Abstract
The problem of how word meaning is processed in the brain has been a topic of intense investigation in cognitive neuroscience. While considerable correlational evidence exists for the involvement of sensory-motor systems in conceptual processing, it is still unclear whether they play a causal role. We investigated this issue by comparing the performance of patients with Parkinson’s disease (PD) with that of age-matched controls when processing action and abstract verbs. To examine the effects of task demands, we used tasks in which semantic demands were either implicit (lexical decision and priming) or explicit (semantic similarity judgment). In both tasks, PD patients’ performance was selectively impaired for action verbs (relative to controls), indicating that the motor system plays a more central role in the processing of action verbs than in the processing of abstract verbs. These results argue for a causal role of sensory-motor systems in semantic processing.
Keywords: Conceptual processing, Embodiment, Language comprehension, Lexical semantics, Parkinson’s disease, Priming
1. Introduction
A large body of evidence now supports the view that semantic processes interact in varied ways with the neural systems that underlie perception and motor control. This has led to a number of theoretical proposals, collectively known as the “embodied semantics” framework, according to which the concepts that underlie word meaning are constituted, at least in part, by the memory traces of past sensory-motor experiences. In this view, a word form acquires at least part of its meaning through modality-specific perceptual, emotional, and motor representations, and its retrieval from memory requires the neural re-enactment of these sensory-motor traces (Binder & Desai, 2011; Barsalou, 1999; Damasio, 1989; Gallese & Lakoff, 2005; Kemmerer & Gonzalez-Castillo, 2010; Pulvermüller & Fadiga, 2010).
An alternative view holds that word meaning is fundamentally abstract and modality-independent, and therefore qualitatively distinct from sensory-motor representations (Burgess & Lund, 2000; Fodor, 1975 and 2000; Landauer & Dumais, 1997; Pylyshyn, 1999). According to this “disembodied” perspective, all concepts are represented in an abstract and symbolic form. The conceptual system can be indirectly influenced by perception and action (and vice-versa), but the two systems are nevertheless separate and independent. In the following, we will briefly review some of the evidence bearing on this issue, focusing on the relationship between the motor system and the meaning of words referring to actions (action words).
Action concepts offer a convenient test bed for the embodied semantics framework partly because of their strong association with the motor system, such that many of these concepts can be mapped onto well-defined bodily actions, which can be easily measured or induced in the laboratory. Furthermore, the somatotopic organization of the motor cortex allows fine-grained hypotheses about the motor representations underlying different action concepts to be investigated with functional neuroimaging or transcranial magnetic stimulation (TMS). Finally, patients with a variety of motor disorders can be examined for connections between their motor impairments and the abnormal processing of action concepts.
Behavioral evidence supporting a role for motor representations in the processing of action concepts comes mainly from studies showing that exposure to words or sentences interferes with a concomitant or immediately subsequent action in a semantically specific way (Borghi, Glenberg & Kaschak, 2004; Bub & Masson, 2010; Glenberg & Kaschak, 2002; Myung, Blumstein & Sedivy, 2006; Zwaan & Taylor, 2006; see Fisher & Zwaan, 2008, for a review). Glenberg and colleagues, for instance, found that when participants make semantic judgments about sentences that imply a motion toward or away from the body, responses are faster when they require a movement in the same direction as that implied in the sentence (action-sentence compatibility effect). Other studies indicate that this interaction between language and action is also body-part specific (Scorolli & Borghi, 2007) and happens very early following word presentation (within 200 ms; Boulenger et al., 2006; Nazir et al., 2008; Sato, Mengarelli, Riggio, Gallese, & Buccino, 2008).
Additional evidence comes from several studies showing that motor cortical areas are selectively activated during processing of action-related words and sentences (for reviews, see Aziz-Zadeh & Damasio, 2008; Fernandino & Iacoboni, 2010; Kemmerer & Gonzalez Castillo, 2010). Some functional MRI studies suggest that verbs related to different body parts (e.g. hand, foot, or mouth) activate the primary motor and the premotor cortex on the left hemisphere in roughly somatotopic fashion, consistent with the idea of motor simulation (Aziz-Zadeh, Wilson, Rizzolatti, & Iacoboni, 2006; Boulenger, Hauk, & Pulvermüller, 2009; Hauk, Johnsrude, & Pulvermüller, 2006; Raposo, Moss, Stamatakis, & Tyler, 2009; Tettamanti et al., 2005). Other studies have shown increased activation in the anterior supramarginal gyrus, which is involved in the control of goal-directed actions, for processing of action-related words or sentences, compared to non-action related stimuli (Desai, Binder, Conant, & Seidenberg, 2010; Desai, Binder, Conant, Mano, & Seidenberg, 2011; Noppeney, Josephs, Kiebel, Friston, & Price, 2005; Rueschemeyer, Van Rooij, Lindemann, Willems, & Bekkering, 2010; Tettamanti et al., 2005). Further evidence of motor cortex activation during action language processing comes from studies employing TMS-induced motor-evoked potentials (Buccino et al., 2005; Glenberg et al., 2008; Oliveri et al., 2004), EEG (Hauk and Pulvermüller, 2004; van Elk, van Schie, H., Zwaan, R., & Bekkering, 2010), and MEG (Boulenger, Shtyrov, & Pulvermüller, 2012; Pulvermüller, Shtyrov and Ilmoniemi, 2005).
As pointed out by skeptics (e.g. Bedny & Caramazza, 2011; Chatterjee, 2010; Mahon & Caramazza, 2008), these studies have not directly demonstrated that motor representations play a causal role in language comprehension. In fact, a crucial prediction of embodied theories of meaning is that disruption of the motor system should selectively disrupt processing of action-related concepts. Preliminary evidence in line with this prediction has been provided by TMS studies and by studies of neurological patients.
Pulvermüller et al. (2005) found that single-pulse TMS over the left primary motor hand area led to faster lexical decision responses to arm-related than to leg-related action words, while stimulation over the leg area produced the opposite result. Similarly, Papeo, Vallesi, Isaja, & Rumiati (2009) found that stimulation over the hand motor area induced an RT advantage for hand-action verbs over non-hand-action verbs, although this effect was present only when stimulation was delayed by 350 ms relative to word onset. When the pulse was delivered at 170 ms post-stimulus onset (similar to the timing used by Pulvermüller et al., 2005), no differences between the two word types were observed. Tomasino, Fink, Sparing, Dafotakis, M., & Weiss (2008) found that single-pulse TMS over the motor hand area led to facilitation of action verb processing (relative to stimulation over the vertex) when participants were required to produce motor imagery in response to the verb, but not when making frequency judgments or simply reading the word. Unfortunately, since participants were tested only on action verbs in this study, it is not possible to conclude that this effect was specific to action words. Finally, a study by Willems, Labruna, D’Esposito, Ivry, & Casasanto (2011) found that theta-burst stimulation (TBS) over the left premotor cortex facilitated processing of manual-action verbs, but not of non-manual-action verbs, in a subsequent lexical decision task. The neurophysiologic effects of single-pulse TMS and TBS are still not fully understood, but to the extent that the behavioral effects in these studies were specific to action verbs, the TMS evidence generally supports an involvement of the motor system in action-verb processing.
Studies of neurological patients can provide more direct evidence for the necessity of the motor system in processing action concepts. Brain systems involved in motor control can be disrupted either by focal brain lesions, such as those resulting from stroke, or by neurodegenerative disorders, such as amyotrophic lateral sclerosis (ALS; also known as motor neuron disease) and Parkinson’s disease. Several studies suggest that both types of disruption lead to deficits in action semantics. For instance, Buxbaum & Saffran (2002) tested a group of chronic stroke patients – half of them presenting with apraxia – on tasks requiring semantic similarity judgments. They found that apraxic patients were more impaired at reasoning about tools than about animals, and also more impaired at judging tool manipulation than tool function, while non-apraxic patients showed the opposite pattern. Furthermore, apraxics performed worse than non-apraxics in reasoning about body parts. Convergent results were reported by Neininger & Pulvermüller (2003), who showed that patients with right frontal lesions (and associated left hemiparesis) were selectively impaired at processing action verbs, whereas patients with right temporo-occipital lesions were more impaired at processing visual nouns.
Similar results were found with ALS patients. Bak, O’Donovan, Xuereb, Boniface, & Hodges (2001), using a word-picture matching task, showed that these patients were more impaired at matching action verbs than concrete nouns, while Alzheimer’s patients and healthy controls showed no difference between these two categories. The same pattern of impairment was obtained in a study of a familial form of movement disorder resembling progressive supranuclear palsy (Bak et al., 2006). Moreover, a recent study by Grossman et al. (2008) found that not only were ALS patients impaired on action semantics, relative to object semantics, but the degree of cortical atrophy in motor and premotor areas correlated with performance on action-verb judgments (and not on judgments of concrete nouns).
Parkinson’s disease (PD) is another neurodegenerative disorder that severely affects the motor system. It is characterized by rigidity, bradykinesia (slowness of movement), postural instability, and tremor during rest, resulting from progressive loss of dopaminergic cells in the substantia nigra (for a review, see Dauer and Przedborski, 2003). Patients can also present with cognitive impairments, particularly deficits in executive functions, which may be related to dysfunction of the frontostriatal circuitry (Koerts, Leenders and Brouwer, 2009; Owen, 2004; Zgaljardic, Borod, Foldi, Mattis, 2003).
The effects of the disruption of the dopaminergic pathways on the various cortical regions are not fully understood, but the motor symptoms of PD have been linked to abnormal activity in the primary motor cortex (M1) and the supplementary motor area (SMA) (Jenkins, Fernandez, Playford, et al., 1992; Pasquereal & Turner, 2011; Rascol, Sabatini, Chollet, et al., 1992; Suppa, Iezzi, Conte, et al., 2010; Wu, Long, Wang, et al., 2011). Thus, while PD certainly does not represent a case of “pure” motor cortex dysfunction, it presents an opportunity to test the integrity of the conceptual system in the face of a disruption of those components of the motor network.
Boulenger et al. (2008a) pursued this goal by testing PD patients and healthy controls with a lexical decision (LD) task and masked priming. Target words were either action verbs or concrete nouns, and they were primed by either the same word (displayed in capitalized letters) or by a sequence of consonants. They found that off-medication PD patients displayed reduced priming for action verbs relative to concrete nouns, compared to healthy controls or to the same patients on medication. Since the prime was not consciously perceived by the participants, this result suggests that motor simulations are automatically activated by action word recognition, in the absence of explicit semantic processing. Another study by Cotelli et al. (2007) showed that PD patients also perform worse in action naming than in object naming, suggesting that their motor impairment also impacts the explicit processing of action semantics.
One major limitation of the aforementioned studies with clinical populations is that – with the exception of Buxbaum & Saffran (2002) – they all contrasted action verbs with non-action nouns. This grammatical class confound represents a problem because verbs are syntactically and semantically more complex than nouns (for reviews, see Druks, 2002, and Levin, 1993). In particular, verbs carry information about argument structure, corresponding to the kinds of entities that can be attached to them in a sentence (i.e., its arguments), how many arguments can be attached, and which thematic roles can be attributed to these arguments. In other words, verbs and nouns differ not only with respect to their “core” meanings, but also with respect to the complexity of their lexical representations. Psycholinguistic research has shown that a verb’s argument structure is an intrinsic part of its lexical representation (Shapiro, Zurif, & Grimshaw, 1987; Ferretti, McRae, & Hatherell, 2001; Trueswell & Kim, 1998), and fMRI studies of verb processing have found increased activation in peri-sylvian language areas as a function of increasing argument structure complexity (den Ouden, Fix, Parrish, Thompson, 2009; Thompson et al., 2007). Therefore, the patients’ lower performance on action verbs may not necessarily be due to a deficit in action semantics, but rather to a deficiency in processing the argument-structure aspects of the verb.
In the present study, we compared PD patients and age-matched controls on their ability to process action-related verbs. Crucially, performance on action verbs was assessed in relation to performance on abstract verbs (matched for argument structure), thus avoiding the grammatical class confound.
Another main goal of our study was to examine the conditions under which sensory-motor systems may be involved in conceptual processing. In principle, the motor system could contribute to action verb processing at an early, automatic stage of word recognition, in tasks where explicit access to word semantics is not required (e.g. LD). Alternatively, the role of the motor system could be conditional on explicit semantic processing. Yet another possibility, as suggested by the results reviewed above, is that the motor system could participate in different stages of word processing, contributing both to automatic word recognition and to controlled, explicit semantic processing. To investigate the impact of motor impairments on different levels of word processing, we used both LD and a semantic similarity judgment (SSJ) task, which requires explicit semantic comparisons. The LD task also included a masked priming manipulation, so that we were able to evaluate motor/semantic interactions at three levels of cognitive control: subliminal activation (masked priming), implicit conscious activation (LD), and explicit comparison (SSJ).
Our main hypotheses pertained to the interactions between group and verb type in each task. We predicted that, relative to healthy controls, PD patients would show lower performance on action verbs than on abstract verbs, in both tasks. We also predicted that, compared to healthy controls, PD patients would show relatively less priming for action than for abstract verbs.
2. Methods
2.1. Participants
Twenty PD patients (mean age = 64.5, 9 females) and 22 healthy older adults (mean age = 65.4, 12 females) participated in the study. PD patients had been previously examined by a movement disorders specialist and met the criteria for idiopathic PD. Seventeen patients were taking dopaminergic medication and were in the ON state during testing. Two patients were in the OFF state (off medication for at least 12 hours) at the time of testing because they were being evaluated for deep brain stimulation surgery. One patient had not yet started taking antiparkinsonian medication (Table 1). All participants were screened for dementia (MMSE2 > 25) and other neurological conditions. Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). Participants received monetary compensation for participation in the study. The study was approved by the institutional review board of the Medical College of Wisconsin, and all participants signed an informed consent form.
Table 1.
Individual patient information and group means (standard deviations) for age (years), education (years), WTAR standard score (max = 34), MMSE2 (max = 30), UPDRS (max = 108), time since diagnosis (years), Hoehn-Yahr stage (max = 4), medication status at time of testing, and daily medication DOPA-equivalent dose (mg).
| Patients | Gender | Age | Education | WTAR-Std | MMSE2 | UPDRS | Years since diagnosis | Hoehn-Yahr | Status at testing | DOPA equivalence |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | M | 75 | 21 | 107 | 27 | 17 | 3.5 | 2 | ON | 750 |
| P2 | F | 77 | 12 | 108 | 30 | 24 | 4.5 | 3 | ON | 350 |
| P3 | M | 60 | 15 | 123 | 30 | 12 | 2 | 1 | OFF | 0 |
| P4 | F | 59 | 16 | 110 | 26 | 21 | 4 | 2 | ON | up to 600 |
| P5 | F | 52 | 16 | 104 | 30 | 25 | 9 | 2 | ON | 700–1000 |
| P6 | F | 63 | 13 | 102 | 29 | 21 | 2 | 2 | ON | 800 |
| P7 | M | 65 | 19 | 104 | 26 | 47 | 14 | 4 | ON | 750 |
| P8 | F | 72 | 14 | 104 | 27 | 22 | 10 | 2 | ON | 600 |
| P9 | F | 68 | 16 | 113 | 30 | 29 | 10 | 2 | ON | 800 |
| P10 | M | 60 | 14 | 107 | 27 | 57 | 2.5 | 3 | OFF | 600 |
| P11 | M | 64 | 12 | 96 | 27 | 45 | 6 | 3 | ON | 150 |
| P12 | M | 67 | 19 | 93 | 28 | 68 | 5 | 4 | OFF | 1550 |
| P13 | M | 74 | 14 | 99 | 28 | 43 | 6 | 2 | ON | 200 |
| P14 | F | 60 | 18 | 102 | 28 | 24 | 7 | 2 | ON | variable |
| P15 | M | 37 | 17 | 113 | 30 | 10 | 5 | 2 | ON | 750 |
| P16 | M | 65 | 18 | 123 | 30 | 26 | 2 | 2 | ON | 200 |
| P17 | F | 62 | 28 | 125 | 30 | 10 | 8 | 1 | ON | 200–500 |
| P18 | M | 80 | 13 | 121 | 28 | 25 | 9 | 2 | ON | 850 |
| P19 | M | 61 | 19 | 123 | 29 | 10 | 1.5 | 1.5 | ON | 100 |
| P20 | F | 69 | 18 | 122 | 26 | 18 | 2.5 | 2 | ON | 200 |
| Patient | 9/20 F | 64.5 (9.5) | 16.6 (3.7) | 110 (9.9) | 28.3 (1.5) | 27.7 (16.1) | 5.7 (3.4) | 2.3 (0.8) | ||
| Control | 12/22 F | 65.4 (6.1) | 16.2 (1.9) | 115.9 (6.6) | 28.9 (0.9) |
2.2. Materials
The lexical decision task used a set of 80 verbs and 80 phonologically legal pseudowords. Pseudowords were selected from the English Lexicon Project (ELP) database (http://elexicon.wustl.edu), Balota et al. (2007), such that verbs and pseudowords were matched in number of letters, bigram frequency, and lexical decision RT and accuracy. Half of the verbs referred to voluntary hand/arm actions (e.g. to grasp, to squeeze), and the others referred to abstract concepts (e.g. to depend, to improve). Action and abstract verbs were matched in number of letters, number of phonemes, number of syllables, bigram frequency, number of orthographic and phonological neighbors, and lemma frequency (see Table 2). The two conditions were also matched in mean naming response time (RT) and in lexical decision RT and accuracy, according to the ELP database (Balota et al., 2007). Verbs were classified in terms of argument structure according to the WebCelex database (http://celex.mpi.nl). All verbs in the LD task were either transitive (T) or admitted both transitive and intransitive argument structures (TI), and their numbers were similar across conditions (action: 16 T, 24 TI; abstract: 20 T, 20 TI; χ2 = 0.45, df = 1, p = 0.5).
Table 2.
Mean (and standard deviation) of the lexical measures for each condition of the lexical decision task.
| Action | Abstract | T-test (p-value) | Pseudo-word | T-test (p-value) | |
|---|---|---|---|---|---|
| Letters | 5.45 (1.13) | 5.27 (1.2) | 0.50 | 5.3 (1.1) | 0.73 |
| Phonemes | 4.22 (1.05) | 4.27 (1.28) | 0.85 | - | - |
| Syllables | 1.27 (0.45) | 1.27 (0.45) | 1.00 | - | - |
| Log Frequency | 1.27 (0.5) | 1.23 (0.69) | 0.78 | - | - |
| Bigram Freq | 1599 (844) | 1627 (682) | 0.87 | 1596 (629) | 0.88 |
| Ortho Neighbor | 3.87 (4.08) | 3.90 (4.47) | 0.98 | 2.56 (2.77) | 0.02 |
| Phono Neighbor | 9.80 (10.63) | 9.45 (9.50) | 0.88 | - | - |
| LD RT | 665 (57) | 661 (67) | 0.77 | 668 (40) | 0.53 |
| LD Acc | 0.95 (0.07) | 0.93 (0.13) | 0.40 | 0.94 (0.08) | 0.93 |
| Naming RT | 640 (48.5) | 629 (50.7) | 0.34 | - | - |
| SemD | 1.73 (0.22) | 1.85 (0.19) | 0.02 | - | - |
The first T-test column shows the p-values of the test between action words and abstract words; the second T-test column shows the p-values for the test between all words combined and pseudo-words. Log frequency values were obtained from the WebCelex database (http://celex.mpi.nl); SemD values obtained from Paul Hoffman and Tim Rogers (personal communication; see Hoffman, Rogers, & Lambon Ralph, 2011). All other measures retrieved from the English Lexicon Project database (http://elexicon.wustl.edu), Balota et al. (2007).
The semantic similarity judgment task used a set of 120 action verbs and a set of 120 abstract verbs (thirty-nine action verbs and 36 abstract verbs also appeared in the LD task). Each set was organized into 40 triplets, such that in each triplet, two of the verbs had similar meanings. The two conditions were matched in number of letters, number of phonemes, number of syllables, number of orthographic and phonological neighbors, and lemma frequency, as well as in mean naming RT and mean RT and accuracy in lexical decision according to the ELP (Table 3). Argument structure was also matched across conditions (action: 1 intransitive [I], 50 T, and 69 TI; abstract: 1 I, 65 T, and 54 TI; χ2 = 3.78, df = 2, p = 0.15).
Table 3.
Mean (and standard deviation) of the lexical measures for each condition of the semantic judgment task. See legend of Table 2 for details.
| Action | Abstract | T-test (p-value) | |
|---|---|---|---|
| Letters | 5.33 (0.18) | 5.32 (0.13) | 0.97 |
| Phonemes | 4.22 (0.15) | 4.25 (0.13) | 0.86 |
| Syllables | 1.33 (0.06) | 1.42 (0.05) | 0.30 |
| Log Frequency | 1.22 (0.06) | 1.27 (0.07) | 0.60 |
| Ortho Neighbor | 4.89 (5.02) | 4.09 (4.78) | 0.21 |
| Phono Neighbor | 10.77 (10.42) | 8.72 (9.44) | 0.11 |
| LD RT | 662 (46) | 650 (40) | 0.21 |
| LDAcc | 0.94 (0.06) | 0.94 (0.07) | 0.97 |
| Naming RT | 637 (46) | 624 (27) | 0.13 |
| SemD | 1.70 (0.15) | 1.84 (0.15) | < .001 |
2.3. Procedure
PD patients were tested immediately after their regular neurologist visit at the hospital. The neurologist administered the Unified Parkinson’s Disease Scale (UPDRS) at the end of the clinical visit. Patients and controls were given the Mini-Mental State Examination - Second Edition (MMSE-2), the Wechsler Test of Adult Reading (WTAR), and the handedness questionnaire at the beginning of the testing session. They then performed the LD and SSJ tasks, with a short break in between. The entire procedure lasted around one hour. For both tasks, a laptop PC running E-prime software (version 1.2, Psychology Software Tools, Inc.) was used for stimulus presentation and response recording. Response buttons were two Ablenet Jelly Bean switches (www.ablenetinc.com) connected to a PST Serial Response Box (Psychology Software Tools, Inc.).
LD task
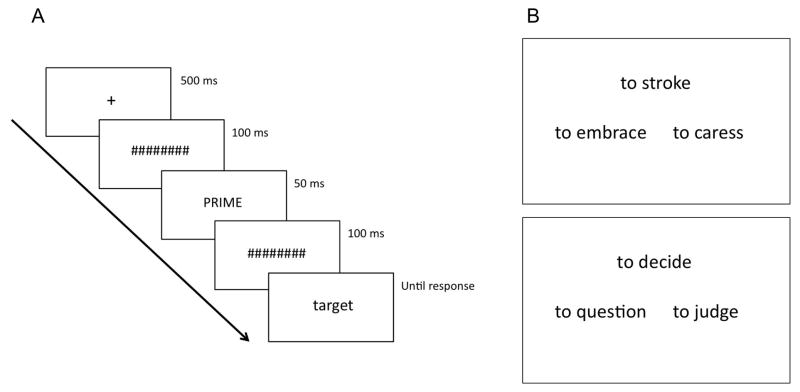
Each trial began with a fixation cross that was presented in the center of the screen for 500 ms (Figure 1A). The fixation was followed by a series of eight hash marks, presented for 100 ms, followed by the prime stimulus (50 ms), another series of eight hash marks (100 ms), and the target stimulus, which remained on the screen until the participant made a response. Each verb and pseudoword was presented with the word “to” to its left (e.g. “to knock”, “to frasp”). The prime was either the same as the target word or a consonant string, also preceded by the word “to”. Primes were presented in upper case font and targets in lower case font to make them perceptually distinct. The priming manipulation was counterbalanced across participants, so that each verb was primed by the capitalized target and by the consonant string an equal number of times. For instance, half of the participants saw the items “to grasp” and “to think” primed with capitalized targets and the items “to pinch” and “to cheat” primed with consonant strings, while the remaining participants saw the items “to grasp” and “to think” primed with consonant strings and the items “to pinch” and “to cheat” primed with capitalized targets. Participants were asked to indicate as quickly and as accurately as possible whether the target was a word or not by pressing one of two response buttons using the preferred hand (all participants used their right hand). The relative position of the two response buttons (left-right to indicate word-nonword) was counterbalanced across subjects. Participants performed six practice trials before beginning the actual task.
Figure 1.
A) Display sequence for each trial of the LD task. B) Display of an action (top) and of an abstract (bottom) trial of the SSJ task.
SSJ task
In each trial, three verbs were presented simultaneously in a triangular arrangement (Figure 1B). Each verb was presented with the word “to” to its left. Participants were instructed to decide which of the two words on the bottom was most similar in meaning to the one on top, and indicate their response by pressing one of two response buttons using whichever hand they preferred (all participants used their right hand). They were instructed to keep their hand just above the buttons, with the forearm resting on the table, and to respond as fast and as accurately as possible. The words remained on the screen until the participant made a response. There were 80 trials, divided equally between action verbs and abstract verbs. The position of the two bottom verbs on the screen (left or right) was counterbalanced across subjects. Participants performed six practice trials before beginning the actual task.
2.4. Data analysis
LD task
Trials with response time (RT) exceeding 4 seconds were discarded from further analyses. For each participant, we also removed trials for which the RTs were identified as outliers according to the adjusted bloxplot (Hubert & Vandervieren, 2008), which is more appropriate to highly skewed distributions than the regular Tukey boxplot or other rules based on the standard deviation or the interquartile range (Seo, 2006). For an initial exploration of the data, we performed a 2 × 2 × 2 ANOVA with RT as the dependent variable, with two within-subjects factors [Verb Type (action, abstract) and Priming (capitalized target, consonant string)] and one between-subjects factor [Group (patient, control)]. We followed up this ANOVA with repeated-measures t tests with Verb Type as the independent variable, separately for the control and the PD groups. As described in the Introduction, however, our original hypotheses concerned only the Group by Verb Type interactions for RT and priming. We predicted that, relative to abstract verbs, the net RT for action verbs would be longer in the patient than in the control group, and that the net priming would be smaller in the patient than in the control group. In order to directly test these hypotheses, we calculated, for each participant, the net RT for the action condition (net RT = mean RT for action verbs minus mean RT for abstract verbs). Priming scores were normalized relative to each participant’s overall RT and converted to Z-scores. The net priming for action verbs (relative to abstract verbs) was calculated for each subject by subtracting the priming Z-score for abstract verbs from the one for action verbs. Net RT and net priming were compared across groups by means of independent-samples t-test (one-tailed) with Group as the independent variable. Note that, while the 3-way ANOVA allows us to explore all possible interactions in the data, its power to detect a pre-specified effect in a particular direction is reduced relative to a directional t-test; only a t-test with net RT and net priming as dependent variables allows us to test our original hypothesis taking into account the predicted direction of the effects. Also note that these t tests are a priori, and are therefore independent of the results of the 3-way ANOVA.
SSJ task
Trials with response time (RT) exceeding 5 seconds were discarded from further analyses. Outlier trials for individual participants were identified with the adjusted boxplot, as described above. We also removed from the analysis trials for which accuracy in the healthy participants group was close to chance level (< .6). The predicted Group by Verb Type interaction was tested as in the LD analysis: for each participant, we calculated the net RT and net accuracy (Acc) for the action trials (net RT = mean RT for action verbs minus mean RT for abstract verbs; net Acc = Acc for action verbs minus Acc for abstract verbs). Since we had specific hypotheses about the direction of the group differences on these variables, net RT and net Acc were entered as dependent variables in one-tailed, independent-samples t-tests, with Group as the independent variable. The effect of Verb Type within each group was assessed by means of separate, two-tailed t-tests.
3. Results
A Wilcoxon rank sum test showed that the mean UPDRS score of the patients off medication (45.7) was not significantly different from that of the patients on medication (24.5), W = 14, p = .25. In order to verify whether these two subgroups displayed similar results, we analyzed them separately at first; since their effects were in the same direction, we grouped all patients together for the main analysis.
3.1. Lexical Decision task
A total of 5.7% of trials were removed in the control group (4.7% for action, 4.1% for abstract, 8.3% for pseudowords), and 6% of trials were removed in the patient group (5.2% for action, 5% for abstract, 7.9% for pseudowords). In the net priming analysis, one participant (patient 13) was identified as an extreme outlier by the Tukey boxplot rule (Tukey, 1977) and was therefore excluded from that analysis.
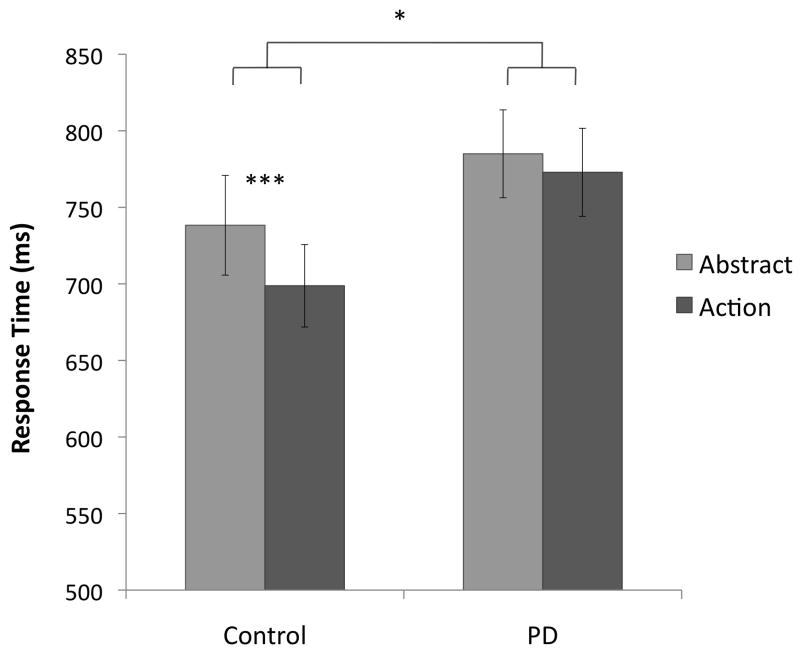
The 3-way ANOVA revealed a main effect of Verb Type, where RTs were longer for abstract (mean RT = 762 ms) than for action verbs (736 ms), F(1, 40) = 12.6, p = .001. There was also a main effect of Priming, in which responses to verbs primed with the capitalized version of themselves (mean RT = 724 ms) were faster than those to verbs primed with a consonant string (773 ms), as expected. There was no main effect of Group (p = .23).
There was a marginally significant interaction between Verb Type and Group, in which, relative to abstract verbs, RTs for action verbs were longer in the patient than in the control group (control-action: 699 ms; control-abstract: 738 ms; patient-action: 773 ms; patient-abstract: 785 ms), F(1, 40) = 3.52, p = .068. No other interactions were found.
In the control group, a paired t-test showed that responses to action verbs (699 ms) were significantly faster than responses to abstract verbs (738 ms), t(21) = 3.42, p = .002, two-tailed (Figure 2). The majority of participants achieved perfect Acc for the word trials, with only one participant making more than one error, so Acc was not further analyzed. Mean Acc was .995 for action and .984 for abstract verbs.
Figure 2.
Response times in the lexical decision (LD) task. Error bars represent the standard error of the mean. * p < .05, *** p < .005.
Unlike controls, PD patients showed no effect of Verb Type in RT, p = .17, two-tailed (Figure 2). As in the control group, patients’ Acc was close to ceiling (action: .992; abstract: .983; mean of less than one error per condition), and was not further analyzed.
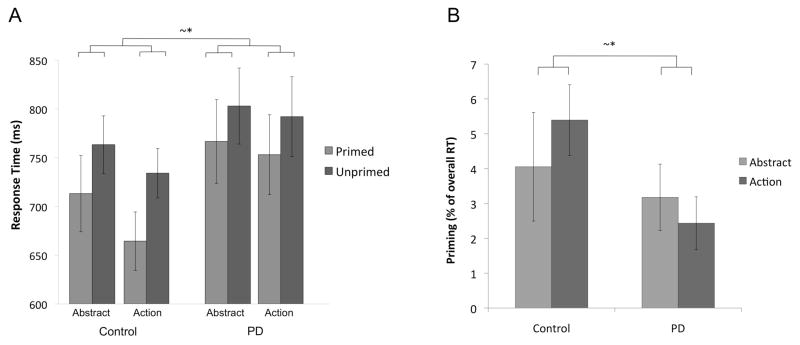
As predicted, net RT was significantly larger in the control group than in the patient group, t(40) = 1.88, p = .034, one-tailed. The group difference in net priming was marginally significant, t(39) = 1.52, p = .068, one-tailed, with the control group showing a trend toward a larger priming effect for action verbs than for abstract verbs, and a trend in the opposite direction for the patient group (Figure 3).
Figure 3.
Priming in the LD task. A) Response times for primed and unprimed targets. B) Priming as percentage of overall RT for words. Error bars represent the standard error of the mean. ~* p < .07.
3.2. Semantic Similarity Judgment task
One trial was removed from the analysis owing to performance in the control group being at chance level (.5). A total of 3.8% of trials were removed in the control group (3.6% for action, 4.0% for abstract), and a total of 2.9% of trials were removed in the patient group (3.6% for action, 2.3% for abstract).
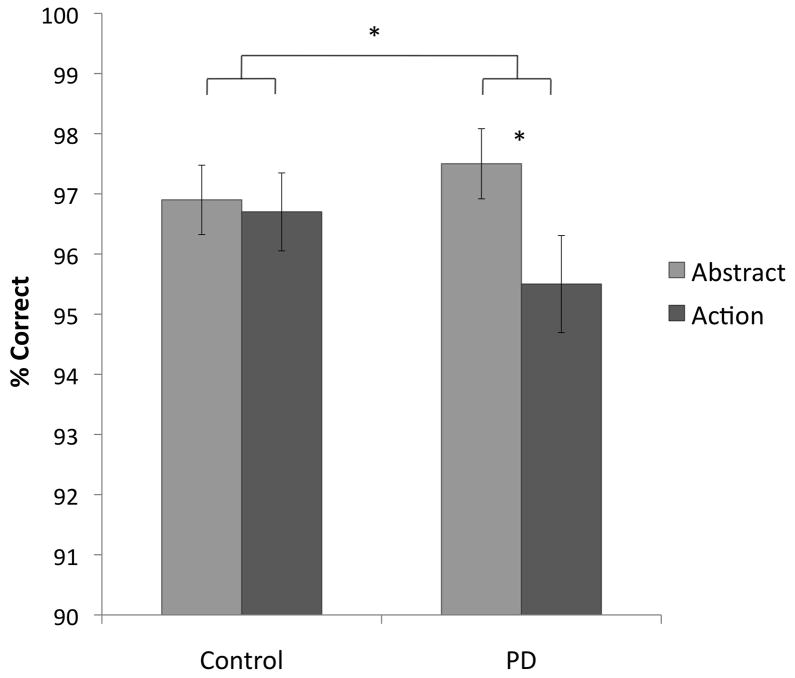
In the control group, responses to action-verb trials (mean RT = 2022 ms) were significantly slower than responses to abstract-verb trials (1890 ms), t(21) = 2.57, p = .018, two-tailed. There was no significant difference in Acc (action: .967, abstract: .969), p = .82, two-tailed (Figure 4).
Figure 4.
Accuracy in the Semantic Similarity Judgment (SSJ) task. Error bars represent the standard error of the mean. * p < .05.
Similar to controls, patients’ responses to action trials (2451 ms) were significantly slower than responses to abstract trials (2332 ms), t(19) = 2.44, p = .025, two-tailed. In contrast to controls, however, patients’ Acc in the action condition (.955) was significantly lower than in the abstract condition (.975), t(19) = 2.68, p = .015, two-tailed (Figure 4).
An independent-samples, one-tailed t-test revealed that net Acc was significantly lower for PD patients than for controls, t(40) = 1.92, p = .031, as predicted. Net RT did not differ between groups (p = .42, one-tailed).
The Acc was well matched between action and abstract conditions for controls, which argues against the possibility of this interaction being driven by greater difficulty of action trials that was amplified for the patient group due to general cognitive decline. The RT for action trials was, however, slightly but significantly higher than that for abstract trials for controls. In order to investigate whether this RT difference could somehow be reflected in Acc, we repeated the analysis after matching the conditions on RT. We excluded the five action trials with the longest RTs and the five abstract trials with the shortest RTs, which resulted in the mean RT being matched across conditions for both controls (action: mean RT = 1964 ms; abstract: mean RT = 1969 ms; p = 0.92) and patients (action: mean RT = 2391 ms; abstract: mean RT = 2403 ms; p = 0.84). This analysis yielded essentially the same results as the original one, with patients showing significantly lower accuracy for action verbs (mean Acc = .956) than for abstract verbs (mean Acc = .974), t(19) = 2.57, p = .019, two-tailed, and no difference in the control group (p = .88). This interaction was significant, t(40) = 1.90, p = .032, one-tailed.
4. Discussion
The embodied approach to conceptual knowledge maintains that the motor system makes a fundamental contribution to the semantic representation of action words. Consistent with this view, evidence for a close relationship between action word processing and action execution has been steadily accumulating over the past several years, spanning a variety of behavioral testing techniques, linguistic stimuli, presentation modalities, and brain activity measures, as reviewed above. However, one crucial prediction of the embodied approach regards the effects of motor system dysfunction on semantic processing, namely that comprehension of action-related words would be more affected than comprehension of words not related to action. The present study provides evidence for this prediction by showing that, relative to controls, PD patients were more impaired in processing action verbs than abstract verbs. This pattern was observed as longer RTs for action verbs in LD and as lower accuracy for action verbs in SSJ.
Although several studies have suggested a relationship between motor disorders and deficits of action word processing, the implications of those results for the embodied semantics debate are limited by the fact that the action-semantic manipulation was almost always confounded with a word class distinction (i.e., verb vs. noun). Our study avoided this problem by assessing participants’ performance on action verbs in relation to abstract verbs, matched in argument structure complexity.
Although the PD patients we tested have clear motor impairments, these were relatively mild, as shown by their low UPDRS score (mean 27.7 on a 0–88 scale) and by the fact that they could still live independently (e.g., they could walk, write, grasp and move objects, and most of them could drive). Furthermore, not all aspects of the motor system are affected in PD (patients do not typically develop apraxias, ataxias, or dyskinesias, for instance, although dyskinesia can appear as a side-effect of the medication). Hence, it should be expected that any impairments in semantic processing due to motor system dysfunction in our patients would not be particularly severe or catastrophic.
4.1. Different stages in word comprehension
As mentioned in the Introduction, behavioral studies have shown that motor processes and word recognition interact very early, within 200 ms of word presentation (e.g. Boulenger et al., 2006; Sato et al., 2008). Furthermore, such interaction is observed in tasks that, in principle, do not require access to semantic representations, such as lexical decision (e.g. Boulenger et al., 2008a and 2008b; Myung et al., 2006; Sato et al., 2008). Other studies, however, have shown that this language/motor crosstalk can depend on sentence-level meaning (e.g. Borghi, Glenberg and Kaschak, 2004; Glenberg and Kaschak, 2002; Glenberg, Sato and Cattaneo, 2008) and lead to relatively late motor cortex activation relative to word onset (Boulenger, Hauk and Pulvermüller, 2009). This raises the issue of whether the contribution of the motor system to word processing is automatic and context-independent, or, instead, modulated by the context in which the word occurs.
Word comprehension likely encompasses different degrees (and possibly different stages) of semantic processing. When an isolated word is subliminally perceived, for example, only a very limited analysis of its meaning probably takes place, such that only its more prominent semantic features are likely to be processed. Arguably, a much more detailed computation is required to allow the identification of the semantic nuances that emerge from a word’s use in context, such as the different senses of the word good in the expressions this is a good knife and this is a good question. At the upper end of the comprehension spectrum, the precise meaning of a word in a particular context may not become entirely clear until the entire discourse is processed, where the relevant discourse may be a sentence, a paragraph, a joke, or a story. In principle, sensory-motor representations could contribute to semantic processing at any level of comprehension, in which case we would expect motor cortical activation to occur at different latencies relative to the presentation of the word. Therefore, tasks that demand different levels of semantic processing probably lead to different temporal and neuroanatomical patterns of motor activation during action verb processing. Our results indicate that the motor system contributes to the semantic processing of action words on (at least) two different levels: a shallow, automatic level, as assessed by priming and lexical decision, and a deeper, controlled level, as assessed by semantic similarity judgment.
Although, in principle, lexical decision could be performed without access to semantic representations, it is well known that response times in this task are affected by semantic factors (e.g. Dunabeitia, Aviles & Carreiras, 2008; James, 1975; Pexman, Lupker & Hino, 2002). Concrete nouns, for instance, are typically processed faster and more accurately than abstract nouns (the “concreteness effect”; e.g., Binder, Westbury, McKiernan, Possing & Medler, 2005; Kroll & Merves, 1986; Samson & Pillon, 2004). We found a similar effect for healthy controls, who responded faster to action than to abstract verbs. The fact that PD patients did not show this advantage for action verbs indicates that they were, at least to some extent, impaired at processing the action-semantic features of the stimuli, as predicted by embodied theories of word meaning.
The LD results also suggest that priming of action verbs was reduced (compared to priming of abstract verbs) in the PD group. This is consistent with the results of Boulenger et al. (2008a), who found that PD patients off dopaminergic medication showed normal priming for concrete nouns, but not for action verbs. When the same patients were tested while on medication, priming was observed in both conditions. In the present study where most patients were on dopaminergic medication, a trend was found toward dissociation in priming for action and abstract verbs, although this result should be interpreted with caution, since the interaction did not reach significance. The smaller sample size in the Boulenger et al. study might explain why they did not find this effect with the medicated patients. The longer average disease duration of their PD sample (10 years) compared to ours (5.7 years) could also help explain why they did not find an interaction between group and word type in RT, since a decline in executive functions is often observed at later stages of the disease (e.g., Ko et al., 2012; Mahieux et al., 1998; Raskin, Borod, & Tweedy, 1992). In fact, the progression of executive dysfunction in PD seems to be non-linear, with acceleration in the rate of decline around 13 years after diagnosis (Aarsland, Muniz, & Matthews, 2011). In the Boulenger et al. study, we conjecture that the decline in executive control in the patients with longer disease duration, especially in the OFF state, may have affected the more controlled task measures such as RT across all conditions, minimizing the difference between conditions, while preserving more automatic processes such as priming. Thus, one possibility is that selective impairment on one condition may be, paradoxically, more difficult to find in more severely affected patients. This speculation awaits further investigation.
The SSJ task required a much deeper level of semantic processing, since participants had to explicitly compare the meanings of the three words in order to make a decision. As expected, given the general slowing of responses typical of PD, patients were slower than controls, while the overall accuracy was similar for both groups. In line with our hypothesis, controls made a similar number of errors in the action and the abstract conditions, but PD patients made significantly more errors in the action verb trials, compared with the abstract ones. In the action verb condition, 14 different trials (out of 39) had errors across PD patients, and no trial had errors from more than eight patients, so the Acc effect in this group likely reflects the semantic manipulation, rather than being driven by a few problematic trials. Thus, our results suggest that the motor system also plays a functional role in the more elaborate, controlled semantic processing of action verbs.
It should be noted that our results only allow us to infer that the motor system contributes to the semantic processing of action verbs, such that a component of action meaning is based on action systems of the brain. Multiple processing streams, with different information sources, are likely to participate in the determination of word meaning. The goal of the present study was to verify whether the motor system plays a causal role in this process (in the case of action words), or whether the motor activations found in previous studies could be considered epiphenomenal or as mere embellishments. Taken together, our results strongly argue for a causal role of the motor system in action word processing.
4.2. Motor vs. Cognitive Impairments in PD
Besides the motor symptoms that constitute the hallmark of the disease, PD patients can also present with impairments in executive functions, including deficits in working memory, attention, and cognitive set shifting (Brown & Marsden, 1990; Emre, 2003; Owen, 2004). Is it possible, then, that the action-verb deficit displayed by PD patients was a consequence of cognitive rather than of motor impairments? That would be a real possibility if (a) the action-verb condition was overall more difficult than the abstract-verb condition, or (b) action verb processing imposed stronger demands on executive functions compared to abstract verb processing. The fact that controls actually performed better on the action than on the abstract condition in the LD task rules out the possibility that the results in the patient group were driven by overall difficulty. In the SSJ task, accuracy scores for the two verb types were virtually identical in the control group, which suggests that the lower accuracy for action verbs displayed by PD patients was not driven by an overall higher error rate for this verb type. Although both groups showed somewhat longer RTs for action than for abstract verbs, it is unlikely that the Group by Condition interaction in accuracy was driven by difficulty as reflected in RT, because the same interaction was found even when the analysis was done on a subset of the stimuli where RT was matched between conditions.
With respect to executive demands, there are reasons to believe that the abstract-verb condition was in fact the one that imposed stronger demands on cognitive control. Compared to concrete words, abstract words are associated with a larger number of senses and linguistic contexts, and therefore require more cognitive control to filter out irrelevant senses during comprehension (Badre & Wagner, 2002; Hoffman, Rogers, & Lambon Ralph, 2011). Furthermore, the inferior frontal gyrus (IFG) -- a region associated with executive processing -- is consistently activated by abstract over concrete words (Binder et al., 2005; Binder, Desai, Graves, & Conant, 2009; Desai et al., 2010; Noppeney & Price, 2004). Further still, neuropsychological and rTMS evidence indicates that the IFG becomes less involved in processing abstract words when more contextual information is available (Hoffman, Jefferies, & Lambon Ralph, 2010).
Using Latent Semantic Analysis (LSA), Hoffman, Rogers, & Lambon Ralph (2011) devised a measure of “semantic diversity” (SemD), i.e., the range of different senses, meanings and linguistic contexts in which a word tends to occur, and found it to be negatively correlated with imageability (which is highly coupled to concreteness). In that study, stroke patients presenting with executive dysfunction performed a synonym judgment task similar to the SSJ task used here, and their accuracy was inversely correlated with SemD. (In fact, SemD predicted this group’s performance better than imageability or word frequency.) In both our tasks, SemD was significantly higher for the abstract- than for action-verb condition (Tables 2 and 3), which makes it very unlikely that the action-verb deficit displayed by PD patients was due to impairment in executive functions.
5. Conclusions
Relative to healthy controls, PD patients were more impaired at processing action verbs than abstract verbs. These results indicate that impairments of the motor system are accompanied by selective impairments in processing action-related verbs, as predicted by the embodiment account of word meaning. Action verb processing was relatively hindered both at the automatic word recognition level and at the controlled semantic judgment level. The design of the study rules out the possibility that the deficit observed in the Parkinson’s group reflects a general impairment in verb processing, rather indicating that the differential effect of motor system impairment on the two conditions was a function of the core meanings associated with the words. These results are difficult to reconcile with the notion that word meaning is based purely on amodal representations.
We investigated the role of the motor system in action-related language processing.
PD patients and controls performed lexical decision and semantic judgment tasks.
The words were either action verbs or abstract verbs.
PD patients were more impaired in action verb processing in both tasks.
The motor system plays a causal role in action language semantics.
Acknowledgments
We thank Vicki Conte for her invaluable help with patient recruitment, and the patients and their families for participation. We also thank Paul Hoffman and Timothy Rogers for providing SemD ratings, and three anonymous reviewers for helpful comments. This work was funded by the grant NIH R01 DC010783 (RD).
References
- Aarsland D, Muniz G, Matthews F. Nonlinear decline of mini-mental state examination in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2011;26(2):334–7. doi: 10.1002/mds.23416. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Damasio A. Embodied semantics for actions: Findings from functional brain imaging. Journal of Physiology, Paris. 2008;102:35–39. doi: 10.1016/j.jphysparis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Current Biology. 2006;16:1818–1823. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Semantic retrieval, mnemonic control, and prefrontal cortex. Behavioral and Cognitive Neuroscience Reviews. 2002;1(3):206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- Bak T, O’Donovan D, Xuereb J, Boniface S, Hodges J. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain. 2001;124(Pt 1):103–20. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- Bak T, Yancopoulou D, Nestor P, Xuereb J, Spillantini M, Pulvermüller F, et al. Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain. 2006;129(Pt 2):321–32. doi: 10.1093/brain/awh701. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, et al. The English Lexicon Project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Barsalou L. Perceptual symbol systems. Behavioral And Brain Sciences. 1999;22:577–660. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A. Perception, action, and word meanings in the human brain: the case from action verbs. Annals of the New York Academy of Sciences. 2011;1224(1):81–95. doi: 10.1111/j.1749-6632.2011.06013.x. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–36. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17(6):905–17. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Borghi A, Glenberg A, Kaschak M. Putting words in perspective. Memory & Cognition. 2004;32(6):863–73. doi: 10.3758/bf03196865. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Hauk O, Pulvermüller F. Grasping ideas with the motor system: semantic somatotopy in idiom comprehension. Cerebral Cortex. 2009;19(8):1905–14. doi: 10.1093/cercor/bhn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir T. Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008a;46(2):743–56. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Roy A, Paulignan Y, Deprez V, Jeannerod M, Nazir T. Cross-talk between language processes and overt motor behavior in the first 200 msec of processing. Journal of Cognitive Neuroscience. 2006;18(10):1607–15. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Silber BY, Roy A, Paulignan Y, Jeannerod M, Nazir T. Subliminal display of action words interferes with motor planning: A combined EEG and kinematic study. Journal of Physiology - Paris. 2008b;102(1–3):130–136. doi: 10.1016/j.jphysparis.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Shtyrov Y, Pulvermüller F. When do you grasp the idea? MEG evidence for instantaneous idiom understanding. Neuroimage. 2012;59(4):3502–3513. doi: 10.1016/j.neuroimage.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends in Neurosciences. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Bub D, Masson M. On the nature of hand-action representations evoked during written sentence comprehension. Cognition. 2010;116(3):394–408. doi: 10.1016/j.cognition.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Brain Research Cognitive Brain Research. 2005;24(3):355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Burgess C, Lund K. The dynamics of meaning in memory. Cognitive Dynamics: Conceptual and representational change in humans and machines. 2000;13:17–56. [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowledge of object manipulation and object function: dissociations in apraxic and nonapraxic subjects. Brain and Language. 2002;82(2):179–99. doi: 10.1016/s0093-934x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Disembodying cognition. Language and cognition. 2010;2(1):79–116. doi: 10.1515/LANGCOG.2010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Zanetti M, Arévalo A, Cappa SF, Padovani A. Action and object naming in Parkinson’s disease without dementia. European Journal of Neurology. 2007;14:632–637. doi: 10.1111/j.1468-1331.2007.01797.x. [DOI] [PubMed] [Google Scholar]
- Damasio A. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- den Ouden D-B, Fix S, Parrish TB, Thompson CK. Argument structure effects in action verb naming in static and dynamic conditions. Journal of Neurolinguistics. 2009;22:196–215. doi: 10.1016/j.jneuroling.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R, Binder J, Conant L, Seidenberg M. Activation of sensory-motor areas in sentence comprehension. Cerebral Cortex. 2010;20(2):468–78. doi: 10.1093/cercor/bhp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R, Binder J, Conant L, Mano Q, Seidenberg M. The neural career of sensorimotor metaphors. Journal of Cognitive Neuroscience. 2011;23(9):2376–2386. doi: 10.1162/jocn.2010.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druks J. Verbs and nouns -- a review of the literature. Journal of Neurolinguistics. 2002;15(3–5):289–315. [Google Scholar]
- Dunabeitia JA, Aviles A, Carreiras M. NoA’s ark: influence of the number of associates in visual word recognition. Psychonomic Bulletin and Review. 2008;15:1072–1077. doi: 10.3758/PBR.15.6.1072. [DOI] [PubMed] [Google Scholar]
- Emre M. Dementia associated with Parkinson’s disease. The Lancet Neurology. 2003;2(4):229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Fernandino L, Iacoboni M. Are cortical motor maps based on body parts or coordinated actions? Implications for embodied semantics. Brain and Language. 2010;112(1):44–53. doi: 10.1016/j.bandl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Ferretti TR, McRae K, Hatherell A. Integrating verbs, situation schemas, and thematic role concepts. Journal of Memory and Language. 2001;44:516–547. [Google Scholar]
- Fischer MH, Zwaan RA. Embodied language: a review of the role of the motor system in language comprehension. Quarterly Journal of Experimental Psychology. 2008;61(6):825–50. doi: 10.1080/17470210701623605. [DOI] [PubMed] [Google Scholar]
- Fodor JA. The Language of Thought. Cambridge, MA: Harvard University Press; 1975. [Google Scholar]
- Fodor JA. The Mind Doesn’t Work That Way: The Scope and Limits of Computational Psychology. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22(3–4):455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Glenberg A, Kaschak M. Grounding language in action. Psychonomic Bulletin & Review. 2002;9(3):558–565. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- Glenberg A, Sato M, Cattaneo L. Use-induced motor plasticity affects the processing of abstract and concrete language. Current Biology. 2008;18(7):R290–1. doi: 10.1016/j.cub.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L, Riggio L, Palumbo D, Buccino G. The Quarterly Journal of Experimental Psychology (2006) 6. Vol. 61. Psychology Press; 2008. Processing abstract language modulates motor system activity; pp. 905–919. [DOI] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71(18):1396–401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2006;41(2):301–7. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermüller F. Neurophysiological distinction of action words in the fronto-central cortex. Human Brain Mapping. 2004;21(3):191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Jefferies E, Lambon Ralph MA. Ventrolateral prefrontal cortex plays an executive regulation role in comprehension of abstract words: convergent neuropsychological and repetitive TMS evidence. Journal of Neuroscience. 2010;30(46):15450–15456. doi: 10.1523/JNEUROSCI.3783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Rogers TT, Lambon Ralph MA. Semantic diversity accounts for the “missing” word frequency effect in stroke aphasia: insights using a novel method to quantify contextual variability in meaning. Journal of Cognitive Neuroscience. 2011;23(9):2432–2446. doi: 10.1162/jocn.2011.21614. doi:10.1162/jocn.2011.21614. [DOI] [PubMed] [Google Scholar]
- Hubert M, Vandervieren E. An adjusted boxplot for skewed distributions. Computational Statistics and Data Analysis. 2008;52 (12):5186–5201. [Google Scholar]
- James CT. The role of semantic information in lexical decisions. Journal of Experimental Psychology: Human Perception and Performance. 1975;104:130–136. [Google Scholar]
- Jenkins I, Fernandez W, Playford ED, et al. Impaired activation of the supplementary motor area in Parkinson’s disease is reversed when akinesia is treated with apomorphine. Annals of Neurology. 1992;32:749–757. doi: 10.1002/ana.410320608. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez-Castillo J. The Two-Level Theory of verb meaning: An approach to integrating the semantics of action with the mirror neuron system. Brain and Language. 2010;112(1):54–76. doi: 10.1016/j.bandl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Antonelli F, Monchi O, Ray N, Rusjan P, Houle S, Lang AE, et al. Prefrontal dopaminergic receptor abnormalities and executive functions in Parkinson’s disease. Human Brain Mapping. 2012 doi: 10.1002/hbm.22006. doi:10.1002/hbm.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerts J, Leenders K, Brouwer W. Cognitive dysfunction in non-demented Parkinson’s disease patients: controlled and automatic behavior. Cortex. 2009;45(8):922–9. doi: 10.1016/j.cortex.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Merves JS. Lexical access for concrete and abstract words. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1986;12:92–107. [Google Scholar]
- Landauer T, Dumais S. A Solution to Plato’s Problem: The Latent Semantic Analysis Theory of Acquisition, Induction, and Representation of Knowledge. Psychological Review. 1997;104(2):211–240. [Google Scholar]
- Levin B. English Verb Classes and Alternations: A Preliminary Investigation. University of Chicago Press; Chicago, IL: 1993. [Google Scholar]
- Mahieux F, Fénelon G, Flahault A, Manifacier MJ, Michelet D, Boller F. Neuropsychological prediction of dementia in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1998;64(2):178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon B, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology, Paris. 2008;102(1–3):59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Myung J-Y, Blumstein S, Sedivy J. Playing on the typewriter, typing on the piano: manipulation knowledge of objects. Cognition. 2006;98(3):223–43. doi: 10.1016/j.cognition.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Nazir T, Boulenger V, Roy A, Silber B, Jeannerod M, Paulignan Y. Language-induced motor perturbations during the execution of a reaching movement. Quarterly Journal of Experimental Psychology. 2008;61(6):933–43. doi: 10.1080/17470210701625667. [DOI] [PubMed] [Google Scholar]
- Neininger B, Pulvermüller F. Word-category specific deficits after lesions in the right hemisphere. Neuropsychologia. 2003;41(1):53–70. doi: 10.1016/s0028-3932(02)00126-4. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Kiebel S, Friston K, Price C. Action selectivity in parietal and temporal cortex. Cognitive Brain Research. 2005;25(3):641–9. doi: 10.1016/j.cogbrainres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Finocchiaro C, Shapiro K, Gangitano M, Caramazza A, Pascual-Leone A. All talk and no action: A transcranial magnetic stimulation study of motor cortex activation during action word production. Journal of Cognitive Neuroscience. 2004;16(3):374–381. doi: 10.1162/089892904322926719. [DOI] [PubMed] [Google Scholar]
- Owen A. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. The Neuroscientist. 2004;10(6):525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Papeo L, Vallesi A, Isaja A, Rumiati RI. Effects of TMS on different stages of motor and non-motor verb processing in the primary motor cortex. PLoS ONE. 2009;4(2):e4508. doi: 10.1371/journal.pone.0004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner R. Primary motor cortex of the parkinsonian monkey: Differential effects on the spontaneous activity of pyramidal tract-type neurons. Cerebral Cortex. 2011;21(6):1362–78. doi: 10.1093/cercor/bhq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pexman PM, Lupker SJ, Hino Y. The impact of feedback semantics in visual word recognition: number-of-features effects in lexical decision and naming tasks. Psychoomic Bulletin and Review. 2002;9:542–549. doi: 10.3758/bf03196311. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Fadiga L. Active perception: sensorimotor circuits as a cortical basis for language. Nature Reviews Neuroscience. 2010;11(5):351–60. doi: 10.1038/nrn2811. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y, Ilmoniemi R. Brain signatures of meaning access in action word recognition. Journal of Cognitive Neuroscience. 2005;17(6):884–92. doi: 10.1162/0898929054021111. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Is vision continuous with cognition? The case for cognitive impenetrability of visual perception. Behavioral and Brain Sciences. 1999;22(3):341–65. doi: 10.1017/s0140525x99002022. discussion 366–423. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss H, Stamatakis E, Tyler L. Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia. 2009;47(2):388–96. doi: 10.1016/j.neuropsychologia.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, Rascol A. Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Archives of Neurology. 1992;49(2):144–148. doi: 10.1001/archneur.1992.00530260044017. [DOI] [PubMed] [Google Scholar]
- Raskin SA, Borod JC, Tweedy JR. Set-shifting and spatial orientation in patients with Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology. 1992;14(5):801–821. doi: 10.1080/01688639208402864. doi:10.1080/01688639208402864. [DOI] [PubMed] [Google Scholar]
- Rueschemeyer SA, Van Rooij D, Lindemann O, Willems RM, Bekkering H. The function of words: distinct neural correlates for words denoting differently manipulable objects. Journal of Cognitive Neuroscience. 2010;22(8):1844–1851. doi: 10.1162/jocn.2009.21310. [DOI] [PubMed] [Google Scholar]
- Samson D, Pillon A. Orthographic neighborhood and concreteness effects in the lexical decision task. Brain and Language. 2004;91(2):252–264. doi: 10.1016/j.bandl.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Sato M, Mengarelli M, Riggio L, Gallese V, Buccino G. Task related modulation of the motor system during language processing. Brain and Language. 2008;105(2):83–90. doi: 10.1016/j.bandl.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Seo S. Masters thesis. University of Pittsburgh; Pennsylvania: 2006. A review and comparison of methods for detecting outliers in univariate data sets. [Google Scholar]
- Scorolli C, Borghi A. Sentence comprehension and action: effector specific modulation of the motor system. Brain Research. 2007;1130(1):119–24. doi: 10.1016/j.brainres.2006.10.033. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Zurif E, Grimshaw J. Sentence processing and the mental representation of verbs. Cognition. 1987;27(3):219–246. doi: 10.1016/s0010-0277(87)80010-0. [DOI] [PubMed] [Google Scholar]
- Suppa A, Iezzi E, Conte A, et al. Dopamine influences primary motor cortex plasticity and dorsal premotor-to-motor connectivity in Parkinson’s disease. Cerebral Cortex. 2010;20(9):2224–2233. doi: 10.1093/cercor/bhp288. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, et al. Listening to action-related sentences activates fronto-parietal motor circuits. Journal of Cognitive Neuroscience. 2005;17(2):273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Shapiro L, Li L, Schendel L. Analysis of verbs and verbargument structure: A method for quantification of aphasic language production. Clinical Aphasiology. 1995;23:121–140. [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam MM. Neural correlates of verb argument structure processing. Journal of Cognitive Neuroscience. 2007;19(11):1753–67. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino B, Fink G, Sparing R, Dafotakis M, Weiss P. Action verbs and the primary motor cortex: a comparative TMS study of silent reading, frequency judgments, and motor imagery. Neuropsychologia. 2008;46(7):1915–26. doi: 10.1016/j.neuropsychologia.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Trueswell JC, Kim AE. How to prune a Garden Path by nipping it in the bud: Fast priming of verb argument structure. Journal of Memory and Language. 1998;39:102–123. [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- van Elk M, van Schie H, Zwaan R, Bekkering H. The functional role of motor activation in language processing: motor cortical oscillations support lexical-semantic retrieval. NeuroImage. 2010;50(2):665–77. doi: 10.1016/j.neuroimage.2009.12.123. [DOI] [PubMed] [Google Scholar]
- Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Human Brain Mapping. 2011;32(9):1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Labruna L, D’Esposito M, Ivry R, Casasanto D. A functional role for the motor system in language understanding: evidence from theta-burst transcranial magnetic stimulation. Psychological Science. 2011;22(7):849–54. doi: 10.1177/0956797611412387. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod J, Foldi N, Mattis P. A Review of the Cognitive and Behavioral Sequelae of Parkinson’s Disease: Relationship to Frontostriatal Circuitry. Cognitive & Behavioral Neurology. 2003;16 (4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zwaan R, Taylor L. Seeing, acting, understanding: motor resonance in language comprehension. Journal of Experimental Psychology: General. 2006;135(1):1–11. doi: 10.1037/0096-3445.135.1.1. [DOI] [PubMed] [Google Scholar]