Abstract
Aim
The goal of these studies was to examine the capacity for glomerular repair after a podocyte depleting injury.
Methods
We created transgenic (TG) mice expressing the yeast enzyme cytosine deaminase specifically in glomerular podocytes. In these TG animals, the prodrug 5-flucytosine (5-FC) is converted to 5-fluorouracil (5-FU) and promotes cell death.
Results
Treatment with increasing dosages of 5-FC caused graded increases in proteinuria 1–2 weeks after treatment, which returned to control levels by the 10-week time point. Light microscopic examination revealed minimal pathology at the 2-week time point, but electron microscopy revealed found foot process effacement as well as focal areas of glomerular basement membrane duplication, and immunohistochemical studies detected podocyte apoptosis and a decrease in the number of Wilms tumor protein 1 (WT1) positive cells. By the 10-week time point, however, the number of WT1 positive cells was similar to controls and a few mice had developed focal areas of glomerulosclerosis. Consistent with the effects of 5-FC on podocyte number, expression of the podocyte mRNAs for nephrin, podocin, synaptopodin and podocalyxin were altered in a similar temporal fashion.
Conclusion
The glomerulus has a significant capacity for repair after a podocyte depleting injury.
Keywords: glomerular disease, mouse model, podocyte
Introduction
Podocytes play a key role in the pathogenesis of glomerular diseases [1–4]. In this regard, a decrease in the number of glomerular podocytes is observed in both animal and human kidney diseases [3,5–15]. Because podocytes are terminally differentiated cells with a limited capacity for replication [3,16], it has been suggested that podocytes which are lost cannot be effectively replaced, causing instability of the glomerular tuft and glomerulosclerosis [3]. As a result, some investigators contend that a reduction in glomerular podocytes may be a final common pathway causing progressive renal injury in glomerular diseases [3]. Although recent studies suggest that a population of renal progenitor cells has the capacity to regenerate podocytes [17–19], in glomerular diseases, this regenerative process is insufficient to prevent a decrease in podocyte number characteristic of some glomerular disease processes [3,5–12]. In these diseases, however, on-going podocyte injury may limit the regenerative potential. We, therefore, created a mouse model of podocyte injury that permitted discrete episodes of podocyte damage that was sufficient to cause a reduction in the number of glomerular podocytes. We then determined the capacity of glomerular repair mechanisms [17–19] to replenish podocytes after a single podocyte depleting injury.
Methods
Creation of cytosine deaminase (CD) TG mice
To create the CD cDNA a reverse transcription reaction (RT) was performed using C. neoformans mRNA (kindly provided by William J. Steinbach at Duke University Medical Center) and then the cDNA was amplified using the high fidelity Pfu DNA polymerase (Stratagene) and primer pairs (CCATGTCCCCCGTAGAAGGAT) and (TTACGATCCTGTGACCTCTCC). The resulting RT-PCR product was ligated into the vector pCR 2.1 (Invitrogen). To create the inducible CD transgene, we subcloned an XhoI/BamHI fragment of the CD cDNA in PCR2.1 into a previously described Tet-On construct [20] in frame with an N-terminal HA epitope. The resulting construct was sequenced at the DNA Analysis Facility at Duke University Medical Center.
The transgene was linearized by cutting with the restriction enzymes ApaLI/ClaI and purified as previously described [20] prior to pronuclear injection. TG mice were created at the Duke University TG facility using an inbred mouse strain (FVB/NJ) as described [20]. For transgene expression, CD TG mice were bred with TG mice expressing the reverse tetracycline transactivator (rtTA) under the control of the podocin promoter [21] (background FVB/NJ) to create TG animals with both transgenes (“double” TG mice”) as well as control animals (non-TG mice and “single” TG mice with either the rtTA or CD transgene). Screening for the CD transgene was performed by the polymerase chain reaction (PCR) using the primer pairs described above and DNA prepared from mouse-tails. Amplification of the rtTA transgene was performed as previously described [21].
Transgene expression
To screen for transgene expression, mice were treated for 1-week prior to study with 2-mg/ml doxycycline in drinking water with 5% sucrose to enhance palatability. After 1 week, mice were sacrificed and tissues were harvested for RT-PCR, immunoblotting and immunohistochemistry as previously described [20]. RT-PCR was performed as described [20] using the primer pairs described above. Control PCR reactions were performed using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (CLONTECH Laboratories, Palo Alto, CA) as described [20]. Immunoblotting was performed using enriched glomerular preparations as previously described [20,22].
Animal Studies
“Double” TG mice were treated with 2-mg/ml doxycycline in drinking water containing 5% sucrose for 1 week and then received 5-FC (100 or 500 mg/kg/day) by once daily subcutaneous injection for 5 days while the doxycycline in drinking water was continued. One week of doxycycline treatment has previously been shown to maximally induce the transgene [21]. Control mice were treated in an identical fashion with 500mg/kg/day 5-FC. As an additional control, “double” TG mice were similarly treated with 5% sucrose water without doxycycline and then 500 mg/kg/day 5-FC. Twenty-four hour urine collections were performed, 4–6 and 8–10 weeks after the last injection of 5-FC. Mice were then sacrificed at the indicated time points and both blood and tissues harvested, and then stored at −70°C for the studies described below. All animal procedures were approved by the Animal Care and Use Committee of Duke University Medical Center.
Because we could not detect apoptotic podocytes at the 1–2 or 8–10 week time points, an additional group of mice was studied using the protocol described above but kidneys were harvested on days 2–4 days during treatment with 5-FC.
Measurement of albuminuria, and proteinuria and urinary nephrin excretion
Albuminuria and proteinuria were measured as previously described [20,22], using a kit from AssayPro (St. Charles, MO) and the Brilliant Blue dye binding assay, respectively.
Immunohistochemistry
Mouse kidney cortex was embedded in OCT (Optimal Cutting Temperature) compound, snap frozen in liquid nitrogen and stored at −70°C until sectioning. Frozen sections were then fixed in acetone and air-dried. Expression of synaptopodin, WT1 and the HA epitope were identified by indirect immunofluorescence using a mouse monoclonal antibody to synaptopodin (Progen, Heidelberg, Germany), a rabbit polyclonal WT1 (Santa Cruz Biotechnology, Santa Cruz, CA) and a rabbit polyclonal antibody to the HA epitope (clone DW2, Upstate Cell Signaling Solutions, Lake Placid, NY), respectively. Ki-67 staining was performed to identify cells that have reentered the cell cycle [23] using 2 different monoclonal antibodies (clone TEC-3 from Dakocytomation, Glostrup, Denmark, or clone B56 from BD Biosciences, Palo Alto, CA). Briefly, slides were blocked with 5% non-fat dry milk in PBS for 30 minutes. For the studies, the WT1, anti-HA and Ki-67 antibodies were added at a dilution of 1:100 and the synaptopodin antibody was added at a dilution of 1:50 in D-PBS with 5% non-fat dry milk. After 1 hour, slides were washed 3 times in D-PBS and then incubated for 1 hour with the secondary antibodies as described [20]. In some studies, nuclei were counter stained with 0.5 μg/ml DAPI (4′,6-diamidino-2-phenylindole). After washing, slides were examined by a Nikon Eclipse TE2000-S fluorescent microscope. To determine podocyte number, podocyte nuclei were stained with a WT1 antibody (rhodamine), the glomerular tuft was stained with a synaptopodin antibody (fluorescein) and nuclei were countered stained with DAPI. Podocyte nuclei were then quantitated by counting nuclei that both co-localized with DAPI and were associated with synaptopodin staining. Data were expressed as the average number of podocyte nuclei per glomerular profile as previously described [24].
Detection of podocyte apoptosis
Acetone fixed frozen sections were stained with the WT1 antibody described above and then apoptotic nuclei were labeled by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) using an ApoTag Red In Situ Apoptosis kit from Millipore (Bedford, MA) according to the directions of the manufacturer. Apoptotic podocytes were detected by co-localization of WT1 and TUNEL labeled nuclei.
Immunoblotting for nephrin, synaptopodin and cleaved caspase 3
Immunoblotting was performed using the synaptopodin antibody described above, a rabbit polyclonal antibody to nephrin (Alpha Diagnostics, San Antonio, TX), a rabbit monoclonal antibody to cleaved caspase-3 (17kD fragment) (Cell Signaling Technology, Danvers, MA) and a mouse monoclonal antibody to actin (Millipore. Bedford, MA) as previously described [25].
Serum albumin and blood urea nitrogen (BUN) measurements
Serum albumin and BUN levels were measured by IDEXX Laboratories, Inc. (Westbrook ME).
Light microscopy
Formalin fixed tissue sections were stained with hematoxylin and eosin and examined by a pathologist blinded to genotype.
Transmission electron microscopy (TEM)
Small blocks of cortical tissue from selected animals were fixed in an aqueous solution of 8% glutaraldehyde (Sigma-Aldrich). Analysis at the electron microscopic level was performed in a qualitative fashion and areas of interest chosen in semithin sections for preparation of ultrathin sections for examination by a pathologist (D.N.H.) blinded to genotype.
Real-time quantitative RT-PCR (Q-RT-PCR)
Total RNA was isolated from enriched glomerular preparations and the reverse transcription reaction was performed as described [20,26]. Real-time quantitative PCR was performed using the ABI PRISM 7700 Sequence Detector System (Perkin-Elmer Applied Biosystems Division, Wellesley, MA, USA) and the universal SYBR Green PCR master Mix Kit (Perkin-Elmer Applied Biosystems Division, Wellesley, MA, USA) as previously described [26]. The amplification signals were analyzed with Perkin-Elmer ABI Prism 7700 Sequence detection software and were normalized to the endogenous β-actin mRNA level. The primers used for the Q-RT-PCR studies have been previously described [26].
Quantitation of podocyte apoptosis by flow cytometry
To quantitate podocyte apoptosis, kidneys were harvested and placed in ice-cold D-PBS. Cell suspensions were prepared by mincing kidney cortex and passing the tissue sequentially through 180μm and 71μm sieves followed by washing with ice-cold D-PBS. The supernatant was then digested for 1 hour in RPMI medium with 750-units/ml collagenase (Sigma-Aldrich, St. Louis, MO) and the cells washed in ice-cold D-PBS. Podocalyxin positive cells were identified by staining for 1 hour with a mouse monoclonal antibody (R&D Systems, Minneapolis, MN) in D-PBS with 10% fetal calf serum at 4°C. Apoptotic cells were identified by annexin V staining using a kit from BD Pharmingen (San Diego, CA) according to the directions of the manufacturer. Quantitation of apoptotic cells was performed by flow cytometric analysis at the Duke Comprehensive Cancer facility by gating on podocalyxin positive cells (fluorescein) and counting the percentage of podocalyxin positive cells stained with annexin V. Because a podocalyxin-like protein is found in peritubular capillaries in the kidney [27], we gated on podocalyxin positive cells with high levels of immunofluorescence based on the observation that podocalyxin staining was either weak or absent in peritubular capillaries of mouse kidney. For the studies, apoptotic cells were differentiated from necrotic cells by staining with 7-amino-actinomycin D according to the directions of the manufacturer. Data are expressed as a percentage of the podocalyxin positive cell population by dividing the number of apoptotic podocalyxin positive cells by the total number of podocalyxin positive cells and expressing the resulting number as a percentage.
Statistical analysis
Data presented as the mean ± standard error of the mean (SEM) and statistical analyses were performed using the InStat computer program (GraphPad Sofware, Inc.). For comparison of continuous variables, a test of normality was performed (Kolmogorov–Smirnov test) prior to assessing statistical significance using the following statistical methods: 1. T-test for variables passing the normality test [28], or 2. Mann-Whitney test for variables that were not normally distributed [28]. For comparisons between more than two groups, statistical analysis included either: 1. A one way Analysis of variance (ANOVA) followed by a Bonferonni multiple comparisons post test for normally distributed variables [28], or 2. A Kruskal-Wallis test followed by a Dunn multiple comparisons post test for variables that were not normally distributed [28].
Results
Creation of CD TG mice
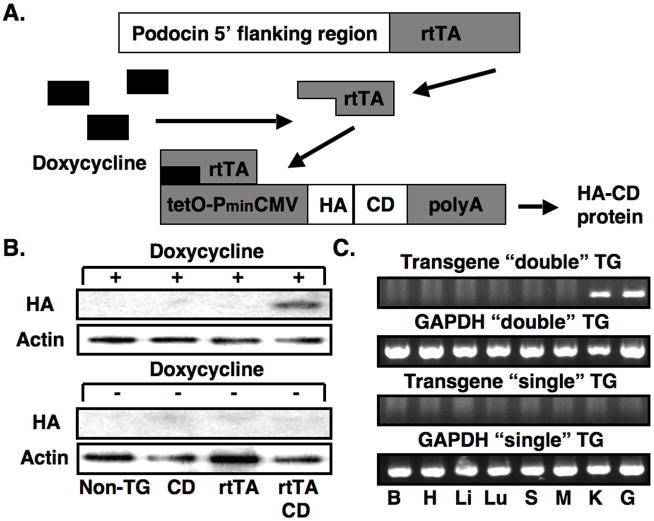
For the experiments, we utilized the Tet-On system, which has been successfully used by this laboratory for inducible transgene expression [20]. We utilized the inducible approach because, in future experiments, this strategy would permit targeting inducible expression of CD to other renal or extrarenal tissues using available Tet-On or Tet-Off mice [29]. The doxycycline inducible system requires two TG mice for podocyte specific expression. As shown in Figure 1A, the first TG animal expresses the reverse tetracycline-controlled transcriptional activator (rtTA) under the control of the human podocin (NPHS2) promoter [21]. The second TG mouse expresses CD under the control of tet operator sequence (tetO) and a minimal CMV promoter (PminCMV) [21]. By breeding the two TG mice, animals are obtained that express both transgenes. In these “double” TG mice, treatment with doxycycline induces CD expression.
Figure 1.
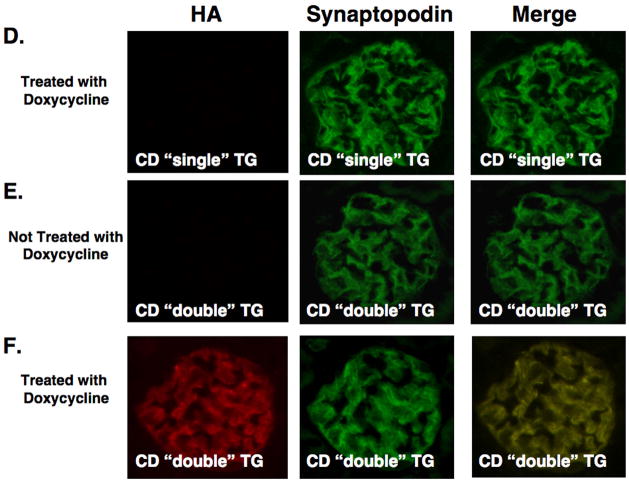
Creation of CD TG mice and induction of the transgene. Panel A shows the inducible TG strategy using the doxycycline inducible Tet-On system. Two TG mice are required for podocyte specific expression. The first TG animal expresses rtTA under the control of the human podocin (NPHS2) promoter. In the second TG mouse, expression of CD is driven by tetO and PminCMV (see text for details). In mice that express both transgenes, treatment with doxycycline induces expression of the HA tagged CD protein. In panel B, induction of the transgene by doxycycline was investigated by immunoblotting for the HA epitope in non-TG mice, “single” TG mice (rtTA or CD) and “double” TG mice (rtTA and CD) in the presence of doxycycline or vehicle (5% sucrose water). Transgene was induced by doxycycline in “double” TG mice but not in control mice (upper panel). Transgene expression was not detectable by immunoblotting in the absence of doxycycline (lower panel). β-actin was used as a loading control. In panel C, tissue specific expression of the transgene was investigated by RT-PCR in “double” TG mouse and a “single” TG CD mouse as indicated after treatment with doxycycline using transgene specific primers. The CD RT-PCR product was detected in both kidney cortex and isolated glomerular preparations from “double” TG mice. No CD RT-PCR products were detected in other tissues from the “double” TG mice (top panel) or any tissues of the “single” TG CD mouse (lower panel). The GAPDH control confirmed that the RT reaction was successful in the tissues examined. B, H, Li, Lu S, M, G, K, G are brain, heart, liver, lung, spleen, muscle (skeletal), kidney cortex and glomeruli, respectively. In panels D, E and F, tissue sections were stained for expression of the HA tagged CD transgene and the podocyte marker synaptopodin. As shown in panel D, synaptopodin, but not the HA epitope, was detected in this “single” TG CD mouse treated with doxycycline. Similarly, synaptopodin, but not the HA epitope, was detected in this “double” TG CD mouse in the absence of doxycycline treatment (Panel E). In contrast, both the HA epitope and synaptopodin were detected in “double” TG mice treated with doxycycline (Panel F). When the two images are merged the HA epitope and synaptopodin had a similar cellular distribution in the “double” TG mouse.
For the experiments, two independent CD TG lines were established that expressed the transgene in an inducible fashion. Experimental results were similar using the progeny from these independent lines. Figure 1B shows inducible expression of the HA-tagged CD transgene by immunoblotting for the HA tagged CD enzyme using glomerular preparations from “double” TG mice (CD and rtTA transgenes) as well as “single” TG mice (CD or rtTA transgenes) and non-TG controls treated with doxycycline. The CD protein was detectable by immunoblotting in “double” TG mice but not in “single” TG or non-TG mice (upper panel). In the absence of doxycycline, the CD protein was not detectable in either non-TG, “single” TG or “double” TG mice (Figure 1B, lower panel).
Figure 1C shows tissue specific expression of the CD transgene by RT-PCR using mRNA prepared from mice treated doxycycline. As shown in the top panel, a RT-PCR product of the appropriate size was detected in both kidney cortex and isolated glomerular preparations from “double” TG mice. No CD RT-PCR products were detected in other tissues from the “double” TG mice. In the lower panel, no RT-PCR products were detected in this “single” TG CD mouse lacking the rtTA transgene. The control GAPDH RT-PCR reaction confirmed that the reverse transcriptase reaction was successful in the tissues examined. Expression of CD was: 1. Not detectable by RT-PCR in any of the tissues from other doxycycline treated “single” TG mice or non-TG controls, and 2. Not detectable by RT-PCR in “double” TG mice in either the absence of doxycycline treatment or in the absence of a RT reaction in doxycycline treated animals.
We next determined cell specific expression of the transgene. For these studies, tissue sections were stained for expression of the HA tagged CD transgene (rhodamine) and the podocyte marker synaptopodin (fluorescein). As shown in the middle panel of Figure 1D and 1E, only synaptopodin was detected in “single” TG CD mice treated with doxycycline as well as in “double” TG CD mice in the absence of doxycycline treatment. Similar results were seen in rtTA “single” TG mice and non-TG controls in the presence of doxycycline. In contrast, Figure 1F shows that both the HA epitope and synaptopodin were detected in “double” TG mice treated with doxycycline. Merging the two images suggested that the HA epitope and synaptopodin shared a similar cellular distribution in doxycycline-treated “double” TG mice.
Effect of treatment with 5-FC on albuminuria and proteinuria
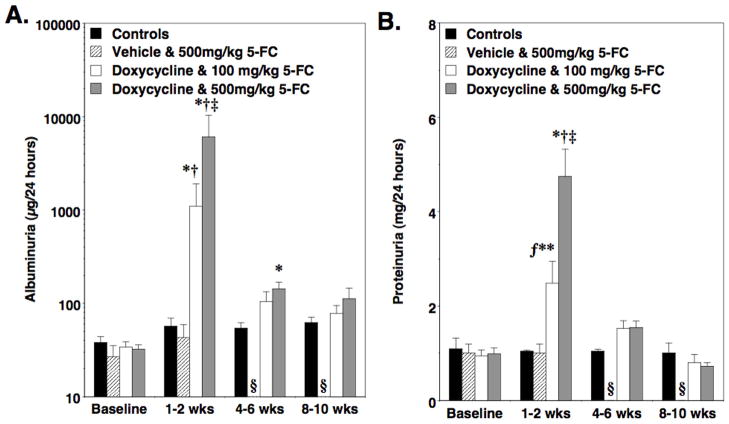
We next determined the effect of 5-FC treatment on albuminuria and proteinuria. For these studies, “double” TG mice were treated with doxycycline or vehicle (sucrose water) and then continued on doxycycline or vehicle while mice received 5-FC at dosages of either 100 or 500mg/kg/day as described in the Methods Section. Control mice were treated in an identical fashion with doxycycline and 500mg/kg/day 5-FC. Twenty-four hour urine samples were collected at baseline and 1–2, 4–6 weeks and 8–10 weeks after treatment with 5-FC was completed. As shown in Figure 2A and 2B, treatment with 5-FC caused graded increases in both albuminuria and proteinuria at the 1–2 week time point in “double” TG mice compared to controls (non-TG and “single” TG mice). This increase in urinary albumin and protein excretion returned toward baseline by the 4–6 and 8–10 week time points. In the absence of doxycycline treatment, treatment with 500mg/kg 5-FC had no significant effect on albuminuria or proteinuria in “double” TG mice. A similar albuminuria pattern was observed when the data was expressed as micrograms albumin per milligram creatinine (Table 1). Serum albumin and BUN levels are shown in Table 2 1–2 weeks after treatment with 5-FC. There was trend toward an increase in BUN levels and a decrease in albumin levels in “double” TG mice treated with 500mg/kg 5-FC compared to controls but these differences did not reach statistical significance.
Figure 2.
Effect of 5-FC on albuminuria and proteinuria. In panel A and B, albuminuria and proteinuria, respectively, were measured at the indicated time points. Treatment with 5-FC caused graded increases in both albuminuria and proteinuria 1–2 weeks after 5–FC treatment. By the 8–10 week time point, however, albuminuria and proteinuria had returned toward baseline. In the absence of doxycycline, treatment with 5-FC had no significant effect on either albuminuria or proteinuria in “double” TG CD mice. Albuminuria data is presented in a log scale. Sixteen to 26 mice were studied in each group and data was analyzed using a Kruskal-Wallis test followed by a Dunn multiple comparisons post test. *P<0.01 vs baseline, †P<0.01 vs controls, ‡P<0.05 vs 100mg/kg/day “double” TG mice, ƒP<0.05 vs baseline, **P<0.05 vs controls, §Not done
Table 1.
Albuminuria (micrograms albumin/milligram creatinine)
| Baseline | 1–2 weeks | 4–6 weeks | |
|---|---|---|---|
| Controls (Doxycycline & 500mg/kg 5FC) | 47 ± 5 | 51 ± 11 | 65 ± 6 |
| “Double” TG (Doxycycline & 100mg/kg 5FC) | 59 ± 7 | 767 ± 485*† | 114 ± 20 |
| “Double” TG (Doxycycline & 500mg/kg 5FC) | 41 ± 7 | 7565 ± 5134‡ | 122 ± 20* |
6–13 mice were studied per group and data was analyzed using a Kruskal-Wallis test followed by a Dunn multiple comparisons post test, some urine samples analyzed in in Figure 3 were not available for the measurements,
P<0.05 vs baseline,
P<0.01 vs controls,
P<0.001 versus either baseline or controls
Table 2.
Serum chemistry values in controls and “double” TG mice
| Group | Controls* | “Double” TG* |
|---|---|---|
| BUN (mmol/L) | 8.57 ± 0.89 | 11.42 ± 1.96 |
| Albumin (g/L) | 25.0 ± 1.3 | 22.5 ± 2.1 |
10–12 mice were studied per group, *controls and “double” TG mice were treated with both doxycycline and 500mg/kg/day 5-FC
Effect of treatment with 5-FC on glomerular histomorphology
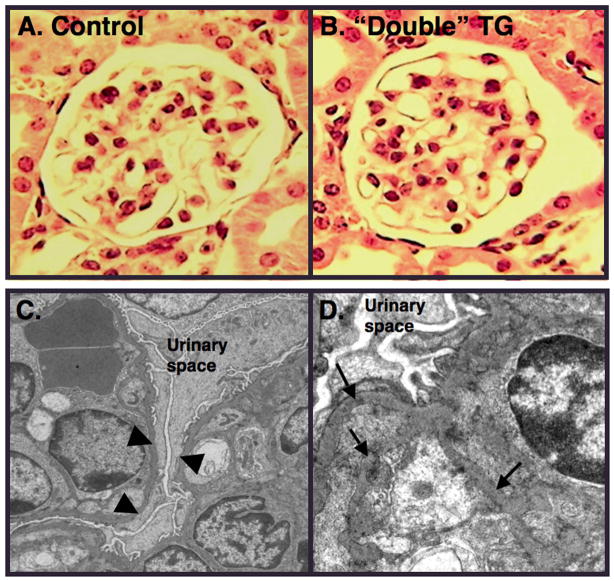
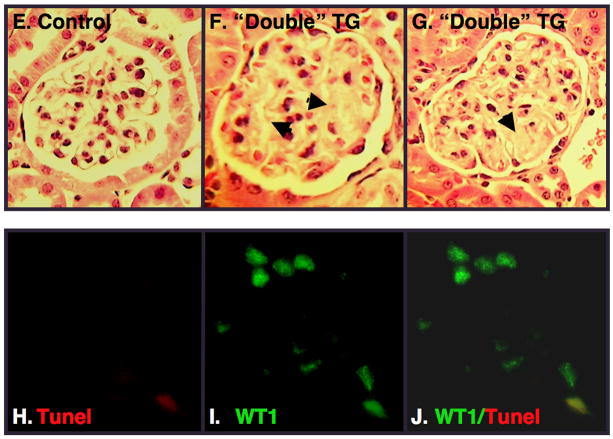
Light microscopic examination of kidney sections revealed minimal histopathologic abnormalities (Figure 3A and 3B). Transmission electron microscopy (TEM), however, revealed prominent foot process (FP) effacement in “double” mice treated with doxycycline and 500-mg/kg/day dosages of 5-FC (Figure 3C). As shown in Figure 3D, focal areas of glomerular basement membrane (GBM) duplication were observed in some animals at the 2-week time point. Ten weeks after treatment with 5-FC, light microscopic studies were normal in most animals but a few of the “double” TG mice treated with both doxycycline and 5-FC (20%) developed focal areas of glomerulosclerosis (Figure 3E, 3F and 3G). To assess podocyte apoptosis, podocyte nuclei were labeled by TUNEL (rhodamine) as well as stained with a WT1 antibody (fluorescein). Apoptotic podocytes were detected by co-localization of WT1 and TUNEL stained nuclei. Using this methodology, we were unable to detect podocyte apoptosis at either the 2-week or 10-week time point. We, therefore, studied a separate group of “double” TG mice treated with doxycycline and either 500-mg/kg/day 5-FC or vehicle while the doxycycline was continued. Kidneys were the harvested 2–4 days after the first dose of 5-FC or vehicle. In these mice, a few apoptotic podocytes were detected in “double” TG mice treated with both doxycycline and 5-FC (Figure 3H, 3I, and 3J). In contrast, apoptotic podocytes were never detected in any of the “double” TG controls treated with doxycycline and vehicle.
Figure 3.
Glomerular pathology. Panels A and B show light microscopic sections from a “single” TG CD mouse and a “double” TG mouse, respectively, 1–2 weeks after treatment with doxycycline and 500mg/kg/day 5-FC. Glomerular histopathology was similar in “double” TG mice and controls 1–2 weeks after treatment with 500mg/kg/day 5-FC at the light microscopic level. Panels C and D show glomerular ultrastructural from a “double” TG mouse treated with doxycycline and 500mg/kg/day 5-FC 1–2 weeks after treatment with 5-FC. Transmission electron microscopy (TEM) revealed prominent FP effacement (panel C, arrowheads) and focal areas of GBM duplication (panel D, arrows). Panels E, F and G show light microscopic studies from a control mouse and “double” TG mice 10 weeks after treatment with both doxycycline and 500-mg/kg/day 5-FC. A few of the “double” TG mice developed focal areas of glomerulosclerosis (arrows). Panels H, I and J are images from a “double” TG mouse during treatment with both doxycycline and 500-mg/kg/day 5-FC. In panel H (left panel), we assessed podocyte apoptosis in tissue sections by TUNEL using a commercially available kit (rhodamine tag). In panel I (middle panel), tissue sections were also stained with an antibody to WT1, which specifically stains podocyte nuclei (fluorescein tag). Podocyte apoptosis was assessed by co-localization of TUNEL stained nuclei with WT1 (Panel J). A few apoptotic podocytes were detected in “double” TG mice treated with both doxycycline and 5-FC. In contrast, no podocyte apoptosis was observed in control mice treated with doxycycline and vehicle.
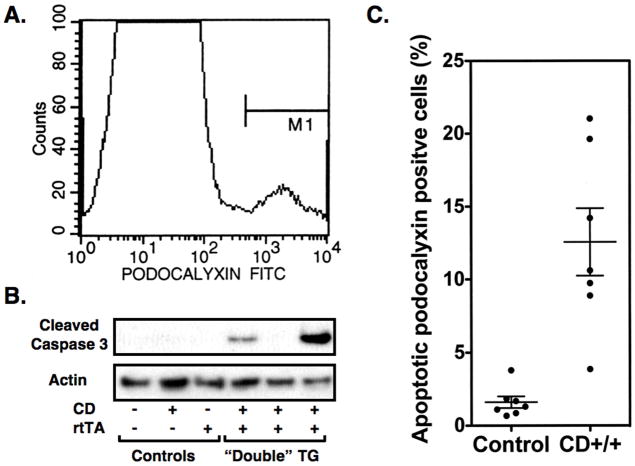
To better quantitate the apoptotic response to 5-FC, we used flow cytometry to identify podocytes in cell suspensions prepared from renal cortex as described in the Methods Section. As shown in Figure 4A, a small population of cells (4.2 ± 0.67%) expressed high levels of the podocyte marker podocalyxin. In controls treated with both doxycycline and 500mg/kg/day 5-FC, there were a few apoptotic podocalyxin positive cells detected (1.6 ± 0.39%), perhaps as a result of the methodology used to prepare of the cell suspensions (Figure 4B). In “double” TG mice treated in an identical fashion, there was a significant increase in the percentage of apoptotic cells detected (12.6 ± 2.3%: P<0.001). The number of apoptotic podocalyxin positive cells was similar to controls in “double” TG mice treated with 5-FC in the absence of doxycycline treatment (2.0 ± 0.8%).
Figure 4.
Quantitation of podocyte apoptosis. Podocytes in cell suspensions of kidney cortex were identified by staining for podocalyxin. As shown in Panel A, a small population of cells stained with the podocalyxin antibody. In controls treated with both doxycycline and 500mg/kg/day 5-FC, a few apoptotic podocytes were detected, perhaps caused by the procedure used to prepare the cell suspensions (Figure 4B). In “double” TG mice treated in an identical fashion, there was a significant increase in the percentage of apoptotic podocytes detected. In panel C, the active 17kD caspase 3 fragment is detected by immunoblotting in glomerular preparations from “double” TG mice treated with both doxycycline and 5-FC but not in glomerular preparations from control animals treated in an identical fashion. In panel B, 7 controls and 7 “double” TG mice (CD++) were studied at each time point (CD++ vs controls; P<0.001). In panel C, similar results were observed in 3 experiments.
As an additional method to assess apoptosis, immunoblotting was performed using glomerular preparations and an antibody to cleaved caspase 3. Caspase 3 is a critical executioner of apoptosis and its activation requires proteolytic processing into an active 17kD fragment [30]. As shown in Figure 4C, the 17kD fragment was detected in glomerular preparations from a few “double” TG mice treated with both doxycycline and 5-FC. The cleaved caspase 3 fragment was not detected in glomerular preparations from control animals treated in an identical fashion.
Effect of treatment with 5-FC on podocyte number
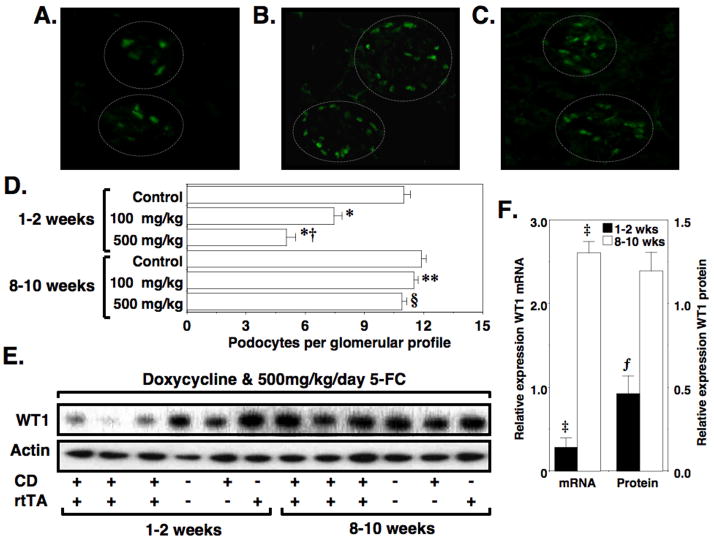
To determine if treatment with 5-FC caused podocyte depletion, podocyte nuclei were quantitated as described in the Methods Section, and data were expressed as the average number of podocyte nuclei per glomerular profile. Figure 5A and 5B show representative WT1 stained images from “double” TG and control mice, respectively, 2 weeks after treatment with 500mg/kg 5-FC. Figure 5C shows a representative WT1 stained image from “double” TG mice 10 weeks after 5-FC treatment, and Figure 5D shows quantitation of podocyte number at the indicated time points. Two weeks after 5–FC treatment, podocyte number was significantly reduced in a graded fashion in “double” TG mice treated with either 100 or 500 mg/kg 5-FC but not in control mice treated with 500mg/kg/5-FC. By the 8–10 week time point, however, podocyte number in “double” TG mice was not significantly different from controls. WT1 staining was not detected outside the glomerular tuft. To determine if expression of WT1 protein and mRNA levels followed a similar temporal pattern, we quantitated WT1 expression by both Q-RT-PCR and immunoblotting using either mRNA or protein lysates prepared from enriched glomerular preparations (Figure 5E and 5F). Data in Figure 5F are expressed relative to control mice at each time point. Both mRNA and protein for WT1 were decreased 1–2 weeks after treatment with 5-FC. By the 8–10 week time point, however, WT1 mRNA levels were significantly increased. WT1 protein levels also tended to be increased at the 8–10 week time point but this difference did not reach statistical significance.
Figure 5.
Restoration of podocyte number after a podocyte depleting injury. Frozen tissue sections were stained with both a WT1 antibody (fluorescein) to stain podocyte nuclei as described in the Methods Section. Panels A and B show representative tissues sections from a “double” TG mouse and a “single” TG CD mouse, respectively, 2 weeks after treatment with doxycycline and 500mg/kg/day 5-FC. Panel C shows a representative tissue section from a “double” TG mouse 10 weeks after treatment with doxycycline and 500mg/kg/day 5-FC. The dashed lines delineate two glomerular tufts in each tissue section. Panel D shows the number of podocytes per glomerular profile for control mice and “double” TG mice at the indicated time points after treatment with doxycycline and the either 100 mg/kg/day or 500mg/kg/day 5-FC as indicated. Treatment with 5-FC induced a graded decrease in podocyte number in “double” TG mice 2 weeks after 5-FC treatment. By the 8–10 weeks time point, however, podocyte number was not significantly different in “double” TG mice treated with either 100 or 500 mg/kg/day 5-FC compared to control animals. Panel E shows WT1 protein levels by immunoblotting of enriched glomerular preparations. Panel F shows quantitation of WT1 mRNA by Q-RT-PCR as well as quantitation of WT1 mRNA levels by densitometry. Data are expressed relative to control mice at each time point (relative expression in control mice is 1.0). As shown in panels E and F, both mRNA and protein for WT1 were decreased 1–2 weeks after treatment with 5-FC. By the 8–10 week time point, however, WT1 mRNA levels were significantly increased. The WT1 protein levels also tended to be increased but this difference did not reach statistical significance. Actin was used as a loading control. *P<0.01 vs control mice 2 weeks after 5-FC treatment, †P<0.05 vs “double” TG mice 2 weeks after treatment with 100mg/kg/day 5-FC, **P<0.01 vs “double” TG mice 2 weeks after treatment with 100mg/kg/day, §P<0.01 vs “double” TG mice 2 weeks after treatment with 500mg/kg/day, ‡P<0.025 vs control mice, ƒP<0.05 vs control mice
A few studies suggest that podocytes may reenter the cell cycle and proliferate [31,32]. To examine this possibility, we stained cell nuclei with the podocyte marker WT1 and a nuclear marker of cells that have reentered the cell cycle (the Ki-67 antigen) [23] using 2 different Ki-67 antibodies and examining multiple time points after treatment with 5-FC. We were, however, unable to detect any nuclei that expressed both WT1 and Ki-67 although we did detect Ki-67 staining in a few renal tubular cells (data not shown).
Expression of glomerular mRNAs and proteins
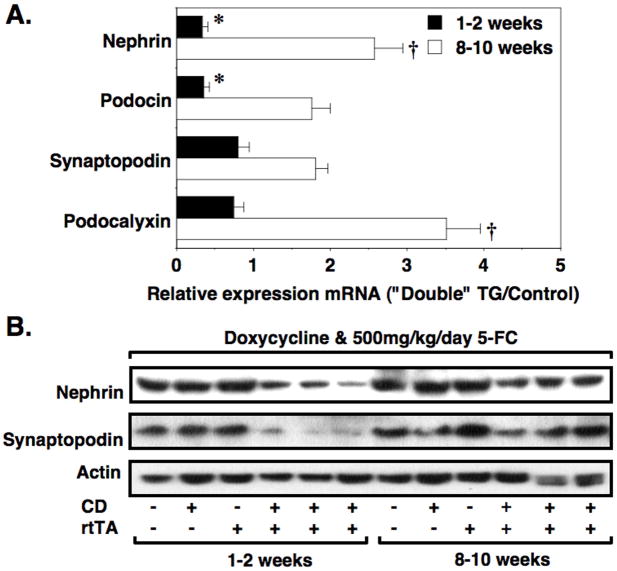
To determine if mRNAs expressed predominantly by podocytes in the kidney [33–36] were altered in a temporal pattern similar to WT1, we performed Q-RT-PCR using a panel of previously described primer pairs [26] for nephrin, podocin, synaptopodin and podocalyxin. Data were expressed relative to control mice at each time point (relative expression in control mice is 1.0). As shown in Figure 6A, the temporal expression pattern suggested that mRNAs expressed predominantly by podocytes were decreased 1–2 weeks after treatment with 5-FC and were increased at the 8–10 weeks time point compared to controls. As a control, we also performed Q-RT-PCR using primer pairs for the mRNAs CD2 adapter protein (CD2-AP) and α-actinin 4. Both CD2-AP and α-actinin 4 are implicated in glomerular disease pathogenesis but, unlike nephrin, podocin, synaptopodin and podocalyxin, are expressed in many cell types within the kidney [37–40]. There was little change in expression of the mRNAs for either CD2-AP (0.85 ± 0.16 [1–2 weeks] vs 0.94 ± 0.36 [8–10 weeks] relative expression; P = NS) or α-actinin 4 (0.83 ± 0.11 [1–2 weeks] vs 1.24 ± 0.23 [8–10 weeks] relative expression; P = NS).
Figure 6.
Temporal expression of podocyte mRNAs and proteins. Glomerular mRNA was prepared from control and “double” TG mice treated with both doxycycline and 500 mg/kg/day 5-FC at either the 1–2 week or 8–10 week time points. Real time quantitative PCR was then performed using primer pairs specific for the indicated mRNAs. Data for “double” TG mice were expressed relative to the control group (relative expression in control mice is 1.0). As shown in Panel A, nephrin and podocin mRNAs in the “double” TG mice were significantly downregulated at the 1–2 week time point compared to controls. In contrast, at the 8–10 week time point, nephrin and podocalyxin mRNAs were significantly upregulated in the “double” TG mice compared to control animals. As shown in Panel B and Table 3, both nephrin and synaptopodin protein levels were significantly decreased at the 1–2-week time point and, unlike the mRNA levels, tended to remain depressed at the 8–10 week time point. For the mRNA studies, 7 to 11 mice per group were studied at each time point. For the immunoblotting studies, 6 mice were studied per group. *P<0.05 vs controls, †P<0.01 vs control
We next investigated expression of the podocyte proteins nephrin and synaptopodin by immunoblotting. As shown in Figure 6B and Table 3, both nephrin and synaptopodin levels were significantly decreased at the 1–2-week time point and, unlike the mRNA levels, tended to remain depressed at the 8–10 week time point.
Table 3.
Effect of 5-FC on expression of the podocyte proteins nephrin and synaptopodin
| Group | Densitometry units
|
|||
|---|---|---|---|---|
| Nephrin/Actin | Synaptopodin/Actin | |||
| Controls* | “Double” TG* | Controls* | “Double” TG* | |
| 2 weeks after treatment | 0.51 ± 0.044 | 0.34 ± 0.003* | 0.46 ± 0.033 | 0.31 ± 0.053* |
| 8 weeks after treatment | 0.56 ± 0.007 | 0.40 ± 0.019* | 0.48 ± 0.047 | 0.37 ± 0.020 |
6 mice were studied per group, *controls and “double” TG mice were treated with both doxycycline and 500mg/kg/day 5-FC,
P<0.05 versus controls
Discussion
In the present studies, we found that treatment with the prodrug 5-FC caused graded increases in albuminuria and proteinuria after transgene induction, which was associated with graded decreases in the number of glomerular podocytes. Light microscopic changes were minimal at the 2-week time point but, ultrastructural examination revealed extensive foot process effacement and focal duplication of the GBM. By the 10-week time point, albuminuria and proteinuria had returned to baseline and a few mice had developed focal glomerulosclerosis. Moreover, at this 10-week time point, podocyte number was not significantly different from control animals. These findings, taken together with published studies [15,18,41], support the notion that the glomerulus has a significant capacity for repair after a discrete, podocyte depleting injury.
A reduction in the number of glomerular podocytes is observed in both animal models and human kidney diseases [3,5–15]. Because podocytes are thought to have a limited capacity for replication [3,16], some investigators contend that podocytes which are lost cannot be effectively replaced, causing instability of the glomerular tuft and glomerulosclerosis [3]. Accumulating evidence, however, suggests that, in some disease processes, podocytes may reenter the cell cycle and proliferate [31,32,41]. To examine this possibility, we stained cell nuclei with the podocyte marker WT1 and a nuclear marker of cells that have reentered the cell cycle (the Ki-67 antigen) [23]. We were, however, unable to detect any nuclei that expressed both WT1 and Ki-67. More recently, several laboratories have suggested that the glomerulus contains a population of renal progenitor cells [17–19] that can be induced to differentiate toward either podocyte or renal tubular cell lineages [18,19]. Moreover, this progenitor cell population has some capacity to repopulate the glomerulus with podocytes following glomerular injury [18]. While additional studies will be necessary to elucidate mechanisms, it is possible that this renal progenitor cell population played a role in replenishing the glomerulus with podocytes after treatment with 5-FC in the current study.
We also found that depletion of glomerular podocytes was also associated with alterations in expression of podocyte mRNAs (Figure 5A) in a temporal fashion that was similar to the tempo of changes in podocyte number. It is possible that these mRNA changes simply reflect alterations in the number of glomerular podocytes. Alternatively, podocytes may undergo a phenotypic change associated with decreased expression of podocyte specific proteins [31,32,42]. In support of this hypothesis, we found that that both nephrin and synaptopodin remained depressed 8–10 weeks after treatment with 5-FC (Figure 5B) despite recovery of podocyte number by this time point. The loss of podocyte WT1 expression at the 1–2 week time point might, therefore, reflect a loss of differentiation markers and not podocyte depletion per se. We did, however, observe apoptosis of glomerular podocytes suggesting at a portion of the podocyte population is lost following treatment with 5-FC. Whether or not this podocyte loss represents a significant percentage of the viable podocytes will require additional study. As mentioned above, one possibility is that a population of renal progenitor cells replenishes the glomerular podocyte population after a podocyte depleting injury [17–19,43]. In this scenario, podocyte depletion mobilizes a population of renal stem cells [17–19] that repopulates the glomerulus and acquires podocyte characteristics which, in turn, alters the profile of mRNAs expressed within the glomerulus.
Lastly, podocyte depletion was induced in the current experiments by expressing the yeast enzyme CD specifically in podocytes using a doxycycline inducible strategy. This approach has several advantages compared to other strategies in which transgene expression is constitutive [7,44–47]. First, the level of transgene expression can be modulated using different doxycycline dosages [21]. Investigators could, therefore, titrate the level of transgene expression to optimize the amount of cellular injury induced following treatment with 5-FC. Secondly, this strategy could be used to target inducible expression of CD to other renal or extrarenal tissues using available Tet-On or Tet-Off mice [29]. Thus, this strategy would permit the animal model to be more broadly applicable to the research needs of a wide variety of investigators. Despite these advantages, one disadvantage is that this approach requires 2 TG animals, which complicates studies using multiple genetically modified mice. As an alternative strategy, we also created TG lines in which CD was targeted specifically to podocytes using the human podocin (NPHS2) promoter [21]. Initial studies with these TG mice were promising. Unfortunately, these initial founder lines are no longer available and, although we are currently creating additional founder animals, these studies are still in their preliminary stages.
In summary, we found that expression of the yeast enzyme CD specifically in glomerular podocytes and treatment with the prodrug 5-FC caused graded degrees of albuminuria and proteinuria. Podocyte loss was associated with FP effacement and duplication of the GBM but minimal histopathological alterations by light microscopic examination. Enhanced albuminuria and abnormalities in glomerular ultrastructure were associated with a decrease the number of glomerular podocytes. This decrease in podocyte number was, however, reversible and was accompanied by resolution of both albuminuria and proteinuria, although some mice developed focal areas of glomerulosclerosis. These data suggest that: 1. The kidney has a significant capacity for glomerular repair following a podocyte depleting injury, and 2. This new mouse model may be useful for investigating repair mechanisms in glomerular disease processes.
Acknowledgments
These studies were supported by grants RO1-DK075688 (R.F.S.) and 3R01DK075688-01A2S1 (R.F.S.) from the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases. Dr. Spurney also received salary support through a grant from the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases (RO1- DK087707). The authors would like to thank Dr. Jeffery B. Kopp (National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD) for providing the rtTA TG mice.
Footnotes
Conflict of interest statement: The results presented in this paper have not been published previously in whole or part, except in abstract format, and the authors have no conflicts of interest to declare with regard to the data presented in this manuscript.
References
- 1.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 4.Winn MP, Daskalakis N, Spurney RF, Middleton JP. Unexpected role of TRPC6 channel in familial nephrotic syndrome: does it have clinical implications? J Am Soc Nephrol. 2006;17:378–387. doi: 10.1681/ASN.2005090962. [DOI] [PubMed] [Google Scholar]
- 5.Chen CA, Hwang JC, Guh JY, Chang JM, Lai YH, Chen HC. Reduced podocyte expression of alpha3beta1 integrins and podocyte depletion in patients with primary focal segmental glomerulosclerosis and chronic PAN-treated rats. J Lab Clin Med. 2006;147:74–82. doi: 10.1016/j.lab.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–1035. doi: 10.2337/diabetes.52.4.1031. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 8.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 9.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 11.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 12.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 13.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 14.Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L, Lauria F, Miji M, Deferrari G, Garibotto G. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 15.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol. 2009;174:797–807. doi: 10.2353/ajpath.2009.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 17.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Flannery PJ, Rosenberg PB, Fields TA, Spurney RF. Gq-dependent signaling upregulates COX2 in glomerular podocytes. J Am Soc Nephrol. 2008;19:2108–2118. doi: 10.1681/ASN.2008010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Ellis MJ, Fields TA, Howell DN, Spurney RF. Beneficial effects of the Rho kinase inhibitor Y27632 in murine puromycin aminonucleoside nephrosis. Kidney Blood Press Res. 2008;31:111–121. doi: 10.1159/000121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Chang JH, Paik SY, Mao L, Eisner W, Flannery PJ, Wang L, Tang Y, Mattocks N, Hadjadj S, Goujon JM, Ruiz P, Gurley SB, Spurney RF. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS One. 2012;7:e33942. doi: 10.1371/journal.pone.0033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, Ruiz P, Fields TA, Spurney RF. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 2012;81:1075–1085. doi: 10.1038/ki.2011.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Fields TA, Pazmino K, Dai Q, Burchette JL, Howell DN, Coffman TM, Spurney RF. Activation of Galpha q-coupled signaling pathways in glomerular podocytes promotes renal injury. J Am Soc Nephrol. 2005;16:3611–3622. doi: 10.1681/ASN.2005020167. [DOI] [PubMed] [Google Scholar]
- 27.Kershaw DB, Thomas PE, Wharram BL, Goyal M, Wiggins JE, Whiteside CI, Wiggins RC. Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J Biol Chem. 1995;270:29439–29446. doi: 10.1074/jbc.270.49.29439. [DOI] [PubMed] [Google Scholar]
- 28.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Gingrich JR, Roder J. Inducible gene expression in the nervous system of transgenic mice. Annu Rev Neurosci. 1998;21:377–405. doi: 10.1146/annurev.neuro.21.1.377. [DOI] [PubMed] [Google Scholar]
- 30.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Kitamura H, Adachi A, Sasaki Y, Ishizaki M, Wakamatsu K, Inoue K, Sugisaki Y, Ghazizadeh M. Reduplicated basal lamina of the peritubular capillaries in renal biopsy specimens. J Submicrosc Cytol Pathol. 2005;37:305–311. [PubMed] [Google Scholar]
- 32.D’Amico G, Ferrario F. Mesangiocapillary glomerulonephritis. J Am Soc Nephrol. 1992;2:S159–166. doi: 10.1681/ASN.V210s159. [DOI] [PubMed] [Google Scholar]
- 33.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 34.Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 36.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 38.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 39.Choi HJ, Lee BH, Cho HY, Moon KC, Ha IS, Nagata M, Choi Y, Cheong HI. Familial focal segmental glomerulosclerosis associated with an ACTN4 mutation and paternal germline mosaicism. Am J Kidney Dis. 2008;51:834–838. doi: 10.1053/j.ajkd.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, Kalluri R, Gerszten RE, Pollak MR. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest. 2003;111:1683–1690. doi: 10.1172/JCI17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shkreli M, Sarin KY, Pech MF, Papeta N, Chang W, Brockman SA, Cheung P, Lee E, Kuhnert F, Olson JL, Kuo CJ, Gharavi AG, D’Agati VD, Artandi SE. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. 2012;18:111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 2000;58:137–143. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 43.Alcazar JM, Marin R, Gomez-Campdera F, Orte L, Rodriguez-Jornet A, Mora-Macia J. Clinical characteristics of ischaemic renal disease. Nephrol Dial Transplant. 2001;16(Suppl 1):74–77. doi: 10.1093/ndt/16.suppl_1.74. [DOI] [PubMed] [Google Scholar]
- 44.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 46.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 47.Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol. 2005;16:2257–2262. doi: 10.1681/ASN.2004121134. [DOI] [PubMed] [Google Scholar]