the fourth generation of progression. Raven; New York: 1995. Pharmacological challenges in anxiety disorders; pp. 1311–23. [Google Scholar]
- [17].Vickers K, McNally RJ. Respiratory symptoms and panic in the National Comorbidity Survey: a test of Klein’s suffocation false alarm theory. Behav Res Ther. 2005;43:1011–8. doi: 10.1016/j.brat.2004.06.019. [DOI] [PubMed] [Google Scholar]
- [18].Wilson KA, Hayward C. A prospective evaluation of agoraphobia and depression symptoms following panic attacks in a community sample of adolescents. J Anxiety Disord. 2005;19:87–103. doi: 10.1016/j.janxdis.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [19].McNally RJ, Riemann BC, Kim E. Selective processing of threat cues in panic disorder. Behav Res Ther. 1990;28:407–12. doi: 10.1016/0005-7967(90)90160-k. [DOI] [PubMed] [Google Scholar]
- [20].van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–33. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- [21].Jensen CF, Peskind ER, Keller TW, McFall ME, Raskind MA. Comparison of sodium lactate-induced panic symptoms between panic disorder and posttraumatic stress disorder. Depress.Anxiety. 1998;7:122–5. [PubMed] [Google Scholar]
- [22].Gorman JM, Liebowitz MR, Fyer AJ, Dillon D, Davies SO, Stein J, et al. Lactate infusions in obsessive-compulsive disorder. Am.J.Psychiatry. 1985;142:864–6. doi: 10.1176/ajp.142.7.864. [DOI] [PubMed] [Google Scholar]
- [23].Liebowitz MR, Fyer AJ, Gorman JM, Dillon D, Davies S, Stein JM, et al. Specificity of lactate infusions in social phobia versus panic disorders. Am.J.Psychiatry. 1985;142:947–50. doi: 10.1176/ajp.142.8.947. [DOI] [PubMed] [Google Scholar]
- [24].Cowley DS, Dager SR, Dunner DL. Lactate infusions in major depression without panic attacks. J.Psychiatr.Res. 1987;21:243–8. doi: 10.1016/0022-3956(87)90025-2. [DOI] [PubMed] [Google Scholar]
- [25].Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am.J.Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- [26].Pratt JA. The neuroanatomical basis of anxiety. Pharmacology & Therapeutics. 1992;55:149–81. doi: 10.1016/0163-7258(92)90014-q. [DOI] [PubMed] [Google Scholar]
- [27].Shekhar A, SG B, TJ S, AW G. Neurobiology of panic disorder. Trends in the Economics of Neurobiology. 2002:36–41. [Google Scholar]
- [28].Watanabe A, Nakao K, Tokuyama M, Takeda M. Prediction of first episode of panic attack among white-collar workers. Psychiatry Clin Neurosci. 2005;59:119–26. doi: 10.1111/j.1440-1819.2005.01345.x. [DOI] [PubMed] [Google Scholar]
- [29].Ballenger JC, Burrows GD, DuPont RL, Jr., Lesser IM, Noyes R, Jr., Pecknold JC, et al. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. I. Efficacy in short-term treatment. Arch Gen Psychiatry. 1988;45:413–22. doi: 10.1001/archpsyc.1988.01800290027004. [DOI] [PubMed] [Google Scholar]
- [30].Charney DS, Heninger GR. Noradrenergic function and the mechanism of action of antianxiety treatment. II. The effect of long-term imipramine treatment. Arch.Gen.Psychiatry. 1985;42:473–81. doi: 10.1001/archpsyc.1985.01790280055005. [DOI] [PubMed] [Google Scholar]
- [31].Tesar GE, Rosenbaum JF. Successful use of clonazepam in patients with treatment-resistant panic disorder. J Nerv Ment Dis. 1986;174:477–82. doi: 10.1097/00005053-198608000-00006. [DOI] [PubMed] [Google Scholar]
- [32].Rifkin A, Klein DF, Dillon D, Levitt M. Blockade by imipramine or desipramine of panic induced by sodium lactate. Am J Psychiatry. 1981;138:676–7. doi: 10.1176/ajp.138.5.676. [DOI] [PubMed] [Google Scholar]
- [33].Kelly D, Mitchell-Heggs N, Sherman D. Anxiety and the effects of sodium lactate assessed clinically and physiologically. Br.J.Psychiatry. 1971;119:129–41. doi: 10.1192/bjp.119.549.129. [DOI] [PubMed] [Google Scholar]
- [34].Cloos JM, Ferreira V. Current use of benzodiazepines in anxiety disorders. Curr Opin Psychiatry. 2009;22:90–5. doi: 10.1097/YCO.0b013e32831a473d. [DOI] [PubMed] [Google Scholar]
- [35].Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Reviews in the neurosciences. 2010;21:119–39. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- [36].Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–9. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- [37].Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Archives of General Psychiatry. 2001;58:556–61. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- [38].Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19:567–96. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- [39].Bandelow B, Zohar J, Hollander E, Kasper S, Moller HJ, Allgulander C, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- [40].Nutt DJ, Ballenger JC, Sheehan D, Wittchen HU. Generalized anxiety disorder: comorbidity, comparative biology and treatment. Int J Neuropsychopharmacol. 2002;5:315–25. doi: 10.1017/S1461145702003048. [DOI] [PubMed] [Google Scholar]
- [41].Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am.J.Physiol. 1928;84:490–515. [Google Scholar]
- [42].Hess WR, Akert K. Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA.Arch.Neurol.Psychiatry. 1955;73:127–9. doi: 10.1001/archneurpsyc.1955.02330080005003. [DOI] [PubMed] [Google Scholar]
- [43].Hess WR, Brugger M. Das subkortikake Zenrrumder affektriven Abwehrreaktion. Helv.Physiol.Acta. 1943;1:33–52. [Google Scholar]
- [44].Lynn R. National rates of economic growth, anxiety and suicide. Nature. 1969;222:494. doi: 10.1038/222494a0. [DOI] [PubMed] [Google Scholar]
- [45].Markgraf CG, Winters RW, Liskowsky DR, McCabe PM, Green EJ, Schneiderman N. Hypothalamic, midbrain and bulbar areas involved in the defense reaction in rabbits. Physiol Behav. 1991;49:493–500. doi: 10.1016/0031-9384(91)90270-x. [DOI] [PubMed] [Google Scholar]
- [46].Duan YF, Winters R, McCabe PM, Green EJ, Huang Y, Schneiderman N. Behavioral characteristics of defense and vigilance reactions elicited by electrical stimulation of the hypothalamus in rabbits. Behav.Brain Res. 1996;81:33–41. doi: 10.1016/s0166-4328(96)00042-3. [DOI] [PubMed] [Google Scholar]
- [47].Di Scala G, Schmitt P, Karli P. Flight induced by infusion of bicuculline methiodide into periventricular structures. Brain Research. 1984;309:199–208. [PubMed] [Google Scholar]
- [48].Olds ME, Olds J. Approach-escape interactions in rat brain. Am J Physiol. 1962;203:803–10. doi: 10.1152/ajplegacy.1962.203.5.803. [DOI] [PubMed] [Google Scholar]
- [49].Schenberg LC, De Aguiar JC, Graeff FG. GABA modulation of the defense reaction induced by brain electrical stimulation. Physiol Behav. 1983;31:429–37. doi: 10.1016/0031-9384(83)90062-8. [DOI] [PubMed] [Google Scholar]
- [50].Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26:407–17. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- [51].Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512:81–8. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- [52].Anderson JJ, DiMicco JA. Effect of local inhibition of gamma-aminobutyric acid uptake in the dorsomedial hypothalamus on extracellular levels of gamma-aminobutyric acid and on stress-induced tachycardia: a study using microdialysis. J.Pharmacol.Exp.Ther. 1990;255:1399–407. [PubMed] [Google Scholar]
- [53].Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J.Physiol. 2002;538:941–6. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512:81–8. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- [55].Soltis RP, DiMicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia in rats. Am.J.Physiol. 1992;262:R689–R97. doi: 10.1152/ajpregu.1992.262.4.R689. [DOI] [PubMed] [Google Scholar]
- [56].DiMicco JA, Abshire VM, Hankins KD, Sample RH, Wible JH., Jr. Microinjection of GABA antagonists into posterior hypothalamus elevates heart rate in anesthetized rats. Neuropharmacology. 1986;25:1063–6. doi: 10.1016/0028-3908(86)90203-0. [DOI] [PubMed] [Google Scholar]
- [57].Wible JH, Jr., DiMicco JA, Luft FC. Hypothalamic GABA and sympathetic regulation in spontaneously hypertensive rats. Hypertension. 1989;14:623–8. doi: 10.1161/01.hyp.14.6.623. [DOI] [PubMed] [Google Scholar]
- [58].Keim SR, Shekhar A. The effects of GABAA receptor blockade in the dorsomedial hypothalamic nucleus on corticotrophin (ACTH) and corticosterone secretion in male rats. Brain Res. 1996;739:46–51. doi: 10.1016/s0006-8993(96)00810-4. [DOI] [PubMed] [Google Scholar]
- [59].Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J.Neurosci. 1996;16:1173–9. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liebowitz MR, Gorman JM, Fyer A, Dillon D, Levitt M, Klein DF. Possible mechanisms for lactate’s induction of panic. Am.J.Psychiatry. 1986;143:495–502. doi: 10.1176/ajp.143.4.495. [DOI] [PubMed] [Google Scholar]
- [61].DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol.Biochem.Behav. 2002;71:469–80. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- [62].Rasche D, Foethke D, Gliemroth J, Tronnier VM. Deep brain stimulation in the posterior hypothalamus for chronic cluster headache. Case report and review of the literature. Schmerz. 2006;20:439–44. doi: 10.1007/s00482-005-0462-3. [DOI] [PubMed] [Google Scholar]
- [63].Wilent WB, Oh MY, Buetefisch CM, Bailes JE, Cantella D, Angle C, et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. J Neurosurg. 2010;112:1295–8. doi: 10.3171/2009.9.JNS09577. [DOI] [PubMed] [Google Scholar]
- [64].Cannon WB, Britton SW. Studies on conditions of activity in endocrine glands. XV. Pseudoaffective medulliadrenal secretion. Am.J.Physiol. 1925;72:283–94. [Google Scholar]
- [65].Bard P, Mountcastle VB. Some forebrain mechanisms involved in the expression of rage with special reference to suppression of angry behavior. Res.Publ.Assoc.Res.Nerv.Ment.Dis. 1948;27:362–404. [PubMed] [Google Scholar]
- [66].Chen P, Smith MS. Suckling-induced activation of neuronal input to the dorsomedial nucleus of the hypothalamus: possible candidates for mediating the activation of DMH neuropeptide Y neurons during lactation. Brain Res. 2003;984:11–20. doi: 10.1016/s0006-8993(03)02999-8. [DOI] [PubMed] [Google Scholar]
- [67].Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Behar KL, et al. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–93. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- [68].Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology. 2008;33:2093–107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Simpson JR, Jr., Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Eren I, Tukel R, Polat A, Karaman R, Unal S. Evaluation of regional cerebral blood flow changes in panic disorder with Tc99m-HMPAO SPECT. Psychiatry Res. 2003;123:135–43. doi: 10.1016/s0925-4927(03)00062-3. [DOI] [PubMed] [Google Scholar]
- [71].Hurley RA, Fisher R, Taber KH. Sudden onset panic: epileptic aura or panic disorder? J Neuropsychiatry Clin Neurosci. 2006;18:436–43. doi: 10.1176/jnp.2006.18.4.436. [DOI] [PubMed] [Google Scholar]
- [72].Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol.Psychiatry. 1999;45:872–82. doi: 10.1016/s0006-3223(98)00348-5. [DOI] [PubMed] [Google Scholar]
- [73].Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJ, Kendler KS, et al. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006 doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- [74].Pohl R, Balon R, Bechou R, Lycaki H. Lactate-induced anxiety after imapramine and diazepam treatment. Anxiety. 1994;1:54–63. doi: 10.1002/anxi.3070010204. [DOI] [PubMed] [Google Scholar]
- [75].Orlowski M, Reingold DF, Stanley ME. D-and L-stereoisomers of allylglycine: convulsive action and inhibition of brain L-glutamate decarboxylase. J Neurochem. 1977;28:349–53. doi: 10.1111/j.1471-4159.1977.tb07754.x. [DOI] [PubMed] [Google Scholar]
- [76].Reingold DF, Orlowski M. Inhibition of human and mouse brain glutamate decarboxylase by the alpha-keto analogs of cysteine and homocysteine. Biochem.Pharmacol. 1978;27:2567–70. doi: 10.1016/0006-2952(78)90328-3. [DOI] [PubMed] [Google Scholar]
- [77].Reingold DF, Orlowski M. Inhibition of brain glutamate decarboxylase by 2-keto-4-pentenoic acid, a metabolite of allylglycine. J Neurochem. 1979;32:907–13. doi: 10.1111/j.1471-4159.1979.tb04574.x. [DOI] [PubMed] [Google Scholar]
- [78].Horton RW, Chapman AG, Meldrum BS. Regional changes in cerebral GABA concentration and convulsions produced by D and by L-allylglycine. Journal of neurochemistry. 1978;30:1501–4. doi: 10.1111/j.1471-4159.1978.tb10484.x. [DOI] [PubMed] [Google Scholar]
- [79].Fernandez-Pascual S, Mukala-Nsengu-Tshibangu A, Martin Del Rio R, Tamarit-Rodriguez J. Conversion into GABA (gamma-aminobutyric acid) may reduce the capacity of L-glutamine as an insulin secretagogue. The Biochemical journal. 2004;379:721–9. doi: 10.1042/BJ20031826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Goldstein DB. D-amino acid oxidase in brain: distribution in several species and inhibition by pentobarbitone. J Neurochem. 1966;13:1011–6. doi: 10.1111/j.1471-4159.1966.tb10299.x. [DOI] [PubMed] [Google Scholar]
- [81].Neims AH, Zieverink WD, Smilack JD. Distribution of D-amino acid oxidase in bovine and human nervous tissues. J Neurochem. 1966;13:163–8. doi: 10.1111/j.1471-4159.1966.tb07508.x. [DOI] [PubMed] [Google Scholar]
- [82].Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, et al. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–15. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–35. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–56. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- [85].Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci. 2006;26:7093–104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nature medicine. 2010;16:111–5. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Molosh AI, Johnson PL, Fitz SD, Dimicco JA, Herman JP, Shekhar A. Changes in Central Sodium and not Osmolarity or Lactate Induce Panic-Like Responses in a Model of Panic Disorder. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shekhar A, Keim SR. LY354740, a potent group II metabotropic glutamate receptor agonist prevents lactate-induced panic-like response in panic-prone rats. Neuropharmacology. 2000;39:1139–46. doi: 10.1016/s0028-3908(99)00215-4. [DOI] [PubMed] [Google Scholar]
- [89].Shekhar A, Johnson PL, Fitz SD, Nakazato A, Chaki S, Steckler T, et al. A selective, non-peptide CRF receptor 1 antagonist prevents sodium lactate-induced acute panic-like responses. Int J Neuropsychopharmacol. 2011;14:355–65. doi: 10.1017/S1461145710001355. [DOI] [PubMed] [Google Scholar]
- [90].Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–3. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- [91].Andreatini R, Blanchard C, Blanchard R, Brandao ML, Carobrez AP, Griebel G, et al. The brain decade in debate: II. Panic or anxiety? From animal models to a neurobiological basis. Braz J Med Biol Res. 2001;34:145–54. doi: 10.1590/s0100-879x2001000200001. [DOI] [PubMed] [Google Scholar]
- [92].Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AA, den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol.Psychiatry. 2002;52:126–35. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- [93].Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural Pathways Underlying Lactate-Induced Panic. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res.Bull. 1986;16:231–48. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- [95].Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res.Brain Res.Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- [96].Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–86. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- [97].Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J.Neurosci. 1997;17:9726–35. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Peskind ER, Jensen CF, Pascualy M, Tsuang D, Cowley D, Martin DC, et al. Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biol.Psychiatry. 1998;44:1007–16. doi: 10.1016/s0006-3223(98)00053-5. [DOI] [PubMed] [Google Scholar]
- [99].Gorman JM, Battista D, Goetz RR, Dillon DJ, Liebowitz MR, Fyer AJ, et al. A comparison of sodium bicarbonate and sodium lactate infusion in the induction of panic attacks. Arch.Gen.Psychiatry. 1989;46:145–50. doi: 10.1001/archpsyc.1989.01810020047008. [DOI] [PubMed] [Google Scholar]
- [100].George DT, Lindquist T, Nutt DJ, Ragan PW, Alim T, McFarlane V, et al. Effect of chloride or glucose on the incidence of lactate-induced panic attacks. Am.J.Psychiatry. 1995;152:692–7. doi: 10.1176/ajp.152.5.692. [DOI] [PubMed] [Google Scholar]
- [101].Hiyama TY, Watanabe E, Okado H, Noda M. The subfornical organ is the primary locus of sodium-level sensing by Na(x) sodium channels for the control of salt-intake behavior. J Neurosci. 2004;24:9276–81. doi: 10.1523/JNEUROSCI.2795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Watanabe E, Fujikawa A, Matsunaga H, Yasoshima Y, Sako N, Yamamoto T, et al. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci. 2000;20:7743–51. doi: 10.1523/JNEUROSCI.20-20-07743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Grob M, Drolet G, Mouginot D. Specific Na+ sensors are functionally expressed in a neuronal population of the median preoptic nucleus of the rat. J Neurosci. 2004;24:3974–84. doi: 10.1523/JNEUROSCI.3720-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010;108:995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J.Appl.Physiol. 1997;82:469–79. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- [106].Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res. 1981;222:373–81. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- [107].Walker BR. Cardiovascular effect of V1 vasopressinergic blockade during acute hypercapnia in conscious rats. Am.J.Physiol. 1987;252:R127–R33. doi: 10.1152/ajpregu.1987.252.1.R127. [DOI] [PubMed] [Google Scholar]
- [108].Cuccheddu T, Floris S, Serra M, Porceddu ML, Sanna E, Biggio G. Proconflict effect of carbon dioxide inhalation in rats. Life Sci. 1995;56:L321–L4. doi: 10.1016/0024-3205(95)00093-3. [DOI] [PubMed] [Google Scholar]
- [109].Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, et al. Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2010 doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- [110].Marotta SF, Sithichoke N, Garcy AM, Yu M. Adrenocortical responses of rats to acute hypoxic and hypercapnic stresses after treatment with aminergic agents. Neuroendocrinology. 1976;20:182–92. doi: 10.1159/000122482. [DOI] [PubMed] [Google Scholar]
- [111].Sithichoke N, Malasanos LJ, Marotta SF. Cholinergic influences on hypothalamic-pituitary-adrenocortical activity of stressed rats: an approach utilizing choline deficient diets. Acta Endocrinol.(Copenh) 1978;89:737–43. doi: 10.1530/acta.0.0890737. [DOI] [PubMed] [Google Scholar]
- [112].Sithichoke N, Marotta SF. Cholinergic influences on hypothalamic-pituitary-adrenocortical activity of stressed rats: an approach utilizing agonists and antagonists. Acta Endocrinol.(Copenh) 1978;89:726–36. doi: 10.1530/acta.0.0890726. [DOI] [PubMed] [Google Scholar]
- [113].Goetz RR, Klein DF, Papp LA, Martinez JM, Gorman JM. Acute panic inventory symptoms during CO(2) inhalation and room-air hyperventilation among panic disorder patients and normal controls. Depress.Anxiety. 2001;14:123–36. doi: 10.1002/da.1054. [DOI] [PubMed] [Google Scholar]
- [114].Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, et al. Response to hyperventilation in a group of patients with panic disorder. Am.J.Psychiatry. 1984;141:857–61. doi: 10.1176/ajp.141.7.857. [DOI] [PubMed] [Google Scholar]
- [115].Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, et al. Ventilatory physiology of patients with panic disorder. Arch.Gen.Psychiatry. 1988;45:31–9. doi: 10.1001/archpsyc.1988.01800250035006. [DOI] [PubMed] [Google Scholar]
- [116].Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch.Gen.Psychiatry. 1993;50:306–17. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- [117].Forster HV, Smith CA. Contributions of Central and Peripheral Chemoreceptors to the Ventilatory Response to Co2/H+ J Appl Physiol. 2010 doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fukuda Y, Sato A, Suzuki A, Trzebski A. Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. J.Auton.Nerv.Syst. 1989;28:61–74. doi: 10.1016/0165-1838(89)90008-8. [DOI] [PubMed] [Google Scholar]
- [119].Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J.Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–90. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–9. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- [122].Johnson PJ, Samuels BC, Fitz SD, Barton S, Lightman SL, Lowry CA, et al. Activation of the orexin 1 receptor is a critical component of CO2 mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.38. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu Z, Song N, Geng W, Jin W, Li L, Cao Y, et al. Orexin-a and Respiration in a Rat Model of Smoke-Induced Chronic Obstructive Pulmonary Disease. Clin Exp Pharmacol Physiol. 2010 doi: 10.1111/j.1440-1681.2010.05411.x. [DOI] [PubMed] [Google Scholar]
- [124].Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–23. [PubMed] [Google Scholar]
- [125].Zhu LY, Summah H, Jiang HN, Qu JM. Plasma orexin-a levels in COPD patients with hypercapnic respiratory failure. Mediators Inflamm. 2011;2011:754847. doi: 10.1155/2011/754847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Shekhar A, Katner JS, Sajdyk TJ, Kohl RR. Role of norepinephrine in the dorsomedial hypothalamic panic response: an in vivo microdialysis study. Pharmacol Biochem Behav. 2002;71:493–500. doi: 10.1016/s0091-3057(01)00688-8. [DOI] [PubMed] [Google Scholar]
- [127].Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J.Neurophysiol. 1997;77:3396–400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- [128].Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- [129].Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393:244–66. [PubMed] [Google Scholar]
- [130].Dai J, van d. V., Swaab DF, Buijs RM. Postmortem anterograde tracing of intrahypothalamic projections of the human dorsomedial nucleus of the hypothalamus. J.Comp Neurol. 1998;401:16–33. doi: 10.1002/(sici)1096-9861(19981109)401:1<16::aid-cne2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [131].Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J.Comp Neurol. 1996;376:143–73. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [132].Yamakawa K, Kudo K, Kanba S, Arita J. Distribution of prolactin-releasing peptide-immunoreactive neurons in the rat hypothalamus. Neurosci Lett. 1999;267:113–6. doi: 10.1016/s0304-3940(99)00346-8. [DOI] [PubMed] [Google Scholar]
- [133].Shekhar A, Hingtgen JN, DiMicco JA. Selective enhancement of shock avoidance responding elicited by GABA blockade in the posterior hypothalamus of rats. Brain Res. 1987;420:118–28. doi: 10.1016/0006-8993(87)90246-0. [DOI] [PubMed] [Google Scholar]
- [134].Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc.Soc Exp.Biol.Med. 1998;218:284–306. doi: 10.3181/00379727-218-44296. [DOI] [PubMed] [Google Scholar]
- [135].Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J.Neurosci. 1994;14:6500–10. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chen T, Hui R, Dong YX, Li YQ, Mizuno N. Endomorphin 1- and endomorphin 2-like immunoreactive neurons in the hypothalamus send axons to the parabrachial nucleus in the rat. Neurosci Lett. 2004;357:139–42. doi: 10.1016/j.neulet.2003.12.079. [DOI] [PubMed] [Google Scholar]
- [137].Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am.J.Physiol Heart Circ.Physiol. 2001;280:H2891–H901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- [138].Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am.J.Physiol Regul.Integr.Comp Physiol. 2004;287:R472–R8. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- [139].Hosoya Y, Ito R, Kohno K. The topographical organization of neurons in the dorsal hypothalamic area that project to the spinal cord or to the nucleus raphe pallidus in the rat. Exp.Brain Res. 1987;66:500–6. doi: 10.1007/BF00270682. [DOI] [PubMed] [Google Scholar]
- [140].McDowall LM, Horiuchi J, Killinger S, Dampney RA. Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1020–6. doi: 10.1152/ajpregu.00541.2005. [DOI] [PubMed] [Google Scholar]
- [141].Crestani CC, Alves FH, Resstel LB, Correa FM. Cardiovascular effects of noradrenaline microinjection in the bed nucleus of the stria terminalis of the rat brain. J Neurosci Res. 2007;85:1592–9. doi: 10.1002/jnr.21250. [DOI] [PubMed] [Google Scholar]
- [142].Sajdyk TJ, Johnson PL, Fitz SD, Shekhar A. Chronic inhibition of GABA synthesis in the Bed Nucleus of the Stria Terminalis elicits anxiety-like behavior. J Psychopharmacol. 2007 doi: 10.1177/0269881107082902. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann.N.Y.Acad.Sci. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- [144].Starcevic V, Kellner R, Uhlenhuth EH, Pathak D. The phenomenology of panic attacks in panic disorder with and without agoraphobia. Compr Psychiatry. 1993;34:36–41. doi: 10.1016/0010-440x(93)90033-z. [DOI] [PubMed] [Google Scholar]
- [145].Starcevic V, Uhlenhuth EH, Kellner R, Pathak D. Comparison of primary and secondary panic disorder: a preliminary report. J Affect Disord. 1993;27:81–6. doi: 10.1016/0165-0327(93)90080-4. [DOI] [PubMed] [Google Scholar]
- [146].Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–12. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- [147].Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur.J.Pharmacol. 1988;151:243–8. doi: 10.1016/0014-2999(88)90804-7. [DOI] [PubMed] [Google Scholar]
- [148].Jordan D, Mifflin SW, Spyer KM. Hypothalamic inhibition of neurones in the nucleus tractus solitarius of the cat is GABA mediated. J.Physiol. 1988;399:389–404. doi: 10.1113/jphysiol.1988.sp017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Potts JT, Paton JF, Mitchell JH, Garry MG, Kline G, Anguelov PT, et al. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience. 2003;119:201–14. doi: 10.1016/s0306-4522(02)00953-3. [DOI] [PubMed] [Google Scholar]
- [150].Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: Subregional distribution in the nucleus tractus solitarius. J.Comp Neurol. 2005;493:274–90. doi: 10.1002/cne.20758. [DOI] [PubMed] [Google Scholar]
- [151].Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J.Neurosci. 2003;23:3844–54. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Sevoz-Couche C, Comet MA, Hamon M, Laguzzi R. Role of nucleus tractus solitarius 5-HT3 receptors in the defense reaction-induced inhibition of the aortic baroreflex in rats. J.Neurophysiol. 2003;90:2521–30. doi: 10.1152/jn.00275.2003. [DOI] [PubMed] [Google Scholar]
- [153].Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. J.Psychosom.Res. 1998;44:133–51. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- [154].Gorman JM, Levy GF, Liebowitz MR, McGrath P, Appleby IL, Dillon DJ, et al. Effect of acute beta-adrenergic blockade on lactate-induced panic. Arch.Gen.Psychiatry. 1983;40:1079–82. doi: 10.1001/archpsyc.1983.01790090041006. [DOI] [PubMed] [Google Scholar]
- [155].Nesse RM, Cameron OG, Curtis GC, McCann DS, Huber-Smith MJ. Adrenergic function in patients with panic anxiety. Arch.Gen.Psychiatry. 1984;41:771–6. doi: 10.1001/archpsyc.1984.01790190045005. [DOI] [PubMed] [Google Scholar]
- [156].Hamada T, Koshino Y, Misawa T, Isaki K, Gejyo F. Mitral valve prolapse and autonomic function in panic disorder. Acta Psychiatr.Scand. 1998;97:139–43. doi: 10.1111/j.1600-0447.1998.tb09976.x. [DOI] [PubMed] [Google Scholar]
- [157].McCraty R, Atkinson M, Tomasino D, Stuppy WP. Analysis of twenty-four hour heart rate variability in patients with panic disorder. Biol Psychol. 2001;56:131–50. doi: 10.1016/s0301-0511(01)00074-6. [DOI] [PubMed] [Google Scholar]
- [158].Shioiri T, Kojima-Maruyama M, Hosoki T, Kitamura H, Tanaka A, Yoshizawa M, et al. Dysfunctional baroreflex regulation of sympathetic nerve activity in remitted patients with panic disorder. A new methodological approach. Eur Arch Psychiatry Clin Neurosci. 2005;255:293–8. doi: 10.1007/s00406-005-0561-2. [DOI] [PubMed] [Google Scholar]
- [159].Soltis RP, DiMicco JA. GABAA and excitatory amino acid receptors in dorsomedial hypothalamus and heart rate in rats. Am.J.Physiol. 1991;260:R13–R20. doi: 10.1152/ajpregu.1991.260.1.R13. [DOI] [PubMed] [Google Scholar]
- [160].Gorman JM. New molecular targets for antianxiety interventions. J.Clin.Psychiatry. 2003;64(Suppl 3):28–35. [PubMed] [Google Scholar]
- [161].Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- [162].Nicoletti F, Bockaert J, Collingridge GL, Conn JP, Ferraguti F, Schoepp DD, et al. Metabotropic glutamate receptors: From the workbench to the bedside In memory of Prof. Erminio Costa. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, et al. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology (Berl) 2005;179:271–83. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- [164].Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–60. [PubMed] [Google Scholar]
- [165].Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, et al. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–37. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- [166].Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacology, Biochemistry & Behavior. 2002;71:379–92. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- [167].Grillon C, Cordova J, Levine LR, Morgan CA., III Anxiolytic effects of a novel group II metabotropic glutamate receptor agonis (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology. 2003:446–54. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- [168].Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–97. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- [169].Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–50. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- [170].Charney DS, Heninger GR, Breier A. Noradrenergic function in panic anxiety. Effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch.Gen.Psychiatry. 1984;41:751–63. doi: 10.1001/archpsyc.1984.01790190025003. [DOI] [PubMed] [Google Scholar]
- [171].Charney DS, Woods SW, Krystal JH, Nagy LM, Heninger GR. Noradrenergic neuronal dysregulation in panic disorder: the effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatrica Scandinavica. 1992;86:273–82. doi: 10.1111/j.1600-0447.1992.tb03266.x. [DOI] [PubMed] [Google Scholar]
- [172].Ko GN, Elsworth JD, Roth RH, Rifkin BG, Leigh H, Redmond DE., Jr. Panic-induced elevation of plasma MHPG levels in phobic-anxious patients. Effects of clonidine and imipramine. Arch Gen Psychiatry. 1983;40:425–30. doi: 10.1001/archpsyc.1983.01790040079011. [DOI] [PubMed] [Google Scholar]
- [173].Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–74. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- [174].Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annual Review of Neuroscience. 1979;2:113–68. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- [175].Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- [176].Shekhar A, Katner JS, Rusche WP, Sajdyk TJ, Simon JR. Fear-potentiated startle elevates catecholamine levels in the dorsomedial hypothalamus of rats. Pharmacol Biochem Behav. 1994;48:525–9. doi: 10.1016/0091-3057(94)90564-9. [DOI] [PubMed] [Google Scholar]
- [177].Przuntek H, Philippu A. Reduced pressor responses to stimulation of the locus coeruleus after lesion of the posterior hypothalamus. Naunyn Schmiedebergs Arch Pharmacol. 1973;276:119–22. doi: 10.1007/BF00501184. [DOI] [PubMed] [Google Scholar]
- [178].Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- [179].Keegan CE, Herman JP, Karolyi IJ, O’Shea KS, Camper SA, Seasholtz AF. Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology. 1994;134:2547–55. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- [180].Swanson S, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J.Comp Neurol. 1989;285:413–35. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- [181].Brown MR, Hauger R, Fisher LA. Autonomic and cardiovascular effects of corticotropin-releasing factor in the spontaneously hypertensive rat. Brain Res. 1988;441:33–40. doi: 10.1016/0006-8993(88)91380-7. [DOI] [PubMed] [Google Scholar]
- [182].Ku YH, Tan L, Li LS, Ding X. Role of corticotropin-releasing factor and substance P in pressor responses of nuclei controlling emotion and stress. Peptides. 1998;19:677–82. doi: 10.1016/s0196-9781(98)00004-7. [DOI] [PubMed] [Google Scholar]
- [183].Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, Salyakina D, et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1196–204. doi: 10.1002/ajmg.b.30750. [DOI] [PubMed] [Google Scholar]
- [184].Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–61. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, et al. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur.J.Pharmacol. 2005;509:145–53. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- [186].Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, et al. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. European Journal of Neuroscience. 2001;13:373–80. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- [187].Keller C, Bruelisauer A, Lemaire M, Enz A. Brain pharmacokinetics of a nonpeptidic corticotropin-releasing factor receptor antagonist. Drug Metab Dispos. 2002;30:173–6. doi: 10.1124/dmd.30.2.173. [DOI] [PubMed] [Google Scholar]
- [188].Steckler T, Nakazato A, Kennis L, Mackie C, Nakamura M, Vinken P, et al. CRF1 antagonists - therapeutic implications for affective and mood disorders. Société de Chimie Thérapeutique. 2006;32:1–19. [Google Scholar]
- [189].Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–25. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- [190].Ferguson AV, Samson WK. The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front Neuroendocrinol. 2003;24:141–50. doi: 10.1016/s0091-3022(03)00028-1. [DOI] [PubMed] [Google Scholar]
- [191].Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- [192].Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur.J.Neurosci. 2002;15:315–28. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- [193].Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, et al. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J.Neurosci. 2003;23:1557–62. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, et al. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J.Physiol. 2005;563:569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress , and anxiety. European Journal of Pharmacology. 2003:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- [196].Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J.Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–97. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- [198].Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul.Pept. 2002;104:131–44. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- [199].Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul.Pept. 2002;104:91–5. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- [200].Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am.J.Physiol. 1999;277:R1780–R5. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- [201].Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am.J.Physiol Regul.Integr.Comp Physiol. 2003;285:R581–R93. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- [202].Furlong TM, Carrive P. Hypocretin/orexin neurons and the perifornical hypothalamus play a more important role in contextual fear than in restraint stress. Society for Neuroscience. 2005 [Google Scholar]
- [203].Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am.J.Physiol Regul.Integr.Comp Physiol. 2000;278:R692–R7. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- [204].Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- [205].Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul.Pept. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- [206].Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am.J.Physiol Regul.Integr.Comp Physiol. 2001;281:R1801–R7. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- [207].Coote JH, Hilton SM, Perez-Gonzalez JF. Inhibition of the baroreceptor reflex on stimulation in the brain stem defence centre. J.Physiol. 1979;288:549–60. [PMC free article] [PubMed] [Google Scholar]
- [208].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed Elsevier Academic Press; 2005. [Google Scholar]
- [209].Segura T, Martin DS, Sheridan PJ, Haywood JR. Measurement of the distribution of [3H]bicuculline microinjected into the rat hypothalamus. J.Neurosci.Methods. 1992;41:175–86. doi: 10.1016/0165-0270(92)90059-m. [DOI] [PubMed] [Google Scholar]
- [210].Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- [211].Balon R, Pohl R, Yeragani VK, Rainey JM, Weinberg P. Lactate- and isoproterenol-induced panic attacks in panic disorder patients and controls. Psychiatry Res. 1988;23:153–60. doi: 10.1016/0165-1781(88)90005-4. [DOI] [PubMed] [Google Scholar]
- [212].Kobelt P, Paulitsch S, Goebel M, Stengel A, Schmidtmann M, van der Voort IR, et al. Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res. 2006;1117:109–17. doi: 10.1016/j.brainres.2006.08.092. [DOI] [PubMed] [Google Scholar]
- [213].Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol.Psychiatry. 2003;53:275–83. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]