Abstract
Background
Blood omega-3 and omega-6 fatty acid levels have been associated with reduced risk for total mortality in patients with stable coronary heart disease (CHD), but their relationships with mortality in the setting of myocardial infarction (MI) are unknown.
Objective
To determine the association between red blood cell (RBC) fatty acid levels measured at admission and 2-year mortality in MI patients, independent of the GRACE risk score, a traditional mode of risk stratification,
Design
Admission RBC fatty acid levels were measured in patients enrolled in a prospective, 24-center MI registry (TRIUMPH). Two-year mortality was modeled with Cox proportional hazards regression to assess the extent to which the inclusion of fatty acid levels would improve, over and above the GRACE score, risk stratification for 2-year mortality.
Results
RBC fatty acid data were available from 1,144 patients who did not report taking fish oil supplements after discharge. Two RBC fatty acids [eicosapentaenoic acid (EPA n-3) and docosapentaenoic n-6 (DPA)] were univariate predictors of total mortality. The combined fatty acid c-statistic (0.60, p<0.001) improved the c-statistic of the GRACE score alone from 0.747 (p<0.001) to 0.768 (p<0.05 vs. GRACE alone). The net reclassification index improved by 31% (95% CI, 15% to 48%) and the relative incremental discrimination index improved by 19.8% (7.5% to 35.7%).
Conclusion
RBC EPA and DPA n-6 levels improved the prediction of 2-yr mortality over and above the GRACE score in MI patients.
Introduction
Identifying patients at higher risk for death after myocardial infarction (MI) is a cornerstone of modern cardiovascular care1. The GRACE (Global Registry of Acute Coronary Events) score was developed to better risk-stratify patients admitted with an acute coronary syndrome (ACS) and identify those at increased risk for death over the next 6 months2. The c-statistic for the GRACE score was originally reported to be 0.75, but the extent to which it applies to longer term mortality or can be improved upon with other risk markers has received relatively little attention.
Several epidemiologic studies have linked higher fish intake, the primary source of the marine omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), with lower risks of sudden cardiac death3 and death from coronary heart disease (CHD)4. Higher blood levels of EPA + DHA are also associated with a lower risk for death from any cause5. In addition, two large post-MI randomized trials found that higher intake of fish6 and fish oil7 reduced all-cause mortality and improved cardiovascular (CV) prognosis. On the other hand, a recent 1-year German study in post-MI patients found no effect of 840 mg/d of omega-3 fatty acids on CV endpoints8, and a study using the same dose in dysglycemic patients reached the same conclusion9.
Heart cell membrane content of omega-3 fatty acids can be estimated by measuring red blood cell (RBC) fatty acid composition10;11 which has been shown to be a more stable marker of omega-3 status than plasma levels12. The primary known determinants of RBC omega-3 levels are EPA+DHA intake, fish oil supplementation, smoking and age with genetic factors explaining about 25% of the variability13. In light of the association between marine omega-3 fatty acid status and CV prognosis, and considering the fact that omega-3 tissue content is modifiable by diet or supplementation14;15, levels of specific fatty acids could be a valuable addition to risk stratification after MI, and could identify patients for novel treatment strategies to improve prognosis. Although a suite of RBC fatty acids (including certain omega-3, omega-6, trans, saturated and monounsaturated species) showed an improved ability to discriminate ACS cases from controls as compared with classic Framingham risk factors,16 the prognostic value of the RBC fatty acid patterns in patients presenting with an acute MI is unknown. The aim of the present study was to determine the extent to which admission RBC fatty acid levels 1) predicted 2-year mortality rates and 2) improved upon the GRACE score prediction in patients with acute MI.
Methods
Participants
The Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction Patient Health status (TRIUMPH) study is a prospective MI registry of 24-centers across the United States17). Inclusion criteria were age ≥ 18 years with biomarker evidence of myocardial injury (positive troponins or elevated creatinine kinase-MB fraction within 24 hours of hospital admission) and supporting evidence of an acute MI (either ischemic symptoms lasting for > 20 minutes or electrocardiographic ST changes). Patients were excluded if they were incarcerated, refused participation, were unable to provide consent, did not speak English or Spanish, were transferred to the participating hospital from another facility more than 24 hours after initial admission, or expired or were discharged prior to being contacted by the investigators.
Patients were enrolled in TRIUMPH from April 11, 2005 to December 31, 2008. Consecutive patients, enrolled between 04/2005 and 10/2007, who consented to supplemental blood work, also had their RBC membrane fatty acid levels measured and were included in this analysis. The study protocol was approved by the individual Institutional Review Boards of the participating centers and all participants provided written informed consent. Detailed clinical and treatment characteristics were collected by chart abstractions and standardized interviews performed between 24 and 72 hours after acute MI admission. Trained data collectors at each site acquired the requisite data. Vital status was determined by phone interviews and queries of the social security death master file. Disease-specific morbidity or mortality data were not available.
GRACE score
The GRACE risk score was developed in 15,007 and subsequently validated in 7,638 MI patients from 94 hospitals in 14 countries. In developing the score, many variables were considered: age, sex, medical history (8 items), prior medications (9 items), signs and symptoms at admission (7 items), and in-hospital medical treatments, procedures and complications (25 items). Of these, nine entered the final model: age; histories of myocardial infarction and heart failure; admission heart rate, systolic blood pressure, serum creatinine level; elevated initial serum cardiac biomarker level; ST-segment depression on presenting electrocardiogram; and percutaneous coronary intervention performed in hospital2. The GRACE risk score has been shown to provide good discrimination of 6-month mortality (c-statistic = 0.75), even with modern cardiac care, and is well-calibrated18.
Red blood cell membrane fatty acid levels
RBCs were obtained from ethylenediaminetetraacetic acid (EDTA) blood samples after removal of the plasma and buffy coat and stored at −70°C until thawed for analysis. Briefly, an RBC aliquot was heated at 100°C for 10 minutes with methanol containing 14% boron trifluoride. The fatty acid methyl esters thus generated were extracted with hexane and water and were analyzed via gas chromatography using a GC2010 (Shimadzu Corporation, Columbia, MD) equipped with a 30m capillary column (Omegawax 250, Supelco, Bellefonte, PA)19. FAs were identified through comparison with a standard fatty acid methyl ester mixture (GLC-727, Nuchek Prep, Elysian, MN). (Note: trans fatty acids were not measurable by this method). The coefficient of variations were <6% for all fatty acids of interest.
Other Laboratory Tests
Plasma lipids and lipoproteins were measured by the VAP test (Atherotech, Inc., Birmingham, AL). Troponin T was measured using Roche Elecsys Troponin T Immunoassay and hs-CRP was measured using Roche Tina-quant CRP (Latex) assay. Serum creatinine levels were determined by the Roche serum creatinine Jaffé method using rate-blanking and compensation.
Statistical Methods
Continuous variables are described as mean ± standard deviation (SD) and were compared using Mantel-Haenszel trend tests. Non-normal data are described as median [interquartile range (IQR)] and are compared using the Kruskal-Wallis test. Categorical variables are described as counts and percentages and were compared using the chi-square test, the Fisher exact test, or the Kruskal-Wallis test as appropriate.
RBC fatty acids of interest were chosen on the basis of their association with 2-year mortality after adjusting for the GRACE score. To best determine the functional relationship between 2-year mortality and RBC FAs we used Martingale residuals calculated from the Proportional Hazard model with the Grace Score as the lone predictor. The Martingale residual can be interpreted as the difference between the observed and the expected number of events under the assumed Cox model across the range of RBC fatty acid levels20. We then performed locally-weighted quadratic regression with a smoothing window of 67% predicting the Martingale residual on fatty acids of interest, as suggested by Klein and Moeschberger20. Based on these plots we determined whether the fatty acids of interest had an association with risk and whether that risk was best described as a linear, polynomial, or categorical relationship. We then plotted Kaplan-Meier curves based upon RBC fatty acid tertiles (for linear variables) or cutpoint (for non-linear variables) and computed a trend test p-value.
To determine the extent to which the fatty acids provided incremental information beyond the GRACE score, we tested nested proportional hazard models. Model 1 contained the GRACE risk score, and Model 2 contained GRACE plus the fatty acids of interest. To evaluate the potential improvement in the GRACE score prediction, we then followed the recommendations of Hlatky et al21. First we computed the independent significance of the fatty acid markers by Hazard Ratios and 95% CI’s. We then computed the survival c-statistics22 and tested them using the methods by Antolini23. Next, we computed the Integrated Discrimination Improvement (IDI) on both the absolute and relative scale, along with the continuous Net Reclassification Improvement (NRI), as described by Pencina24. Bootstrapping was used to obtain 95% confidence intervals. The IDI combines the increase in mortality probability provided by including the fatty acid marker for those experiencing an event plus the decrease in mortality probability for those not experiencing an event. Similarly, the continuous NRI is the net proportion of subjects getting correctly reclassified relative to the GRACE score alone. Lastly, we graphically inspected and computed calibration measures using deciles of predicted risk according to the approach of D’Agostino25. Statistical significance was defined as P<0.05. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
Results
A total of 4277 patients were enrolled in TRIUMPH, and the first 1517 had their admission omega-3 fatty acid levels measured. Baseline characteristics of the patients who had their fatty acids levels measured were similar to those who did not. Of the 1517 patients, 21% (n=320) reported commencing omega-3 supplementation after discharge and were removed from the analysis so that the baseline assessment would be a more accurate reflection of their chronic state. This resulted in a final cohort of 1,197 patients. Vital status at 2 years was available for 1,144 of which 135 (11.8%) had died.
GRACE Risk Score and 2-year Mortality
Although originally validated for 6-month survival, in this study the GRACE score was a significant predictor of 2-year mortality with a c-statistic of 0.75 (p<0.001), with a calibration chi square of 12.53 (p=0.13) indicating good calibration. A comparison of all demographic, behavioral, medical and treatment variables according to mortality status at 2 years is presented in Supplementary Table 1.
RBC Fatty Acids and 2-Year Mortality
RBC fatty acid differences between surviving and dead patients are shown in Table 1. Three fatty acids were significantly different in unadjusted analyses, EPA, the n-6 fatty acid docosapentaenoic acid (DPA), and the n-6 fatty acid dihomo-γ-linolenic acid (DGLA). Of these only EPA and DPA were significantly related to 2-year mortality after adjustment for the GRACE score (Table 1; see below), hence subsequent analyses focused only on these two.
Table 1.
Red blood cell fatty acids [median (IQR)] as predictors of 2 year mortality, alone and adjusted for GRACE score
| Status at 2 years | Fatty Acids Alone | With GRACE Score | ||||
|---|---|---|---|---|---|---|
| RBC Fatty Acid | Dead n = 135 | Alive n = 1009 | Hazard Ratio Unadjusted | Prop Hazard p-value Unadjusted | Hazard Ratio Adjusted | Prop Hazard p-value Adjusted |
| Myristic | 0.188 (0.148, 0.264) | 0.222 (0.148, 0.312) | 0.273 (0.073, 1.018) | 0.0531 | 0.443 (0.119, 1.64) | 0.2225 |
| Palmitic | 20.119 (18.510, 21.451) | 20.004 (18.477, 21.380) | 0.989 (0.923, 1.059) | 0.7504 | 0.992 (0.923, 1.066) | 0.8207 |
| Palmitoleic | 0.421 (0.300, 0.604) | 0.471 (0.331, 0.667) | 0.817 (0.596, 1.12) | 0.2097 | 0.861 (0.647, 1.146) | 0.3052 |
| Stearic | 15.558 (14.517, 16.725) | 15.815 (14.785, 17.002) | 0.942 (0.863, 1.028) | 0.1769 | 0.96 (0.882, 1.045) | 0.3455 |
| Oleic | 14.761 (13.621, 16.169) | 14.592 (13.379, 15.816) | 1.06 (0.992, 1.134) | 0.0869 | 1.028 (0.959, 1.102) | 0.4437 |
| Linoleic | 12.879 (11.789, 13.826) | 13.287 (11.803, 14.661) | 0.91 (0.842, 0.983) | 0.0169 | 0.957 (0.883, 1.037) | 0.2795 |
| γ-Linolenic | 0.080 (0.053, 0.116) | 0.091 (0.061, 0.132) | 2.158 (0.446, 10.434) | 0.3386 | 2.901 (0.705, 11.942) | 0.1401 |
| α-Linolenic | 0.159 (0.116, 0.191) | 0.158 (0.118, 0.215) | 1.123 (0.283, 4.456) | 0.8694 | 1.025 (0.224, 4.693) | 0.9746 |
| Eicosenoic | 0.148 (0.110, 0.214) | 0.135 (0.102, 0.186) | 2.38 (0.748, 7.567) | 0.1418 | 1.173 (0.318, 4.32) | 0.8108 |
| Eicosadienoic | 0.292 (0.217, 0.376) | 0.262 (0.211, 0.345) | 1.217 (0.756, 1.957) | 0.4190 | 1.334 (0.8, 2.224) | 0.2686 |
| Dihomo-γ-linolenic | 1.562 (1.284, 1.809) | 1.639 (1.388, 1.996) | 0.51 (0.346, 0.752) | 0.0007 | 0.678 (0.455, 1.008) | 0.0547 |
| Arachidonic | 20.195 (18.675, 22.039) | 19.959 (18.340, 21.470) | 1.07 (0.999, 1.146) | 0.0543 | 1.041 (0.973, 1.113) | 0.2486 |
| Eicosapentaenoic | 0.552 (0.382, 0.746) | 0.607 (0.408, 0.858) | 0.508 (0.302, 0.854) | 0.0106 | 0.524 (0.312, 0.88) | 0.0145 |
| Docosatetraenoic | 4.164 (3.480, 4.669) | 3.942 (3.329, 4.497) | 1.13 (0.99, 1.29) | 0.0697 | 1.109 (0.957, 1.285) | 0.1679 |
| Docosapentaenoic n-6 | 0.835 (0.654, 1.004) | 0.767 (0.616, 0.942) | 1.599 (1.038, 2.465) | 0.0334 | 2.002 (1.257, 3.187) | 0.0035 |
| Docosapentaenoic n-3 | 2.793 (2.368, 3.298) | 2.824 (2.400, 3.254) | 0.971 (0.772, 1.222) | 0.8029 | 1.041 (0.825, 1.313) | 0.7357 |
| Docosahexaenoic | 4.364 (3.469, 5.442) | 4.267 (3.445, 5.306) | 1.073 (0.958, 1.201) | 0.2246 | 1.004 (0.889, 1.134) | 0.9438 |
| Omega-3 Index* | 4.921 (3.994, 6.164) | 4.859 (3.981, 6.055) | 1.021 (0.924, 1.13) | 0.6788 | 0.968 (0.868, 1.08) | 0.5643 |
| n3/n6 ratio | 0.289 (0.239, 0.346) | 0.286 (0.245, 0.349) | 0.701 (0.123, 3.994) | 0.6887 | 0.479 (0.078, 2.953) | 0.4279 |
| n3HUFA/total HUFA† | 0.224 (0.193, 0.257) | 0.222 (0.197, 0.259) | 0.464 (0.018, 12.235) | 0.6455 | 0.214 (0.007, 6.402) | 0.3741 |
sum of eicosapentaenoic and docosahexaenoic acids;
highly unsaturated fatty acids, i.e., >C18 and >2 double bonds.
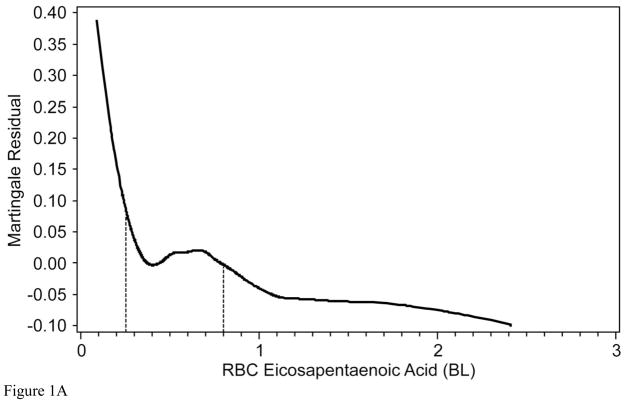
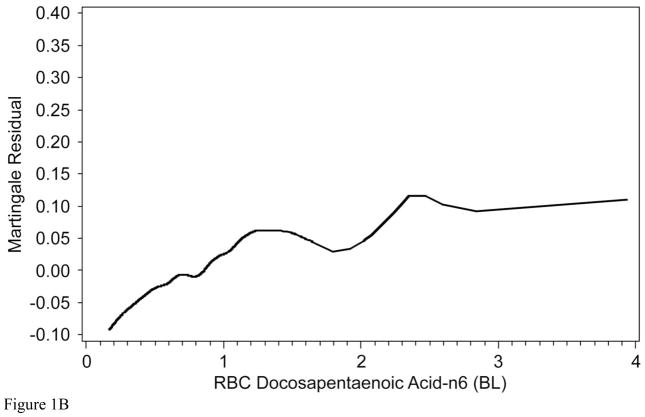
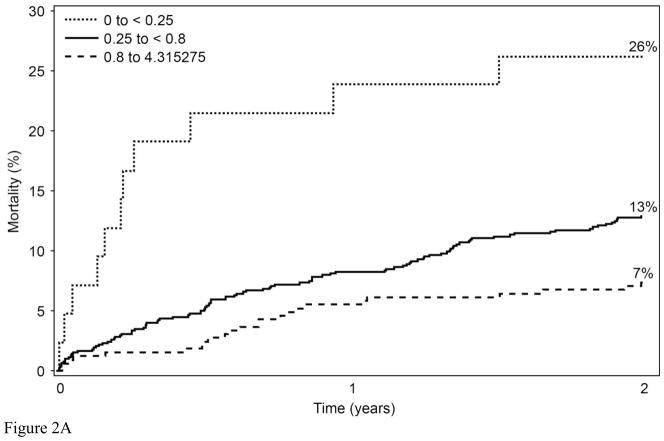
To determine whether the relationships with 2-year mortality for EPA and DPA were continuous or not, Martingale residuals were constructed (Figures 1a and 1b). Higher levels of EPA and lower levels of DPA were associated with a lower risk of death within 2 years (Table 1). For EPA the relationships were non-linear and suggested high-, intermediate-, and low-risk cutpoints (<0.25%, 0.25%–0.8%, and >0.8%, respectively). For DPA, the relationships were approximately linear. Based on these findings, patient characteristics (including demographics, clinical, lipid factors and GRACE score components) by category of RBC EPA (Table 2) and by tertile of RBC DPA (Table 3) were examined. The two fatty acids themselves were inversely related to each other. Kaplan-Meier curves for admission levels of both EPA by categories (Figure 2a) and DPA by tertiles (Figure 2b) showed significant unadjusted associations with 2-yr mortality. The GRACE score was not significantly related to levels of RBC EPA or DPA. EPA levels were inversely related to a history of chronic heart failure and directly related to high-density lipoprotein cholesterol levels and education. DPA was directly associated with serum creatinine and African American race and inversely with education. (A comparison of all demographic, behavioral, medical and treatment variables according to RBC EPA categories and to DPA tertiles is presented in Supplementary Tables 2 and 3, respectively).
Figure 1.
The Martingale residual plots show the difference between the observed and predicted (by GRACE score) 2-year mortality as a function of two RBC fatty levels: A) eicosapentaenoic acid (EPA) and B) n-6 docosapentaenoic acid (both expressed as a % of total RBC fatty acids). Values above 0 represent more deaths than predicted, and those below, fewer deaths than predicted. The relationship between EPA and risk was non-linear, therefore cut points (vertical lines) were selected to estimate high (<0.25%), intermediate (0.25% and 0.8%) and low risk categories (>0.8%). Therefore, EPA was modeled as a categorical variable. Relationships with DPA were relatively linear, therefore this fatty acid was modeled as a continuous variable.
Table 2.
Subject characteristics by category of Red Blood Cell (RBC) Eicosapentaenoic Acid (EPA, % total fatty acids)
| Total n = 1144 | 0 to <0.25% n = 42 | 0.25 to <0.8% n = 776 | 0.8 to 4.32% n = 326 | P-value | |
|---|---|---|---|---|---|
|
| |||||
| GRACE 6-mo Mortality Risk Score | 101.3 ± 30.6 | 102.7 ± 33.4 | 100.6 ± 31.4 | 102.8 ± 28.2 | 0.975 |
|
| |||||
| Age (yr) | 59.5 ± 12.5 | 58.6 ± 11.2 | 58.8 ± 12.9 | 61.3 ± 11.7 | 0.198 |
|
| |||||
| History of Chronic Heart Failure | 107 (9.4%) | 7 (16.7%) | 77 (9.9%) | 23 (7.1%) | 0.036 |
|
| |||||
| History of Myocardial Infarction | 244 (21.3%) | 9 (21.4%) | 169 (21.8%) | 66 (20.2%) | 0.613 |
|
| |||||
| Heart Rate (beats/min) | 82.6 ± 21.3 | 82.1 ± 18.9 | 83.5 ± 22.4 | 80.6 ± 18.7 | 0.666 |
|
| |||||
| Systolic Blood Pressure (mm Hg) | 141.2 ± 29.2 | 142.6 ± 24.2 | 140.7 ± 29.7 | 142.0 ± 28.6 | 0.906 |
|
| |||||
| ST-Depression on Admitting EKG | 419 (38.4%) | 15 (36.6%) | 281 (38.3%) | 123 (38.9%) | 0.773 |
|
| |||||
| Creatinine (mg/dL) | 1.2 ± 1.1 | 1.4 ± 1.5 | 1.2 ± 1.0 | 1.2 ± 1.2 | 0.249 |
|
| |||||
| In-Hospital PCI | 759 (66.3%) | 27 (64.3%) | 511 (65.9%) | 221 (67.8%) | 0.494 |
|
| |||||
| Sex | 0.708 | ||||
| Male | 753 (65.8%) | 30 (71.4%) | 503 (64.8%) | 220 (67.5%) | |
| Female | 391 (34.2%) | 12 (28.6%) | 273 (35.2%) | 106 (32.5%) | |
|
| |||||
| Race | 0.106 | ||||
| White/Caucasian | 809 (70.8%) | 28 (66.7%) | 535 (69.0%) | 246 (75.7%) | |
| Black/African-American | 250 (21.9%) | 10 (23.8%) | 186 (24.0%) | 54 (16.6%) | |
| Other | 83 (7.3%) | 4 (9.5%) | 54 (7.0%) | 25 (7.7%) | |
|
| |||||
| Education > High School | 589 (51.7%) | 21 (50.0%) | 372 (48.2%) | 196 (60.3%) | < 0.001 |
|
| |||||
| Body Mass Index (kg/m2) | 29.6 ± 6.5 | 28.9 ± 7.2 | 29.7 ± 6.5 | 29.5 ± 6.4 | 0.583 |
|
| |||||
| Ejection Fraction (%) | 48.6 ± 13.4 | 45.3 ± 13.6 | 48.2 ± 13.5 | 50.0 ± 13.0 | 0.049 |
|
| |||||
| RBC EPA (M-IQR) | 0.6 (0.4, 0.8) | 0.2 (0.2, 0.2) | 0.5 (0.4, 0.6) | 1.1 (0.9, 1.3) | < 0.001 |
|
| |||||
| RBC DPA (M-IQR) | 0.8 (0.6, 1.0) | 0.9 (0.7, 1.1) | 0.8 (0.6, 0.9) | 0.7 (0.5, 0.9) | < 0.001 |
|
| |||||
| RBC EPA + DHA (M-IQR) | 4.9 (4.0, 6.1) | 3.6 (3.1, 4.7) | 4.6 (3.9, 5.5) | 6.2 (4.8, 7.5) | < 0.001 |
|
| |||||
| Taking fish oil on admission | 133 (19.9%) | 1 (5.6%) | 58 (12.9%) | 74 (36.5%) | < 0.001 |
|
| |||||
| C-reactive protein | 16.7 ± 22.7 | 52.2 ± 42.7 | 16.2 ± 22.8 | 9.5 ± 9.9 | 0.015 |
|
| |||||
| Statin at discharge n (%) | 984 (86.0%) | 41 (97.6%) | 663 (85.4%) | 280 (85.9%) | 0.378 |
|
| |||||
| HDL (mg/dL) | 40 ± 11 | 36 ± 8 | 40 ± 11 | 40 ± 11 | 0.006 |
|
| |||||
| LDL (mg/dL) | 96 ± 32 | 94 ± 29 | 97 ± 33 | 94 ± 31 | 0.989 |
|
| |||||
| Triglycerides (mg/dL) (M-IQR) | 130 (100, 175) | 128 (99, 176) | 132 (101, 178) | 128 (95, 171) | 0.730 |
|
| |||||
| Total Cholesterol (mg/dL) | 157 ± 38 | 154 ± 40 | 157 ± 38 | 156 ± 37 | 0.794 |
Continuous variables compared using linear trend test. Categorical variables compared using Mantel-Haenszel trend test, or for data presented as medians and interquartile ranges (M-IQR), Kruskal-Wallis test. DHA, docosahexaenoic acid; EPA, Eicosapentaenoic Acid; DPA, Docosapentaenoic acid; PCI, percutaneous coronary intervention; LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol.
Table 3.
Patient characteristics by tertile of Red Blood Cell (RBC) Docosapentaenoic Acid n-6 (DPA; % of fatty acids)
| Tertile 1 (0.12 to <0.66%) n = 380 |
Tertile 2 (0.66 to <0.88%) n = 379 |
Tertile 3 (0.88 to 3.93%) n = 385 |
P-value | |
|---|---|---|---|---|
|
| ||||
| GRACE 6-mo Mortality Risk Score | 103.6 ± 31.5 | 97.9 ± 30.1 | 102.5 ± 30.0 | 0.632 |
|
| ||||
| Age (yr) | 61.0 ± 12.4 | 58.2 ± 12.8 | 59.2 ± 12.2 | 0.055 |
|
| ||||
| History of Chronic Heart Failure | 37 (9.7%) | 26 (6.9%) | 44 (11.4%) | 0.417 |
|
| ||||
| History of Myocardial Infarction | 82 (21.6%) | 72 (19.0%) | 90 (23.4%) | 0.541 |
|
| ||||
| Heart Rate (beats/min) | 81.7 ± 20.4 | 81.6 ± 19.7 | 84.6 ± 23.5 | 0.063 |
|
| ||||
| Systolic Blood Pressure (mm Hg) | 141.2 ± 28.6 | 139.6 ± 25.9 | 142.6 ± 32.5 | 0.511 |
|
| ||||
| ST-Depression on Admitting EKG | 142 (39.4%) | 145 (40.3%) | 132 (35.6%) | 0.280 |
|
| ||||
| Creatinine (mg/dL) | 1.2 ± 0.6 | 1.2 ± 0.8 | 1.4 ± 1.6 | 0.002 |
|
| ||||
| In-Hospital PCI | 261 (68.7%) | 259 (68.3%) | 239 (62.1%) | 0.053 |
|
| ||||
| Sex | 0.122 | |||
| Male | 261 (68.7%) | 248 (65.4%) | 244 (63.4%) | |
| Female | 119 (31.3%) | 131 (34.6%) | 141 (36.6%) | |
|
| ||||
| Race | < 0.001 | |||
| White/Caucasian | 299 (78.9%) | 277 (73.1%) | 233 (60.7%) | |
| Black/African-American | 50 (13.2%) | 84 (22.2%) | 116 (30.2%) | |
| Other | 30 (7.9%) | 18 (4.7%) | 35 (9.1%) | |
|
| ||||
| Education greater than High School | 212 (56.1%) | 199 (52.5%) | 178 (46.6%) | 0.009 |
|
| ||||
| Body Mass Index (kg/m2) | 29.5 ± 6.0 | 30.0 ± 6.7 | 29.3 ± 6.6 | 0.742 |
|
| ||||
| Ejection Fraction (%) | 47.9 ± 13.5 | 49.5 ± 13.0 | 48.5 ± 13.8 | 0.551 |
|
| ||||
| RBC EPA (M-IQR) | 0.7 (0.4, 1.0) | 0.5 (0.4, 0.8) | 0.6 (0.4, 0.8) | < 0.001 |
|
| ||||
| RBC DPA (M-IQR) | 0.5 (0.4, 0.6) | 0.8 (0.7, 0.8) | 1.0 (0.9, 1.2) | < 0.001 |
|
| ||||
| RBC EPA+DHA (M-IQR) | 5.3 (4.0, 6.7) | 4.5 (3.8, 5.4) | 5.0 (4.1, 5.9) | < 0.001 |
|
| ||||
| Taking fish oil on admission | 81 (33.6%) | 31 (14.2%) | 21 (10.0%) | < 0.001 |
|
| ||||
| C-reactive protein | 17.7 ± 21.3 | 8.6 ± 9.1 | 19.2 ± 31.4 | 0.888 |
|
| ||||
| Statin at discharge n (%) | 325 (85.5%) | 328 (86.5%) | 331 (86.0%) | 0.859 |
|
| ||||
| HDL (mg/dL) | 40 ± 11 | 40 ± 10 | 40 ± 11 | 0.762 |
|
| ||||
| LDL (mg/dL) | 94 ± 30 | 99 ± 32 | 94 ± 33 | 0.993 |
|
| ||||
| Triglycerides (mg/dL) (M-IQR) | 134 (104, 182) | 133 (103, 181) | 124 (93, 172) | 0.091 |
|
| ||||
| Total Cholesterol (mg/dL) | 156 ± 37 | 160 ± 38 | 154 ± 39 | 0.725 |
See Table 2 for Total column. Continuous variables compared using linear trend test.
Categorical variables compared using Mantel-Haenszel trend test, or for data presented as medians and interquartile ranges (M-IQR), Kruskal-Wallis test. DHA, docosahexaenoic acid; EPA, Eicosapentaenoic Acid; DPA, Docosapentaenoic acid; PCI, percutaneous coronary intervention; LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol.
Figure 2.
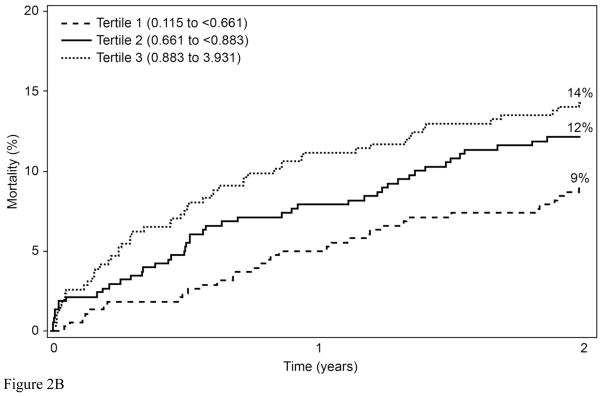
Kaplan-Meier curves describing the unadjusted mortality by A) RBC EPA cutpoints (log rank p<0.001), and B) RBC DPA tertiles (log rank p=0.018).
Improvements in GRACE Score Discrimination of 2-Year Mortality by RBC Fatty Acids
When adjusted for the GRACE score, an EPA level in the lowest category (<0.25%) had a hazard ratio (95% CI) of 3.71 (1.81, 7.61; p<0.001) relative to the highest category (≥ 0.8%), and the intermediate level (0.25% to 0.8%) had a hazard ratio of 1.76 (1.13, 2.75; p=0.013) relative to the highest. A 1-SD increase in RBC DPA had a mortality hazard ratio of 1.232 (1.056, 1.438; p=0.008). Two factors not included in the GRACE score (HDL-cholesterol and C-reactive protein) were also entered into the model. Neither one modified the GRACE score prediction nor the improvement with EPA and DPA (data not shown).
The c-statistic for the model including only EPA and DPA was 0.60 (95% CI=0.55, 0.65). The inclusion of these two RBC fatty acids with the GRACE score improved the discrimination of the GRACE score’s c-statistic from 0.75 to 0.77 (p<0.05) (Table 4). The relative incremental discrimination index improved by 19.8% (95% CI, 7.5%, 35.7%) and the absolute IDI improved by 2.2% (0.87%, 3.92%). The improvement in IDI was due almost completely to an improvement in prediction of those that died (2%) whereas the change in probability for survival was more negligible (0.2%). Adding the RBC fatty acids improved the classification of true events by 26% and of true non-events by 5.5%. Together these resulted in a net reclassification index of 31% (15%, 48%). Lastly, calibration chi-squares for the GRACE + fatty acids model [11.67 (p=0.17)] indicated that it, like the GRACE model alone, was well calibrated.
Table 4.
Model Parameters comparing GRACE alone to GRACE+EPA+DPA
| Metric | GRACE vs GRACE+EPA+DPA |
|---|---|
| C stat from Grace alone to + FAs | 0.747 vs 0.768 (p=0.047) |
| Incremental Discrimination Index (IDI) | 0.022 (0.0087,0.0392) |
| Relative IDI | 0.198 (0.079, 0.357) |
| Net Reclassification Index | 0.314 (0.152, 0.481) |
| % events correctly reclassified | 25.9% |
| % non-events correctly reclassified | 5.46% |
| Calibration Chi-sq | 12.53 and 11.67 (p=0.129 and 0.167) |
| Proportional Hazards Regression Model HR (Hazard Ratio with 95% CI) |
GRACE score alone (p<0.001) For EPA: <0.25% vs >0.8%; HR=3.71 (1.816,7.61) p=0.0004 0.25%–0.8% vs >0.8%; HR=1.76 (1.13,2.75) p=0.013 For DPA (per 1 SD increase): HR=1.23 (1.06, 1.44) p=0.0081 |
Discussion
Our purpose in this study was not to develop a new GRACE score, but to determine the extent to which information derived from RBC fatty acid analysis might improve upon an already well-validated risk prediction algorithm for MI patients. Our primary finding was that RBC fatty acid data improved the c-statistic and reduced GRACE score misclassification rates. Secondarily, we found that the ability of the GRACE score alone to discriminate those likely to survive (i.e., the c-statistic) can be extended from the originally-validated 6 months to 2 years2. Although our findings imply that risk-stratification in MI patients may be improved by adding RBC EPA and DPA levels to the GRACE risk score, our primary point was to demonstrate that potentially important information about health status may be derived from (at least 2 components of) the fatty acid profile of RBC membrane.
Much research is now focused on improving the prognostic accuracy of validated prediction models by including novel risk markers. It has, however, proven difficult to not only improve the c-statistic, but to also improve classification24;26;27. In the current study, we have taken the approach recommended by an expert committee of the American Heart Association21 in which effects on discrimination, IDI, NRI, and calibration were all evaluated. We found that all aspects of the GRACE score prediction were improved by the addition of RBC fatty acids. Importantly, 31% of our population were correctly reclassified by the inclusion of the fatty acids, primarily driven by the 26% of the cohort that GRACE predicted would be alive but who, in fact, died. Some previous attempts to improve on the GRACE score prediction with novel markers have been successful28;29 whereas others have not30;31.
Our study confirms and extends the results of the Infarction Prognosis Study from Japan, where plasma EPA levels were found to be predictors of 16-month total mortality in MI patients32. Although not directly compared with the GRACE score, several components of the GRACE score were included in their multivariable models. Ueeda et al. reported similar findings for cardiovascular endpoints33.
In our previous work, we proposed that an RBC EPA+DHA level (the omega-3 index) of at least 8% offer the greatest protection against subsequent death from CHD34. Mortality data from the Heart and Soul study supported this view5 as do data from case-control studies of ACS and acute MI patients35;36. Our data are also consistent with other studies showing an association between lower omega-3 index and sudden cardiac death and/or non-fatal MI35;37;38. Our findings support those of a Norwegian study showing that a low omega-3 index was associated with increased risk for in-hospital ventricular fibrillation in MI patients39, and, among frail40 elderly patients (mean age 82 years) acutely admitted to the hospital for any cause, 3-year mortality was higher among patients with plasma EPA levels in the lowest quartile41. Although our findings agree with those of another Norwegian study in showing no significant relationship between 2-year mortality and the omega-3 index in post-MI patients42, the Norwegian study did not further examine individual fatty acids (e.g., EPA) nor compare them with GRACE score predictions. In patients without known history of CV disease, lower blood levels of long-chain n-3 fatty acids are associated with an increased risk of sudden death37;38.
A particularly compelling aspect of using the EPA level to risk-stratify patients is that it is modifiable. A recent study in patients after coronary artery bypass surgery reported by Benedetto et al. found that in those prescribed omega-3 fatty acids at discharge, 2.5-year mortality was reduced by almost 50%43. Higher plasma EPA levels (caused by 1.8 g/d EPA supplementation for 5 years) were also associated with reduced major cardiac events in the Japan EPA Lipid Intervention Study44. Similarly, treatment with about 1 g/d of EPA+DHA in the GISSI-Prevenzione 7 and GISSI Heart Failure45 trials also significantly reduced all-cause mortality, although a recent study from Germany reported no benefit on total mortality in post-MI patients after 1 year of treatment with the same dose of EPA+DHA.8 This latter study, however, was severely underpowered, suggesting that the preponderance of evidence still supports the potential value of EPA+DHA supplementation in post-MI patients. Our findings suggest that this benefit may be most beneficial in those with an EPA level below 0.25%.
The median omega-3 index and EPA levels in our study were 4.9% and 0.6%, respectively, which are consistent with other studies from the US16;19;46. In Japan, where fish and seafood are more common components of the diet, the average omega-3 index is twice as high and the RBC EPA level is 2–3 times higher47;48. Compared to an estimated life expectancy of 78.4 years in the US, life expectancy in Japan is 82.3 years; 2011 world rankings of 50th and 5th, respectively 49. The reasons for this 4-year difference in longevity are undoubtedly numerous, however, higher plasma omega-3 levels have been identified as an important correlate of reduced atherosclerotic burden among Japanese men 50.
The mechanisms through which omega-3 fatty acids reduce mortality after MI are not entirely clear. However, a direct cardio-protective effect of omega-3 fatty acids by their incorporation into myocardial cell membranes is thought to play a role51. Mechanisms suggested to mediate this cardio-protective effect have recently been reviewed52 and include altering membrane potentials; affecting sodium and calcium channels, which can reduce susceptibility to arrhythmias); reducing platelet aggregation and thrombin generation; lowering levels of circulating inflammatory markers; and affecting vagal tone, which can reduce heart rate. Recent mouse studies from our lab found potential anti-fibrotic effects53. In acute MI patients from Japan, there was a negative correlation between serum levels of omega-3 fatty acids and coronary artery plaque burden54, and omega-3 supplementation reduces the inflammatory cell burden of carotid plaques55;56. Omega-3 fatty acids blood levels have been inversely associated with the rate of telomere attrition57, a putative measure of cellular aging.
The mechanism(s) by which increased levels of DPA may influence mortality risk are far less clear. DPA n6 is the ultimate fatty acid metabolite of arachidonic acid (C20:4 n6), and is present, like EPA, in the cell membranes in quite low quantities. For example, DPA comprises 0.8% of the fatty acid composition of the RBC cell membrane, compared to 0.6%for EPA, 4.4% for DHA and 20% for arachidonic acid. DPAn6 tends to be inversely associated with DHA and has been proposed as a marker of functional DHA deficiency58. There are no known functional metabolites of DPA (such as prostaglandins, leukotrienes, resolvins, neuroprotectins, etc.), and it is not known to be a substrate for cyclooxygenase, lipoxygenase, or cytochrome P450. So the effects of higher (vs lower) DPA levels on inflammatory processes is unknown. In the current study we speculate that DPA may be a marker of functional omega-3 deficiency; whether DPA is a CV risk predictor independent of EPA/DHA is uncertain.
Strengths and Limitations
The main strengths of this study were its relatively large sample size, multi-center design, long-term follow-up, focus on total mortality, comparison with an established risk prediction model (GRACE), and the use of a validated biomarker of fatty acid status. Its limitations were its observational design, the lack of disease-specific endpoints, and the potential for unmeasured confounding. Further studies are required to determine whether utilizing RBC fatty acid measures in risk stratification will lead to increased recommendations for raising omega-3 intakes in the high risk groups or more aggressive treatment (e.g. intra-cardiac defibrillators or revascularization), and more importantly, whether improved risk stratification can reduce mortality.
In conclusion, we found that levels of two relatively minor fatty acid components of RBC membranes (EPA and DPAn6) added significantly to the ability of the GRACE score to predict risk for 2 year mortality in MI patients.
Supplementary Material
Acknowledgments
Funding Sources:
The TRIUMPH study was supported by grants from the National Heart, Lung, and Blood Institute Specialized Center of Clinically Oriented Research in Cardiac Dysfunction and Disease (grant no. P50 HL077113) and Cardiovascular Outcomes, Kansas City, MO.
Abbreviations
- CAD
coronary artery disease
- CHD
coronary heart disease
- CV
cardiovascular
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EDTA
ethylenediaminetetraacetic acid
- EPA
eicosapentaenoic acid
- GRACE
Global Registry of Acute Coronary Events
- MI
myocardial infarction
- RBC
red blood cells
- TRIUMPH
Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction
- US
United States
Footnotes
Authors’ potential conflicts of interest:
WSH: Ownership interest in OmegaQuant Analytics, LLC; employment by Health Diagnostic Laboratory, Inc.; a consultant to Aker Biomarine, Amarin, and Omthera; and a past speaker for GlaxoSmithKline.
JAS: None declared.
KK: None declared.
JHO: Ownership interest in CardioTabs, and a speaker for GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 3.Albert CM, Hennekens CH, O’Donnell CJ, et al. Fish Consumption and Risk of Sudden Cardiac Death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Eng J Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 5.Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood Eicosapentaenoic and Docosahexaenoic Acids Predict All-Cause Mortality in Patients With Stable Coronary Heart Disease: The Heart and Soul Study. Circ Cardiovasc Qual Outcomes. 2010;3:406–412. doi: 10.1161/CIRCOUTCOMES.109.896159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 7.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 8.Rauch B, Schiele R, Schneider S, et al. Highly purified omega-3 fatty acids for secondary prevention of sudden cardiac death after myocardial infarction-aims and methods of the OMEGA-study. Cardiovasc Drugs Ther. 2010;20:365–375. doi: 10.1007/s10557-006-0495-6. [DOI] [PubMed] [Google Scholar]
- 9.The ORIGIN Investigators. N-3 Fatty Acids and Cardiovascular Outcomes in Patients with Dysglycemia. N Eng J Med. 2012 doi: 10.1056/NEJMoa1203859. Published online June 12. [DOI] [PubMed] [Google Scholar]
- 10.Harris WS, Sands SA, Windsor SL, et al. Omega-3 Fatty Acids in Cardiac Biopsies from Heart Transplant Patients: Correlation with Erythrocytes and Response to Supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf RG, Cleland LG, Gibson RA, et al. Relation between blood and atrial fatty acids in patients undergoing cardiac bypass surgery. Am J Clin Nutr. 2010;91:528–534. doi: 10.3945/ajcn.2009.28302. [DOI] [PubMed] [Google Scholar]
- 12.Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem. 2009;43:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG, Robins SJ. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012 doi: 10.1016/j.atherosclerosis.2012.05.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86:1621–1625. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 15.Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302:1651–1657. doi: 10.1001/jama.2009.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shearer GC, Pottala JV, Spertus JA, Harris WS. Red blood cell fatty acid patterns and acute coronary syndrome. PLoS ONE. 2009;4:e5444. doi: 10.1371/journal.pone.0005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Assi E, Ferreira-Gonzalez I, Ribera A, et al. Do GRACE (Global Registry of Acute Coronary events) risk scores still maintain their performance for predicting mortality in the era of contemporary management of acute coronary syndromes? Am Heart J. 2010;160:826–834. doi: 10.1016/j.ahj.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 20.Klein J, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. 2. Springer-Verlag; 2003. [Google Scholar]
- 21.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 23.Antolini L, Nam BH, D’Agostino RB. Inference on correlated discrimination measures in survival analysis: a nonparametric approach. Commun Stat Theory Meth. 2004;33:2117–2135. [Google Scholar]
- 24.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, Rao CR, editors. Handbook of Statistics. Vol. 23. London: Elsevier; 2004. [Google Scholar]
- 26.Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem. 2008;54:17–23. doi: 10.1373/clinchem.2007.096529. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Chambless LE, Ballantyne CM, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med. 2006;166:1368–1373. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 28.Lorgis L, Cottin Y, Danchin N, et al. Impact of obesity on the prognostic value of the N-terminal pro-B-type natriuretic peptide (NT-proBNP) in patients with acute myocardial infarction. Heart. 2011;97:551–556. doi: 10.1136/hrt.2010.213041. [DOI] [PubMed] [Google Scholar]
- 29.Narayan H, Dhillon OS, Quinn P, et al. C-Terminal Provasopressin (Copeptin) as Prognostic Marker after Acute Non ST Elevation Myocardial Infarction - Leicester Acute Myocardial Infarction Peptide II (LAMP II) study. Clin Sci (Lond) 2011 doi: 10.1042/CS20100564. [DOI] [PubMed] [Google Scholar]
- 30.Timoteo AT, Toste A, Ramos R, et al. Does admission NT-proBNP increase the prognostic accuracy of GRACE risk score in the prediction of short-term mortality after acute coronary syndromes? Acute Card Care. 2009;11:236–242. doi: 10.1080/17482940903177036. [DOI] [PubMed] [Google Scholar]
- 31.Meune C, Drexler B, Haaf P, et al. The GRACE score’s performance in predicting inhospital and 1-year outcome in the era of high-sensitivity cardiac troponin assays and B-type natriuretic peptide. Heart. 2011 doi: 10.1136/hrt.2010.220988. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Shin MJ, Kim JS, et al. Blood eicosapentaenoic acid and docosahexaenoic acid as predictors of all-cause mortality in patients with acute myocardial infarction--data from Infarction Prognosis Study (IPS) Registry. Circ J. 2009;73:2250–2257. doi: 10.1253/circj.cj-09-0327. [DOI] [PubMed] [Google Scholar]
- 33.Ueeda M, Doumei T, Takaya Y, et al. Association of serum levels of arachidonic acid and eicosapentaenoic acid with prevalence of major adverse cardiac events after acute myocardial infarction. Heart Vessels. 2011;26:145–152. doi: 10.1007/s00380-010-0038-8. [DOI] [PubMed] [Google Scholar]
- 34.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in Blood Cell Membranes from Acute Coronary Syndrome Patients and Controls. Atherosclerosis. 2007;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 36.Park Y, Lim J, Lee J, Kim SG. Erythrocyte fatty acid profiles can predict acute non-fatal myocardial infarction. Br J Nutr. 2009;102:1355–1361. doi: 10.1017/S0007114509990298. [DOI] [PubMed] [Google Scholar]
- 37.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 38.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. J Am Med Assoc. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 39.Aarsetoy H, Ponitz V, Nilsen OB, Grundt H, Harris WS, Nilsen DW. Low levels of cellular omega-3 increase the risk of ventricular fibrillation during the acute ischaemic phase of a myocardial infarction. Resuscitation. 2008 Sep;78(3):258–64. doi: 10.1016/j.resuscitation.2008.04.007. Epub 2008 Jun 16, 2008, 78, 258–264. [DOI] [PubMed] [Google Scholar]
- 40.Winograd CH, Gerety MB, Chung M, Goldstein MK, Dominguez F, Jr, Vallone R. Screening for frailty: criteria and predictors of outcomes. J Am Geriatr Soc. 1991;39:778–784. doi: 10.1111/j.1532-5415.1991.tb02700.x. [DOI] [PubMed] [Google Scholar]
- 41.Lindberg M, Saltvedt I, Sletvold O, Bjerve KS. Long-chain n-3 fatty acids and mortality in elderly patients. Am J Clin Nutr. 2008;88:722–729. doi: 10.1093/ajcn/88.3.722. [DOI] [PubMed] [Google Scholar]
- 42.Aarsetoey H, Ponitz V, Grundt H, Staines H, Harris WS, Nilsen DW. (n-3) fatty acid content of red blood cells does not predict risk of future cardiovascular events following an acute coronary syndrome. J Nutr. 2009;139:507–513. doi: 10.3945/jn.108.096446. [DOI] [PubMed] [Google Scholar]
- 43.Benedetto U, Melina G, di Bartolomeo R, et al. n-3 Polyunsaturated Fatty Acids After Coronary Artery Bypass Grafting. Ann Thorac Surg. 2011;91:1169–1175. doi: 10.1016/j.athoracsur.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki M, Yokoyama M, Saito Y, et al. Incremental Effects of Eicosapentaenoic Acid on Cardiovascular Events in Statin-Treated Patients With Coronary Artery Disease. Circ J. 2009 May 8; doi: 10.1253/circj.cj-08-1197. [DOI] [PubMed] [Google Scholar]
- 45.GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 46.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 47.Itomura M, Fujioka S, Hamazaki K, et al. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]
- 48.Yanagisawa N, Shimada K, Miyazaki T, et al. Polyunsaturated fatty acid levels of serum and red blood cells in apparently healthy Japanese subjects living in an urban area. J Atheroscler Thromb. 2010;17:285–294. doi: 10.5551/jat.2618. [DOI] [PubMed] [Google Scholar]
- 49.2011
- 50.Sekikawa A, Curb JD, Ueshima H, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massaro M, Scoditti E, Carluccio MA, De Caterina R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:109–115. doi: 10.1016/j.plefa.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Shearer GC, Chen Q, et al. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;123:584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueeda M, Doumei T, Takaya Y, et al. Serum N-3 polyunsaturated fatty acid levels correlate with the extent of coronary plaques and calcifications in patients with acute myocardial infarction. Circ J. 2008;72:1836–1843. doi: 10.1253/circj.cj-08-0249. [DOI] [PubMed] [Google Scholar]
- 55.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 56.Cawood AL, Ding R, Napper FL, et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.