Abstract
Background
The malaria parasites Plasmodium falciparum and Plasmodium vivax generate significant concentrations of free unbound ferrous iron heme as a side product of hemoglobin degradation. The presence of these chemically reactive forms of iron, rare in healthy cells, presents an opportunity for parasite-selective drug delivery. Accordingly, our group is developing technologies for the targeted delivery of therapeutics to the intra-erythrocytic malaria parasite. These so-called ‘fragmenting hybrids’ employ a 1,2,4-trioxolane ring system as an iron(II)-sensing ‘trigger’ moiety and a ‘traceless’ retro-Michael linker to which a variety of partner drug species may be attached. After ferrous iron-promoted activation in the parasite, the partner drug is released via a β-elimination reaction.
Methods
In this report, we describe three orthogonal experimental approaches that were explored in order to generate in vitro proof-of-concept for ferrous iron-dependent drug delivery from a prototypical fragmenting hybrid.
Conclusion
Studies of two fragmenting hybrids by orthogonal approaches confirm that a partner drug species can be delivered to live P. falciparum parasites. A key advantage of this approach is the potential to mask a partner drug’s intrinsic bioactivity prior to release in the parasite.
Malaria caused by the parasites Plasmodium falciparum and Plasmodium vivax remains one of the major infectious disease problems in the world. Due to resistance to older agents, treatment of malaria is now highly dependent on analogs of the sesquiterpene lactone artemisinin, which are the key components of current multi-drug regimens – the so-called artemisinin-based combination therapies (ACTs). The life cycle of the malaria parasite is complex. Only the erythrocytic stage of infection causes illness and this stage is the primary target of most antimalarials, including the artemisinins.
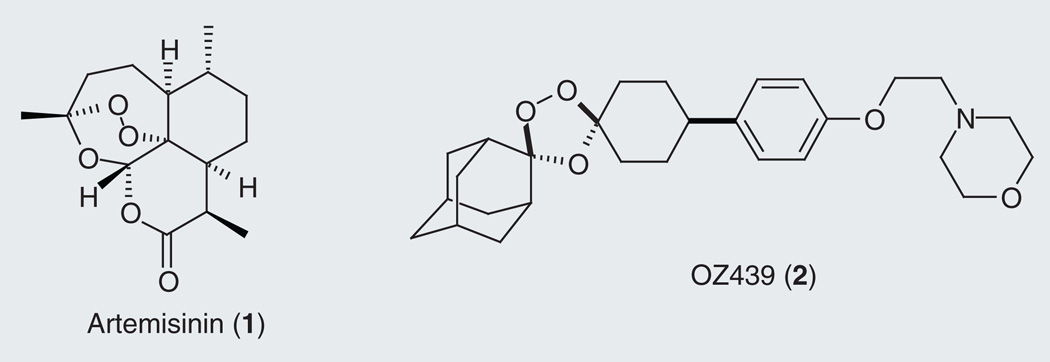
In a process that is now well characterized, erythrocytic malaria parasites take up large quantities of erythrocyte hemoglobin and transport this material to a large acidic organelle, the digestive vacuole (DV), where hemoglobin is processed to individual amino acids that are then utilized by the parasite [1]. As parasites hydrolyze hemoglobin and utilize the resultant amino acids, the unbound, redox-active heme byproduct places the parasite under severe oxidative stress. Free heme is therefore converted into an insoluble biocrystalline form called hemozoin in a process that is thought to be disrupted by aminoquinoline antimalarials [2]. The presence of unbound ferrous iron (heme) in the food vacuole is a unique and exploitable feature of the parasite, as the concentration of such species in human plasma is vanishingly small (~10−16 M) [3]. Artemisinin (1; Figure 1) and newer peroxide-containing antimalarials such as the trioxolanes (2, OZ439 [4]) are widely thought to be activated by heme-mediated peroxide bond cleavage (Fenton-type reaction) in the parasite DV, generating oxygen and carbon-centered radical species [5] that confer parasite toxicity, possibly via the formation of covalent heme adducts [6–8].
Figure 1. Artemisinin (1) and the new investigational trioxolane antimalarial agent OZ439 (2).
The hindered peroxide function in these molecules is implicated in their antimalarial activity.
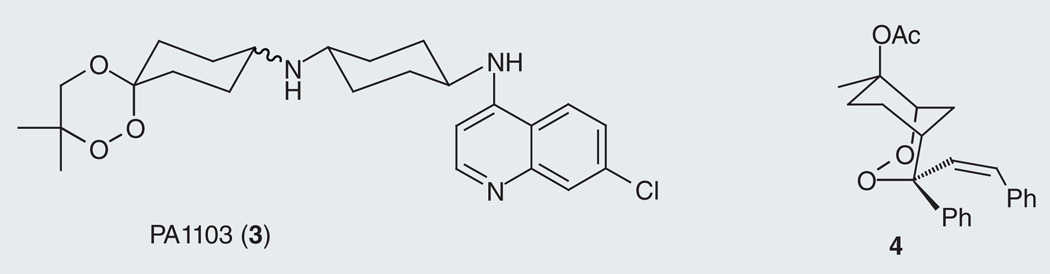
Current ACT regimens combine a rapid-acting artemisinin activity with a longer-lasting partner drug. The use of drug combinations is important, both as a hedge against the generation of resistant parasites, and to address the short-lasting activity of artemisinins, which allows frequent recrudescence following mono-therapy. Given the current emphasis on combination therapy, it is unsurprising that researchers have explored ‘hybrid’ drug species intended to confer both artemisinin- and quinoline-like activities in a single chemical entity. Most notable among these efforts are the so-called trioxaquines (3; Figure 2), examples of which have progressed into clinical development [9]. A number of groups [10–15] have been engaged in this area, employing a variety of peroxidic chemotypes and quinoline activities, as reviewed recently by Posner [16].
Figure 2. Trioxaquine PA1103 (3) and an endoperoxide (4).
Both molecules are intended to confer multiple antimalarial activities.
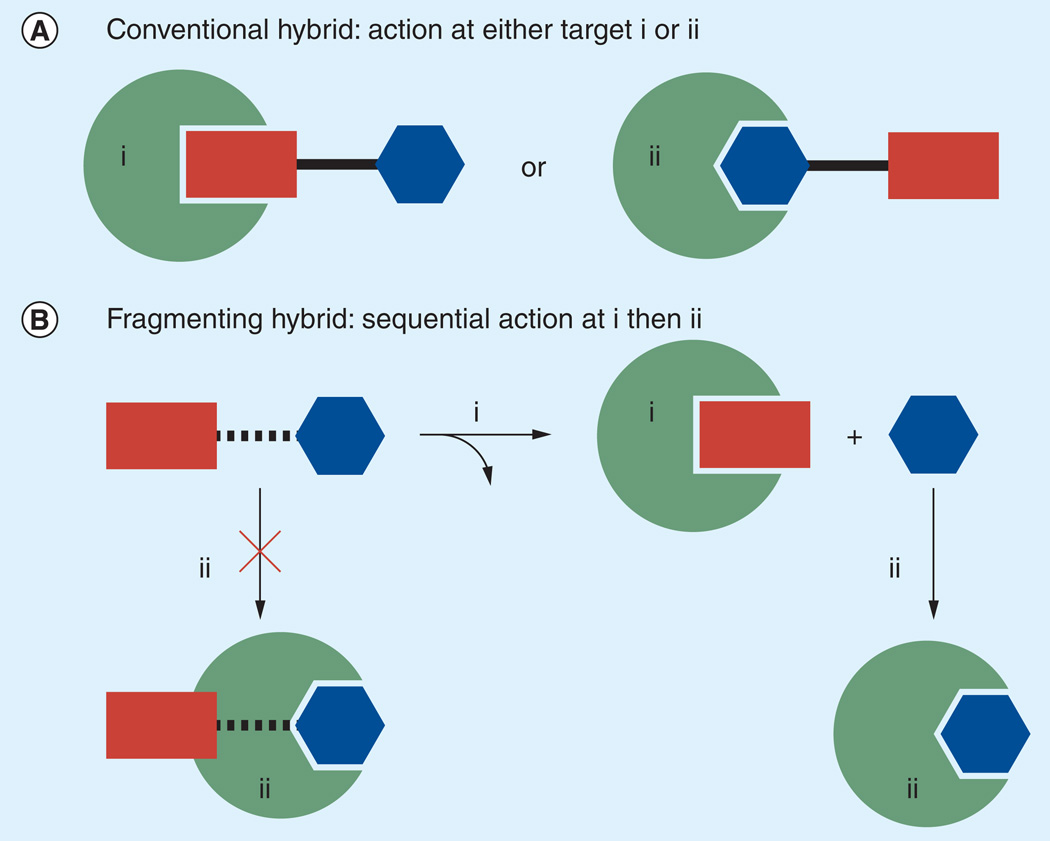
The trioxaquines and related drug conjugates are representative of conventional hybrid drugs, in which the activity of both components must be retained in the hybrid form if dual-pharmacology is to be realized. A consequence of this arrangement is that an individual hybrid molecule can only interact with one of its intended targets at any given time (Figure 3). Nature has not been so kind as to provide biological targets in tethered forms, ready to be simultaneously inhibited by hybrid drugs. These conventional hybrids can be distinguished from what we have termed ‘fragmenting hybrids’ [17], in which activity at one target leads to release of the second agent, which is then free to act independently at its intended target (Figure 3). Such systems might also be viewed as prodrugs [18], with the additional benefit that the ‘pro’ moiety confers a biological activity of its own. The term ‘fragmenting hybrid’ is intended to capture both the dual-action and parasite-targeting aspect of such molecules and this term will be employed in the discussion that follows.
Figure 3. Hybrid drugs are intended to act at multiple targets, in this case represented schematically by ‘i’ and ‘ii’.
(A) At the single-molecule level, a conventional hybrid cannot act simultaneously at both targets. (B) In a fragmenting hybrid, action at one target (e.g., ii) is made contingent on prior action at the other (e.g., i).
In addition to realizing action at both targets, fragmenting hybrids possess other potential advantages when compared with conventional hybrids or multidrug combination therapy. Significantly, the activity of the partner drug while tethered is unimportant, and thus there is no need to preserve its activity while in the conjugated form. Indeed, with a fragmenting hybrid it becomes advantageous to conjugate the partner drug so as to ablate its intrinsic activity, for example, by conjugation at a chemical function that is essential for activity. The ability to mask the intrinsic activity/toxicity of a partner drug, and to then release it only at its intended site of action, has clear implications for drug efficacy, safety and the emergence of drug resistance. In principle, the fragmenting hybrid approach might allow the safe delivery of agents that would be unsuitable for use in their parent form due to pharmacokinetic or toxicological liabilities.
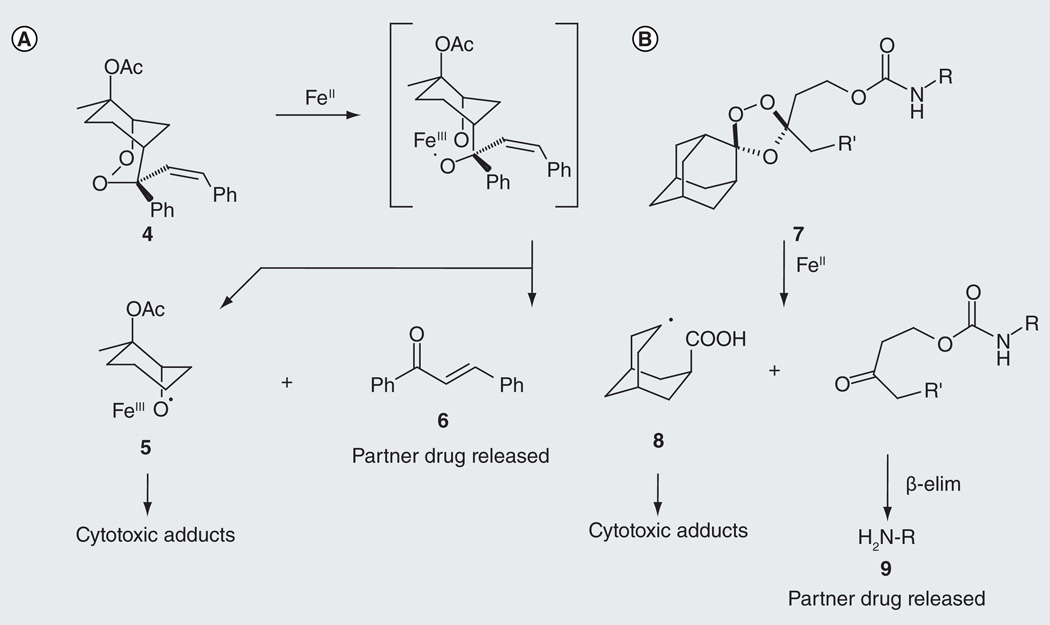
In the context of the malaria parasite, the production of redox-active ferrous iron heme provides the clearest opportunity to realize parasite-selective drug delivery via a fragmenting hybrid. The first fully formulated example of a fragmenting hybrid can be found in the work of O’Neill and Stocks with the endoperoxide prodrug 4 [18] and related 1,2,4-trioxane-based systems [19] in which a chalcone pharmacophore is embedded within a peroxidic ring system. Thus, 4 possesses both a peroxidic activity and a latent chalcone-derived activity, which should be unleashed only following reaction of the peroxide moiety and generation of the active chalcone (Figure 4A). Reaction of 4 in vitro with iron(II) salts indeed produced the chalcone 6 in reasonable chemical yields (45%) [18]. This species was also detected in the ethyl acetate extract of isolated DVs from parasites treated with 50 µM of 4, a concentration that is much higher than that required for antimalarial activity (EC50 = 34 nM for 4 vs the K1 strain of P. falciparum). Although the relative contributions of peroxide- and chalcone-based activities in 4 were not addressed, the work is important as it provides the first cogent description of the fragmenting hybrid concept in antimalarial therapy.
Figure 4. Two examples of fragmenting hybrids.
(A) Early example of a fragmenting hybrid [18]. Iron(II)-promoted fragmentation of endoperoxide 4 produces a cytotoxic carbon-centered radical species (5) while simultaneously leading to hybrid fragmentation and release of a chalcone partner drug (6). (B) Fragmenting hybrid systems 7 described herein [17]. Iron(II)-promoted fragmentation of trioxolane 7 produces a carbon-centered radical (8) while simultaneously unveiling a ketone that undergoes β-elimination to release a partner drug (9).
Challenges
At least two major challenges are presented when considering the implementation of hybrid drug approaches for malaria. The first concerns synthetic and structural complexity, both of which are likely to increase as one joins together multiple drug species into single entities. For example, the stereochemical complexity of early trioxaquine analogs hindered their further development, although later efforts to identify simplified, achiral analogs produced the clinical candidate PA1103 (3) [9,16]. A second and perhaps more significant challenge is the unambiguous demonstration of dual modes of antimalarial action for a hybrid. While multiple activities can be readily demonstrated in vitro, the same feat becomes much more challenging in whole parasites. Very often, investigations of hybrid drug action have involved comparisons of antimalarial potency for the hybrid species versus the individual components. The problem with this approach is that the bioactivity of a molecule is rarely the sum of its parts; very small structural changes can have dramatic effects on potency. Thus, a peroxide-containing hybrid drug may be more potent than its component parts simply because the peroxide-based effect is especially pronounced in the hybrid form. Potency-based arguments can be more compelling if drug resistant strains are employed to tease out different mechanisms, but in the case of peroxidic compounds, such resistant strains are not generally available. Thus, many claims in the literature of dual action for hybrid drugs are less than convincing.
The case of fragmenting hybrids is different because the intrinsic activity of the partner drug species can by blocked while in the hybrid from (Figure 3). Thus, detection of effects resulting from a partner drug can be taken as evidence for release of the free (active) inhibitor from the (inactive) fragmenting hybrid form. Thus, in validating our early fragmenting hybrids (7–9; Figure 4B) we sought to mask the intrinsic bioactivity (or fluorescence) of a partner drug species in the hybrid form, and then to detect its successful release in parasites using microscopy or activity-based probes.
Results & discussion
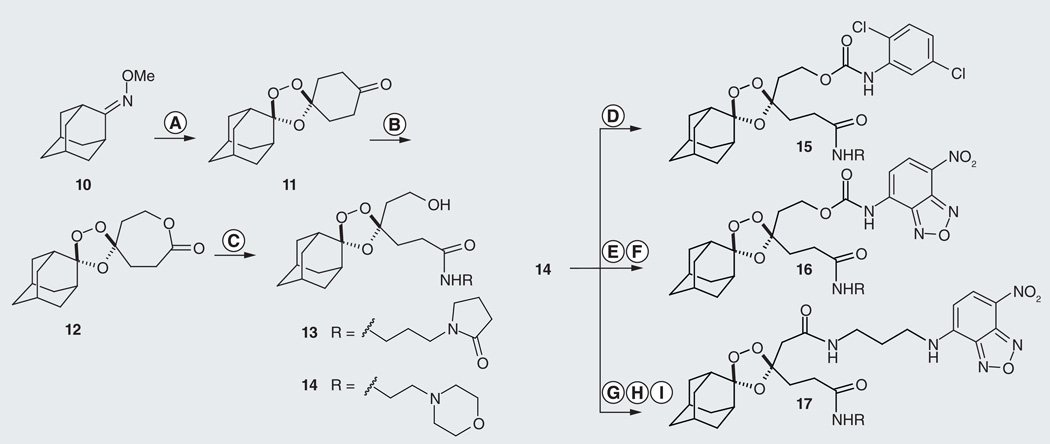
Our design of a prototypical fragmenting hybrid called for a simple structure that would be readily available via chemical synthesis. We chose to employ the 1,2,4-trioxolane ring system as the iron(II)-reactive species because of its synthetic accessibility and its successful implementation in the investigational antimalarial agents arterolane and OZ439 [4,20]. Rather than directly embedding the partner drug species in the peroxidic ring system as O’Neill and co-workers have done (i.e., as in 4), we chose instead to employ a ‘traceless’ retro-Michael linker, the carbonyl function of which would be revealed only upon iron(II)-mediated cleavage of the trioxolane ring (Figure 4B). The addition of this linker greatly broadens the scope of application to any partner drug species with a reactive amine or alcohol function. Many existing ACT partner drugs possess such functionality and therefore these existing drugs become potential candidates for delivery as fragmenting hybrids. In short order, we found that prototypical fragmenting hybrids incorporating the desired traceless linker could be prepared on gram-scale and in only four synthetic steps (10–14; Figure 5) [17,101].
Figure 5. Synthesis of prototypical fragmenting hybrid species 15–17.
Hybrid 15 bears an inactive surrogate for partner drug while analogs 16 and 17 are conditionally and constitutively fluorescent, respectively. Conditions: (A) O3, 1,4-cyclohexadione, pentane, CCl4, 0°C, 1 h, 30–40%; (B) mCPBA, NaHCO3, CH2Cl2, 48 h, 60%; (C) N-3-(aminopropyl)-2-pyrrolidinone (for 13), toluene, 50°C, 95%; or 4-(2-aminoethyl)-morpholine (for 14), toluene, 50°C, 70%; (D) Ar-NCO, toluene, 18 h, 87%; (E) 4-nitrophenylchloroformate, Et3N, DMAP, CH2Cl2, 10 h, 70%; (F) Ar-NH2, DMAP, CH2Cl2, 18 h, 47%; (G) Dess-Martin, CH2Cl2; (H) NaClO2, Na2HPO4, isobutylene, t-BuOH-water-THF, 0°C, 58% over two steps; (I) N-(7-nitro-[1,2,5]benzoxadiazol-4-yl)propane-1,3-diamine, EDC, HOBt, DIEA, DMF, 55%.
Our next objective was to evaluate the ability of these new species to undergo iron(II)-promoted trioxolane fragmentation and subsequent β-elimination. Gratifyingly, reaction of model systems such as 15 with ferrous iron salts in vitro led to rapid (minutes) and clean formation of the expected carbonyl (retro-Michael) species, which then underwent a much slower (hours) β-elimination reaction to release the tethered ‘drug’ species, as detected by LC-MS analysis. In model systems such as 15, the partner ‘drug’ (2,5-dichloroaniline) and enone side product of the traceless linker are without antimalarial activity [17]. Thus, the potent anti-malarial activity of 15(Table 1) can be attributed to a trioxolane-based effect, and this in turn indicates that such model systems can cross erythrocyte and parasite membranes to access the DV of the parasite.
Table 1.
Antimalarial and biochemical activities of compounds 13–22.
| Compound | W2 Plasmodium falciparum EC50 (µM) |
D10 P. falciparum EC50 (µM) |
Falcipain-2 IC50(µM) |
DPAP-1 IC50(µM) |
|---|---|---|---|---|
| 13 | 0.014 (0.002) | 0.029 (0.013)† | n.d. | >10† |
| 14 | 0.040 (0.001) | n.d. | n.d. | n.d. |
| 15 | 0.024 | n.d. | n.d. | n.d. |
| 16 | 0.049 (0.004) | n.d. | n.d. | n.d. |
| 17 | 0.063 (0.029) | n.d. | n.d. | n.d. |
| 18 | 0.180 (0.002) | n.d. | 1.02 (0.02) | n.d. |
| 19 | 0.60 (0.07) | n.d. | >50 | n.d. |
| 20 | n.d. | 0.0052 (0.0004)† | n.d. | 0.070 (0.013)† |
| 21 | n.d. | 0.0040 (0.0002)† | n.d. | 10† |
| 22 | n.d. | 0.052 (0.007)† | n.d. | >10† |
With in vitro ‘chemical’ proof-of-concept established, we faced the much more challenging problem of demonstrating fragmentation and drug release in live parasites. Various approaches were explored and are described here. The first approach involved synthesis of the conditionally fluorescent analog 16, which was expected to produce fluorescence only following trioxolane fragmentation and β-elimination of the fluorescent 4-nitrobenzo-2-oxa-1,3,-diazole (NBD) species. The dye NBD was selected for this work because it had been used previously in studies of artemisinin and other peroxides [21] and remains fluorescent over a wide pH range, including the acidic environment (pH ~5) of the P. falciparum DV. As a control, we also prepared analog 17, which is constitutively fluorescent.
Compounds 16 and 17 retained nanomolar antimalarial activity (Table 1), suggesting that both species are able to cross erythrocyte and parasite membranes to exert a peroxide-based effect. The presence of constitutively fluorescent compound 17 within intra-erythrocytic P. falciparum parasites was easily visualized by fluorescence microscopy (Figure 6). In contrast, the caged fluorophore 16 did not produce any detectable fluorescence in similar experiments. We attribute this result to the slow rate of β-elimination of the NBD dye (several hours) and a correspondingly low concentration produced over the course of the experiment. The problem of slow release would be exacerbated if the dye were able diffuse out of the parasite, a possibility that cannot be excluded. Therefore, while the antimalarial activity of 16 and 17 suggested successful entry and iron(II)-promoted activation within live parasites, a different approach was required to detect the release of a partner drug species in live parasites.
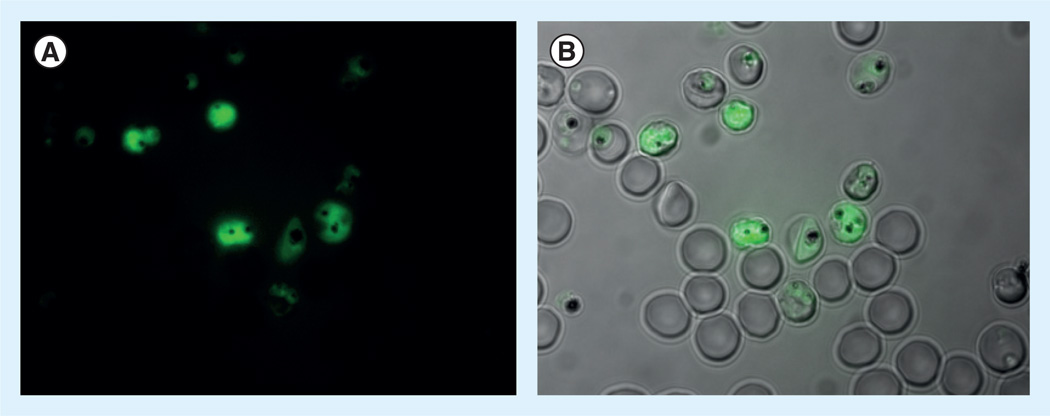
Figure 6. Fluorescence microscopy images of intra-ertyhrocytic 3D7 Plasmodium falciparum parasites treated with 10 µM of 17.
The compound appears to localize in parasites and is distributed in both the digestive vacuole and cytosol. (A) Fluorescence channel. (B) Overlay of bright field and fluorescence channels. Conditionally fluorescent probe 16 showed no detectable fluorescence under comparable conditions (not shown).
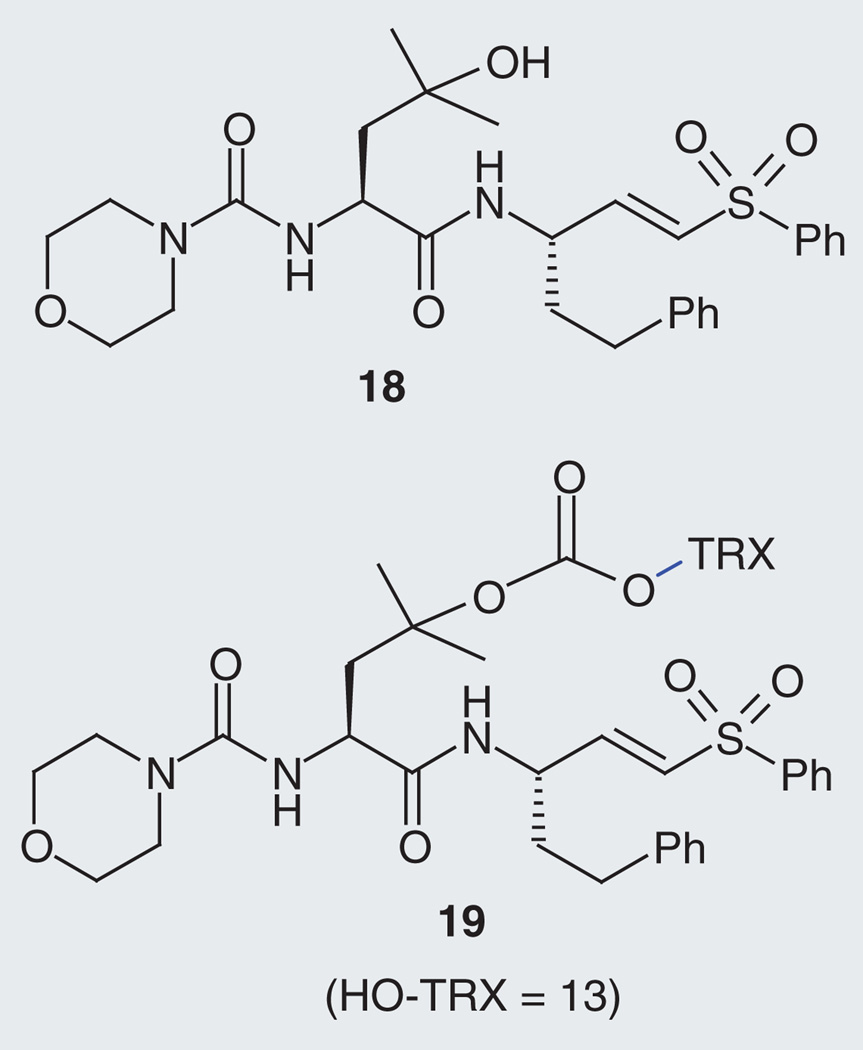
A second approach was based on the observation that P. falciparum parasites treated with cysteine protease inhibitors exhibit a characteristic swollen and dark-staining DV phenotype [22]. This phenotype results from inhibition of hemoglobin-digesting falcipain proteases and a consequent inability of parasites to degrade scavenged hemoglobin in the DV [22]. Importantly, this phenotype typically appears within hours and at concentrations near a compound’s EC50 value; it can be observed visually with a bright field microscope. Thus, release of a falcipain inhibitor from its fragmenting hybrid form could be detected by the appearance of this characteristic DV phenotype. However, for this approach to work the falcipain inhibitor would need to be inactived while in the intact hybrid form (i.e., prior to fragmentation). With these requirements in mind, we synthesized a vinyl-sulfone-based inhibitor (18) in which a hydroxy-leucine side chain at P2 would serve as the site for conjugation into the hybrid form (Figure 7 & Supplementary Data). Importantly, we found that the new inhibitor 18 retained a modest inhibitory effect against falcipain-2 (FP-2 IC50 = 1.02 µM) while its fragmenting hybrid form 19 had no detectable FP-2 inhibitory activity (IC50 >50 µM), as desired. Presumably the bulky trioxolane/linker moiety cannot be accommodated by the S2 pocket of falcipains, which prefer smaller amino acid side chains such as leucine at the P2 position [23].
Figure 7. Falcipain inhibitor 18 and its fragmenting hybrid form 19.
We next examined the effects on parasites of compound 18, its fragmenting hybrid form 19, the protease inhibitor control E64, and trioxolane control 13 (Figure 8). Trioxolane 13 is approximately ten fold more potent than falcipain inhibitor 18 against W2 parasites (EC50 = 0.014 and 0.18 µM, respectively). Fragmenting hybrid 19 retained an antimalarial effect (EC50 = 0.60 µM) that was approximately three fold weaker than that produced by 18 itself. Hybrid 19 is admittedly a very large and not particularly ‘drug-like’ molecule; its modest potency in cells may reflect reduced membrane permeability as compared with 13 or 18. Still 19 did prove very useful for proof-of-concept studies, since its modest antimalarial effect suggests the potential to release active 18 via the expected fragmentation mechanism (Figure 4B).
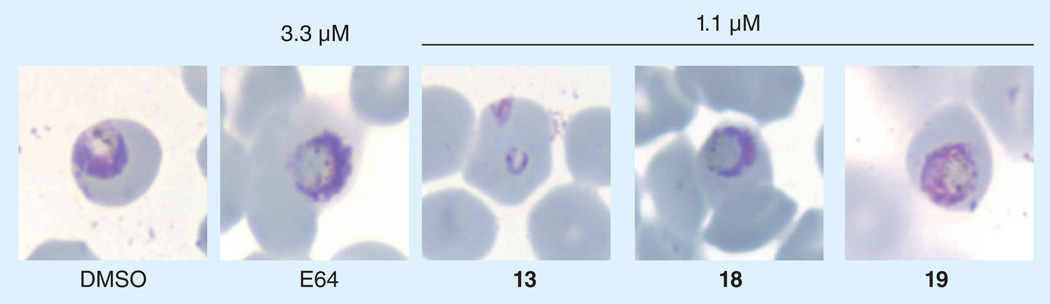
Figure 8. Light microscopy images of Giemsa-stained parasites after treatment with test compounds at the indicated concentrations.
Active protease inhibitors such as E64 and 18 show a characteristic DV phenotype. Fragmenting hybrid 19 also produces this phenotype, suggesting release of active 18.
To determine whether any 18 was in fact released from 19 in parasites, we next looked for the expected dark-staining and swollen DV phenotype, which is expected to be produced only if free 18 is released from 19 (recall that 19 itself does not inhibit FP-2). In these experiments, vehicle (DMSO) treated parasites exhibited a normal DV, whereas parasites treated with 3.3 µM of the cysteine protease inhibitor E64 showed the characteristic swollen and dark staining DV phenotype characteristic of falcipain inhibition (Figure 8). The trioxolane control 13 had no effect on DV morphology over the course of the experiment, as expected. Parasites treated with the falcipain inhibitor 18 at 1.1 µM (a concentration equivalent to its in vitro FP-2 IC50 value) produced the expected swollen DV phenotype. Significantly, the fragmenting hybrid 19 at 1.1 µM also produced a dark staining and swollen DV phenotype (Figure 8). Since hybrid 19 itself has no inhibitory effect on falcipain-2 at concentrations up to 50 µM (Table 1), the appearance of a swollen DV phenotype in 19 treated parasites strongly suggests that active 18 has been released within parasites, as desired. In a dose titration experiment, we found that the lowest concentration of 18 that reliably produced the swollen DV phenotype was approximately 250 nM. Thus, in the experiment employing 1.1 µM of 19, one concludes that at least 25% (i.e., ~250 nM) of active 18 was released from 19 during the course of the experiment. While qualitative in nature, these experiments provided an important, initial indication that a secondary drug payload could indeed be delivered in live parasites from a fragmenting hybrid.
A third approach to validate our fragmenting hybrids entailed the use of activity based probes (ABPs). The past decade has seen an explosion in the availability of ABPs for various classes of enzymes, and in particular, proteases [24,25]. The ability of ABPs to quantitatively measure specific enzymatic activities in cells suggested this as a powerful approach for the study of fragmenting hybrids in live parasites. Specifically, the inhibitory effects of a partner drug species in parasites (as detected with ABPs) would be taken as evidence of successful drug release, since partner drug has no intrinsic activity while in the intact hybrid form. These experiments, conducted in collaboration with the Bogyo laboratory (Stanford University, USA), were described in our recent communication [17], and are summarized below.
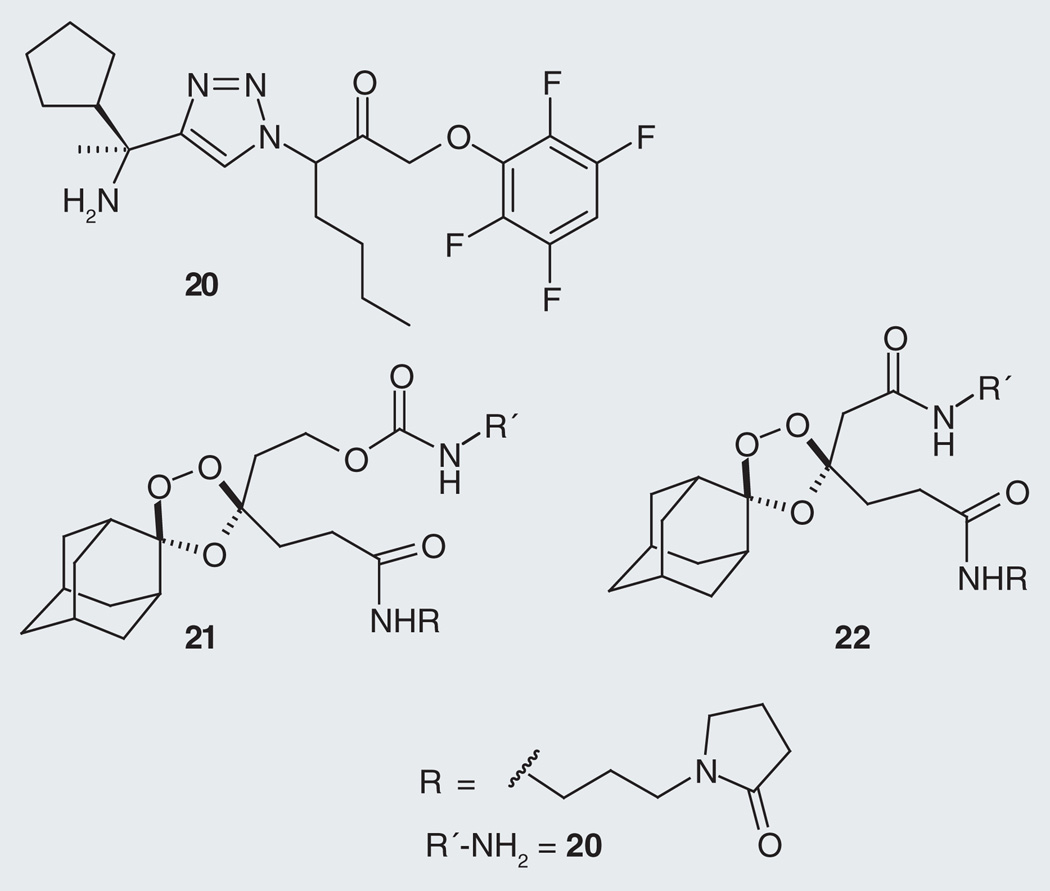
Much as in our work with 18 and its hybrid form 19, our studies employing ABPs involved a protease inhibitor and its inactivated fragmenting hybrid form. In these studies we employed compound 20, a recently described [26] inhibitor of DPAP1 and its fragmenting hybrid form (21; Figure 9). The additional control 22 was also employed in these studies as it confers a competent peroxide-based effect but cannot undergo β-elimination and thus cannot release free 20. We first showed that the DPAP1-inhibitory effects of 20 are reduced approximately 150-fold while in the fragmenting hybrid form 21 (Table 1) [17].
Figure 9. DPAP1 inhibitor 20 and its fragmenting hybrid form 21.
Compound 22 is a control that can exert a trioxolane-based effect but cannot release free 20 as β-elimination is precluded.
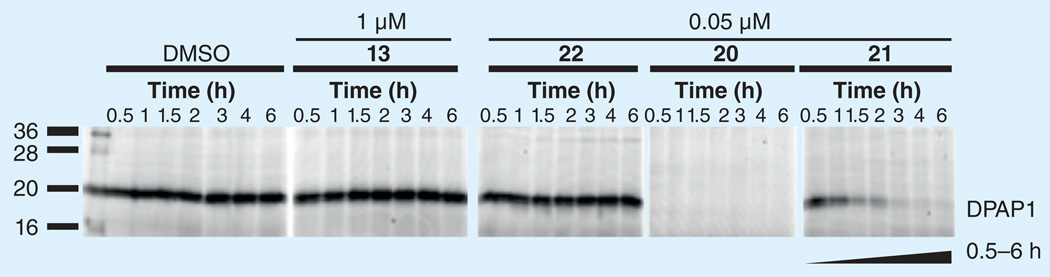
We next demonstrated the iron(II)-dependent release of 20 from 21 within live P. falciparum parasites by probing parasites for residual DPAP1 activity at various time points following treatment with 20 and 21, or the controls 13 or 22 (Figure 10). [17] These studies revealed that DPAP1 was fully inhibited by 50 nM 20 at all time points studied, while its fragmenting hybrid form 21 exhibited time-dependent inhibition of DPAP1, consistent with slow release of 20. Control compounds 13 and 22, while potent antimalarial compounds, did not measurably affect DPAP1 activity in parasites, suggesting they confer only peroxide-based effects, as expected. It was possible from these experiments to estimate a half-life of approximately 1.5 h for release of 20 from 21 in parasites, a therapeutically relevant time-scale given that complete ablation of DPAP1 activity was achieved with 50 nM of 21 [17]. Of course 21 was intended for proof-of-concept studies only and the future identification of true drug candidates will likely require some optimization of release kinetics. Such kinetic fine tuning should be achievable in light of recent work with related linkers, where the rates of β-elimination could be readily altered over several orders of magnitude [27].
Figure 10. An activity-based probe and SDS-PAGE were used to detect residual DPAP1 activity in Plasmodium falciparum parasites treated for 0.5–6 h with test compounds at the indicated concentrations.
DPAP1 is inhibited in a time dependent fashion by fragmenting hybrid 21, an observation that is consistent with the slow release of active DPAP1 inhibitor 20 from 21, as desired.
Conclusion
Three different experimental approaches were explored to validate a new method for targeted drug delivery to the malaria parasite. An approach based on the caged fluorophore 16 was unsuccessful, possibly due to insufficient rates of β-elimination and/or escape of the released dye NBD from the parasite. More successful were approaches based on the inactivation of protease inhibitors 18 or 20 while in the targeted forms 19 and 21. Thus, phenotypic studies involving 18 and 19 were in agreement with our studies of 20–22 employing activity-based probes, providing two orthogonal lines of evidence that partner drug species can be successfully delivered from fragmenting hybrids within live P. falciparum parasites. Moreover, the ability to mask a partner drug’s intrinsic bioactivity prior to its release in the parasite represents a key advantage of the delivery approach described herein.
Future perspective
The field of antimalarial drug discovery is no longer a neglected one, with a large number of academic and industrial groups engaged in work spanning new target identification through lead optimization and candidate selection. Drug-development organizations such as Medicines for Malaria Venture now have a robust pipeline of structurally diverse antimalarial agents in preclinical and clinical development. Despite this success, the long-term goal of developing a single-dose cure effective against all parasite life-cycle stages remains elusive. Whether such a drug (or drug combination) can be identified by the established drug discovery paradigm is an open question. We would argue that more innovative (if risky) approaches to antimalarial therapy should be pursued in parallel with standard approaches. Our group [17,101] and others [18,19,28] have advanced parasite-targeted drug delivery as a new approach to antimalarial therapy that merits further investigation.
Supplementary Material
Executive summary.
Background
-
▪
The production of free, reactive forms of ferrous iron in the malaria parasite can potentially be exploited for parasite-selective delivery of therapeutic agents.
-
▪
Fragmenting hybrids can be distinguished from conventional drug hybrids by the ability to mask a partner drug’s activity or toxicity prior to release within the parasite.
Challenges
-
▪
The evaluation of hybrid drug species requires careful consideration of experimental design, such that dual modes of action can be established unambiguously.
Results & discussion
-
▪
Subcellular phenotypic effects and the use of activity-based probes can be powerful approaches to evaluate the activity of hybrid drug species in parasites.
Acknowledgements
The authors acknowledge experimental assistance provided by V Newman and F Caro (DeRisi Laboratory, UCSF), and K Thorn (Nikon Imaging Center, UCSF).
AR Renslo acknowledges research support from the Bill and Melinda Gates Foundation, the Sandler Foundation and National Institutes of Health (AI094433). PJ Rosenthal is a Doris Duke Charitable Foundation Distinguished Clinical Scientist.
Key Terms
- Digestive vacuole
Organelle of the malaria parasite wherein hemoglobin is digested by various proteases, producing free amino acids and free, potentially reactive heme. The purported site of action of many peroxidic antimalarial drugs.
- Ferrous iron
Iron in the +2 oxidation state. In most organisms, iron is transported and stored in the less reactive ferric (+3) state. The reactivity of ferrous iron is exploited in biology but is highly regulated, as in the ferrous iron heme found in hemoglobin and cytochrome P450 enzymes.
- Prodrugs
Modified form of a drug species that is converted to the parent species in vivo. Can be employed to improve permeability, solubility, or as in the current work, to more selectively deliver a drug species to its intended site of action.
- Activity-based probes
Small-molecules designed to detect a specific enzymatic activity in cells. Typically these are suicide substrates that also possess a chemical handle for conjugation to fluorescent dyes or biotin.
- Traceless linker
Chemical linker that leaves no chemical trace on the tethered species after its release. This is an important consideration for drug-delivery approaches because any application to an approved drug would require that the drug be released in an unmodified state from the linker.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/full/10.4155/FMC.12.174
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Rosenthal PJ. Cysteine proteases of malaria parasites. Int. J. Parasitol. 2004;34(13–14):1489–1499. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Yayon A, Timberg R, Friedman S, Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J. Protozool. 1984;31(3):367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 3.Robert A, Benoit-Vical F, Meunier B. The key role of heme to trigger the antimalarial activity of trioxanes. Coord. Chem. Rev. 2005;249(17–18):1927–1936. [Google Scholar]
- 4. Charman SA, Arbe-Barnes S, Bathurst IC, et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl Acad. Sci. USA. 2011;108(11):4400–4405. doi: 10.1073/pnas.1015762108. ▪▪ Discloses the structure and improved properties of trioxolane drug candidate OZ439.
- 5. O’Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 2004;47(12):2945–2964. doi: 10.1021/jm030571c. ▪ Excellent review of mechanistic aspects of peroxide action.
- 6.Hartwig CL, Rosenthal AS, D’angelo J, Griffin CE, Posner GH, Cooper RA. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem. Pharmacol. 2009;77(3):322–336. doi: 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartwig CL, Lauterwasser EM, Mahajan SS, Hoke JM, Cooper RA, Renslo AR. Investigating the antimalarial action of 1,2,4-trioxolanes with fluorescent chemical probes. J. Med. Chem. 2011;54(23):8207–8213. doi: 10.1021/jm2012003. ▪ Mechanistic study demonstrating the peroxide-dependence of trioxolane action.
- 8.Creek DJ, Charman WN, Chiu FC, et al. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob. Agents. Chemother. 2008;52(4):1291–1296. doi: 10.1128/AAC.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 2008;41(1):69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 10.Robert A, Benoit-Vical F, Claparols C, Meunier B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl Acad. Sci. USA. 2005;102(38):13676–13680. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousejra-El Garah F, Claparols C, Benoit-Vical F, Meunier B, Robert A. The antimalarial trioxaquine DU1301 alkylates heme in malaria-infected mice. Antimicrob. Agents Chemother. 2008;52(8):2966–2969. doi: 10.1128/AAC.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradines V, Portela J, Boissier J, Cosledan F, Meunier B, Robert A. Trioxaquine PA1259 alkylates heme in the blood-feeding parasite Schistosoma mansoni. Antimicrob. Agents Chemother. 2011;55(5):2403–2405. doi: 10.1128/AAC.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadwick J, Amewu RK, Marti F, et al. Antimalarial mannoxanes: hybrid antimalarial drugs with outstanding oral activity profiles and a potential dual mechanism of action. ChemMedChem. 2011;6(8):1357–1361. doi: 10.1002/cmdc.201100196. [DOI] [PubMed] [Google Scholar]
- 14.Feng TS, Guantai EM, Nell M, et al. Effects of highly active novel artemisinin-chloroquinoline hybrid compounds on beta-hematin formation, parasite morphology and endocytosis in Plasmodium falciparum. Biochem. Pharmacol. 2011;82(3):236–247. doi: 10.1016/j.bcp.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Capela R, Cabal GG, Rosenthal PJ, et al. Design and evaluation of primaquineartemisinin hybrids as a multistage antimalarial strategy. Antimicrob. Agents Chemother. 2011;55(10):4698–4706. doi: 10.1128/AAC.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slack RD, Jacobine AM, Posner GH. Antimalarial peroxides: advances in drug discovery and design. MedChemComm. 2012;3(3):281–297. [Google Scholar]
- 17. Mahajan SS, Deu E, Lauterwasser EM, et al. A fragmenting hybrid approach for targeted delivery of multiple therapeutic agents to the malaria parasite. ChemMedChem. 2011;6(3):415–419. doi: 10.1002/cmdc.201100002. ▪ First description of trioxolane-based delivery systems incorporating traceless linkers.
- 18. O’Neill PM, Stocks PA, Pugh MD, et al. Design and synthesis of endoperoxide antimalarial prodrug models. Angew. Chem. Int. Ed. Engl. 2004;43(32):4193–4197. doi: 10.1002/anie.200453859. ▪▪ Describes the concept of peroxide-based drug delivery for malaria therapy.
- 19.O’Neill PM, Mukhtar A, Ward SA, et al. Application of thiol-olefin co-oxygenation methodology to a new synthesis of the 1,2,4-trioxane pharmacophore. Org. Lett. 2004;6(18):3035–3038. doi: 10.1021/ol0492142. [DOI] [PubMed] [Google Scholar]
- 20. Vennerstrom JL, Arbe-Barnes S, Brun R, et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430(7002):900–904. doi: 10.1038/nature02779. ▪▪ Describes the discovery of OZ277, the first trioxolane drug candidate.
- 21.Stocks PA, Bray PG, Barton VE, et al. evidence for a common non-heme chelatable-iron-dependent activation mechanism for semisynthetic and synthetic endoperoxide antimalarial drugs. Angew. Chem. Int. Ed. Engl. 2007;46(33):6278–6283. doi: 10.1002/anie.200604697. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal PJ, Mckerrow JH, Aikawa M, Nagasawa H, Leech JH. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J. Clin. Invest. 1988;82(5):1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenai BR, Lee BJ, Alvarez-Hernandez A, et al. Structure–activity relationships for inhibition of cysteine protease activity and development of Plasmodium falciparum by peptidyl vinyl sulfones. Antimicrob. Agents Chemother. 2003;47(1):154–160. doi: 10.1128/AAC.47.1.154-160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonovic M, Bogyo M. Activity-based probes as a tool for functional proteomic analysis of proteases. Expert Rev. Proteomics. 2008;5(5):721–730. doi: 10.1586/14789450.5.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DS, Weerapana E, Cravatt BF. Strategies for discovering and derisking covalent, irreversible enzyme inhibitors. Future Med. Chem. 2010;2(6):949–964. doi: 10.4155/fmc.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deu E, Leyva MJ, Albrow VE, Rice MJ, Ellman JA, Bogyo M. Functional studies of Plasmodium falciparum dipeptidyl aminopeptidase I using small molecule inhibitors and active site probes. Chem. Biol. 2010;17(8):808–819. doi: 10.1016/j.chembiol.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santi DV, Schneider EL, Reid R, Robinson L, Ashley GW. Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates. Proc. Natl Acad. Sci. USA. 2012;109:6211–6216. doi: 10.1073/pnas.1117147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbons P, Verissimo E, Araujo NC, et al. Endoperoxide carbonyl falcipain 2/3 inhibitor hybrids: toward combination chemotherapy of malaria through a single chemical entity. J. Med. Chem. 2010;53(22):8202–8206. doi: 10.1021/jm1009567. [DOI] [PubMed] [Google Scholar]
Patent
- 101.Renslo A, Mahajan S. US0951304. 2010
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.