Abstract
Major Depressive Disorder is a devastating mental disorder. Current antidepressant medications can be effective for some patients with depression, however these drugs exert mood-elevating effects only after prolonged administration and a sizable fraction of the patient population fails to respond to treatment. There is an urgent need for faster-acting antidepressants with reliable treatment outcomes and sustained efficacy that could impact individuals with depression in particular those contemplating suicide. Recent clinical studies report that ketamine, an ionotropic glutamatergic N-methyl-d-aspartate (NMDA) receptor blocker, shows fast-acting antidepressant action, thus bringing fresh insight into preclinical studies investigating novel antidepressant targets and treatments. Our recent studies show that the effects of ketamine are dependent on brain-derived neurotrophic factor (BDNF) and subsequent activation of the high affinity BDNF receptor, TrkB. Our findings also suggest that the fast-acting antidepressant effects of ketamine require rapid protein translation, but not transcription, resulting in robust increases in dendritic BDNF protein levels that are important for the behavioral effect. These findings also uncover eukaryotic elongation factor 2 kinase (eEF2K), a Ca2+/calmodulin dependent serine/threonine kinase that phosphorylates eEF2 and regulates the elongation step of protein translation, as a major molecular substrate mediating the rapid antidepressant effect of ketamine. Our results show that ketamine-mediated suppression of resting NMDA receptor activity leads to inhibition of eEF2 kinase and subsequent dephosphorylation of eEF2 and augmentation of BDNF synthesis. This article will outline our recent studies on the synaptic mechanisms that underlie ketamine action, in particular the properties of eEF2K as a potential antidepressant target.
Keywords: Antidepressant, NMDA receptors, neuronal signaling, glutamate, spontaneous neurotransmission, eEF2
Major Depressive Disorder and the need for faster-acting antidepressants
Major Depressive Disorder is one of the most common mental disorders. Current estimates suggest that the 12-month prevalence of depression is ~6.7% of the US adult population with a larger percentage of the population affected by milder forms of the disorder [1]. Depression can be effectively treated with any of several antidepressant medications or electroconvulsive shock (ECS) in some individuals. Serotonin selective reuptake inhibitors (SSRIs) are the most widely prescribed antidepressants but require prolonged administration (several weeks to months) to trigger antidepressant efficacy in treatment responders. The mechanism of action of SSRIs, and other conventional antidepressants, is not well understood past their acute effects, which has impacted the development of new and improved antidepressants. There is an urgent need for faster-acting antidepressants that could impact not only individuals with depression but also other patient populations including individuals with bipolar illness and those contemplating suicide. Indeed, suicide is a major public health problem in the US accounting for over 34,000 deaths in 2007 with an overall rate of 11.3 completed suicides per 100,000 people [2]. A drug with rapid antidepressant action would be an improvement to traditional antidepressants in treating suicide.
A treatment advance for antidepressant medication would be expected to fulfill three main criteria for effective use. First, antidepressants should act fast, delivering symptomatic relief within hours to days. This issue is especially important for suicidal patients who typically need emergency attention. Second, medications with antidepressant action should produce their effects in a predictable manner by producing positive outcomes in a large fraction of the patient population within a well-defined time frame with limited side effects. Finally, these medications would ideally provide sustained relief over the long-term to help patients successfully reintegrate into society.
Recent studies offer hope with respect to the future development of faster-acting antidepressants with reliable treatment outcomes and sustained efficacy. Clinical studies report that ketamine, an ionotropic glutamatergic NMDA receptor antagonist, and scopolamine, a muscarinic acetylcholine receptor blocker show rapidly acting antidepressant action, thus bringing fresh insight into preclinical studies investigating novel antidepressant targets and treatments [3-6]. While there is a great deal of enthusiasm about these compounds, and importantly they provide a proof of principle that it is possible to generate a rapid antidepressant response in a patient population, there are also questions about their abuse liability and psychomimetic effects. This review will outline our recent studies focused on understanding the synaptic mechanisms that underlie these fast-acting antidepressant effects, in particular the molecular basis of ketamine action.
Ketamine as a fast-acting antidepressant
An exciting and rather unexpected finding in the field of depression has been the demonstration that ketamine has rapid and long-lasting antidepressant effects in depressed individuals [3, 5, 6]. A single, low-dose intravenous infusion of ketamine has been shown to alleviate symptoms of depression within two hours with effects lasting up to two weeks in patients with Major Depressive Disorder as well as bipolar patients. In these studies ketamine appeared to be relatively safe and well tolerated by patients with no evidence of the treatment producing psychomimetic effects. However, it remains unclear whether ketamine can be used routinely without encountering potential adverse effects.
It is intriguing that an NMDA receptor antagonist would mediate an antidepressant response since NMDA receptor activation is typically critical for triggering various forms of synaptic plasticity, including long-term potentiation, and learning processes [7]. While there is a large body of literature suggesting increased baseline glutamatergic transmission may be involved in depression and stress-related behaviors [8], it remains unclear whether suppression of this increase in baseline glutamatergic neurotransmission is the main end point of ketamine in triggering an antidepressant effect. In recent work we set out to investigate the mechanism that triggers ketamine's fast-acting antidepressant effects. Our studies have revealed that the mechanism necessary to elicit the effects of ketamine share a number of strong similarities to mechanisms that underlie homeostatic synaptic plasticities triggered after blockade of glutamatergic neurotransmission in central synapses [9]. Importantly, the mechanisms that triggers ketamine's antidepressant effect and those involved in homeostatic synaptic plasticities elicit augmentation of synaptic efficacy that compensates for the suppression of neurotransmission induced by activity blockade and/or inhibition of postsynaptic ionotropic receptors. In our recent work [10] we found that ketamine administration to hippocampal slices in the absence of stimulation elicited a potentiation of synaptic transmission mediated by AMPA receptors. A better understanding of the mechanism that underlies this synaptic potentiation may uncover a novel target for future antidepressant design.
eEF2 kinase as a target of ketamine action
We have recently demonstrated that ketamine elicits a fast-acting antidepressant response in mice following chronic unpredictable stress [10]. We also found that ketamine as well as other NMDA receptor antagonists, MK801 or CPP, produce fast-acting antidepressant behavioral effects in naïve mice [10]. The effects of these NMDA receptor antagonists are dependent on BDNF and the subsequent activation of the high affinity BDNF receptor, TrkB, as these effects are lost in inducible BDNF knockout mice as well as in conditional TrkB knockout mice. Our findings also suggest that the fast-acting antidepressant effects require protein translation, but not transcription, resulting in rapid increases in dendritic BDNF protein levels that are important for the behavioral effect. BDNF is a well-characterized neurotrophin linked to the action of traditional antidepressant compounds as BDNF expression in the hippocampus is increased by antidepressant treatment [11] and BDNF deletion in the hippocampus impairs behavioral responses elicited by administration of classical antidepressants [12, 13]. Moreover, a single intraventricular or intrahippocampal BDNF infusion causes rapid and sustained antidepressant effects in the forced swim test lasting up to 6 days [14, 15]. These observations implicate BDNF-dependent signaling as a common pathway where classical antidepressant action and the fast-acting effects of ketamine merge. However, a single application of ketamine can elicit an elevation in BDNF in a short time frame (~ 30 min.) [10], while classical antidepressants require repeated administration to reach the same endpoint [16]. BDNF, in turn, may act on several downstream signaling cascades that impact synaptic plasticity as well as overall neuronal activity [10, 16].
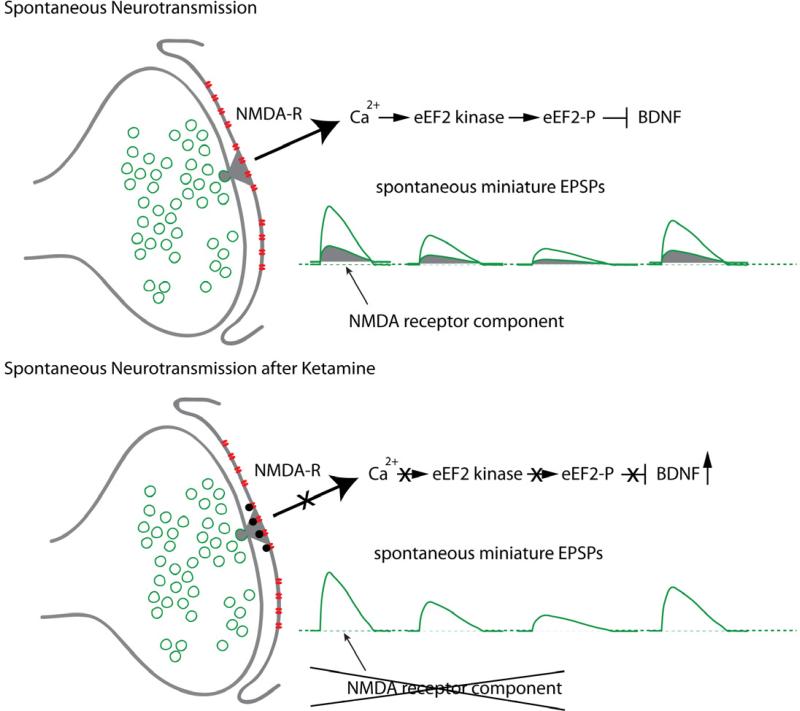
This rather swift action of ketamine on BDNF translation is in line with recent work which suggests a strong causal link between blockade of resting NMDA receptor activation and rapid increases in local dendritic protein translation [17, 18] (Figure 1). In agreement with these in vitro studies, we find that ketamine causes a decrease in phosphorylation of eukaryotic elongation factor 2 (eEF2), which normally impedes protein translation, suggesting translational de-repression of BDNF mRNA. Importantly, we find that inhibitors of the eEF2 kinase (e.g., rottlerin or NH125), which prevent eEF2 phosphorylation, also trigger a fast-acting antidepressant-like effect in mice. These findings suggest a behavioral and clinically relevant correlate of dendritic translational de-repression through blockade of NMDA receptors at rest and highlight eEF2-kinase-dependent regulation of BDNF transmission as a potential target for antidepressant action [10].
Figure 1.
Synaptic mechanism underlying ketamine action. Top: Under resting conditions, spontaneous glutamate release and NMDA receptor activation leads to activation of eEF2 kinase triggering eEF2 phosphorylation and silencing BDNF translation. Bottom: Ketamine-mediated use-dependent blockade of tonic NMDA receptor activity at rest ceases activation of eEF2 kinase resulting in a gradual loss of eEF2 phosphorylation and de-suppression of BDNF translation.
A key aspect of NMDA receptor activity and eEF2 kinase regulation is its specificity toward distinct forms of neurotransmission. Within the brain there are two forms of neurotransmission, evoked transmission in which neurotransmitter is released in response to action potentials, and spontaneous transmission that occurs independent of action potentials. Evoked neurotransmission is presynaptic action potential firing driven neurotransmitter release that then acts on postsynaptic receptors to mediate specific effects on intracellular signaling cascades. Spontaneous neurotransmission occurs as a result of a low but not negligible probability that a synaptic vesicle will fuse with the presynaptic membrane. Recent work has suggested that spontaneous neurotransmitter release activates postsynaptic signaling pathways and postsynaptic receptors distinct from evoked neurotransmission [19]. Moreover, NMDA receptor antagonists have been shown to augment dendritic protein synthesis via blockade of spontaneous but not evoked transmission. Spontaneous NMDA receptor activation triggers eEF2K to phosphorylate eEF2 and releases this factor from the translational machinery thereby effectively halting translation, whereas acute NMDA antagonist treatment inhibits this tonic eEF2K activity leading to dephosphorylation of eEF2 thus increasing translation of target transcripts [10, 20].
eEF2 kinase as regulator of dendritic protein translation
Our recent studies highlight eEF2K as a major molecular substrate mediating the rapid antidepressant effect of ketamine. eEF2K is a Ca2+/calmodulin-dependent serine/threonine kinase important for the regulation of elongation of protein translation. eEF2K is a member of the atypical alpha-kinase family [21]. The alpha-kinase family is unusual because members of this family can phosphorylate serine and threonine residues found in α-helices on the substrate protein [22] whereas typical kinases phosphorylate residues within loops, β-turns, or unstructured domains. eEF2K contains three functional domains, an N-terminal calmodulin-binding domain (aa51-96), an α-kinase catalytic domain immediately downstream (aa100-350), and an eEF2 binding domain in the C-terminus (aa521-725) [23]. eEF2K specifically phosphorylates eEF2, which represses eEF2 activity. Active eEF2 is important for the elongation step of protein translation; it catalyzes the translocation of peptidyl-tRNA from the A-site to the P-site on the eukaryotic ribosome to allow for the addition of a new amino acid to a growing polypeptide strand. The phosphorylation of eEF2 at threonine (Thr) 56 by eEF2K causes eEF2 to release from the ribosome, halting translation elongation of most proteins [24, 25]. However, increased translation of αCaMKII, MAP1B and other proteins has been associated with phosphorylation of eEF2 [26-30]. There are many ways in which eEF2K activity is regulated (see Table 1).
Table 1.
Regulation of eEF2K by phosphorylation and the effect on eEF2K kinase activity
| Stimulus | Phosphorylation of eEF2K | Effect on eEF2K kinase activity | Reference |
|---|---|---|---|
| Ca2+/CaM binding | Yes, autophosphorylation at Thr348, Ser500 | Increase | [38] |
| AMPK | Yes, phosphorylation at Ser398 | Increase | [36] |
| PKA | Yes, phosphorylation at Ser499 | Increase | [37] |
| Decrease pH (7.4 to 6.8) | Yes, increased autophosphorylation | Increase | [21] |
| p70 S6 Kinase | Yes, phosphorylation at Ser366 | Decrease | [40] |
| p90 ribosomal S6 kinase | Yes, phosphorylation at Ser366 | Decrease | [40] |
Ca2+/CaM: calcium/calmodulin; AMPK: AMP-activated protein kinase; PKA: cAMP-dependent protein kinase A; Thr: Threonine; Ser: Serine
How does synaptic activity regulate eEF2K function and ultimately protein translation? eEF2K and eEF2 are both found in the post-synaptic compartment of dendrites, along with many other proteins necessary for protein translation, initiation, and elongation [27, 28, 31]. The presence of translational machinery at the synapse enables local protein synthesis following synaptic activity and mediates synapse specific long-term potentiation and long-term depression [31]. During synaptic transmission and plasticity a major source of calcium influx in dendrites is mediated by activation of NMDA receptors. Calcium entry from NMDA receptors has been well characterized as a key activator of numerous calcium calmodulin dependent substrates (e.g., CaMKII, calcineurin, nitric oxide synathase). Accordingly, earlier studies have shown that direct NMDA receptor activation elicits eEF2K function leading to eEF2 phosphorylation and inhibition of elongation [29]. Interestingly, inhibition of elongation by phospho-eEF2 can paradoxically increase translation of certain mRNAs but suppress others [29].
Synaptic activation of NMDA receptors can also increase eEF2K activity and lead to the inhibition of protein translation [27]. Calcium influx following NMDA receptor activation and NMDA receptor-mediated miniature excitatory post-synaptic currents (mEPSC) have been shown to cause an increase in eEF2 phosphorylation [20, 27, 32, 33]. Therefore, blocking NMDA receptor-mediated miniature neurotransmission reduces eEF2 phosphorylation in a synapse-specific fashion and mediates increases in local protein translation [20]. Another way the phosphorylation status of eEF2 is affected is by metabotropic glutamate receptor (mGluR) signaling [30]. eEF2K is physically associated with the group I mGluRs, including mGluR1 and mGluR5, by its binding to the postsynaptic scaffolding protein Homer [28]. It is important to note that eEF2K knockout mice are viable and fertile [21] supporting the notion that targeting eEF2K function for treatment advance may result in limited peripheral side-effects. These eEF2K knockout mice show a deficit in phosphorylated eEF2 coupled with deficits in mGluR receptor activation-mediated long-term synaptic depression [28], which requires dendritic protein translation [34]. These examples highlight the complexity of how synaptic activity can regulate eEF2 function and impact dendritic protein translation. Future studies that utilize a combination of both presynaptic and postsynaptic approaches are needed to better delineate the mechanisms controlling the eEF2 kinase pathway and ultimately its role in mediating rapid antidepressant effects.
Implications and Future Directions
The finding that ketamine elicits its antidepressant effect via its blockade of resting NMDA receptor-mediated neurotransmission has several implications for fundamental synaptic mechanisms underlying neuronal signaling. In particular, studies to date highlight a key role for spontaneous glutamate release mediated activation of eEF2 kinase as a substrate for ketamine action as well as an important regulator of synaptic efficacy. These findings bolster the need to investigate neuronal signaling at the level of single synapses to uncover previously untapped novel targets for potential treatment advance.
Despite the robust effect of currently available eEF2K inhibitors in preclinical animal models, their low specificity raises significant concerns about their clinical potential. Therefore, future design of more specific eEF2K inhibitors may alleviate some of the specificity concerns and result in drugs with better efficacy and fewer side effects. It is important to note that eEF2K is closely related to the alpha-kinase family that can exert other neuronal effects [21], which further emphasizes the need to specifically target eEF2 kinase and avoid impairing the functions other related kinases.
One advantage of directly targeting eEF2K is that it may bypass some side effects of NMDA receptor antagonists like ketamine, which at high dose or after repeated administration will lead to cognitive impairments including memory problems. The development of a fast-acting antidepressant without these potential caveats is a research area of critical importance. However, since eEF2K itself also has a broad range of substrates including but not limited to BDNF, this specific target may cause unforeseen side effects although the eEF2K knockout mice appear largely normal [21].
While there is a clear need for faster-acting antidepressants, there is also a need for these drugs to have sustained antidepressant efficacy. However, it is unclear whether other fast-acting antidepressants use a similar mode of action as ketamine to exert their effect. A salient example of a relatively rapidly acting antidepressant with a sustained effect is scopolamine. Scopolamine is muscarinic antagonist that produces rapid antidepressant effects in depressed patients with initial improvement observed within 3-5 days after acute treatment, although there were mild side effects reported. Importantly, in patients administered scopolamine the antidepressant effects persisted for at least 12-16 days after the final scopolamine administration. The fact that scopalamine blocks muscarinic acetylcholine receptors, which are G-protein coupled receptors, instead of ionotropic NMDA receptors, like ketamine, suggests that the molecular trigger for the behavioral effect is likely different. However, one would predict a downstream point of convergence between the effects of ketamine and scopolamine. Better understanding the synaptic mechanisms underlying the action of ketamine as well as scopolamine, including their potentially converging targets as well as differences, can guide future design of much needed rapidly acting, reliable antidepressants that can provide sustained relief to patients suffering from this currently intractable condition.
Acknowledgements
We thank members of the Monteggia and Kavalali laboratories for discussions and comments on the manuscript. This work was supported by NIH grants MH070727 (LMM) and MH066198 (ETK), as well as funding from the Brain & Behavior Research Foundation (LMM) and the International Mental Health Research Organization (LMM). Lisa Monteggia has been on speakers bureaus for Sepracor and Roche. Ege Kavalali and Erinn Gideons report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, N.C.f.I.P.a.C. Web-based Injury Statistics Query and Reporting System (WISQARS) www.cdc.gov/ncipc/wisqars.
- 3.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 7.Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 8.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 12.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 19.Kavalali ET, Chung C, Khvotchev M, Leitz J, Nosyreva E, Raingo J, Ramirez DM. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology (Bethesda) 2011;26:45–53. doi: 10.1152/physiol.00040.2010. [DOI] [PubMed] [Google Scholar]
- 20.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Ryazanov AG. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 2002;514:26–29. doi: 10.1016/s0014-5793(02)02299-8. [DOI] [PubMed] [Google Scholar]
- 22.Middelbeek J, Clark K, Venselaar H, Huynen MA, van Leeuwen FN. The alpha-kinase family: an exceptional branch on the protein kinase tree. Cell Mol Life Sci. 2010;67:875–890. doi: 10.1007/s00018-009-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pigott CR, Mikolajek H, Moore CE, Finn SJ, Phippen CW, Werner JM, Proud CG. Insights into the regulation of eukaryotic elongation factor 2 kinase and the interplay between its domains. Biochem J. 2012;442:105–118. doi: 10.1042/BJ20111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryazanov AG, Rudkin BB, Spirin AS. Regulation of protein synthesis at the elongation stage. New insights into the control of gene expression in eukaryotes. FEBS Lett. 1991;285:170–175. doi: 10.1016/0014-5793(91)80798-8. [DOI] [PubMed] [Google Scholar]
- 25.Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- 26.Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin P, Nastiuk KL, Daniel N, Girault JA, Czernik AJ, Glowinski J, Nairn AC, Premont J. Glutamate-dependent phosphorylation of elongation factor-2 and inhibition of protein synthesis in neurons. J Neurosci. 1997;17:3445–3454. doi: 10.1523/JNEUROSCI.17-10-03445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 30.Verpelli C, Piccoli G, Zibetti C, Zanchi A, Gardoni F, Huang K, Brambilla D, Di Luca M, Battaglioli E, Sala C. Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J Neurosci. 2010;30:5830–5842. doi: 10.1523/JNEUROSCI.0119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T. Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res. 2003;972:168–176. doi: 10.1016/s0006-8993(03)02523-x. [DOI] [PubMed] [Google Scholar]
- 32.Barrera I, Hernandez-Kelly LC, Castelan F, Ortega A. Glutamate-dependent elongation factor-2 phosphorylation in Bergmann glial cells. Neurochem Int. 2008;52:1167–1175. doi: 10.1016/j.neuint.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Scheetz AJ, Constantine-Paton M. NMDA receptor activation-responsive phosphoproteins in the developing optic tectum. J Neurosci. 1996;16:1460–1469. doi: 10.1523/JNEUROSCI.16-04-01460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 35.Arora S, Yang JM, Hait WN. Identification of the ubiquitin-proteasome pathway in the regulation of the stability of eukaryotic elongation factor-2 kinase. Cancer Res. 2005;65:3806–3810. doi: 10.1158/0008-5472.CAN-04-4036. [DOI] [PubMed] [Google Scholar]
- 36.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 37.Diggle TA, Subkhankulova T, Lilley KS, Shikotra N, Willis AE, Redpath NT. Phosphorylation of elongation factor-2 kinase on serine 499 by cAMP-dependent protein kinase induces Ca2+/calmodulin-independent activity. Biochem J. 2001;353:621–626. doi: 10.1042/0264-6021:3530621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyr Dit Ruys S, Wang X, Smith EM, Herinckx G, Hussain N, Rider MH, Vertommen D, Proud CG. Identification of autophosphorylation sites in eukaryotic elongation factor-2 kinase. Biochem J. 2012;442:681–692. doi: 10.1042/BJ20111530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavares CD, O'Brien JP, Abramczyk O, Devkota AK, Shores KS, Ferguson SB, Kaoud TS, Warthaka M, Marshall KD, Keller KM, et al. Calcium/calmodulin stimulates the autophosphorylation of elongation factor 2 kinase on Thr-348 and Ser-500 to regulate its activity and calcium dependence. Biochemistry. 2012;51:2232–2245. doi: 10.1021/bi201788e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]