Abstract
The prevalence of major depressive disorder (MDD) in adult men is roughly half that of women. Clinical evidence supports a protective effect of androgens against depressive disorders in men. The developing brain is subject to androgen exposure but a potential role for this in depression during adulthood has not been considered. In order to explore this question we treated newborn male rat pups with the androgen receptor antagonist flutamide to block endogenous androgen action and then conducted behavioral tests prior to puberty. Depression-like behaviors were assessed with the Forced Swim Test (FST) and the Sucrose Preference Test (SPT), and anxiety-like behaviors were assessed with the Open Field Test (OFT) and the Novelty-Suppressed Feeding Test (NSFT). Compared to the vehicle-treated controls, neonatal-flutamide treatment caused a significant increase in depression-like behaviors in preadolescent male rats but did not cause any significant difference in anxiety-like behaviors.
In separate experiments, male pups with and without flutamide treatment were injected with 5-bromo-2’-deoxyuridine-5’-monophosphate (BrdU) from postnatal day (PND) 1 to 4 to label newly produced cells or the hippocampi were Golgi-Cox imbedded and pyramidal neurons visualized. Three lines of evidence indicate neonatal flutamide treatment inhibits hippocampal neurogenesis and neuronal dendritic spine formation in preadolescent male rats. Compared to vehicle controls, flutamide treatment significantly decreased 1) the number of microtubal associated protein-2+ (MAP-2) neurons in the CA1 region, 2) the number of MAP-2+ neurons in the dentate gyrus (DG) region of the hippocampus, and 3) the density of dendritic spines of pyramidal neurons in the CA1 region. However, there was no effect of flutamide treatment on the number of GFAP+ or GFAP+/BrdU+ cells in the hippocampus. This study suggests that the organizational effect of androgen-induced hippocampal neurogenesis is antidepressant.
Keywords: Depression, Androgen, Flutamide, Hippocampus, Neurogenesis
Introduction
The prevalence of major depressive disorder (MDD) in adult men is roughly half that of women. Clinical evidence supports a protective effect of androgens against depressive disorders. Lower androgen levels are associated with an increase in the prevalence of depressive disorders in adult males (Seidman et al, 2002, 2003, Shores et al, 2004), and androgen replacement improves depressive symptoms in hypogonadal male patients (Perry et al, 2002, Pope et al, 2003). Interestingly, the prevalence of MDD in males and females is not significantly different during childhood but emerges in the age range of 11–14 years (Angold et al, 1998), paralleling the rise of androgens in adolescent males. This has led to a general assumption that it is the post-pubertal onset of androgen synthesis that underlies the sex difference in the prevalence of depression. However, it is well established in studies of reproductive endpoints that perinatal gonadal steroids act to organize the neural substrate and this changed neuroarchitecture is then activated by gonadal steroids post-puberty in a directed manner. Codified as the classic Organizational / Activational Hypothesis of gonadal steroid action (Phoenix et al, 1959), this tenet has not generally been applied to more complex emotion-based behaviors controlled outside the hypothalamus, such as depression-like behaviors controlled in part by the hippocampus.
A large body of literature suggests that sex steroid hormones, including estrogens and androgens, play important roles in hippocampal dimorphism both anatomically and functionally (McEwen et al, 1983, 1999; Pilgrim et al, 1994; Cooke et al, 1999). Estrogens are the major focus of these studies (Dawson et al, 1975; Madeira et al, 1991, 1995; Stromstedt, and Waterman, 1995; Wood et al, 1997; Daniel et al, 1999; Luine et al, 2003; Zhu et al, 2003). Much less research has been done with respect to the effects of androgens on the hippocampus. Testosterone and its 5-alpha reduced metabolite, dihydrotestosterone (DHT), are the major circulating androgenic hormones in the male. In the newborn rat, the testosterone level is 5–6 times higher in males than in females (Forest, 1979; Weisz and Ward, 1980; Rhode et al, 1984). For female rats, testosterone levels remain relatively flat and low during the same period (Forest, 1979). The fetal rat brain expresses androgen receptors (AR) as early as embryonic day (E)12, the expression peaks on E17-18 and then gradually declines during adulthood (Brannvall et al, 2005). The distribution of AR in the brain is broad and includes the hippocampus, cortex, and lateral septum (Sar and Stumpf, 1973; Lieberburg et al, 1977; Handa et al, 1986; Roselli, 1991). In the hippocampus, AR expression is at a much higher density in CA1 than CA3 or DG (Kerr et al, 1996).
Few studies have examined steroid hormone effects on the developing hippocampus, and even fewer have looked at the potential effect of androgens (McEwen, 1983; Pilgrim and Hutchison, 1994). Perinatal androgen treatment increases CA3 pyramidal cell layer volume and neuronal soma size, neuronal dendritic length, the number of dendritic branches, and the overall volume of the CA3 region (Isgor and Sengelaub, 1998, 2003; Forgie and Kolb, 2003). These hippocampal structural changes are associated with functional changes. When androgens are eliminated by neonatal gonadectomy, there is a decrease in dendritic spine density in the hippocampus, and poor spatial navigation in adulthood. However, when neonatally gonadectomized rats are treated with testosterone or DHT during pre- or neonatal life, hippocampal dendritic spine density is increased and the spatial navigation performance is significantly improved (Dawson et al, 1995; Isgor and Sengelaub, 1998, 2003).
In the present study we antagonized the androgen receptor in order to examine the neonatal organizational effects of androgens on depression-like behaviors, anxiety-like behaviors, hippocampal neurogenesis, and synaptogenesis during the adolescent period. Our data indicate neonatal androgens play an important role in protecting male rats from depression-like behaviors, and this protection is correlated with hippocampal neurogenesis and an increased dendritic spine density on pyramidal neurons in the hippocampus.
Experimental procedures
Animals
Timed-pregnant Sprague-Dawley rats were purchased from Charles River Laboratory (Wilmington, MA). Individually housed pregnant females were checked every morning for the appearance of pups in the nest. The day of birth was defined as postnatal day 0 (PND0). Animals were housed under a 12:12 hour light/dark cycle, with food and water freely available. All animal procedures were approved by the University of Maryland at Baltimore Institutional Animal Care and Use Committee.
Flutamide and BrdU Treatment (Table 1)
Table 1. Groups and treatments.
The flutamide or sesame oil vehicle was injected subcutaneously on PND 0 and PND1, respectively. The BrdU was injected peritoneally daily from PND1 to PND 4 (0.05ml distilled water containing 50mg /kg BrdU).
| Groups | Doses (PND0,1) | BrdU (PND1-4) |
|---|---|---|
| Male pups: vehicle | sesame oil (0.1ml) | 50mg/kg 0.05ml H2O |
| Male pups: flutamide (Flu) | 50 mg/kg (0.1ml) | 50mg/kg 0.05ml H2O |
Neonatal treatment with flutamide, an androgen receptor antagonist, was conducted as described previously (Zhang et al, 2008). Briefly, rat pups from multiple dams were randomly distributed into either vehicle or flutamide treatment groups and marked by subcutaneous ink injection in either the front or hind paws for different experimental groups. Pups were removed from dams and placed on a heated pad (37°C) to maintain temperature during the injection procedure. Flutamide (FLU, 250 ug/0.1ml, 50mg/kg) was dissolved in sesame oil. Pups were injected subcutaneously with either flutamide or vehicle on PND 0 and PND 1. The BrdU was injected peritoneally daily from PND1 to 4 (0.05ml distilled water containing 50mg /kg BrdU) to label newly produced cells during neonatal development. After each injection, the injection sites were sealed with cyanoacrylate Vetbond Surgical Adhesive (3M Animal Care Product, St. Paul, MN). Pups were randomly distributed to different mother rats after each treatment.
Behavioral tests
Behavioral tests were conducted in a sequence from the least to the most stressful procedures: the Sucrose Preference Test, the Novelty Suppressed Feeding Test, and the Forced Swim Test. The Open Field Test was conducted in a separate batch of rats.
The Sucrose Preference Test (SPT)
The SPT was performed as described by Banasr and colleagues (Banasr and Duman, 2008). Briefly, on PND 22, rats were habituated with 1% sucrose for 48 hours without any food or water. Then, after 24 hours of deprivation, each rat was provided two identical bottles containing either water or 1% sucrose solution in an individual cage for one hour. After one hour, the amount of water and 1% sucrose intake were recorded. Data were expressed as percentage of sucrose intake from total intake (sucrose/sucrose + water).
The Novelty-Suppressed feeding test (NSFT)
The NSF was conducted as described (Briton and Briton 1981; Bodnoff et al, 1988; Satarelli et al, 2003) with modifications. Briefly, the test was conducted in an open field box measured 76 ×76 ×46 cm. All food was removed from the home cages 24 hours before the test. Two grams of food pellets were placed on a white round paper (d=6.25 cm) in the center of the open field box. During the test, the rat was put at the corner of the testing box for 5 min to measure the latency to bite the food pellets. The rat was then put back in its cage with food. The amount of food the rat ate during the next 5 minute period was measured.
The Forced Swim Test (FST)
The test was conducted accounting to previously established protocols (Porsolt et al, 1977; Detke and Lucki, 1996; Siuciak et al, 1997; Shirayama et al, 2002; Pechnick et al, 2008; Reed et al, 2008). Briefly, on the first day (PND 27), rats swam for 15 min in a 22.5 cm diameter glass cylinder filled with 25 °C water up to 30 cm high. On the second day (PND 28), rats swam for 5 min and were video-taped. Three behaviors were scored automatically with the Forced SwimScan system (Cleversys Inc, Reston, VA, Detke et al, 1995; Tonelli et al, 2008): 1) climbing, rat made an active attempt to escape from the container; 2) swimming, rat stayed afloat, pedaling, and making circular movements around the tank; and 3) immobility, rat did not make any active movements.
The Open Field Test (OFT)
Testing followed the standard procedure described by Lacroix et al, 1998; (Lacroix et al, 1998). The test room was dimly illuminated (two 60 W lights, indirectly), and rats placed in a square arena (76.5 × 76.5 × 49 cm), divided into two areas: a peripheral area and a square center (40 × 40 cm), and allowed to explore for 5 min. The measurement parameters included the number of square-crossings and the time spent in the peripheral and central areas of the open field.
Immunohistochemistry
Brain tissue treatment
On PND 28, rats were deeply anesthetized with ketamine (10mg, intraperitoneal), weighed and transcardially perfused with 0.9% saline until there was no blood trace and then perfused with 4% paraformaldehyde with 2.5% acrolein for about 3 minutes. Brains were collected, weighed, and further fixed for 24 hours in 4% paraformaldehyde. Brains were stored in 30% sucrose for 72 hours and then sectioned into 45 µm sections on a cryostat.
Double-label Immunohistochemistry
MAP-2+ neurons
Newly-produced mature neurons were detected with BrdU/MAP-2 double labeling immunochemical techniques (Yang et al, 2009). Briefly, tissue sections were treated with 1% sodium borohydride in phosphate buffered saline (PBS) for 20 minutes, rinsed, and incubated with 0.04% phenylhydrazine in PBS for 20 minutes. Tissue sections were then incubated with monoclonal antibody against MAP-2 in PBS with 0.4% Triton X-100 (PBS-T) overnight. After being thoroughly rinsed, tissue sections were incubated with biotinylated secondary antibody, followed by rinses and addition of Vectastain Elite ABC reagents (Vector). MAP-2 positive neurons were detected by the diaminobenzidine (DAB) method. Then, tissue sections were further incubated with 2N HCL for one hour at 37°C to denature DNA. After a thorough rinse, tissue sections were incubated with 5% goat serum in PBS-T for 60 minutes. Monoclonal antibody against BrdU (Caltag Lab, 1:10000 in PBS-T) was applied in PBS-T for one hour at room temperature and then for 48 hours at 4°C. The next day, tissue sections were incubated with biotinylated secondary antibody against mouse IgG, followed by rinses and addition of Vectastain Elite ABC reagents (Vector). BrdU positive cells were detected with Nickle-diaminobenzidine in sodium acetate, giving a deep blue color to BrdU-positive nuclei (Figure 2 B). After the reaction, tissue sections were rinsed and mounted onto gelatin-subbed slides, dehydrated, and coverslipped. The number of MAP-2 positive and BrdU/MAP double positive neurons were counted in the CA1 and DG regions of the rostral hippocampus. For each region, the number of neurons was determined in a 5×10 µm counting frame with a 40 X objective. Ten to 15 sections were collected throughout the rostral hippocampus from each animal. The number of MAP-2 positive and BrdU/MAP double positive neurons were counted bilaterally only in whole sections (free of any tears or other physical defects) that matched anatomically across all animals, resulting in four sections per animal. Numbers were averaged to give one final value per animal. The treatment conditions of the animals from which the sections were generated were unknown to the investigator doing the analysis.
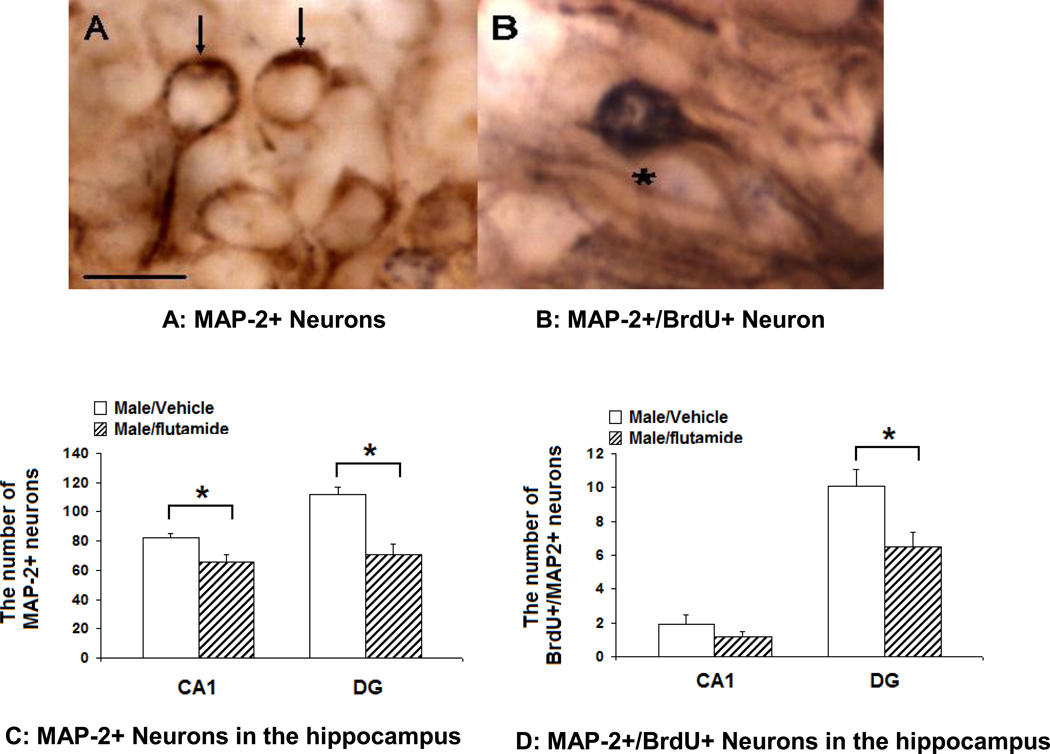
Figure 2.
The effects of neonatal treatment with flutamide on MAP-2+ neurons in the hippocampus of PND 28 male rats: A: MAP-2+/BrdU- neurons. Arrows: MAP-2+ neurons, Scale bar: 25 µm; B: Map-2+/BrdU+ neuron; compared to the vehicle controls, in neonatally flutamide-treated male rats, the number of MAP-2+ neurons was significantly decreased in the CA1 and DG regions (C), and the number of MAP-2+/BrdU+ neurons was significantly decreased only in the DG but not in CA1 region of the hippocampus (D). *: p<0.05.
GFAP+ cells
Newly-produced glial cells were detected with GFAP/BrdU double labeling immunochemical technique as described (Zhang et al, 2008). Briefly, the tissue sections were treated as described above. Sections were incubated with antibody (goat anti-mouse monoclonal antibody) against BrdU and sequentially with biotin-labeled secondary antibody (rabbit anti-goat). BrdU posititive cells were detected with Nickle-diaminobenzidine (DAB) in sodium acetate, giving a deep blue color to BrdU-positive nuclei (Figure 4 B). After being thoroughly rinsed overnight, the tissue sections were sequentially incubated with antibodies against GFAP (Sigma, 1:10,000), and incubated with biotin-labeled secondary antibodies. The GFAP positive cells were detected with DAB. The number of GFAP+ cells was counted in the CA1 and DG regions of the rostral hippocampus, using the Neurolucida program package (Microbrightfield version 2.01) and a counting frame (80 × 80 µm with a 60 X objective. The final value of each animal was averaged from 4 sections and expressed as mean ± SEM.
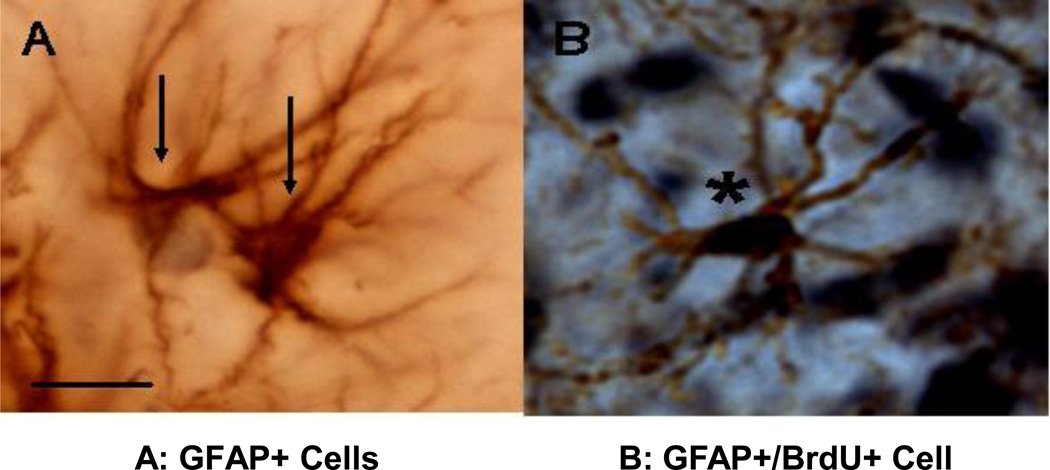
Figure 4.
GFAP+ cells in the hippocampus of PND 28 male rats. A: GFGAP+ Glial cells; B : BrdU+/GFAP+ cell. GFAP: glial fibrillary acidic protein. Scale bar: 25 µm.
Golgi-Cox Method
The Golgi-Cox stain was conducted as described with modifications (Glaser and Van der Loos, 1981; Mong et al, 1999; Mong and McCarthy, 1999; Shors et al, 2001; Amateau and McCarthy, 2004). Briefly, rats were deeply anesthetized with ketamine and fresh brain tissue was collected for impregnation in potassium dichromate solution and incubated in a dark box for 2 weeks. Impregnated brains were then sectioned (100 µm) and mounted for development. The Golgi-Cox staining was developed in 1% Kodak Dektol for 5 minutes and 17.9% Kodak Fix for 10 minutes. Then, the sections were counterstained with 0.2% Methylene Blue for 10 minutes. Finally, brain sections were dehydrated and cover-slipped. Spine counting was conducted with a 60X objective using the Neurolucida program package (Microbrightfield version 2.01). We counted 5 pyramidal neurons in the CA1 region for each rat. Each pyramidal neuron was thoroughly impregnated and clearly distinguishable from other cells. The number of spines was counted in a 10 µm segment which was at least 50 µm away from the soma. For each pyramidal neuron, spines were averaged from six segments including 3 apical and 3 basal segments. The final value for each animal was averaged from 5 pyramidal neurons and expressed as the number of spines/1 µm.
Data analysis
All data were expressed as mean ± SEM. Two-tailed Student’s t test was used to compare the mean between groups, with p<0.05 required for statistical significance.
Results
Neonatal flutamide-treatment and brain and body weights in PND 28 rats
Neonatal flutamide treatment did not affect either brain (t=0.84, df=23, p=0.41) or body weights (t=1.69, df=24, p=0.10) in PND 28 rats as indicated in Table 2.
Table 2. Body and brain weights in PND 28 rats.
Male pups were collected from 4 dams, and distributed randomly to different groups. The pups were treated with flutamide (50mg/kg) or sesame oil vehicle on PND 0 and PND 1 as indicated. All rats were euthanized on PND 28. Body and brain weights were obtained, and expressed as mean ± SEM.
| Groups | Treatment | Mean Body Weight (g) (±SEM) |
Mean Brain Weight (g) (±SEM) |
|---|---|---|---|
| Male | Vehicle | 75.97 ± 2.2 (N=20) | 1.35 ± 0.03 (N=16) |
| Male | Flutamide | 68.70 ± 3.7 (N=15) | 1.31 ± 0.04 (N=13) |
Neonatal flutamide-treatment increased the expression of depression-like behaviors in PND 25–28 rats
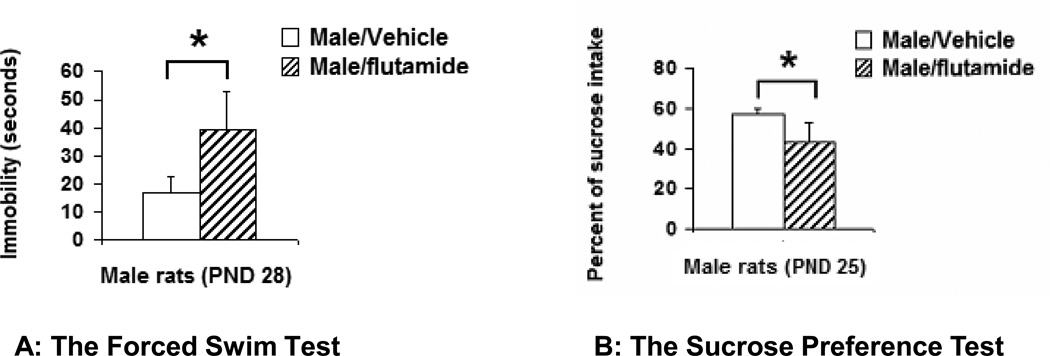
The level of depression-like behaviors was significantly increased in both the FST and the SPT after male pups were treated with flutamide neonatally. In the first day of FST, there was no significant difference in the immobility between the vehicle- and flutamide-treated male rats. During the first 5 minutes, the duration of immobility was 21.6 ± 4.4 (n=5) in vehicle-treated and 19.7 ± 6.9 seconds (n=6) in flutamide-treated males (t=0.237, df=8, p=0.82). However, on the second day of the test, during a 5 minute recording session the duration of immobility was 15.9 ± 5.5 (n=16) in vehicle-treated and 40.1 ± 10.9 (n=11) in flutamide-treated male rats (t=2.16, df=15, p=0.047, Figure 1 A). In the SPT, the flutamide-treated male rats consumed significantly less sucrose than the vehicle-treated ones. The percentage of consumed-sucrose from total amount (water + 1% of sucrose) was 56.8 ± 3.0 (n=10) in vehicle-treated and 43.6 ± 3.4 (n=8) in flutamide-treated male rats (t=2.90, df=15, p=0.01, Figure 1 B).
Figure 1.
The effect of antagonizing AR neonatally on preadolescent rats: Compared to the controls, the duration of immobility was significantly increased (p=0.047) in the Forced Swimming Test (A) and the percentage of sucrose intake was significantly decreased in the Sucrose Preference Test (B) in neonatally flutamide-treated male rats (p=0.01). Data were presented as mean ± SEM. *: p<0.05.
Higher doses of flutamide (30 to 100 mg/kg) have been used in other studies with no adverse effects (Goto et al, 2004; Seale et al, 2005). In a separate experiment, female rats treated with the same dose as males showed no change in depression-like behaviors at PND 28. In the SPT, the percentage of sucrose intake was 57.1 ± 4.8 (n=10) in vehicle- vs 65.3 ± 2.5 (n=4) in flutamide-treated females (t=1.51, df=11, P=0.16); on the second day of the FST, the duration of immobility was 24.9 ± 4.6 (n=10) vs 27.3 ± 4.9 (n=4) seconds (t=0.354, df=8, P=0.73), suggesting 50 mg/kg of flutamide was not toxic in our study.
Neonatal flutamide-treatment did not affect the expression of anxiety-like behaviors in PND 26 to 28 rats
Anxiety-like behaviors were assessed using the OFT and the NSFT. The latency to eat for vehicle-, and flutamide-treated male rats was 133.2 ± 29.3 seconds (n=11), and 161.0 ± 28.0 seconds (n=8), respectively (t=0.90, df=16, p=0.38). Although flutamide-treated rats took a longer time to find and eat the food, the difference between these two groups was not statistically significant (p=0.38). In the OFT, the number of crossings of outer squares was 98.7 ± 9.3 (n=17) in vehicle-treated and 77.8 ± 11.4 (n=9) in flutamide-treated male rats (t=1.20, df=19, p=0.17); the number of inner square crossings was was 9.6 ± 1.8 (n=8) in vehicle-treated and 8.0 ± 2.1 (n=9) in flutamide-treated male rats (t=0.58, df=18, p=0.56). The percentage of time in inner squares was 5.3 ± 1.1 % (n=17) in vehicle-treated and 6.8 ± 1.9% (n=9) in flutamide-treated male rats (t=0.70, df=13, p=0.50). There were no significant differences between treatment groups on any measured parameters (data not shown).
Neonatal flutamide-treatment decreased the number of MAP2+ neurons in the hippocampus
Representative photomicrographs of neurons of MAP-2+/BrdU- neurons are shown in Figure 2 A and neurons double labeled for MAP2 and BrdU are shown in Figure 2 B. Compared to the vehicle-treated controls, the number of MAP-2+ neurons was significantly decreased in both CA1 and DG regions of the hippocampus of flutamide-treated male rats. In the CA1 region, the mean number of MAP-2+ neurons was significantly higher in vehicle-treated (n=8) than in flutamide-treated (n=6) male rats (t=2.69, df=7, p=0.03); likewise in the DG region of the same animals there were significantly more MAP2+ neurons in vehicle-treated versus flutamide-treated male rats (t=4.71, df=9, p=0.001, Figure 2C). A significant difference in the number of BrdU+/MAP-2+ neurons (Figure 2 A) was observed in the DG region with vehicle treated males having more double labeled neurons compared to flutamide-treated male rats (t=2.75, df=12, p=0.02). There was no effect of treatment on double labeled neurons in the CA1 region (t=0.57, df=10, p=0.27, Figure 2 D).
Neonatal flutamide-treatment decreased the density of dendritic spines on pyramidal neurons in CA1 hippocampus
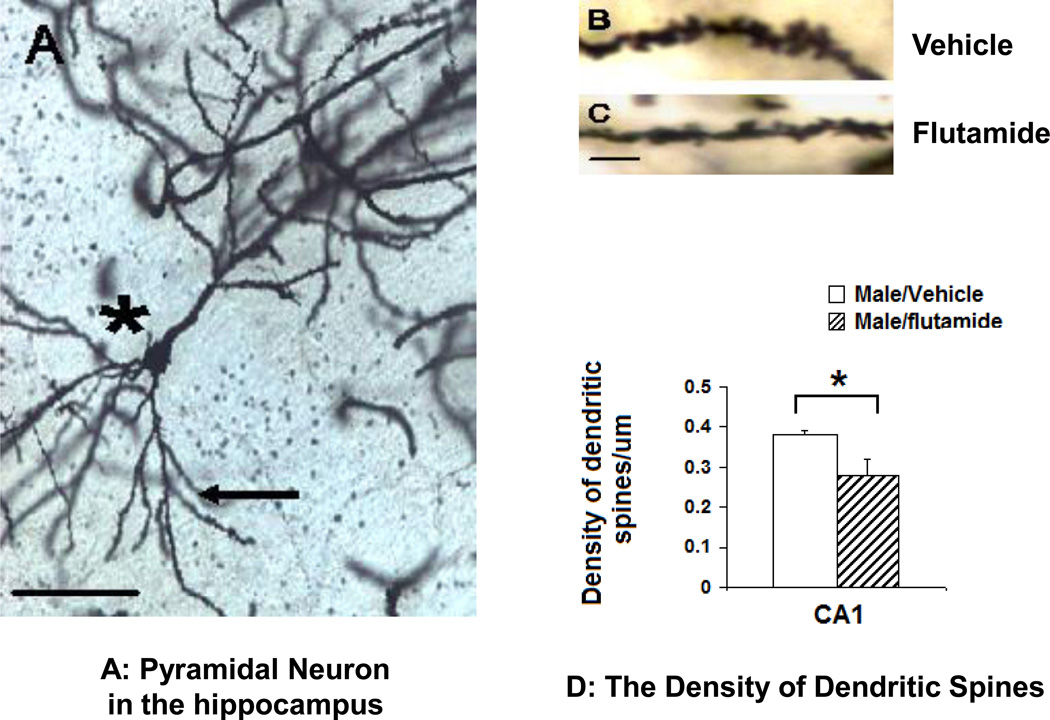
Representative photomicrographs of Golgi-Cox impregnated pyramidal neurons are shown in Figures 3A, C &D. The density of dendritic spines was significantly decreased in neonatally flutamide-treated males compared to vehicle-treated controls. The data were expressed as the number of spines per µm (t=2.58, df=8, p=0.03, n = 7–8; Figure 3 B)
Figure 3.
The effect of neonatal flutamide treatment on the density of dendritic spines in the CA1 region of the hippocampus. A: Golgi-Cox impregnated pyramidal neurons. *: Pyramidal neuron, arrow: basal spines, scale bar: 50 µm. B: high modification of a dendritic spines from vehicle-treated and C: flutamided-treated male rats. Scale bar: 10 µm; D: the density of dendritic spines in the pyramidal neurons in the CA1 hippocampus was significantly decreased (p=0.016) in neonatally flutamide-treated male rats compared to their counterparts. * : p<0.05.
Neonatal flutamide treatment did not affect hippocampal gliogenesis in PND 28 male rats
Representative photomicrographs for GFAP+ cells (Figure 4 A) and GFAP+/BrdU+ cells (Figure 4 B) are shown. In the CA1 region, the number of GFAP+ cells was 22.5 ± 10.7 (n=9) in vehicle-treated and 20.5 ± 2.2 (n=6) in flutamide-treated male rats (t=0.86, df=6, p=0.42), whereas the number of BrdU+/GFAP+ cells was 13.5 ± 1.0 (n=9) in vehicle-treated and 13.6 ± 2.5 (n=6) in flutamide-treated male rats (t=0.03, df=7, p=0.98). In the DG region, the number of GFAP+ cells was 24.1 ± 1.1 (n=9) in vehicle-treated and 23.2 ± 2.4 (n=6) in flutamide-treated male rats (t=0.36, df=7, p=0.73); whereas the number of BrdU+/GFAP+ cells was 13.9 ± 0.8 (n=9) in vehicle-treated and 14.5 ± 2.4 (n=6) in flutamide-treated male rats (t=0.27, df=6, p=0.80).
Discussion
The biological basis of gender bias in the prevalence of major depressive disorder remains unknown. We present evidence here that male rats exhibited more depression-like but not anxiety-like behaviors during preadolescence when they were treated with flutamide, an androgen receptor antagonist, neonatally. This increase in depression-like behaviors was associated with a decrease in the number of neurons and a decrease in the density of dendritic spines on the pyramidal neurons, but not with gliogenesis, in the hippocampus. To our knowledge, this is the first exploration of a potential contributing effect of neonatal androgen exposure on the adult onset of depressive-like symptoms.
There are organizational effects of androgens on depression-like behaviors
Androgens begin to affect hippocampal development prenatally (Bayer, 1980; Bingaman et al, 1994; Isgor and Sengelaub, 1998; Brannvall et al, 2005). Circulating androgens are synthesized in the fetal testis beginning around E16, peaking at E18 and rising again four days later at birth (Weisz and Ward, 1980). During this critical time androgens not only affect fetal brain development but can also determine how the brain functions during adulthood. In this study, we observed that flutamide administration in neonatal male rats resulted in an increase in depression-like behaviors during preadolescence in both the FST and the SPT, suggesting that perinatal androgens are protective against a later onset of depression. Behavioral tests were conducted prior to PND 28 to avoid the surge of androgen production at puberty. The FST has been successfully conducted using perinatal rats in antidepressant studies (Pechnick et al, 2008; Reed et al, 2008). Our results are therefore consistent with an organizational effect of androgens on depression-like behaviors. Organizational effects of androgens have been reported for the hypothalamic-pituitary-adrenal axis (McCormick et al, 1998), spatial memory (Isgor and Sengelaub, 1998), and adult social behaviors (Schulz et al, 2009). Flutamide is a specific androgen receptor antagonist and widely used to block androgen’s effect both in vivo and in vitro (Isgor and Sengelaub, 1998; Naghdi et al, 2001, 2003; Casto et al, 2003; Goto et al, 2004, 2005; Dominguez-Salazar et al, 2005; Edinger et al, 2006 Zhang et al, 2008).
There are no organizational effects of androgens on anxiety-like behaviors
Acute anti-anxiety effects of androgens are reported in both human (Kessler, 2003; Cloitre et al, 2004) and animal studies (Bitran et al, 1993; Bing et al, 1998; Osborne et al, 2009). Our goal was to explore the organizational actions of androgens. We used the open field test (OFT) and the Novelty Food Suppressed test (NFST) to assess anxiety-like behaviors, but did not observe any effects of neonatal flutamide treatment. This may be a result of the behavioral tests we employed. Zuloaga and colleagues (2008), using mice with the testicular feminization mutation (tfm) which renders androgen receptors non-functional throughout life, observed an increase in anxiety-like behaviors as detected in the novel object test (NOT) and the Light/Dark (LD) Box, but not in the OFT or the elevated plus maze (EPM) test. In contrast, Stewart and others (1975) found that injections of high doses of testosterone to neonatal females decreased anxiety-like behavior in the OFT. Other investigators have reported that neonatal castration decreases anxiety behaviors in the EPM in adult male rats (Lucion et al, 1996). These differences in animal models, behavioral tests, and treatment paradigms preclude making definitive conclusions regarding the organizational effects of androgens on adult anxiety-like behavior. The lack of effect may be also related with the neural substrates involved in the expression of emotional behaviors revealed by these tests. While the FST is sensitive to manipulations affecting hippocampal structure and function, the OFT is not. This provide additional evidence of a relationship between the effects of Flu in the hippocampus and the expression of depressive-like behavior
There are organizational effects of androgens on hippocampal neurogenesis
We have previously reported that neonatal testosterone administration increases the number of hippocampal neurons in female rats (Zhang et al, 2008). In the current study, the number of mature (MAP-2+) neurons was significantly decreased in flutamide-treated male rats compared to controls in the DG region. Based on co-labeling with the cell division marker, BrdU, some of these neurons were born during the first four postnatal days and persisted until PND 28. Androgens may promote hippocampal neurogenesis by at least three different routes: the proliferation of new cells, the survival of new cells, and the differentiation of new cells into neurons. Androgens increase new cells via both androgen receptor (AR) as well as estrogen receptor (ER) mediated mechanisms since both DHT and estradiol increase the number of BrdU+ cells in the neonatal hippocampus (Zhang et al, 2008) and estradiol can be synthesized from testosterone in vivo. Androgens may promote neurogenesis via indirect mechanisms. Both testosterone and estradiol up-regulate the expression of brain derived neurotrophic factor (BDNF) in the brain (Rasika and Alvares-Buylla, 1999) including in the hippocampus (Solum and Handa, 2002). BDNF promotes neuronal survival and differentiation (Alderson et al, 1990; Ghosh et al, 1994; Jones et al, 1994). Therefore, it is possible that androgens may program undifferentiated neuronal progenitor cells to respond to local cues such as BDNF to become mature neurons in their later development, an organizational effect of androgens. It is also possible that androgens may cause an increase in neurons by preventing neuronal apoptosis or death (Hammond et al., 2001; Hsu et al., 2001).
In the current study the number of mature neurons (MAP-2+ neurons) in the CA1 region was significantly decreased in flutamide-treated male rats compared to controls. However, in contrast to our findings in the DG region, neonatal flutamide treatment did not affect the number of BrdU+/MAP-2+ neurons. This may be a simple artifact of the smaller number of neurons being born in CA1 at this time, versus the large number proliferating in the DG. Regardless, our observation that there were fewer overall MAP-2+ neurons in the CA1 of flutamide treated males is consistent with an overall decrease in neurons in this region when androgen action is antagonized.
There are no organizational effects of androgens on gliogenesis
In postmortem studies of patients with MDD, a loss of glial cells is detected in fronto-limbic regions (Ongur et al, 1998; Rajkowska et al, 1999; Cotter et al, 2001, 2002), but not in the hippocampus (Stockmeier et al, 2004). Our data indicate that neonatal flutamide administration does not affect the number of glial cells in both the CA1 and DG regions of the hippocampus, suggesting that androgens may not be the major contributor to gliogenesis during neonatal hippocampal development. It also suggests that gliogenesis may not be the major mechanism for the antidepressant effects of androgens observed in this study.
There are organizational effects of androgens on synaptic formation
The density of dendritic spines on pyramidal neurons in the CA1 region of the hippocampus was significantly decreased in flutamide-treated preadolescent rats, suggesting neonatal androgen exposure is important for dendritic spine formation in the developing hippocampus. Multiple studies demonstrate that androgens promote and enhance dendritic spine formation in the adult hippocampus (Leranth et al, 2003, MacLusky et al, 2006, Parducz et al, 2006). However, our data now indicate that neonatal effects of androgens on dendritic spines can be extended into preadolescence. There are two possible explanations for our observation. The first one is that neonatal androgens promote dendritic spine formation and flutamide blocked this effect. Under this hypothesis, the decrease in dendritic spines occurred during the neonatal stage when flutamide was administrated and blocked the action of endogenous androgen. The flutamide-induced spine deficiency sustained into preadolescence, most likely due to lack of endogenous androgens before adolescence. The second possibility is that neonatal androgens prepare hippocampal neurons to respond to environmental cues for more spine formation in later stages of development. Under this hypothesis, neonatal flutamide administration blocked the organizational effect of androgens on dendritic spine formation in the hippocampus. This possibility needs to be further explored.
As the primary loci of excitatory synaptic transmission in the central nervous system (CNS), a change in the density of dendritic spines is associated with many functional changes in the CNS (Colonnier, 1968; Jones et al, 1997; Sorra and Harris, 2000). The density of dendritic spines can be regulated by many factors. For example, the spine density of neurons can be increased by learning and training (Jones et al, 1997; Moster et al, 1997; O’Malley et al, 2000), by estrogens in female rats (Gould et al, 1990; Woolley et al, 1990; Woolley and McEwen, 1992, 1993), and by androgens in male rats (Leranth et al, 2003; MacLusky et al, 2006; Parducz et al, 2006). Accumulated evidence suggests that the plasticity of dendritic spine may associate with depressive disorders and their treatment. For example, chronic administration of fluoxetine, an antidepressant, induces a significant increase in the density of dendritic spines in the rat hippocampus (Hajszan et al, 2005).
Hippocampal neurogenesis and depression
Major Depressive Disorder is a complicated brain disorder. Structural changes have been detected in depressive patients using MRI (Sheline, 1996; Bremmer et al, 2000) and postmortem studies (Rajkowska, 2000, 2002), including orbital and medial prefrontal cortex, amygdala, ventral striatum, inferior anterior cingulate, and the hippocampus (Sheline et al, 1998; Bremmer et al, 2000; Drevets, 2001; Beyer and Krishnan, 2002). Hippocampal atrophy is consistently detected in patients with recurrent depression (Sheline et al, 1996) as well as in patients with first episode of depression (Fordl et al, 2002), suggesting that reduced hippocampal volume may be a risk factor for depression. In the adult, hippocampal neurogenesis is inhibited by stress or glucocorticoids, and reduced neurogenesis is associated with depressive behaviors both in animals and humans (Brown et al, 1999; McEwen et al, 1999; McEwen, 2000; Sapolsky, 2000). In animal studies, effective antidepressants, such as desipramine, fluoxetine, and electroconvulsive therapy, enhance hippocampal neurogenesis (Malberg et al, 2000; Scott et al, 2000). Interestingly, by blocking hippocampal neurogenesis using radiation, Santarelli et al (2003) indicated that the antidepressant effect of fluoxetine is dependent on hippocampal neurogenesis. Developmentally, prenatal stress significantly inhibits hippocampal neurogenesis, and this effect persists until adolescence (Coe et al, 2003) with the affected offspring exhibiting higher rates of depression-like behaviors (Sapolsky, 2001; Schmitz et al, 2002). Interestingly, the increase in depression-like behaviors following prenatal stress is seen only in females (Zhu et al, 2004), suggesting androgens may protect the hippocampus either by promoting hippocampal neurogenesis or preventing its atrophy.
Acknowledgement
We would like to thank Dr. Todd Gould and Dr. Istvan Merchenthaler for their critical reading of this manuscript. JMZ is a BIRCWH scholar. This work was supported by a NIH K-12 Building Interdisciplinary Research Careers in Women's Health (BIRCWH, JMZ) and NIH grant R01 NS050525-02 (M.M.M.).
Comprehensive list of abbreviations
- AR
androgen receptor
- BDNF
brain derived neurotrophic factor
- BrdU
5-bromo-2’-deoxyuridine-5’-monophosphate
- CA1
Cornu Ammonis 1
- DAB
diaminobenzidine
- DG
dentate gyrus
- DHT
dihydrotestosterone
- E
embryonic day
- EPM
the elevated plus maze test
- ER
estrogen receptor
- FLU
flutamide
- FST
the Forced Swim Test
- GFAP
Glial fibrillary acidic protein
- LD
the Light/Dark (LD) box
- MAP-2
microtubal associated protein-2
- MDD
major depressive disorder
- NOT
the novel object test
- NSFT
the Novelty-Suppressed Feeding Test
- OFT
the Open Field
- PBS
phosphate buffered saline
- PND
postnatal day
- SPT
the Sucrose Preference Test
- Tfm
the testicular feminization mutation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alderson RF, Alterman AL, Barde AY, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated function of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- 2.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nature Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 3.Andre´anne Be´dard Æ Claude Gravel Æ Andre´ Parent. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- 4.Angold A, Costello EJ, Worthman CW. Puberty and depression: The roles of age, pubertal status, and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- 5.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. J Biopsych. 2008 doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer SA. Development of the hippocampal region in the rat II. Morphogenesis during embryonic and early postnatal life. J Comp Neurology. 1980;190:87–114. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- 7.Bayer SA. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res. 1982;46:315–323. doi: 10.1007/BF00238626. [DOI] [PubMed] [Google Scholar]
- 8.Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 9.Bing O, Heilig M, Kakoulidis P, Sundblad C, Wikland L, Eriksson E. High doses of testosterone increase anticonflict behavior in rat. Eur. Neuropsychopharmacology. 1998;8:321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 10.Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increase in hypothalamic-corticotrophin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- 11.Bitran D, Kellog CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm. Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 12.Brannvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- 13.Bremmer JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 14.Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21:174–184. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 15.Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiology & Behavior. 2003;79:633–664. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 16.Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fucks E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile Rhesus Monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 17.Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- 18.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. PNAS. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex. 2002;12:386. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 20.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry. 2001;58:545. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 21.Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 22.Dawson JL, Cheung YM, Lau RT. Developmental effects of neonatal sex hormones on spatial and activity skills in the white rat. Biol Psychol. 1975;3:213–229. doi: 10.1016/0301-0511(75)90036-8. [DOI] [PubMed] [Google Scholar]
- 23.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez-Salazar E, Camacho FJ, Paredes RG. Prenatal blockade of androgen receptors reduces the number of intromissions needed to induce conditioned place preference after paced mating in female rats. Pharmacology Biochemistry and Behavior. 2005;81:871–878. doi: 10.1016/j.pbb.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 26.Fordl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 27.Forest MG. Plasma androgens (testosterone and 4-androstenedione in neonatal, prepubertal and peripubertal periods in the human and rat: differences between species. J Steroid Biochem. 1979;11:543–548. doi: 10.1016/0022-4731(79)90080-3. [DOI] [PubMed] [Google Scholar]
- 28.Forgie ML, Kolb B. Manipulation of gonadal hormones in neonatal rats alters the morphological response of cortical neurons to brain injury in adulthood. Behav Neurosci. 2003;117:257–262. doi: 10.1037/0735-7044.117.2.257. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 30.Goto K, Koizumi K, Takaori H, Fujii Y, Furuyama Y, Saika O, Suzuki H. Effects of flutamide on sex maturation and behavior of offspring born to female rats treated during late pregnancy. J Toxicological Sciences. 2004;29:517–534. doi: 10.2131/jts.29.517. [DOI] [PubMed] [Google Scholar]
- 31.Goto K, Koizumi K, Ohta Y, Hashi M, Fujii Y, Ohbo K, Saika O, Suzuki H. Evaluation of general behavior, memory, learning performance, and brain sexual differentiation in F1 offspring males of rats treated with flutamide during late gestation. J Toxicological Sciences. 2005;30:249–259. doi: 10.2131/jts.30.249. [DOI] [PubMed] [Google Scholar]
- 32.Gould E, Woolley CS, Frankfurd M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. European J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 34.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 35.Handa RJ, Reid DL, Resko JA. Androgen receptors in the brain and pituitary of female rats: cyclic changes and comparisons with the male. Biol Reprod. 1986;34:293–303. doi: 10.1095/biolreprod34.2.293. [DOI] [PubMed] [Google Scholar]
- 36.Heyns CF, Simonin MP, Grosgurin P, Schall R, Porchet HV. Comparative efficacy of triptorelinpamoate and leuprolide acetate in men with advanced prostate cancer. Br J Urol Int. 2003;92:226–231. doi: 10.1046/j.1464-410x.2003.04308.x. [DOI] [PubMed] [Google Scholar]
- 37.Howell S, Shalet S. Testosterone deficiency and replacement. Horm Res. 2001;56:86–92. doi: 10.1159/000048142. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HK, Yang RC, Shih HC, Hsieh YL, Chen UY, Hsu C. Prenatal exposure of testosterone prevents SDN-POA neurons of postnatal male rats from apoptosis through NMDA receptor. J. Neurophysiol. 2001;86:2374–2380. doi: 10.1152/jn.2001.86.5.2374. [DOI] [PubMed] [Google Scholar]
- 39.Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- 40.Isgor C, Sengelaub DR. Effects of neuronal gonadal steroidal on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;5 doi: 10.1002/neu.10200. 179-9. [DOI] [PubMed] [Google Scholar]
- 41.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones TA, Klintsova AY, Klilman VL, Siervaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- 43.Kaminetsky JC. Benefits of a new testosterone gel formulation for hypogonadal men. Clin Cornerstone. 2005;7:8–12. doi: 10.1016/s1098-3597(05)80091-2. [DOI] [PubMed] [Google Scholar]
- 44.Kerr JE, Beck SG, Handa RJ. Androgens modulate glucocorticoid receptor mRNA, but not mineralocorticoid receptor mRNA levels, in the rat hippocampus. J Neuroendocrinology. 1996;8:439–447. doi: 10.1046/j.1365-2826.1996.04735.x. [DOI] [PubMed] [Google Scholar]
- 45.Lacroix L, Broersen LM, Weiner I, Feldon J. The effects of excitotoxic lesion of the medical prefrontal cortex on latent inhibition, prepulse inhibition, food hoarding, elevated plus maze, active avoidance and locomotor activity in the rat. Neuroscience. 1998;84:431–442. doi: 10.1016/s0306-4522(97)00521-6. [DOI] [PubMed] [Google Scholar]
- 46.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lieberburg E, Maclusky NJ, McEwen BS. 5a-Dihydrotestosterone (DHT) receptors in brain and pituitary cell nuclei. Endocrinology. 1977;100:598–607. doi: 10.1210/endo-100-2-598. [DOI] [PubMed] [Google Scholar]
- 48.Lucion AB, Charchat H, Periira GM, Rasia-Filho AA. Influence of early postnatal gonadal hormones on anxiety in adult male rats. Physiol and Behav. 1996;60:1419–1423. doi: 10.1016/s0031-9384(96)00246-6. [DOI] [PubMed] [Google Scholar]
- 49.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 50.MacLusky NJ, Hajszan T, Praange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neurosci. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 51.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ”organizational” effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 53.Madeira MD, Lieberman AR. Sex dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- 54.Madeira MD, Sousa N, Paula-Barbosa MM. Sexual dimorphism in the mossy fiber synapses of the rat hippocampus. Ext Brain Res. 1991;87:537–545. doi: 10.1007/BF00227079. [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS. Gonadal steroid influences on brain development and sexual differentiation. In: Greep R, editor. Reproductive physiology IV. Baltimore: University Park; 1983. pp. 99–145. [PubMed] [Google Scholar]
- 56.McEwen BS. Permanence of brain sex differences and structural plasticity of the adult brain. PNAS. 1999;96:7128–7130. doi: 10.1073/pnas.96.13.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McEwen BS, de Leon MJ, Lupien SJ, Meaney MJ. Corticosteroids, the aging brain and cognition. Trends Endocrinol Metab. 1999;10:92–96. doi: 10.1016/s1043-2760(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 58.McEwen BS. Effects of adverse experiences for human structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 59.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mong JA, McCarthy MM. Gonadal steroid-induced astrocyte differentiation in the neonatal rat arcuate nucleus is mediated by GABA. Society for Neuroscience Abstracts. 1999a;25:505. [Google Scholar]
- 61.Moster MB, Trommald M, Egeland T, Andersen P. Spatial training in a complex environment and isolation alter the spine distribution differentially in rat CA1 pyramidal cells. J Comp Neurol. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 62.Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897:44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- 63.Naghdi N, Oryan S, Etemadi R. The study of spatial memory in adult male rats with injection of testosterone enanthate and flutamide into the basolateral nucleus of the amygdale in Morris water maze. Brain Res. 2003;972:1–8. doi: 10.1016/s0006-8993(03)02227-3. [DOI] [PubMed] [Google Scholar]
- 64.O’Malley AOC, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompanies consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 65.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA. 1995;95:13290. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. AGE. 2009;31:191–198. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palomba S, Orio F, Falbo A, Oppedisano R, Tolino A, Zullo F. Tibolone reverses the cognitive effects caused by leuprolide acetate administration, improving mood and quality of life in patients with symptomatic uterine leiomyomas. Fertil Steril. 2008;90:165–173. doi: 10.1016/j.fertnstert.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 68.Parducz A, Hajszan T, MacLusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neurosci. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Pechnick RN, Bresee CJ, Manalo CM, Poland RE. Comparison of the effects of desmethylimipramine on behavior in the forced swim test in peripubertal and adult rats. Bahavioral Pharmacology. 2008;19:81–84. doi: 10.1097/FBP.0b013e3282f3d07f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav. Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- 71.Perry PJ, Yates WR, Williams RD, Anderson AE, MacIndoe JH, Lund BC, Holman TL. Testosterone therapy in late-life major depression in males. J Clin Psychiatry. 2002;63:1096–1101. doi: 10.4088/jcp.v63n1202. [DOI] [PubMed] [Google Scholar]
- 72.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 73.Pilgrim C, Hutchison JB. Developmental regulation of sex differences in the brain: can the role of gonadal steroids be redefined? Neuroscience. 1994;60:843–855. doi: 10.1016/0306-4522(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 74.Pope HG, Cohane GH, Kanayama G, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:105–111. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- 75.Porsolt R, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatment. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 76.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 77.Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 2002;4:105–116. doi: 10.1034/j.1399-5618.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 78.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry. 1999;45:1085. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 79.Rasika S, Alvarez-Buylla A. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 80.Reed AL, Happe HK, Petty F, Bylund DB. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology. 2008;197:433–441. doi: 10.1007/s00213-007-1052-0. [DOI] [PubMed] [Google Scholar]
- 81.Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization to 17β-estradiol. Endocrinology. 1984;114:1754–1760. doi: 10.1210/endo-114-5-1754. [DOI] [PubMed] [Google Scholar]
- 82.Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- 83.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 84.Sapolsky Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 85.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA. 2001;98:12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sar M, Stumpf WE. Autographic localization of radioactivity in the rat brain after the injection of 1,2-3H-testosterone. Endocrinology. 1973;92:251–256. doi: 10.1210/endo-92-1-251. [DOI] [PubMed] [Google Scholar]
- 87.Schmitz C, Rhodes ME, Bludau M, Ong P, Ueffing I, Veholff J, Korr, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Molecular Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- 88.Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;10:1780. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scott BM, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- 90.Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational Role for Testosterone and Estrogen on Adult Hypothalamic-Pituitary-Adrenal Axis Activity in the Male Rat. Endocrinology. 2005;146:1973–1982. doi: 10.1210/en.2004-1201. [DOI] [PubMed] [Google Scholar]
- 91.Seidman SN, Araujo AB, Roose SP, Devanand DP, Xie S, Cooper TB, McKinlay JB. Low testosterone levels in elderly men with dysthymic disorder. Am J Psychiatry. 2002;159:456–459. doi: 10.1176/appi.ajp.159.3.456. [DOI] [PubMed] [Google Scholar]
- 92.Seidman SN. The aging male: androgens, erectile dysfunction, and depression. J Clin Psychiatry. 2003;64(suppl 10):31–37. [PubMed] [Google Scholar]
- 93.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 94.Sheline YI, Wang PW, Gado MH, Csernasky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shirayama Y, Chen ACH, Nakagawa S, Russell D, Duman RS. Rain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stree on dendritic spine density in the male verse female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry. 2004;61:162–167. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- 98.Siuciak Y, Lewis DR, Wiegand SJ, Lindsay R. Antidepressant-like effect of brain derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 99.Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 101.Stewart J, Skvarenina A, Pottier J. Effects of neonatal androgens on open-field behavior and maze learning in the prepubescent and adult rat. Physiology and Behavior. 1975;14:291–295. doi: 10.1016/0031-9384(75)90036-0. [DOI] [PubMed] [Google Scholar]
- 102.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry. 2004;56:640. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stromstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res Mol Brain Res. 1995;34:75–88. doi: 10.1016/0169-328x(95)00140-n. [DOI] [PubMed] [Google Scholar]
- 104.Tonelli LH, Holmes A, Postolache TT. Intranasal Immune Challenge Induces Sex-Dependent Depressive-Like Behavior and Cytokine Expression in the Brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female and neonatal offspring. Endocrinology. 1980;106:306–313. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 106.Wood GK, Lipska BK, Weinberger DR. Behavioral changes in rats with early ventral hippocampal damage varies with age at damage. Brain Res Dev Brain Res. 1997;101:17–25. doi: 10.1016/s0165-3806(97)00050-3. [DOI] [PubMed] [Google Scholar]
- 107.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuations in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal synapse density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 110.Yang C, Wanga G, Wanga H, Liua ZB, Wang X. Cytoskeleton alterations in rat hippocampus following chronic unpredictable mild stress and re-exposure to acute and chronic unpredictable mild stress. Behavioural Brain Research. 2009;205:518–524. doi: 10.1016/j.bbr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu Z, Li X, Chen W, Zhao Y, Li H, Qing C, Jia N, Bai Z, Liu J. Prenatal stress causes gender-dependent neuronal loss and oxidative stress in rat hippocampus. J Neurosci Res. 2004;78:837–844. doi: 10.1002/jnr.20338. [DOI] [PubMed] [Google Scholar]
- 113.Zhu M, Ling Y, Handratta LX, Brodie AM. Novel P450(17alpha) inhibitors: 17-(2"-oxazolyl)- and 17-(2'-thiazolyl)-androgen derivatives. Steroids. 2003;68:603–611. doi: 10.1016/s0039-128x(03)00082-5. [DOI] [PubMed] [Google Scholar]