Abstract
New antibacterial agents are urgently needed for the elimination of biofilm-forming bacteria that are highly resistant to traditional antimicrobial agents. Proliferation of such bacteria can lead to significant economic losses in the agri-food sector. This study demonstrates the potential of the bacteriophage-derived peptidase, CHAPK, as a biocidal agent for the rapid disruption of biofilm-forming staphylococci, commonly associated with bovine mastitis. Purified CHAPK applied to biofilms of Staphylococcus aureus DPC5246 completely eliminated the staphylococcal biofilms within 4 h. In addition, CHAPK was able to prevent biofilm formation by this strain. The CHAPK lysin also reduced S. aureus in a skin decolonization model. Our data demonstrates the potential of CHAPK as a biocidal agent for prevention and treatment of biofilm-associated staphylococcal infections or as a decontaminating agent in the food and healthcare sectors.
1. Introduction
Staphylococcal species commonly colonise the skin and mucosal membranes of both humans and animals. They are a significant causative agent of bovine mastitis in dairy herds [1] and are also associated with a number of diseases in humans, ranging from a variety of skin conditions to more serious infections such as septicemia [2]. Staphylococcal food poisoning is among the most common food-borne microbial diseases [3] and contamination of food industrial surfaces with staphylococcal species has been demonstrated to be a considerable risk factor [4–6]. Along with the urgent requirement for novel antibacterials to combat the prevalence of antibiotic/disinfectant resistant staphylococci in food processing, veterinary and healthcare settings, there is an increasing need for effective antimicrobial agents which can prevent and treat staphylococcal biofilm-associated infections [7–11].
Biofilms are multilayered communities of sessile cells protected by an extracellular matrix, which often adhere to food contact surfaces, damaged tissue and indwelling medical devices [12–14]. Once formed, biofilms may be up to 1,000 times more resistant to antimicrobial agents than planktonic cells alone making them particularly difficult to eliminate [15]. This can ultimately lead to increased risk of persistent infections, as is commonly the case with bovine mastitis [16]. In addition, because of their increased levels of resistance, biofilm-associated infections can result in a need for explantation of medical devices in human healthcare settings [17, 18]. Although the precise mechanisms of biofilm antibiotic resistance have yet to be fully resolved, failure to successfully treat infections with conventional therapies necessitates the investigation and development of novel treatment strategies [9, 18, 19].
In recent years, bacteriophage endolysins (phage lysins) have been the focus of research into combatting antibiotic resistance in Gram-positive pathogens [20–24]. These cell wall peptidoglycan hydrolases possess a number of advantages over conventional antibiotics including, rapid lytic activity against bacterial cells, low probability of developing bacterial resistance, and significantly lower chance of disrupting commensal microflora, due to the enzymes' specificity [25–28].
Our group previously reported the ability of phage K and modified derivatives to prevent biofilm formation and to reduce established biofilm density [29]. However, endolysins have a number of advantages over using whole phage as antimicrobial agents. In the case of whole phage, resistance arising from either adsorption inhibition, restriction modification and abortive infection have been reported in many genera [30–32]. Bacteriophage and their hosts have coevolved over millions of years. The equilibrium in this relationship has been maintained by continual development of resistance and counter resistance. In contrast, with the use of phage endolysins, there has been no report of bacteria developing resistance to these lytic agents even after extensive growth of the bacterium in the presence of sublethal levels of enzyme [25, 33, 34]. In addition, by using phage endolysins instead of whole phage the risk of horizontal gene transfer of virulence genes is avoided. Phage preparations also have the possibility of containing exo- and endotoxins from their respective bacterial host. By overexpressing an endolysin in well-characterised avirulent laboratory strains this risk is circumvented.
To date a number of phage lysins have been described which demonstrate activity against staphylococci including LysK [35], LysWMY [36], Φ11 lysin [37], MV-L [38], LysH5 [39], LysGH15 [40], PlyV12 [41], SAL-2 [42], and SAL-1 [43]. Producing tailor-made proteins by combining domains from phage lysins with various other domains has also been investigated[28, 44, 45]. Multidomain lysins have previously been examined for control of biofilms namely Φ11 lysin [37] and SAL-2 [42]. This is the first report of a single domain lysin being used for this purpose. Cysteine, histidine dependant amidohydrolase/peptidase CHAP K (18.6 kDa) is a truncated derivative of a phage lysin (LysK, 54 kDa) from the myoviridae staphylococcal phage K [35, 46]. This endopeptidase specifically cleaves the peptide bond between D-alanine and the first glycine in the pentaglycine cross-bridge of staphylococcal cell wall peptidoglycan [47]. We have previously reported that purified CHAP K exhibits stronger activity than the original multidomain lysin (LysK) [46] and displays rapid lytic activity against a variety of pathogenic staphylococcal species including Staphylococcus epidermidis and methicillin-resistant S. aureus (MRSA) strains [46, 48]. Here we examine the efficacy of the phage-derived CHAP K enzyme in eliminating Staphylococcus aureus biofilms and demonstrate its efficacy for removal of S. aureus from skin.
2. Materials and Methods
2.1. Bacterial Strains
The strains used in this study were S. aureus strain DPC5246 (Moorepark Food Research Centre, Teagasc, Fermoy, Cork, Ireland) and S. aureus strain Xen29 (Caliper Lifesciences, UK). Strain DPC5246 is an S. aureus bovine mastitis isolate [49]. Strain Xen29 is a confirmed biofilm producing pleural fluid isolate derived from the parental strain S. aureus ATCC 12600 [50]. It has been engineered to emit luminescence when metabolically active [51, 52]. Both strains were stored at −80°C and routinely grown on tryptic soya agar (TSA), in tryptic soya broth (TSB) or TSB supplemented with 1% D-(+)-glucose (TSBg) at 37°C. All media was supplied by Sigma-Aldrich.
2.2. Production of CHAPK
CHAP K is comprised solely of the lytic CHAP domain of the anti-staphylococcal bacteriophage endolysin, LysK [35]. In a previous study by our group, the truncated phage lysin gene was cloned untagged into a pQE60 expression vector (Qiagen) and overexpressed in Escherichia coli (E. coli) XL1-Blue [46]. Highly active CHAP K (18.6 kDa) was purified to >90% homogeneity by cation exchange chromatography. The protein was then desalted and concentrated using an amicon ultra centrifugal filter (Milipore) with 10 kDa cut-off and subsequently stored at −80°C in 25 mM Tris pH 7. The lytic activity of CHAP K against live planktonic cells of staphylococci including multi-antibiotic resistant strains of clinical origin has been demonstrated previously [46, 53].
2.3. Staphylococcal Biofilm Reduction Using CHAPK
2.3.1. Plate Staining Assay
A modified static microtitre plate assay, based on previous studies [54], was used to analyse biofilm formation and treatment with CHAP K . Briefly, overnight (18–24 hr) colonies of S. aureus DPC5246 from a TSA plate were suspended in sterile ringers to an optical density equivalent to 0.5 McFarland standard and subsequently diluted 1 : 100 in TSBg to give a starting inoculum of 1.29 × 106 CFU mL−1. In the biofilm disruption assay, 200 μL volumes of the prepared culture were aliquoted into wells of a sterile 96-well microtitre plate (Sarstedt) and incubated at 37°C for 24 h. After this incubation period, wells were washed three times with 200 μL of sterile ringers using a multichannel pipette (Gilson) to remove media and planktonic cells. Biofilm containing wells were then treated with 200 μL of various concentrations of CHAP K (3.91–500 μg mL−1) in sterile 25 mM Tris pH 7 or with 200 μL of sterile 25 mM Tris pH 7 alone (control), at 37°C for 4 h. At the end of treatment all wells were washed again before the plate was inverted and left to dry for 1 h at 60°C. The biofilms were then stained with 200 μL of 0.5% crystal violet solution for 15 min. The stain solution was removed and the wells were gently washed as before. The plate was left to dry, after which, 30% acetic acid were added to solubilise the stain. The biofilm disrupting ability of CHAP K was determined by examining the optical density of the wells spectrophotometrically.
2.3.2. Viability Plate Count Assay
A 96-well microtitre plate/peg-lid assay, based on the method used by Moskowitz et al. [55] was used to investigate if CHAP K can completely eliminate a staphylococcal biofilm. A peg-lid plate was used in order to ensure that the maximum number of cells were removed from experimental wells with the same efficiency. This method permits removal of the biofilm matrix by centrifugation prior to plating. Briefly, an overnight colony (18–24 h) of S. aureus DPC5246 was suspended in sterile Ringers to an optical density equivalent to 0.5 McFarland standard and subsequently diluted 1 : 100 in TSBg to give a starting inoculum of 1.29 × 106 CFU/mL. 200 μL of the TSBg cell suspension was transferred to the wells of a 96-well plate. As a negative control for biofilm formation, 200 μL of TSBg was used. A peg lid was added and the plate was incubated statically for 24 h at 37°C [56]. After incubation the peg lid was removed and washed three times by placing it in a 96-well plate containing sterile ringers for 30 sec each time. 200 μL of CHAP K at concentrations ranging from 125–1000 μg mL−1 (diluted in 25 mM Tris pH7) was added to treatment wells. 200 μL 25 mM Tris pH 7 was added to the control wells. The biofilm peg lid was placed on the antimicrobial challenge plate and incubated for 4 h at 37°C. After incubation the peg lid was washed three times in sterile ringers as before. Finally the lid was placed in a plate containing 200 μL sterile ringers in each well and centrifuged at 800 g for 20 mins to remove any biofilm remaining on the pegs. Serial dilutions were performed on the contents of each well and a viable plate count was performed using Baird Parker Agar supplemented with egg yolk tellurite.
2.4. Biofilm Prevention by CHAPK
2.4.1. Plate Staining Assay
In order to investigate the ability of CHAP K to prevent the formation of S. aureus biofilms on artificial surfaces, the staining assay, as described previously for the biofilm reduction assay, was carried out with the following modification. At the beginning of the assay 100 μL of CHAP K , at concentrations ranging from 0.78 to 125.0 μg mL−1, were added to 100 μL of TSBg with 1.3 × 106 CFU mL−1 of DPC5246 cells, in a sterile 96-well microtitre plate and incubated for 24 h at 37°C.
2.4.2. Viable Plate Count Assay
The ability of CHAP K to prevent biofilm formation was also investigated using a method similar to the viable plate count method described previously with the following changes. At the beginning of the assay 100 μL of CHAP K , at concentrations ranging from 0.78 to 125.0 μg mL−1, were added to 100 μL of TSBg with 1.3 × 106 CFU mL−1 of DPC5246 cells, in a sterile 96-well microtitre plate.
2.5. Skin Decolonization Assay
This study was carried out using a modified version of the spray test of Hoopes et al. [57]. Briefly, three individual areas, 25 cm2 in size, were marked out on a section of porcine skin (obtained fresh from an abattoir). Each area was disinfected with 70% isopropyl alcohol wipes and allowed to dry at room temperature for up to 30 min. All three marked areas were then seeded with 100 μL of 6.2 × 107 CFU mL−1 (2.5 × 105 CFU cm−2) of S. aureus DPC5246 by pipette, distributed evenly within each area with a sterile plastic spreader (Sarstedt) and allowed to dry for 30 min. CHAP K (200 μg mL−1 in sterile H2O) was then misted 20 cm above one of the 25 cm2 areas, in two passes. The two remaining sections served as controls where one 25 cm2 area was misted with sterile H2O and the other was left untreated. The skin was then left to dry at room temperature for 30 min. Sterile cotton tipped swabs (Deltalab sterile swabs, Fisher Scientific, Ireland) were moistened in sterile Ringer's solution and used to sample each section of skin by rotating and rubbing the swab, in a zigzag pattern, and repeating at right angles. The tips of each swab were placed in 10 mls of Ringers solution and vigorously mixed using a vortex mixer to dislodge cells. The suspensions were serially diluted and plated on Baird Parker agar supplemented with egg yolk tellurite for enumeration of surviving cells. The work was also similarly done using the bioluminescent producing S. aureus Xen29 strain.
3. Results
3.1. Staphylococcal Biofilm Reduction Using CHAPK
3.1.1. Plate Staining Assay
A strong biofilm of S. aureus DPC 5246 was routinely formed when the strain was grown in TSB supplemented with 1% D-(+)-glucose for 24 hr at 37°C. This is represented by the strong staining seen in the untreated well in Figure 1. Solubilising of crystal violet stain and subsequent measurement of OD590 nm allowed accurate quantification of staining and comparison between control and enzyme-treated wells. The data shown in the bar chart in Figure 1 represents the OD590 nm of triplicate wells ± standard error. Mature biofilms (24 h) were treated with enzyme at concentrations ranging from to 3.91–500 μg mL−1, for 4 h at 37°C. The OD590 nm data for the biofilm disruption staining assay demonstrated that at all concentrations tested, CHAP K treatment reduced biofilm formation relative to the untreated control well (Figure 1). A one-way ANOVA indicated that CHAP K treatment caused a statistically significant change in biofilm formation (P value < 0.001). It is clear from the graph in Figure 1 that CHAP K successfully disrupted the S. aureus biofilms in a concentration dependant manner. Visual inspection of the degree and intensity of staining in the CHAP K treated wells compared to untreated biofilm wells indicated that even at a concentration as low as 3.91 μg mL−1, CHAP K caused a visible reduction in biofilm mass. At a concentration of 62.5 μg mL−1 CHAP K there was little or no visibly detectable staining of the wells (Figure 1).
Figure 1.
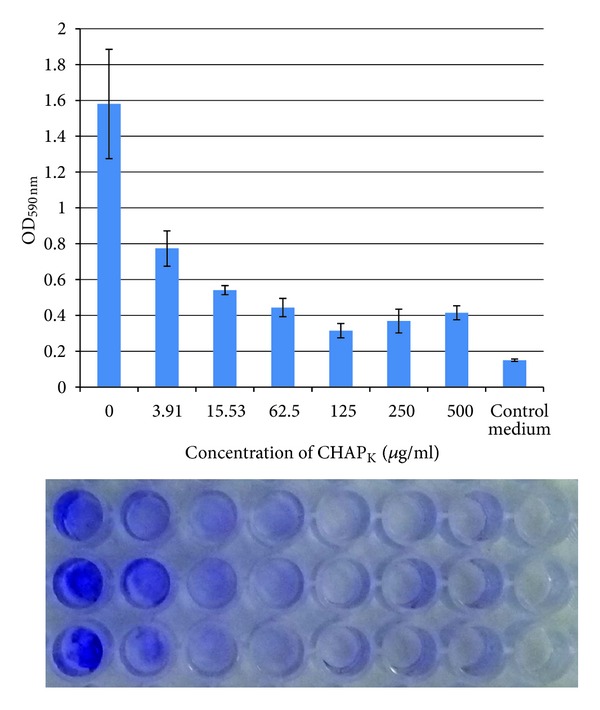
Biofilm staining assay demonstrating disruption of 24 h biofilms of S. aureus DPC5246 after 4 h incubation with CHAP K at concentrations ranging from 3.91–500 μg/mL. The assay was carried out in triplicate in a flat bottomed 96-well plate. Absorbance readings at 590 nm (OD590 nm) are represented in the graph as the mean ± SE. Below the graph is a picture of the stain in the wells prior to solubilisation with acetic acid. The wells correspond to the data represented directly above each set.
3.1.2. Viable Plate Count Assay
Purified CHAP K ranging in concentration from 125–1000 μg/mL, was used to treat a 24 h staphylococcal biofilm. The results of the viable plate counts are summarised in the bar chart in Figure 2, where each bar represents the average of triplicate plate counts ± standard error. After treatment with 125 μg mL−1 a 2-log decrease was seen in the number of cells in the biofilm matrix on the pegs. The average plate count from the wells with untreated DPC5246 biofilms was 2.7 × 104 CFU mL−1. The average viable plate count for the wells treated with 125 μg mL−1 was 2.2 × 102 CFU mL−1. After treatment with CHAP K at concentrations of 500 μg mL−1 or higher there was complete eradication of the biofilm which corresponded to a 4-log drop in CFU mL−1 when compared to the untreated control wells.
Figure 2.
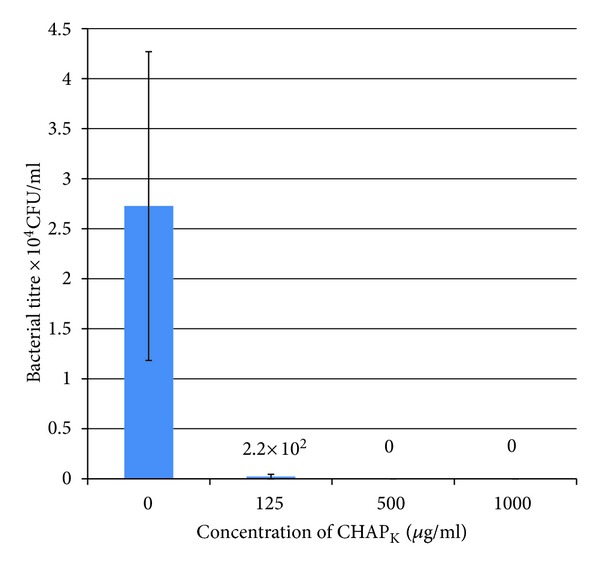
Viable plate count assay demonstrating the antimicrobial effect of a CHAP K treatment on 24 h biofilms of S. aureus DPC5246. 24 h staphylococcal biofilms were grown on peg lids in microtitre plates. The bar chart shows the CFU mL−1 that were retrieved from the pegs after 4 h treatment with CHAP K , at the concentrations outlined below each bar. The assay was performed in triplicate and each bar is a representation of the mean ± SE. The values indicated above the bars are the mean CFU/mL after treatment.
3.2. Biofilm Prevention by CHAPK
3.2.1. Plate Staining Assay
To investigate the capacity of CHAP K to inhibit the formation of S. aureus biofilms, various concentrations of the enzyme were incubated with strain DPC5246 for 24 h at 37°C in a microtitre plate assay. After staining and subsequent solubilisation of stain, OD590 nm measurements were recorded and used to assess the ability of CHAP K to prevent biofilm formation. This data is presented in Figure 3. A one-way ANOVA indicated that CHAP K treatment caused a statistically significant change in biofilm formation (P value < 0.001). Increasing degrees of biofilm prevention were evident in the presence of increasing concentrations of enzyme. At 15.63 μg mL−1 a considerable decrease in optical density is seen when compared with the untreated control wells. A concentration of 31.25 μg mL−1 indicated complete prevention as the mean OD590 nm value (0.14) is exactly the same as that of the control medium.
Figure 3.
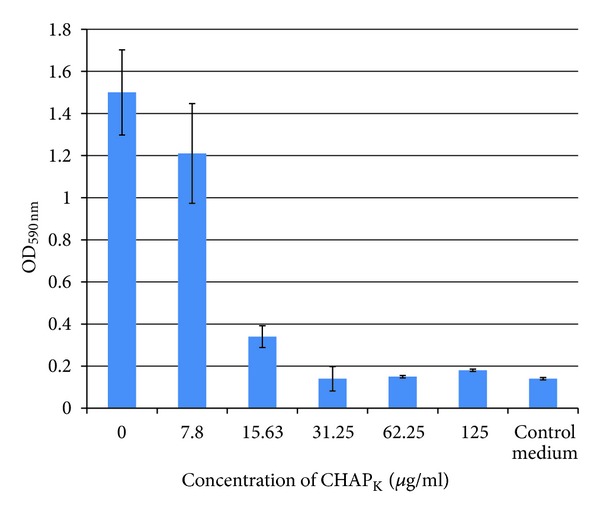
CHAP K biofilm prevention assay: staining. Concentrations of enzyme ranging from 0–125 μg mL−1 were incubated with S. aureus DPC5246 in TSBg at 37°C for 24 h. The wells were stained with crystal violet (1%) and subsequently the stain was solubilised with acetic acid. Optical density readings at 590 nm (OD590) of all wells were recorded in a microtitre plate reader and displayed on the bar chart. Assays were carried out in triplicate and OD590 data was expressed as the mean ± SE.
3.2.2. Viable Plate Count Assay
To confirm that CHAP K is able to completely prevent biofilm formation a plate count assay was performed on wells in which S. aureus was grown in TSBg at various concentrations of CHAP K . The bar chart in Figure 4 represents averages of triplicate values ± standard error. Growth of bacteria in the presence of 7.8 μg mL−1 CHAP K caused a 2-log drop in biofilm formation and at 15.63 μg mL−1 a 4-log reduction was evident. Complete prevention corresponding to a 6-log drop was achieved when the DPC 5246 was incubated with CHAP K at a concentration of 31.25 μg mL−1 or higher.
Figure 4.
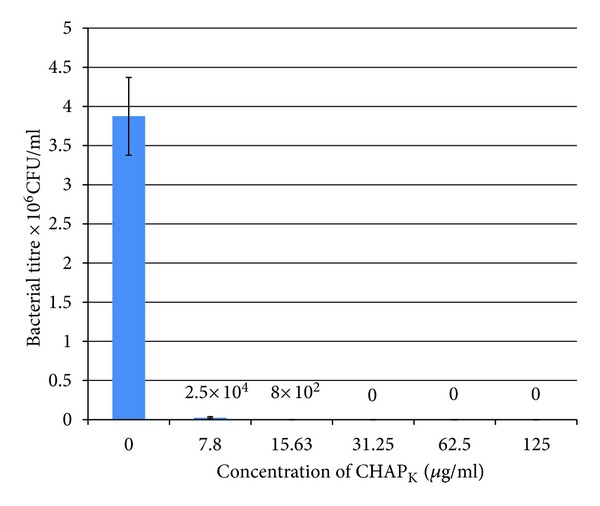
CHAP K biofilm prevention assay: viable plate count. Concentrations of enzyme ranging from 7.8−125 μg mL−1 were incubated with S. aureus DPC5246 in TSBg at 37°C for 24 h in a 96 well peg plate. The pegs were washed after incubation and subsequently any cells remaining adhered to the pegs were removed by centrifugation at 800 rpm for 20 mins. Assays were carried out in triplicate and OD590 data was expressed as the mean ± SE. The values indicated above the bars are the mean CFU/mL after treatment.
3.3. Removal of S. aureus from Skin Using CHAPK
The potential of CHAP K as a skin decolonization agent was assessed by incorporation of the enzyme into a spray. Sections of porcine skin (25 cm2) were seeded with 2.5 × 105 CFU cm−2 of S. aureus strain DPC5246 and misted with 200 μg mL−1 solution of CHAP K for two seconds. Water-treated and untreated skin sections had similar CFU values when enumerated after 30 minutes. This contrasted with a significant reduction in CFUs on the CHAP K -treated skin, which received approximately 60 μg of enzyme. CHAP<?brm?>K<?erm?> treatment was found to be sufficient to remove >99% of S. aureus DPC5246 in 30 min when compared with treatment with water, i.e., reduced from 3.7 × 103 CFU mL−1 when treated with water to 1.7 × 101 CFU mL−1 when treated with the CHAPK solution. Similar results were achieved when CHAP K was employed against the bioluminescent strain S. aureus Xen29 (Figure 5).
Figure 5.
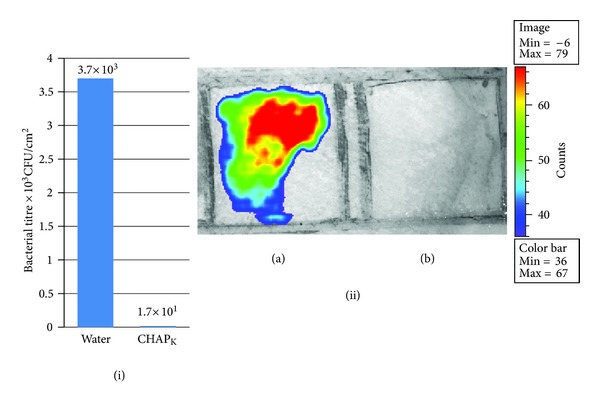
Skin decolonisation. Reduction of S. aureus on porcine skin using CHAP K . (i) Two sections of porcine skin were seeded with 2.5 × 105 CFU cm−2 of S. aureus DPC 5246. Plate counts were performed to enumerate the S. aureus present on porcine skin 30 min after being sprayed with either sterile water or CHAP K solution. (ii) Two sections of skin were seeded with 2.4 × 106 CFU cm−2 of S. aureus Xen29. One area of skin was treated with sterile water (a) and the other area treated with CHAP K solution (b). Skin was imaged (exposure: 15 seconds, binning: medium, colour-scale 36–67) 30 min after treatment with CHAP K /H2O using the in vivo Imaging System (IVIS) (Xenogen, Ca). This system enabled direct monitoring of the effect of CHAP K treatment in real time. The colour bar represents bacterial bioluminescence signal intensity, with red to blue indicating high and low bioluminescence, respectively.
4. Discussion
Biofilms are recognised as a significant problem in the food industry. Biofilm-forming bacterial strains are generally much more difficult to kill than their planktonic counterparts. They survive in sub-optimal environmental conditions, display widespread resistance to antibiotics and disinfectants and often lead to persistent infections such as is commonly seen with bovine mastitis [16]. Biofilms may also interfere with various processes in food technology and engineering. For example, biofilms can impede liquid flow and heat transfer and lead to increased corrosion rates which can lead to economic losses [58].
This study demonstrates that the phage-derived peptidase, CHAP K , can completely remove a mature staphylococcal biofilm in under 4 h and can also prevent establishment of a staphylococcal biofilm. In the biofilm context it is likely that CHAP K rapidly lyses sessile staphylococcal cells with an efficiency that brings about destabilization of the biofilm matrix leading to their subsequent detachment from solid surfaces. For formation of a mature staphylococcal biofilms a 24 h incubation period is commonly used [37, 56, 59, 60]. In the results presented in this study the untreated wells in the biofilm prevention assay show that bovine mastitis isolate DPC5246 is capable of forming a mature biofilm of 3.9 × 106 CFU/mL in under 24 h.
Disruption of staphylococcal biofilms by phage lysins has previously been reported by Sass and Son [37, 42]. While these studies involved the complete endolysin protein with multiple domains, CHAP K is a truncated form of a natural endolysin (Lys K). It contains one lytic domain and only 33% of the original protein. Due to its lower molecular weight, CHAP K is predicted to have a lower chance of inducing a humoral immune response [46]. Also, because it is smaller CHAP<?brm?>K<?erm?> is more efficiently over expressed in the recombinant E. coli strain compared with the full endolysin; problems of aggregation, which have been encountered with over-expression of cow udders full endolysins are greatly reduced. Lysins with just one catalytic domain, such as CHAP K , may run the risk of being more susceptible to development of host resistance, than a protein with multiple catalytic domains. However, because of the position at which CHAP K cleaves the peptidoglycan (between the characteristic S. aureus pentaglycine bridge and the D-alanine of the tetrapeptide crossink [47]), the possibility of developing resistance seems unlikely but cannot be ruled out. In the earlier studies which reported staphylococcal biofilm disruption by phage lysins, biofilm staining was the sole method used to estimate the efficacy of the enzymatic treatment. Our study combines the staining approach with viable plate counting in order to more accurately represent the effect of the lysin on biofilm forming cells.
At a concentration of 31.25 μg mL−1, CHAP K completely prevented the formation of S. aureus biofilms. This result demonstrates the potential of applying CHAP K as a spray for decontamination of food contact surfaces or of cow udders as a preventative measure for bovine mastitisCHAP K . could also be employed as a coating agent on medical implants such as catheters to prevent the adherence of staphylococci and subsequent biofilm formation and infection. Previous studies have shown that coating medical implants with antibacterial agents can be effective in preventing formation of biofilms [61–63]. Previous work by our group demonstrated that CHAP K is also effective as a biocidal agent against several pathogenic species of Staphylococcus including the well known biofilm former S. epidermidis and all known clonal types of MRSA, and thus can also be considered as a useful antimicrobial agent for prevention or treatment of infections caused by these species. A previous publication by our group on the characterisation of CHAP K demonstrated that the lysin is active over a broad range of temperatures and pH and was not seen to be susceptible to degradation by multiple freeze thawing steps [48]. The robustness of the lysin makes it attractive for commercialisation and utilisation as a decontaminating agent.
The two main reservoirs of S. aureus on animals are the skin and mucosal membranes. Infection can often originate from commensal flora, especially in veterinary and hospital settings, as is the case with both bovine and human mastitis [64, 65]. The present study demonstrated the potential of CHAP K as a decolonisation agent for the removal of S. aureus from the surface of mammalian skin. When applied as a spray, CHAP K eliminated 99% of S. aureus DPC5246 from skin in 30 min. The results of the experiment suggested that CHAP K could be included in bovine teat-dip solution for reduction of mastitis causing staphylococci on the udder prior to and after milking in dairy farms. In addition, treatment of human skin with CHAP K prior to surgery may help prevent serious nosocomial infections.
In conclusion, our data demonstrates the potential of a novel but natural anti-staphylococcal agent to prevent economically important veterinary infections, nosocomial staphylococcal infections and also reduce biofilm formation in processing systems.
Authors' Contribution
M. Fenton and R. Keary contributed equally to this work.
Acknowledgment
This work was supported by Science Foundation Ireland Ref 06/RF/BIM004.
References
- 1.Gruet P, Maincent P, Berthelot X, Kaltsatos V. Bovine mastitis and intramammary drug delivery: review and perspectives. Advanced Drug Delivery Reviews. 2001;50(3):245–259. doi: 10.1016/s0169-409x(01)00160-0. [DOI] [PubMed] [Google Scholar]
- 2.Lowy FD. Staphylococcus aureus infections. The New England Journal of Medicine. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Holečková B, Holoda E, Fotta M, Kalináčová V, Gondol’ J, Grolmus J. Occurrence of enterotoxigenic Staphylococcus aureus in food. Annals of Agriculture and Environmental Medicine. 2002;9(2):179–182. [PubMed] [Google Scholar]
- 4.Gutierrez D, Delgado S, Vazquez-Sanchez D, et al. Incidence of Staphylococcus aureus and analysis of bacterial-associated communities on food industry surfaces. Applied and Environmental Microbiology. 2012;78(24):8547–8554. doi: 10.1128/AEM.02045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrera JJR, Cabo ML, González A, Pazos I, Pastoriza L. Adhesion and detachment kinetics of several strains of Staphylococcus aureus subsp. aureus under three different experimental conditions. Food Microbiology. 2007;24(6):585–591. doi: 10.1016/j.fm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Palá TR, Sevilla A. Microbial contamination of carcasses, meat, and equipment from an Iberian pork cutting plant. Journal of Food Protection. 2004;67(8):1624–1629. doi: 10.4315/0362-028x-67.8.1624. [DOI] [PubMed] [Google Scholar]
- 7.Eady EA, Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Current Opinion in Infectious Diseases. 2003;16(2):103–124. doi: 10.1097/00001432-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus . Journal of Clinical Investigation. 2003;111(9):1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langsrud S, Sidhu MS, Heir E, Holck AL. Bacterial disinfectant resistance—a challenge for the food industry. International Biodeterioration and Biodegradation. 2003;51(4):283–290. [Google Scholar]
- 11.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 12.Dunne WM., Jr. Bacterial adhesion: seen any good biofilms lately? Clinical Microbiology Reviews. 2002;15(2):155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infection Control and Hospital Epidemiology. 2002;23(12):759–769. doi: 10.1086/502007. [DOI] [PubMed] [Google Scholar]
- 14.Marques SC, Rezende JDGOS, Alves LADF, et al. Formation of biofilms by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitizers. Brazilian Journal of Microbiology. 2007;38(3):538–543. [Google Scholar]
- 15.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Canadian Journal of Veterinary Research. 2002;66(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Melchior MB, Vaarkamp H, Fink-Gremmels J. Biofilms: a role in recurrent mastitis infections? Veterinary Journal. 2006;171(3):398–407. doi: 10.1016/j.tvjl.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Aparna MS, Yadav S. Biofilms: microbes and disease. Brazilian Journal of Infectious Diseases. 2008;12(6):526–530. doi: 10.1590/s1413-86702008000600016. [DOI] [PubMed] [Google Scholar]
- 18.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 19.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. International Journal of Medical Microbiology. 2002;292(2):107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DC, Schmelcher M, Rodriguez-Rubio L, et al. Endolysins as antimicrobials. Advances in Virus Research. 2012;83:299–365. doi: 10.1016/B978-0-12-394438-2.00007-4. [DOI] [PubMed] [Google Scholar]
- 21.Fenton M, Ross P, Mcauliffe O, O’Mahony J, Coffey A. Recombinant bacteriophage lysins as antibacterials. Bioengineered Bugs. 2010;1(1):9–16. doi: 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Rubio L, Martinez B, Donovan DM, Rodriguez A, García P. Bacteriophage virion-associated peptidoglycan hydrolases: potential new enzybiotics. Critical Reviews in Microbiology. 2012 doi: 10.3109/1040841X.2012.723675. [DOI] [PubMed] [Google Scholar]
- 23.Fischetti VA. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. International Journal of Medical Microbiology. 2010;300(6):357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szweda P, Schielmann M, Kotlowski R, Gorczyca G, Zalewska M, Milewski S. Peptidoglycan hydrolases-potential weapons against Staphylococcus aureus . Appl Microbiol Biotechnol. 2012;96:1157–1174. doi: 10.1007/s00253-012-4484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418(6900):884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 26.Loessner MJ. Bacteriophage endolysins—current state of research and applications. Current Opinion in Microbiology. 2005;8(4):480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends in Microbiology. 2005;13(10):491–496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrobial Agents and Chemotherapy. 2011;55(2):738–744. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly D, McAuliffe O, Ross RP, Coffey A. Prevention of Staphylococcus aureus biofilm formation and reduction in established biofilm density using a combination of phage K and modified derivatives. Letters in Applied Microbiology. 2012;54(4):286–291. doi: 10.1111/j.1472-765X.2012.03205.x. [DOI] [PubMed] [Google Scholar]
- 30.Lenski RE. Dynamics of interactions between bacteria and virulent bacteriophage. Advances in Microbial Ecology. 1988;10:1–44. [Google Scholar]
- 31.Coffey A, Ross RP. Bacteriophage-resistance systems in dairy starter strains: molecular analysis to application. Antonie van Leeuwenhoek. 2002;82(1–4):303–321. [PubMed] [Google Scholar]
- 32.Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nature Reviews Microbiology. 2010;8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 33.Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294(5549):2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 34.Fischetti VA. Novel method to control pathogenic bacteria on human mucous membranes. Annals of the New York Academy of Sciences. 2003;987:207–214. doi: 10.1111/j.1749-6632.2003.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus . Journal of Bacteriology. 2005;187(20):7161–7164. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoi KJ, Kawahigashi N, Uchida M, et al. The two-component cell lysis genes holWMY and lysWMY of the Staphylococcus warneri M phage ϕWMY: cloning, sequencing, expression, and mutational analysis in Escherichia coli . Gene. 2005;351:97–108. doi: 10.1016/j.gene.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus . Applied and Environmental Microbiology. 2007;73(1):347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashel M, Uchiyama J, Ujihara T, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ϕMR11. Journal of Infectious Diseases. 2007;196(8):1237–1247. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 39.Obeso JM, Martínez B, Rodríguez A, García P. Lytic activity of the recombinant staphylococcal bacteriophage ΦH5 endolysin active against Staphylococcus aureus in milk. International Journal of Food Microbiology. 2008;128(2):212–218. doi: 10.1016/j.ijfoodmicro.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Gu J, Xu W, Lei L, et al. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. Journal of Clinical Microbiology. 2011;49(1):111–117. doi: 10.1128/JCM.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoong P, Schuch R, Nelson D, Fischetti VA. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium . Journal of Bacteriology. 2004;186(14):4808–4812. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son JS, Lee SJ, Jun SY, et al. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Applied Microbiology and Biotechnology. 2010;86(5):1439–1449. doi: 10.1007/s00253-009-2386-9. [DOI] [PubMed] [Google Scholar]
- 43.Jun SY, Jung GM, Son JS, Yoon SJ, Choi YJ, Kang SH. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrobial Agents and Chemotherapy. 2011;55(4):1764–1767. doi: 10.1128/AAC.01097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmelcher M, Powell AM, Becker SC, Camp MJ, Donovan DM. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Applied and Environmental Microbiology. 2012;78:2297–2305. doi: 10.1128/AEM.07050-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idelevich EA, von Eiff C, Friedrich AW, et al. In vitro activity against Staphylococcus aureus of a novel antimicrobial agent, PRF-119, a recombinant chimeric bacteriophage endolysin. Antimicrobial Agents and Chemotherapy. 2011;55(9):4416–4419. doi: 10.1128/AAC.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horgan M, O’Flynn G, Garry J, et al. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Applied and Environmental Microbiology. 2009;75(3):872–874. doi: 10.1128/AEM.01831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG, Donovan DM. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiology Letters. 2009;294(1):52–60. doi: 10.1111/j.1574-6968.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- 48.Fenton M, Ross RP, McAuliffe O, O'Mahony J, Coffey A. Characterization of the staphylococcal bacteriophage lysin CHAPK . Journal of Applied Microbiology. 2011;111:1025–1035. doi: 10.1111/j.1365-2672.2011.05119.x. [DOI] [PubMed] [Google Scholar]
- 49.O’Flaherty S, Coffey A, Meaney WJ, Fitzgerald GF, Ross RP. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Letters in Applied Microbiology. 2005;41(3):274–279. doi: 10.1111/j.1472-765X.2005.01762.x. [DOI] [PubMed] [Google Scholar]
- 50.Kadurugamuwa JL, Sin L, Albert E, et al. Direct continuous method for monitoring biofilm infection in a mouse model. Infection and Immunity. 2003;71(2):882–890. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadurugamuwa JL, Sin LV, Yu J, et al. Rapid direct method for monitoring antibiotics in a mouse model of bacterial biofilm infection. Antimicrobial Agents and Chemotherapy. 2003;47(10):3130–3137. doi: 10.1128/AAC.47.10.3130-3137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadurugamuwa JL, Sin LV, Yu J, Francis KP, Purchio TF, Contag PR. Noninvasive optical imaging method to evaluate postantibiotic effects on biofilm infection in vivo. Antimicrobial Agents and Chemotherapy. 2004;48(6):2283–2287. doi: 10.1128/AAC.48.6.2283-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fenton M, Casey PG, Hill C, et al. The truncated phage lysin CHAPk eliminates Staphylococcus aureus in the nares of mice. Bioengineered Bugs. 2010;1(6):1–4. doi: 10.4161/bbug.1.6.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu JA, Kusuma C, Mond JJ, Kokai-Kun JF. Lysostaphin Disrupts Staphylococcus aureus and Staphylococcus epidermidis Biofilms on Artificial Surfaces. Antimicrobial Agents and Chemotherapy. 2003;47(11):3407–3414. doi: 10.1128/AAC.47.11.3407-3414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. Journal of Clinical Microbiology. 2004;42(5):1915–1922. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. Journal of Clinical Microbiology. 1999;37(6):1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoopes JT, Stark CJ, Kim HA, Sussman DJ, Donovan DM, Nelson DC. Use of a bacteriophage lysin, PlyC, as an enzyme disinfectant against Streptococcus equi . Applied and Environmental Microbiology. 2009;75(5):1388–1394. doi: 10.1128/AEM.02195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar CG, Anand SK. Significance of microbial biofilms in food industry: a review. International Journal of Food Microbiology. 1998;42(1-2):9–27. doi: 10.1016/s0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 59.Hell É, Giske CG, Nelson A, Römling U, Marchini G. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis . Letters in Applied Microbiology. 2010;50(2):211–215. doi: 10.1111/j.1472-765X.2009.02778.x. [DOI] [PubMed] [Google Scholar]
- 60.Furukawa S, Akiyoshi Y, Komoriya M, Ogihara H, Morinaga Y. Removing Staphylococcus aureus and Escherichia coli biofilms on stainless steel by cleaning-in-place (CIP) cleaning agents. Food Control. 2010;21(5):669–672. [Google Scholar]
- 61.Bruellhoff K, Fiedler J, Moller M, Groll J, Brenner RE. Surface coating strategies to prevent biofilm formation on implant surfaces. The International Journal of Artificial Organs. 2010;33:646–653. doi: 10.1177/039139881003300910. [DOI] [PubMed] [Google Scholar]
- 62.van Heerden J, Turner M, Hoffmann D, Moolman J. Antimicrobial coating agents: can biofilm formation on a breast implant be prevented? Journal of Plastic, Reconstructive and Aesthetic Surgery. 2009;62(5):610–617. doi: 10.1016/j.bjps.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 63.Bernthal NM, Stavrakis AI, Billi F, et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PloS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012580.e12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. British Journal of Dermatology. 2006;155(4):680–687. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 65.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews. 1997;10(3):505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]


