Abstract
This study investigates the role of basolateral amygdala (BLA) dopamine in heroin-induced conditioned immunomodulation. Animals underwent conditioning in which heroin administration was repeatedly paired with placement into a conditioning chamber. Six days after the final conditioning session animals were returned to the chamber and received intra-BLA microinfusions of dopamine, D1 or D2, antagonist. Antagonism of D1, but not D2, receptors within the BLA blocked the suppressive effect of heroin-associated environmental stimuli on iNOS, TNF-α and IL-1β. This study is the first to demonstrate that the expression of heroin’s conditioned effects on proinflammatory mediators require dopamine D1 receptors within the BLA.
Keywords: Rat, nitric oxide, basolateral amygdale, TNFα, IL-1β, dopamine
1. Introduction
The high rate at which heroin users contract opportunistic infections suggests that heroin use may impair the ability of the host to combat infectious disease (Luttgens, 1949; Hussey & Katz, 1950; Louria et al., 1967). Although it has been postulated that this high rate of infection amongst heroin users results from increased contact with pathogens, possibly through needle sharing or impurities in street grade drugs, there is evidence that heroin may directly impact the clinical course of infection (Kreek, 1990; Farizio et al., 1992; McLachlan et al., 1993) and diseases associated with intravenous heroin use may be influenced by the ability of opioids to modulate immune function (McDonough et al., 1980; Donahoe et al., 1986; Novick et al., 1989; Ochshorn et al., 1990). In fact, clinical studies report abnormalities in basic immune parameters in heroin users, including decreased numbers of circulating lymphocytes, decreased NK cell activity, and reduced antibody-dependent cellular cytotoxicity (Nair et al., 1986; Govitrapong et al., 1998), suggesting that heroin may further contribute to increased infection susceptibility among users, independently of concurrent risk factors. In further support of this, studies have indicated that human heroin users exhibit poorer immune responses following hepatitis vaccination (Quaglio et al. 2004; Rodrigo et al., 1992).
New studies in our laboratory have indicated that heroin induces alterations in a number of immunological parameters including alterations in natural killer cell activity, T- and B-lymphocyte proliferation, and nitric oxide production (Fecho et al. 2000; Lysle & How 2000). Nitric oxide is produced by many cells of the immune system and it mediates diverse biological functions including vasodilation, the cytotoxic activity of macrophages, and the inhibition of platelet adhesion and aggregation (Suschek et al., 2004; Tuteja et al., 2004). Opiate drug administration has also been found to alter the production of several proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and interleukin-one beta (IL-1β) (Chao et al 1993; Bencsics et al 1997; Pacifici et al 2000; Clark et al., 2007). IL-1β and TNF-α both play critical roles in the body’s defense against infectious challenge, while also serving to regulate the immune response to these challenges.
Previous research has shown that the effects of heroin on proinflammatory mediators (nitric oxide, IL-1β and TNF-α) may be conditioned to environmental stimuli that have been associated with drug administration (e.g., Lysle & Ijames, 2002; Szczytkowski & Lysle, 2007) and that these conditioned effects are attenuated by inhibition of the basolateral amygdala (Szczytkowski & Lysle, 2008). These findings suggest that the basolateral amygdala is an integral component of the circuit mediating the conditioned effects of heroin on proinflammatory mediators. The current study further explores the role of basolateral amygdala by examining the dopaminergic inputs to this brain region and their involvement in the expression of heroin-induced conditioned immunomodulation. Interestingly, the dopaminergic system within the BLA has been shown to be involved in learning and memory and specifically the associative learning that underlies classical conditioning. For example, intra-amygdalar 6-hydroxydopamine injections impaired (Ashford & Jones, 1976), while post-training intra-BLA dopamine infusions enhanced (LaLumiere et al., 2004) memory consolidation of a conditioned avoidance task. In addition, rats with 6-hydroxydopamine lesions of the amygdala exhibit impaired acquisition of conditioning to explicit cues (Selden et al., 1991). Microdialysis studies revealed an increase in dopamine and its metabolites during the learning of a discriminative task in rats (Hori et al., 1993) further supporting the importance of this neurotransmitter in BLA-mediated learning and memory. Furthermore,Macedo et al. (2007) demonstrated that antagonism of dopamine D1 receptors within the BLA reduces the expression of conditioned, but not unconditioned, fear.
Dopaminergic signaling within the BLA appears to be particularly important for the associative learning processes that accompany the acquisition and expression of conditioned responses to drugs of abuse. For example, intra-BLA administration of D-amphetamine potentiates cue-induced cocaine seeking possibly by increasing monoamine tone in this region (Ledford et al., 2003). In addition, site specific adminstration of a dopamine D1/D2/D3 antagonist into the basolateral amygdala, but not the central amygdala, blocks alcohol-induced conditioned place preference in mice (Gremel & Cunningham, 2008). More specifically, stimulation of dopamine D1 receptors in the BLA was found to be necessary for the expression of cue-induced reinstatement of cocaine-seeking behavior in rats (See et al., 2001; Yun & Fields, 2003). In contrast, dopamine D2 receptors in the BLA appear to be involved in the acquisition of associations between drugs of abuse and the conditioned cues that guide subsequent cue-induced drug-seeking behavior (Berglind et al., 2006). These findings provide a strong rationale for examining the involvement of the dopaminergic system within the BLA on the expression of heroin-conditioned immunomodulation.
The current set of experiments sought to establish the role of the dopaminergic system within the basolateral amygdala in the conditioned effects of heroin on proinflammatory mediators. Rats received five training sessions in which heroin was administered immediately upon placement into a standard conditioning chamber which served as the conditioned stimulus (CS). On the test day, the animals were re-exposed to the chamber for 60-min without drug administration and given a subcutaneous injection of LPS (1000 µg/kg) upon removal. Immediately prior to CS reexposure, animals received intra-BLA infusion of either saline vehicle, SCH23390 (dopamine D1 receptor antagonist) or raclopride (D2 antagonist) to determine the role of dopamine signaling in heroin-induced conditioned immunomodulation. The findings reported here are important because they indicate that previously heroin-associated environmental stimuli are not only capable of inducing alterations in proinflammatory mediators but that these effects may be modified by antagonism of specific dopaminergic receptors within the BLA.
2. Materials and Methods
Animals
Male Lewis rats, weighing 225–250 g, were purchased from Charles River Laboratories (Raleigh, N.C., USA). Upon arrival, animals were housed individually in plastic cages in a colony room with a reversed light-dark (12h) cycle maintained through artificial illumination. Animals were allowed access to food and water ad libitum throughout the experiment except for the time spent in the conditioning chambers when food and water were not available. All animals were given a 2-week habituation period before the start of experimental manipulations and were handled regularly during this time. All procedures described were approved by the IACUC of the University of North Carolina at Chapel Hill and conformed to National Institutes of Health (NIH) Guidelines on the Care and Use of Laboratory Animals.
Drug Administration
Heroin (diacetylmorphine) was obtained from NIDA (Bethesda, MD) and dissolved in 0.9 % sterile saline. For all experiments, animals received a subcutaneous injection of heroin at a dose 1 mg/kg immediately prior to placement in the conditioning chamber on each of the five conditioning trial days. This dose was selected based on prior experiments in our laboratory showing that a 1 mg/kg dose of heroin alters LPS-induced iNOS, IL-1β and TNF-α mRNA expression in spleen and liver tissue and induces conditioning (Lysle & How, 2000; Lysle & Ijames, 2002; Szczytkowski & Lysle, 2007).
Surgery and microinjection
Animals were anesthetized with 0.35 ml intramuscular injections of 1:1 (vol/vol) ketamine hydrochloride (100 mg/ml) mixed with xylazine (20 mg/ml) and placed into the stereotaxic apparatus. Animals were implanted with 26-gauge bilateral guide cannula (Plastics One, Roanoke, VA) directed towards the BLA (AP −2.5, ML±5.0, DV −6.6). Coordinates are expressed as millimeters from bregma (Paxinos & Watson, 1986). Animals were given a two week recovery period before the start of conditioning trials. On test day, animals received bilateral intracranial injections (0.5 µl/side infused over 1 min) of saline vehicle, the D1 antagonist, SCH23390 (2 µg/0.5 µl/side) or the D2 antagonist, raclopride (5 µg/0.5 µl/side) 30 minutes prior to re-exposure to the conditioning chambers. Antagonist doses were chosen based upon previous behavioral studies that had shown reductions in cue-induced drug seeking following administration of these doses into the BLA (Berglind et al, 2006; See et al, 2001). Injectors extended 2 mm beyond the tip of the cannula and were left in place for 1 minute after the injection to allow for proper infusion.
Histology
To confirm proper cannula placement Alcian blue dye was infused via the cannula following sacrifice. Brains were then extracted and post-fixed in a 4% paraformaldehyde solution. Following fixation the brains were transferred to a 30% sucrose solution for cryoprotection and then frozen at −80° C until further analysis. Coronal sections (50 µm) were taken and stained with cresyl violet for verification of cannula placement. Animals with cannula placement outside of the targeted region were removed from the analyses.
Procedures
Acquisition of conditioned response
To condition heroin’s effects on iNOS expression, all animals received five 60-min training sessions in which they received a subcutaneous injection of heroin upon placement into a standard conditioning chamber. Training sessions were separated by 48 h. The conditioning chambers (BRS/LVE, Laurel, MD, USA) were contained in a room separate from the animal colony and contained a metal grid floor design and cedar bedding to create an environment distinct from that of the home cage. All conditioning took place during the dark phase of the light cycle, and the conditioning chambers were kept dark.
Testing of expression of conditioned response
The test day took place six days following the final conditioning session. To test the expression of the conditioned response, animals were re-exposed to the conditioned stimulus (i.e.; the conditioning chambers) without drug to determine whether the conditioned stimulus alone would induce alterations in pro-inflammatory mediators. Thirty minutes prior to testing, animals received intra-BLA microinfusions of either the D1 antagonist, SCH23390, or the D2 antagonist, raclopride. Control animals received intra-BLA microinfusions of saline vehicle. Two groups of animals were then re-exposed to the chambers (conditioned stimulus, CS) for 60-min without further administration of heroin. The remaining animals (home cage, HC) were returned to the home cage following microinfusions and served as controls. After the 60-min re-exposure, the animals were removed from the chambers and given a subcutaneous injection of LPS (1000 µg/kg) to induce iNOS, TNF-α and IL-1β production. Home cage animals also received LPS at this time. Six hours after LPS administration all animals were sacrificed and samples of spleen, liver and blood were collected for analysis. Spleen and liver tissue were collected as immunohistochemical localization of iNOS in rats exposed to LPS has shown the presence of the iNOS enzyme in a number of immune cells in a variety of tissues including eosinophils within the spleen and Kupffer’s cells and hepatocytes within the liver (Bandaletova et al., 1993). The 6-hr timepoint was selected based on previous research in our laboratory showing maximal iNOS induction at six hours following LPS administration (Lysle & How, 2000).
Real Time RT-PCR
To determine iNOS expression, real time RT-PCR was performed on tissue samples from the spleen and liver. Total RNA was extracted from a section of each of the tissues using TRI-Reagent (Molecular Research Center, Cincinnati, OH), a modification of the original method described by Chomczynski and Sacchi (1987). RNA was quantified spectrophotometrically (GeneQuant II, Pharmacia-Biotech, Piscataway, NJ, USA). For the RT-PCR, reverse transcription is performed using Oligo(dT)18 primer and Moloney Murine Leukemia Virus-Reverse transcriptase following the protocol of the Advantage RT-for-PCR Kit from Clontech (Palo Alto, CA, USA).
PCR amplifications were performed using the Fast Start™ DNA Master SYBR Green I Real-Time PCR Kit (Roche) and the LightCycler instrument (Roche). A master mix containing all reaction components was prepared for all reactions, with each reaction using a 20 ml mix placed in glass capillary tubes specifically designed for use in the LightCycler system. The PCR primer set for iNOS, 5’-CCCTTCCGAAGTTTCTGGCAGCAGC-3’ and 5’-GGGTGTCAGAGTCTTGTGCCTTTGG-3’ was synthesized by the Nucleic Acids Core Facility (Lineberger Cancer Center, UNC-Chapel Hill). Copy numbers were generated from an external standard curve. Amplifications were carried out for 40 cycles and curves showing fluorescence at each cycle were determined by the computer software (Roche). Samples were pre-incubated for 10 minutes at 95° C to activate the Fast-Start Taq DNA polymerase. The cycle temperatures were 95, 60, and 72°C for the denaturing, annealing, and extending, respectively. The cycle times were 15, 5, and 25 s for the denaturing, annealing, and extending, respectively. Flourescence level was determined at the end of the extending phase for each cycle of PCR. The analysis of the fluorescence level in standards and samples over the course of 40 cycles was used to derive the number of copies of the target molecule in each sample. Additionally, assessments of housekeeping gene expression, cyclophilin, were made to assure comparable quality of RNA among samples. The sequence of the cyclophilin primers was 5’-CCAAGACTGAGTGGCT-3’ and 5’-AGATTACAGGGTATTGCG-3’. The data are expressed as a copy number of iNOS (per 10ng cDNA) based on the standard curve using the Lightcycler software (Roche).
Furthermore, to confirm the nature of amplification product, a melt curve analysis was conducted after the final PCR cycle. This analysis involved denaturing the products by slowly heating them to 95°C, during which fluorescence is continuously measured.
ELISA
For IL-1β and TNF-α protein determinations, protein was extracted from a section of each homogenized tissue using freeze/thaw lysis in tris-buffer containing antiproteinases. Protein was quantified spectrophotometrically (Bio-Tek, Model EL312 kinetic reader, Winooski, VT, USA) using Bio-Rad protein dye. To account for variability in tissue size, samples were normalized per unit protein based on the results of the spectrophotometric analysis. The BioSource International, Inc. (Carlsbad, CA) rat IL-1β and TNF-α ELISA test kits were used to determine the levels of IL-1β or TNF-α protein in each tissue sample. Briefly, samples and standards were added to microtiter wells coated with antibody that recognizes IL-1β or TNF-α and incubated at room temperature. Wells were washed extensively and then incubated with biotinylated antibody, followed by a second wash and then incubation with Streptavidin-HRP. After the final washing, a chromagen substrate solution was added which reacted with the bound enzyme to produce color. The color intensity developed proportionally to the amount of IL-1β or TNF-α present in each sample. The enzyme reaction was stopped after 30 minutes, and the absorbance at 450 nm was measured with a Bio-Tek (Winooski, VT) Model EL312 kinetic reader. A standard curve was obtained by plotting the absorbance versus the corresponding concentrations of the supplied standards.
Nitrite/Nitrate Assay
The level of nitrite/nitrate in plasma samples was assessed using the Greiss reagent assay. Nitrate and nitrite are formed non-enzymatically when nitric oxide is exposed to oxygen, thus plasma levels of these products indicate the level of nitric oxide production. Total nitrite/nitrate levels is determined by the conversion of nitrate to nitrite utilizing nitrate reductase in the presence of NADPH and flavin adenine dinucleotide, and then an assessment using Greiss reagent. Briefly, 6 µl of plasma diluted in 44 µl of dH2O is incubated for in the dark for 90-min with 10 µl of nitrate reductase (1.0 unit/ml), 20 µl of a 0.31 M phosphate buffer (ph 7.5), 10 µl of 0.86 mM NADPH (Sigma), and 10 µl of a 0.11 mM flavin adenine dinucleotide in individual wells of a 96-well plate. Then, 200 ml of Griess reagent consisting of a 1:1 (v/v) solution 1% sulfanilamide in 5.0% phosphoric acid and 0.1% N-(1-napthyl)ethyl-enedamine dihydrochloride in distilled water was added to the samples. The color developed for ten minutes at room temperature after which the absorbance was determined using a spectrophotometer set at 550 nm. All reactions were carried out in triplicate. The total micromolar concentration of nitrite is determined for each sample based on a standard curve. Recovery of nitrate is greater than 95% using this assay.
Statistical Analysis
Analysis of variance was performed on all data sets. When the overall ANOVA showed significant effects, post hoc comparisons were made using Tukey’s test to compare individual treatment groups to the home cage control. All analyses were conducted with the level of significance set at p < 0.05.
3. Results
D1 Receptor Antagonism
The first study investigated the effect of intra-BLA microinfusion of the D1 receptor antagonist, SCH23390, on the expression of heroin-induced conditioned suppression of proinflammatory mediators. Six days following the final conditioning session, animals were divided into groups with one group being re-exposed to the previously heroin-paired environment for 60 min (CS Exposed) while the control groups remained in the home cage. In order to temporarily block the receptors under investigation, animals in the SCH23390 groups received microinfusions of the SCH23390 compound directly into the BLA 30 minutes prior to testing of the conditioned response. The animals in the saline-treated groups received microinfusions of saline into the BLA.
Figure 1 shows the mean levels of LPS-induced iNOS mRNA expresssion in the spleen and liver for each group of animals. Analysis of iNOS copy number in spleen and liver, respectively, revealed a significant main effect of group [F(3,15)=9.885, P<0.01; F(3,15)=16.883, p<0.01]. Moreover, post-hoc analyses revealed a significant difference in iNOS mRNA copy number between the saline-treated animals exposed to the conditioned stimulus on test day (Saline, white bar) and the animals who remained in the home cage on test day (Saline, black bar). These differences were evident in both tissues (p<0.05) which reconfirms our earlier findings indicating that exposure to a previously heroin-paired environment suppresses the expression of iNOS mRNA. Most importantly, there were no significant differences between the saline-treated control group (Saline, black bar) and the SCH23390-treated CS-exposed group (SCH23390, white bar) for either tissue. These results demonstrate that antagonism of dopamine D1 receptors in the BLA blocks the conditioned effects of a previously heroin-paired environment on iNOS mRNA expression.
Figure 1.
Effect of treatments on LPS-induced expression of iNOS mRNA in the spleen and liver as determined by real-time RT-PCR. Exposure to the conditioned stimulus (Saline, white bar) resulted in a conditioned suppression of iNOS mRNA in both the spleen and liver. Intra-BLA microinfusion of the D1 receptor antagonist SCH23390 blocked the effect of the conditioned stimulus (SCH23390, white bar). Data are expressed as iNOS copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software.
The data in Figure 2 show levels of serum nitrite/nitrate levels for each group. The ANOVA revealed a significant main effect of procedure [F(3,15) = 10.789, P<0.01] on the levels of nitrite/nitrate in the serum. Post-hoc analyses revealed a significant difference between the saline-treated animals exposed to the conditioned stimulus on test day (Saline, white bars) and the animals who remained in the home cage on test day (Saline, black bars) (p<0.05). These results further support our earlier findings that exposure to a previously heroin-paired environment decreases serum nitrite/nitrate levels, indicating a reduction in nitric oxide production. There were no significant differences between the SCH23390-treated group exposed to the conditioned stimulus on test day (SCH23390, white bar) and the saline-treated control group (Saline, black bar) indicating that the antagonism of dopamine D1 receptors in the BLA attenuated the conditioned response. There were also no differences between the saline-treated control group (Saline, black bar) and the SCH23390-treated control group (SCH23390, black bar) indicating that administration of SCH23390 alone does not alter nitrate levels in the serum.
Figure 2.
Effect of treatments on serum nitrite/nitrate levels as determined by the Greiss reagent assay. Animals exposed to the conditioned stimulus on test day (Saline, white bar) exhibit a conditioned suppression of serum nitrite/nitrate concentration. This suppression is blocked by intra-BLA infusion of the dopamine D1 receptor antagonist SCH23390 (SCH23390, white bar). The data are expressed as the micromolar concentration of nitrite/nitrate.
Figure 3 illustrates that the suppressive effect of heroin on mRNA and protein levels of the pro-inflammatory cytokine, IL-1β, can be conditioned to environmental stimuli and this conditioned effect may be reduced by antagonism of dopamine D1 receptors within the BLA. Analysis revealed a significant main effect of treatment on mRNA levels in the spleen [F(3,15)=6.794, p<0.01] and liver [F(3,15)=8.929, p<0.01] as well as a main effect of treatment on protein levels in the spleen [F(3,15)=7.873, p<0.01] and liver [F(3,15)= 3.694, p<0.05]. In line with our previous experiments, the saline-treated conditioned group exposed to the CS (Saline, white bar) exhibited significantly lower levels of IL-1β mRNA and protein in both the spleen and liver (p<0.05) as compared to the saline-treated home cage control group (Saline, black bar). Most importantly, the SCH23390-treated CS-exposed group (SCH23390, white bar) was not significantly different from the saline-treated home cage control group (Saline, black bar) for either tissue indicating that inactivation of the BLA was able to attenuate the conditioned effects of heroin on IL-1β. There were also no significant differences between the SCH23390-treated control group and the saline-treated control group indicating that SCH23390-treatment alone did not have an effect on IL-1β mRNA or protein levels in the spleen or liver.
Figure 3.
Effect of treatments on LPS-induced expression of IL-1β mRNA and protein in the spleen and liver as determined by real-time RT-PCR and ELISA. CS-exposed animals (Saline, white bar) exhibit a conditioned suppression in both IL-1β mRNA and protein in both the spleen and liver that is blocked by intra-BLA administration of the D1 receptor antagonist, SCH23390 (SCH23390, white bar). mRNA data are expressed as IL-1β copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. ELISA data are expressed as pg of cytokine protein per mg of total protein.
Figure 4 shows that the suppressive effect of heroin on the proinflammatory cytokine, TNF-α, may be conditioned to environmental stimuli and, again, this conditioned effect is reduced by antagonism of dopamine D1 receptors within the BLA. This effect was evident at the mRNA and protein level in both spleen and liver tissue. Analysis revealed a main effect of treatment on mRNA and protein, respectively, in both spleen [F(3,15)=6.415, p<0.01; F(3,15)=9.027, p<0.01] and liver [F(3,15)=7.313, p<0.01; F(3,15)=9.996, p<0.01]. In line with our previous experiments, the saline-treated group exposed to the conditioned stimulus on test day exhibited significantly lower levels of TNF-α mRNA and protein in the spleen and liver (p<0.05) when compared to the saline-treated home cage control group. In addition, there were no significant differences between the saline-treated home cage control group and either of the SCH23390-treated groups.
Figure 4.
Effect of treatment on LPS-induced expression of TNF-α mRNA and protein in the spleen and liver as determined by real-time RT-PCR. Animals re-exposed to the CS on test day (Saline, white bars) exhibited a conditioned suppression of TNF-α mRNA and protein that was blocked by D1 receptor antagonism within the BLA (SCH23390, white bars). PCR data are expressed as TNF-α copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. ELISA data are expressed as pg of cytokine protein per mg of total protein.
D2 Receptor Antagonism
The second study investigated the effect of intra-BLA microinfusion of the D2 receptor antagonist, raclopride, on the expression of heroin-induced conditioned suppression of proinflammatory mediators. The experimental design followed that of the D1 receptor antagonist study with one group being re-exposed to the previously heroin-paired environment for 60 min on test day while the control groups remained in the home cage. In order to temporarily block the dopamine D2 receptors within the BLA, animals in the raclopride-treated groups received microinfusions of the D2 antagonist directly into the BLA 30 minutes prior to testing of the conditioned response. The animals in the saline-treated groups received microinfusions of saline into the same area.
Figure 5 shows the mean levels of LPS-induced iNOS mRNA expresssion in the spleen and liver for each group of animals. Analysis of iNOS copy number in spleen and liver, respectively, revealed a significant main effect of group [F(3,15)=7.173, P<0.01; F(3,15)=7.816, p<0.01]. Moreover, post-hoc analyses revealed a significant difference in iNOS mRNA copy number between the saline-treated animals exposed to the conditioned stimulus on test day (Saline, white bars) and the animals who remained in the home cage on test day (Saline, black bars). These differences were evident in both tissues (p<0.05) which reconfirms our earlier findings indicating that exposure to a previously heroin-paired environment suppresses the expression of iNOS mRNA. Interestingly, there was also a significant difference between the saline-treated home cage control group (Saline, black bars) and the raclopride-treated CS-exposed group (Raclopride, white bars) for both tissues. These results indicate that antagonism of dopamine D2 receptors in the BLA does not alter the conditioned effects of a previously heroin-paired environment on iNOS mRNA expression.
Figure 5.
Effect of treatments on LPS-induced expression of iNOS mRNA in the spleen and liver as determined by real-time RT-PCR. CS exposure (Saline, white bars) significantly reduced iNOS mRNA in both the spleen and liver and this effect was not blocked by intra-BLA D2 receptor antagonist administration (Raclopride, white bars). Data are expressed as iNOS copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software.
The data in Figure 6 show the mean levels of serum nitrite/nitrate for each experimental group. The ANOVA revealed a significant main effect of procedure [F(4,15) = 8.295, P<0.05] on serum levels of nitrite/nitrate. Post-hoc analyses revealed a significant difference between the saline-treated animals exposed to the conditioned stimulus on test day (Saline, white bar) and the animals who remained in the home cage (Saline, black bar) (p<0.05). These results are in line with our earlier experiments demonstrating that the suppressive effect of heroin on serum nitrite/nitrate levels may be conditioned to environmental stimuli. There was also a significant difference between the raclopride-treated group exposed to the conditioned stimulus on test day (Raclopride, white bar) and the saline-treated home cage control group (Saline, black bar) indicating that antagonism of dopamine D2 receptors within the BLA does not have an effect on the conditioned response to a previously heroin-paired environment.
Figure 6.
Effect of treatments on serum nitrite/nitrate levels as determined by the Greiss reagent assay. Exposure to the CS on test day resulted in a conditioned suppression of serum nitrite/nitrate levels (Saline, white bar) that was not affected by intra-BLA raclopride (D2 antagonist) administration (Raclopride, white bar). Data are expressed as the micromolar concentration of nitrite/nitrate.
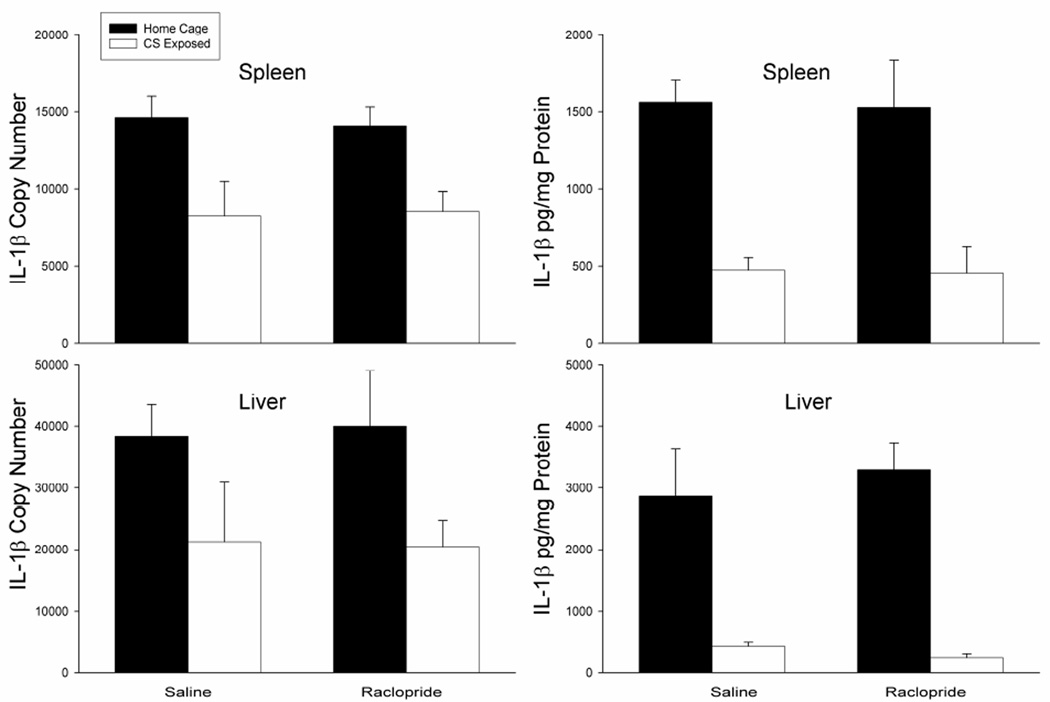
Figure 7 shows that the suppressive effect of heroin on mRNA and protein levels of the pro-inflammatory cytokine, IL-1β, can be conditioned to environmental stimuli and this conditioned response is not altered by antagonism of dopamine D2 receptors within the BLA. Analysis revealed a main effect of treatment on IL-1β mRNA levels in the spleen [F(3,15)=18.94, p<0.01] and liver [F(3,15)=5.601, p<0.05] as well as protein levels in the spleen [F(3,15)=34.440, p<0.01] and liver [F(3,15)=50.416, p<0.01]. In line with our previous experiments, the saline-treated CS-exposed group exhibited significantly lower levels of IL-1β mRNA and protein in the spleen and liver (p<0.05) as compared to the saline-treated home cage control group. Most importantly, the raclopride-treated CS-exposed group (Raclopride, white bars) also displayed signicantly lower levels of IL-1β mRNA in the spleen and liver as compared to the saline-treated home cage control group (Saline, black bars). These data suggest that antagonism of doapmine D2 receptors within the BLA does not alter the conditioned effects of heroin on IL-1β mRNA levels. There were also no significant differences between the raclopride-treated home cage control group and the saline-treated home cage control group indicating that raclopride-treatment alone did not have an effect on IL-1β mRNA or protein levels in the spleen or liver.
Figure 7.
Effect of treatments on LPS-induced expression of IL-1β mRNA in the spleen and liver as determined by real-time RT-PCR and IL-1β protein levels as determined by ELISA. Exposure to the CS on test day resulted in a suppression of IL-1b copy number and protein levels in both the spleen and liver (Saline, white bars). Antagonism of dopamine D2 receptors within the BLA did not block this effect (Raclopride, white bars). PCR data are expressed as IL-1β copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. ELISA data are expressed as pg of cytokine protein per mg of total protein.
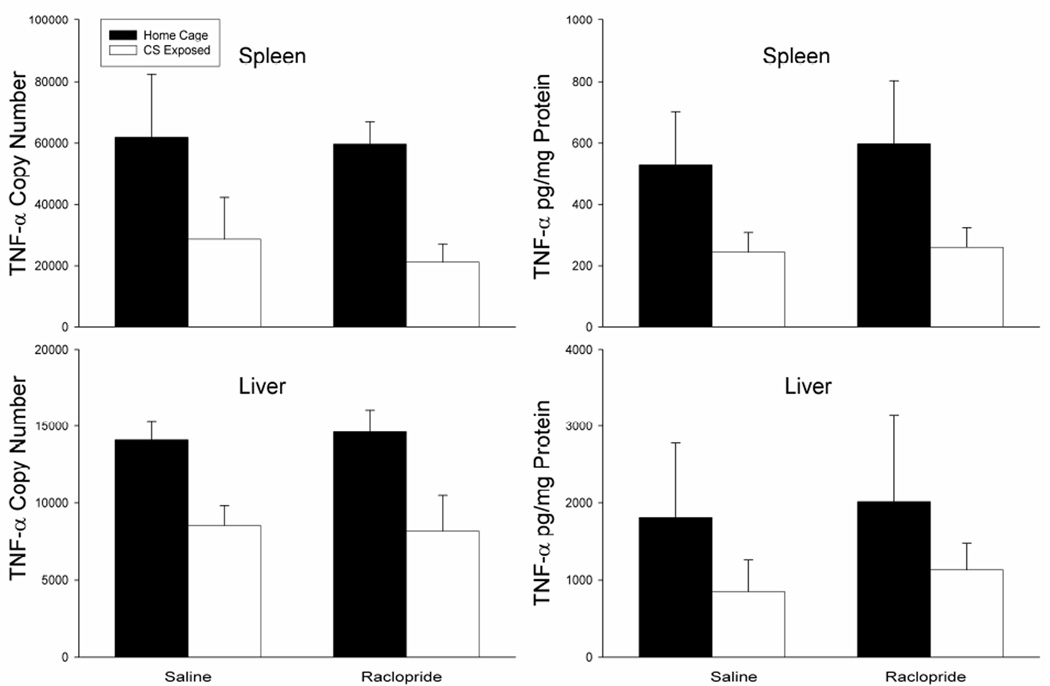
Figure 8 shows that the heroin-induced conditioned suppression of the proinflammatory cytokine, TNF-α, is not affected by antagonism of dopamine D2 receptors within the BLA. Analysis revealed a main effect of treatment on mRNA and protein in the spleen [F(3,15)=10.23, p<0.01; F(3,15)=6.567, p<0.05] and on mRNA in the liver [F(3,15)=18.936, p<0.01]. In line with our previous experiments, the saline-treated group exposed to the conditioned stimulus on test day (Saline, white bars) exhibited significantly lower levels of TNF-α mRNA and protein in the spleen and TNF-α mRNA in the liver (p<0.05) when compared to the saline-treated home cage control group (Saline, black bars). In addition, the raclopride treated group that was re-exposed to the conditioned stimulus on test day (Raclopride, white bars) also exhibited a conditioned reduction in TNF-α mRNA and protein in the spleen and mRNA in the liver as compared to the saline-treated control group (p<0.05). TNF-α mRNA levels in the liver showed this same trend, however, ANOVA revealed this was not statistically significant.
Figure 8.
Effect of treatments on LPS-induced expression of TNF-α mRNA and protein in the spleen and liver as determined by real-time RT-PCR. Animals re-exposed to the conditioned stimulus (Saline, white bars) showed a conditioned suppression in TNF Effect of treatments on LPS-induced expression of TNF-α mRNA and protein that was not altered by intra-BLA raclopride treatment (Raclopride, white bars). The PCR data are expressed as TNF-α copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. ELISA data are expressed as pg of cytokine protein per mg of total protein.
4. Discussion
The findings presented here demonstrate that dopamine within the basolateral amygdala plays a critical role in the conditioned effects of heroin on proinflammatory mediators. Specifically, these results revealed that antagonism of dopamine D1 receptors within the BLA blocks the expression of heroin-induced conditioned suppression of nitric oxide, TNF-α and IL-1β. The SCH23390 compound has been used extensively as a selective D1 receptor antagonist and the dose of 2.0 µg/side was chosen based upon an established literature showing an effect of this dose administered intra-BLA on cue-induced reinstatement to drug seeking (Berglind et al., 2006; Alleweireldt et al., 2006). Interestingly, selective antagonism of dopamine D2 receptors within this same region does not appear to have any effect as the animals exposed to the conditioned stimulus and administered the antagonist still exhibited a conditioned suppression in proinflammatory mediators. Collectively, these data indicate a role for dopamine D1, but not D2, receptors in the expression of heroin-induced conditioned immunomodulation. These data are in line with similar studies demonstrating that intra-accumbens SCH23390 blocks the effects of opioid drugs, and stimuli associated with opioid drugs, on NK cell activity and nitric oxide production (Saurer et al., 2006; Saurer et al., 2009).
The mesolimbic dopamine system is known to be critically involved in the expression of the rewarding properties of drugs of abuse, including opiates. In fact, heroin administration indirectly induces increased dopamine by the binding of its metabolites to mu-opioid receptors on GABA-ergic neurons within the ventral tegmental area. Dopamine has also been widely implicated in the formation of memories underlying associative learning processes. Specifically, dopamine and its receptors have been shown to mediate the learning of associations between drugs of abuse and their conditioned cues and antagonism of dopamine receptors may interfere with the acquisition or retrieval of reward-related memories. For example, microdialysis studies revealed an increase in dopamine and its metabolites during the learning of a discriminative task in rats (Hori et al., 1993) and upon the presentation of a stimulus that had previously been paired with cocaine administration (Weiss et al., 2000). In addition, intra-amygdalar 6-hydroxydopamine injections impaired (Ashford & Jones, 1976), while post-training intra-BLA dopamine infusions enhanced (LaLumiere et al., 2004) memory consolidation of a conditioned avoidance task. Furthermore, intra-BLA infusions of amphetamine were found to potentiate cue-induced drug-seeking behavior following extinction (Ledford et al., 2003). Taken together, these data suggest a role for dopamine in the BLA in heroin-induced classically conditioned immune alterations either through the mediation of neuroimmune pathways or indirectly through the alteration of learning/memory processes.
The study of classical or Pavlovian conditioning allows for the dissociation of the associative processes underlying the acquisition of a learned response from the mechanistic control of the expression of the response itself. In many cases these two phenomena are controlled via separate mechanisms. For example, antagonism of beta-adrenergic receptor activity disrupts the expression but not the acquisition of conditioned morphine-induced immune alterations (Coussons-Read et al., 1994). The data reported here suggest that dopamine D1 receptors within the BLA are required for the expression of heroin’s conditioned effects on proinflammatory mediators. However, it is unknown whether D1 receptors within the BLA might also be involved in the acquisition of these responses and this presents an important area for future investigation. Interestingly, there appears to be differential involvement of dopamine receptor subtypes within the BLA in the acquisition and the expression of responses to conditioned drug cues. Several subtypes of dopamine receptors have been characterized and the BLA has been shown to express both D1- and D2-like receptors (Meador-Woodruff et al, 1991; Scibilia et al, 1992).Macedo et al. (2007) demonstrated that antagonism of dopamine D1 receptors within the BLA reduces the expression of conditioned, but not unconditioned, fear. In addition, stimulation of dopamine D1 receptors in the BLA is necessary for the expression of cue-induced reinstatement of cocaine-seeking behavior in rats (e.g., See et al., 2001; Yun & Fields, 2003). In contrast to the role of D1 receptors, D2 receptors in the BLA appear to be involved in the acquisition of associations between drugs and their cues. For example, blocking dopamine D2 receptors in the BLA during the acquisition phase of conditioning results in alterations in the reinstatement of cue-induced cocaine-seeking behavior (Berglind et al., 2006). There has also been evidence for the involvement of dopamine D3 receptors within the BLA in reward learning as antagonism of these receptors was found to block reinstatement of drug seeking under a second-order schedule of reinforcement (Di Ciano, 2008). Based on the evidence from these studies it might also be important to examine the potential role of dopamine D2 and D3 receptors in the acquisition of associations between heroin and heroin-related cues that elicit immunomodulation.
The BLA receives many of its dopaminergic inputs from the ventral tegmental area (VTA) and substantia nigra as one of the main targets of the mesolimbic dopamine system (Rosenkranz & Grace, 1999). These inputs appear to be important for learning to associate the rewarding properties of drugs of abuse with external cues resulting in the ability of these drug cues to induce relapse to drug-seeking and other conditioned behaviors (DiCiano & Everitt, 2005; See, 2005). The data reported here suggest that opioids and opioid-associated drug cues induce dopamine release from neurons originating in the VTA which then binds to D1 receptors located within the BLA to influence the expression of conditioned responses to heroin related cues. Research has shown that the primary targets of mesolimbic dopaminergic neuron inputs to the BLA are the dendrites of the pyramidal neurons (Muller et al., 2009). Within the BLA there exist two distinct neuronal cell populations, pyramidal and non-pyramidal neurons. The pyramidal neurons of the BLA primarily utilize glutamate as their neurotransmitter and exhibit spiny projections while the non-pyramidal cells rely heavily upon inhibitory GABA signals and show fewer spines (McDonald, 1992). Dopamine selectively increases the excitability of BLA pyramidal neurons allowing for potentiation of the inputs from other brain regions, such as the cortex (Pickel et al., 2006). Interestingly, the pyramidal cell glutamatergic projections from the BLA have been shown to synapse in close proximity to dopamine axons on medium spiny neurons of the nucleus accumbens (Johnson et al., 1994; Kelley et al., 1982; Robinson & Beart, 1988) thus giving rise to a complex interaction between dopamine and glutamate within these two brain regions. In addition, electrophysiological studies have shown that tetanic stimulation of the BLA evokes dopamine efflux in the nucleus accumbens via glutamate receptor-dependent mechanisms localized within the accumbens (Floresco et al., 1998). Furthermore, either exogenously applied or synaptically released dopamine can modulate excitatory responses of nucleus accumbens neurons evoked by low frequency stimulation of the BLA (Yim & Mogenson, 1982; 1986). Collectively, these data suggest that the circuit encompassing the VTA, BLA and nucleus accumbens may be involved in the conditioned effects of heroin on immune measures.
While it appears that the BLA does not directly impact immune functioning it is highly plausible that conditioned immune alterations may be induced through the connections of the BLA with the nucleus accumbens.Saurer et al. (2006a) demonstrated that intra-accumbens shell administration of a dopamine D1 agonist resulted in decreased NK cell activity indicating that dopamine activity within the accumbens is sufficient for expression of heroin-induced conditioned immunomodulation. It is as yet unknown what factors may be mediating these effects in the periphery but there is a wealth of data suggesting that the sympathetic nervous system may be involved. For example, both primary and secondary lymphoid tissues have been found to be innervated by the sympathetic nerve fibers (Williams et al., 1981; Felten et al., 1984) and adrenergic receptors have been identified on the surface of immune cells (Livnat et al., 1985; Hori et al., 1995; Maestroni, 2006). More specifically, peripheral administration of β-adrenergic antagonists has been shown to block the expression of the conditioned effects of morphine on several measurements of immune status (Coussons-Read et al., 1994). However, it appears that at least some of these immune alterations are mediated peripherally by neuropeptide Y as systemic administration of a neuropeptide Y Y1 receptor antagonist dose-dependently attenuates morphine-induced alterations in NK cell activity while β-adrenergic antagonism had no effect (Saurer et al., 2006b). These data seem to suggest differential mechanisms for the control of specific opioid-induced immune alterations. It is imperative to understand the mechanisms by which heroin-induced conditioned immunomodulation occurs as these effects may have widespread implications for the health of recovering opiate users. Nitric oxide, TNF-α and IL-1β are all components of the innate immune response that play critical roles in the host’s initial response to pathogenic challenge. In the absence of a proper immune response, such as occurs following exposure to drug-related cues, the host’s immune system may not be able to adequately deal with infection. This disruption of immune functioning may allow for increased pathogenic proliferation which may progress to sepsis. The exact neural mechanism through which heroin-induced conditioned immunomodulation occurs is still not fully understood, but the data presented here suggests that the BLA plays a critical role. The experiments reported here are the first to identify the dopaminergic D1 receptors within the BLA as crucial for the expression of the effects of heroin-related cues on immune functioning.
Acknowledgement
This research was supported by National Institute on Drug Abuse grant DA25667. JLS was also supported by a National Research Service Award (DA21467) from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31(2):363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Ashford J, Jones BJ. The effects of intra-amygdaloid injections of 6-hydroxydopamine on avoidance responding in rats. Br J Pharmacol. 1976;56(3):255–261. doi: 10.1111/j.1476-5381.1976.tb07636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaletova T, Brouet I, Bartsch H, Sugimura T, Esumi H, Ohshima H. Immunohistochemical localization of an inducible form of nitric oxide synthase in various organs of rats treated with Propionibacteriumacnes and lipopolysaccharide. Apmis. 1993;101:330–336. [PubMed] [Google Scholar]
- Benscsics A, Elenkov IJ, Vizi ES. Effect of morphine on lipopolysaccharide-induced tumor necrosis factor-alpha in vivo:involvement of the sympathetic nervous system. J Neuroimmunol. 1997;73(1–2):1–6. doi: 10.1016/s0165-5728(96)00163-4. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the BLA differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137(2):699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Chao CC, Molitor TW, Close K, Hu S, Peterson PK. Morphine inhibits the release of tumor necrosis factor in human peripheral blood mononuclear cell cultures. Int J Immunopharmacol. 1993;15:447–453. doi: 10.1016/0192-0561(93)90057-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acide guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain. 2007;3:28. doi: 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons ME, Dykstra LA, Lysle DT. Pavlovian conditioning of morphine-induced alterations of immune status: Evidence for peripheral β-adrenergic receptor involvement. Brain, Behavior, & Immunity. 1994;8:204–217. doi: 10.1006/brbi.1994.1019. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol. 2005;526:186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122(1):129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Nicholson JKA, Madden JJ, Donahoe F, Shafer DA, Gordon D, Bokos P, Falek A. Coordinate and independent effects of heroin, cocaine and alcohol abuse on T-cell E-rosette formation and antigenic marker expression. Clin Immunol. 1986;41:254–264. doi: 10.1016/0090-1229(86)90109-1. [DOI] [PubMed] [Google Scholar]
- Farizio KM, Buehler JW, Chamberland ME, Whyte BM, Froelicher ES, Hopkin SG, Reed CM, Mokotoff ED, Cohn DL, Troxler S, Phelps AF, Berkelman RL. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA. 1992;267:1798–1805. [PubMed] [Google Scholar]
- Fecho K, Nelson CJ, Lysle DT. Phenotypic and functional assessments of immune status in the rat spleen following acute heroin treatment. Immunopharmacology. 2000;46:193–207. doi: 10.1016/s0162-3109(99)00175-7. [DOI] [PubMed] [Google Scholar]
- Felten DL, Livnat S, Felten SY, Carlson SL, Bellinger DL, Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13(6):693–699. doi: 10.1016/0361-9230(84)90230-2. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdale stimulation evokes glutamate receptor-dependent dopamine efflux in the NAC of the anesthetized rat. European Journal of Neuroscience. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Govitrapong P, Suttitum T, Kotchabhakdi N, Uneklabh T. Alterations of immune functions in heroin addicts and heroin withdrawal subjects. J Pharmacol Exp Ther. 1998;266:417–423. [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Tanaka J, Nomura M. Effects of discrimination learning on the rat amygdala dopamine release: a microdialysis study. Brain Res. 1993;621(2):296–300. doi: 10.1016/0006-8993(93)90119-8. [DOI] [PubMed] [Google Scholar]
- Hori T, Katafuchi T, Take S, Shimizu N, Niijima A. The autonomic nervous system as a communication channel between the brain and immune system. Neuroimmunomodulation. 1995;2(4):203–215. doi: 10.1159/000097198. [DOI] [PubMed] [Google Scholar]
- Hussey HH, Katz S. Infections resulting from narcotic addiction: report of 102 cases. Am J Med. 1950;9:186–193. doi: 10.1016/0002-9343(50)90021-0. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat NAC: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61(4):851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta JH. The amygdalo-striatial projection in the rat: An anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Immune function in heroin addicts and former heroin addicts in treatment: Pre- and post-AIDS epidemic. NICA Res. Mono. 1990;96:192–219. [PubMed] [Google Scholar]
- Lalumiere RT, Nguyen LT, McGaugh JL. Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. Eur J Neurosci. 2004;20(10):2804–2810. doi: 10.1111/j.1460-9568.2004.03744.x. [DOI] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28(10):1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- Livnat S, Felten SY, Carlson SL, Bellinger DL, Felten DL. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985;10(1):5–30. doi: 10.1016/0165-5728(85)90031-1. [DOI] [PubMed] [Google Scholar]
- Louria DB, Hensle T, Rose J. The major medical complication of heroin addiction. Ann Int Med. 1967;67:1–27. doi: 10.7326/0003-4819-67-1-1. [DOI] [PubMed] [Google Scholar]
- Luttgens WF. Endocarditis in “main line” opium addicts. Arch Int Med. 1949;83:653–664. doi: 10.1001/archinte.1949.00220350063005. [DOI] [PubMed] [Google Scholar]
- Lysle DT, How T. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharmacology. 2000;46:181–192. doi: 10.1016/s0162-3109(99)00172-1. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Ijames SG. Heroin-associated environmental stimuli modulate the expression of inducible nitric oxide synthase in the rat. Psychopharmacology. 2002;164:416–422. doi: 10.1007/s00213-002-1208-x. [DOI] [PubMed] [Google Scholar]
- Macedo CE, Martinez RC, Albrecht-Souza L, Molina VA, Brandão ML. 5-HT2- and D1-mechanisms of the basolateral nucleus of the amygdale enhance conditioned fear and impair unconditioned fear. Behav Brain Res. 2007;177(1):100–108. doi: 10.1016/j.bbr.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann NY Acad Sci. 2006;1069:195–207. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res Bull. 1992;28(2):179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonough RJ, Madden JJ, Falek A, Shafer DA, Pline M, Gordon D, Bokos P, Kuehnle JC, Mendelson J. Alteration of T and null lymphocyte frequencies in the peripheral blood of human opiate addicts: in vivo evidence for opiate receptor sites on T lymphocytes. J Immunol. 1980;125:2539–2543. [PubMed] [Google Scholar]
- McLachlan C, Crofts N, Wodak A, Crowe S. The effects of methadone on immune function among injecting drug users: A review. Addiction. 1993;88:257–263. doi: 10.1111/j.1360-0443.1993.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5(4):231–242. [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Struct Funct. 2009;213(3):275–288. doi: 10.1007/s00429-008-0196-y. [DOI] [PubMed] [Google Scholar]
- Nair MPN, Laing TJ, Schwartz SA. Decreased natural and antibody-dependent cellular cytotoxic activities in intravenous drug users. Clin Immunol. 1986;38:68–78. doi: 10.1016/0090-1229(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Novick DM, Oschorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, Kreek MJ. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J Pharmacol Exp Ther. 1989;250:606–610. [PubMed] [Google Scholar]
- Oschorn M, Novick DM, Kreek MJ. In vitro studies of the effects of methadone on natural killer cell activity. Isr J Med Sci. 1990;26:421–425. [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P. Pharmocokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol. 2000;22:603–614. doi: 10.1016/s0192-0561(00)00023-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pickel VM, Colago EE, Mania I, Molosh AI, Rainnie DG. Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically evoked N-methyl-D-aspartic acid currents in rat basolateral amygdala. Neuroscience. 2006;142(3):671–690. doi: 10.1016/j.neuroscience.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Quaglio G, Pajusco B, Civitelli P, Migliozzi S, Des Jarlais D, Romano L, Lechi A, Mezzelani P, Lugoboni F. Immunogenicity, reactogenicity and adherence with hepatitis A vaccination among drug users. Drug and Alchol Dependence. 2004;74(1):85–88. doi: 10.1016/j.drugalcdep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Robinson TG, Beart PM. Excitant amino acid projections from rat amygdala and thalamus to NAC. Brain Res Bull. 1988;20(4):467–471. doi: 10.1016/0361-9230(88)90136-0. [DOI] [PubMed] [Google Scholar]
- Rodrigo JM, Serra MA, Aparisi L, Escudero A, Gilabert MS, Garcia F, Gonzalez R, del Olmo JA, Wassel AH, Artero A. Immune response to hepatitis B vaccine in parenteral drug abusers. Vaccine. 1992;10(11):798–801. doi: 10.1016/0264-410x(92)90516-m. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19(24):11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Morphine-induced alterations of immune status are blocked by the dopamine D2-like receptor agonist 7-OH-DPAT. Journal of Neuroimmunology. 2004;148:54–62. doi: 10.1016/j.jneuroim.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. J Neuroimmunol. 2006a;173(1–2):3–11. doi: 10.1016/j.jneuroim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT. Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity. J Neuroimmunol. 2006b;177(1–2):18–26. doi: 10.1016/j.jneuroim.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT. Evidence for the nucleus accumbens as a neural substrate of heroin-induced immune alterations. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibilia RJ, Lachowicz JE, Kilts CD. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992;11(2):146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the BLA attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154(3):301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everritt BJ, Jarrard LE, Robbins TW. Complementary roles for amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neursoscience. 1991;42(2):335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Suschek CV, Schnorr O, Kolb-Bachofen V. The role of iNOS in chronic inflammatory processes in vivo: Is it damage-promoting, protective, or active at all? Curr Mol Med. 2004;4:763–765. doi: 10.2174/1566524043359908. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT. Conditioned effects of heroin on the expression of inducible nitric oxide synthase in the rat are susceptible to extinction and latent inhibition. Psychopharmacology. 2007;191:879–889. doi: 10.1007/s00213-006-0673-z. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT. Conditioned effects of heroin on proinflammatory mediators require the basolateral amygdala. European Journal of Neuroscience. 2008;28(9):1867–1876. doi: 10.1111/j.1460-9568.2008.06472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, Chandra M, Tuteja R, Misra MK. Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. J. Biomed Biotechnol. 2004;2004:227–237. doi: 10.1155/S1110724304402034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonada-Vlaar CS, Parson LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97(8):4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Peterson RG, Shea PA, Schmedtje JF, Bauer DC, Felten DL. Sympathetic innveration of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull. 1981;6(1):83–94. doi: 10.1016/s0361-9230(81)80072-x. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Mesolimbic dopamine projection modulates amygdale-evoked EPSP in NAC neurons: An in vivo study. Brain Research. 1986;369:347–352. doi: 10.1016/0006-8993(86)90548-2. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. BLA lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]