Abstract
Purpose
Tumor derived cytokines play a significant role in the progression of head and neck squamous cell carcinoma (HNSCC). Targeting proteins, such as tristetraprolin (TTP), that regulate multiple inflammatory cytokines, may inhibit HNSCC progression; however, its role in cancer is poorly understood. The goal of this study was to determine if TTP regulates inflammatory cytokines in HNSCC.
Methods
TTP mRNA and protein expression were determined by quantitative RT-PCR and western blot, respectively. mRNA stability and cytokine secretion were evaluated by quantitative RT-PCR and ELISA, respectively, after overexpression or knockdown of TTP in HNSCC. HNSCC tissue microarrays were immunostained for IL-6 and TTP.
Results
TTP expression in HNSCC cell lines was inversely correlated with secretion of IL-6, VEGF and PGE2. Knockdown of TTP increased mRNA stability and secretion of cytokines. Conversely, overexpression of TTP in HNSCC cells led to decreased secretion of IL-6, VEGF and PGE2. Immunohistochemical staining of tissue microarrays for IL-6 demonstrated that staining intensity is prognostic for poor disease-specific survival (p=0.023), tumor recurrence and second primary tumors (p=0.014), and poor overall survival (p=0.019).
Conclusions
Our findings show that downregulation of TTP in HNSCC enhances mRNA stability and promotes secretion of IL-6, VEGF and PGE2. Furthermore, high IL-6 in HNSCC tissue is a biomarker for poor prognosis. In as much as enhanced cytokine secretion is associated with poor prognosis, TTP may be a therapeutic target to reduce multiple cytokines concurrently in HNSCC.
Keywords: cytokines, IL-6, VEGF
Introduction
Cytokines have a significant role in cancer, including roles in malignant transformation, tumor growth, survival, invasion, angiogenesis, and metastasis1–3. Several cytokines and pro-inflammatory factors, including interleukin-1 (IL-1), IL-4, IL-6, IL-8, IL-10, granulocyte-macrophage colony stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), and cycloxygenase-2 (COX-2), are upregulated in cancer1,4–6. Some of these molecules mediate the role of inflammation in cancer. By promoting cell proliferation, invasion, and survival, cytokines facilitate tumor progression in multiple cancers, including colorectal, breast, head and neck and non-small cell lung cancers3,7,8. Since cytokines have a critical role in tumor progression, expression is highly regulated at many levels: mRNA transcription, post-transcription, and initiation of protein translation. Post-transcriptional regulation of cytokines occurs at different stages such as nuclear export, cytoplasmic localization, stability/degradation, and translation of RNA into protein9. RNA binding proteins (RNA-BPs) are trans-acting factors that regulate these events by binding cis elements on target mRNA. Cis elements such as adenine and uridine (AU)-rich elements (AREs) are located in the 5’- and 3’-untranslated region (UTR) of mRNA transcripts. Since cytokine transcripts contain AREs in their 3’UTR, proteins that promote degradation or stability of transcripts by binding to this region have therapeutic potential.
Expression of RNA-BPs that regulate cytokine mRNA stability may be altered in cancer10,11. In breast cancer, overexpression of human related antigen R (HuR), an RNA-BP that stabilizes mRNA, contributes to poor prognosis and increased secretion of VEGF12. Conversely, in colon cancer, tristetraprolin, (TTP), which decreases mRNA stability, is downregulated, correlating with increased COX-2 expression13. These changes in RNA-BPs underscore their significance in cancer progression.
HNSCC is the sixth most common cancer, annually affecting approximately 500,000 individuals worldwide14. The 5-year survival rate is about 50%, primarily due to late detection. HNSCCs secrete multiple cytokines including VEGF, IL-6, and COX-215–18. Since COX-2 and IL-6 inhibitors decrease cell proliferation and invasion in HNSCC in vitro, targeted cancer therapy towards tumor secreted inflammatory cytokines may inhibit tumor progression3,17–19. Treating HNSCC with agents directed at a single cytokine is unrealistic due to secretion of multiple cytokines with overlapping functions20–22. Given the importance of cytokines such as IL-6, PGE2, TNFα, VEGF and EGF in HNSCC progression3,5,6, downregulation of multiple cytokines simultaneously by targeting a common upstream protein may inhibit HNSCC progression. However, the role of TTP in regulation of multiple cytokines has not been investigated in HNSCC. Furthermore, the relationship between TTP expression and cytokine secretion, in HNSCC progression has not been established. In this study, we investigated whether TTP regulates multiple inflammatory cytokines in HNSCC and the significance of overexpression of one of these cytokines on tumor progression.
Methods
Cell culture
Human HNSCC cell lines, UM-SCC-(1, -5, -11A, -14A, -17B -22B, -74A, -81B) and OSCC3 cells were cultured as described23, 24. UM-SCC-(1, -5, -11A, -14A, -17B -22B, -74A, -81B) were validated by genotyping. OSCC3 cells were obtained from Dr. Peter Polverini and were genotyped in the Sequencing Core at the University of Michigan. The HPV16 immortalized human oral keratinocyte cell line (HOK 16B, generous gift from Dr. No-Hee Park, University of California, Los Angeles) was maintained in low-Ca2+ keratinocyte growth medium (Cascade Biologics, Portland, OR)25. Primary human oral keratinocytes (NHK) were cultured in oral keratinocyte growth medium (Sciencell, Carlsbad, CA).
Western blot analysis
Whole-cell lysates were prepared by sonicating cell suspensions on ice, and western blot analysis was performed as described24. Membranes were blocked and incubated in the primary antibody overnight at 4°C or 1 hour at room temperature. Antibody concentrations were as follows: rabbit anti-TTP (Abcam) 1:2000, actin (Cell Signaling) 1:3000, horseradish peroxidase conjugated donkey anti-rabbit IgG and goat anti-mouse secondary antibodies (1:2000 to 1:5000; Jackson ImmunoResearch Laboratories). Immunoreactive proteins were visualized by SuperSignal West Pico Chemiluminescent system (Pierce) and exposed to X-ray film.
Real-time PCR
Total RNA was isolated from cells witha Qiazol (Invitrogen). cDNA was synthesized using SuperScript II (Invitrogen). Quantitative Real time PCR was performed with SYBR Green Master mix on an Applied Biosystems 7600HT Real Time PCR machine. Data were analyzed using the delta-delta Ct method with normalization to GAPDH and then expressed relative to NHK.
Adenoviral infection and IL-1β induction
A DL3 replication-deficient serotype-5 adenovirus containing CMV -β-gal and human TTP were generated through the Vector Core at the University of Michigan as described26. UM-SCC-11A and -17B and OSCC3 cells (35×104) were transduced with 1,000 MOI Ad5-β-gal or Ad5-TTP in serum-free media. Three hours after transduction, 10% FBS was added. Twenty-one hours later, HNSCC cell lines were serum-starved for 4 hours and incubated with 1 ng/ml IL-1β or PBS in serum-free media. Conditioned media was collected after 24 hours.
ELISA for IL-6, VEGF, and PGE2
Conditioned medium from NHK, HOK16B, OSCC3, and HNSCC cell lines (UM-SCC-1 -11A, -14A, -17B, -22B, -74A, and -81B) was processed as described27. The total cell number was measured with a trypan blue enumeration assay. IL-6 and VEGF secreted into the medium was measured by a non-competitive ELISA whereas PGE2 was measured by competitive ELISA (R & D Systems).
Transient TTP knockdown
UM-SCC-1, -22B and -81B were transfected with on target plus TTP smart pool (Dharmacon) siRNA. UM-SCC-1 cells were transfected with Nucleofectin (Lonza) as described23,28. UM-SCC-22B and -81B cells (25×104 in 6 well dishes) were transfected with 1 µg of siRNA using Lipofectomine RNAimax (Invitrogen), as described29. Four hours after transfection, 10% FBS was added. Conditioned media and whole-cell lysates were collected as described above.
Stable TTP knockdown and mRNA decay
For stable knockdown of TTP, UM-SCC-1 and -22B were infected with short hairpin RNA (shTTP and shVSVG control) in lentiviral particles in a VSVG backbone (University of Michigan Vector Core). Stable cell lines were selected in 10µg/ml puromycin (Sigma). For mRNA decay experiments, 35×104 cells were plated in 60mm dishes. The next day, the cells were serum starved for four hours and treated with 10 nM actinomycin D for 0, 1, 2 and 3 hours (Sigma). After incubation, cells were washed with PBS and RNA was harvested.
Invasion assay
In vitro cell invasion was determined according to the manufacturer’s instructions (BD Biosciences), as described previously23,28. Cells were washed and plated on Matrigel coated inserts at 2.5–3.5×104 cells in DMEM medium in triplicate. For control experiments, cells were plated in DMEM medium on identical inserts that were not coated with Matrigel. The lower chamber contained DMEM medium with 10% FBS as a chemoattractant. After 24h incubation, cells that had migrated to the lower surface of the membrane were fixed and stained with 10% methanol and crystal violet. To determine percent invasion, the average number of cells per field on the invasion inserts was divided by the average number of cells per field on the control inserts and multiplied by 100.
Migration assay
Cells were plated at 95% density in 24 well plates in triplicate. 24 hours after plating, cells were serum starved for 6 hours, then treated with 25 mmol of Mitomycin C (Sigma) for 2 hours in serum free conditions. Media was removed and a scratch was made with 200 µl pipette tip. Immediately following the scratch, complete media with 25 mmol of Mitomycin C was added and three fields in each replicate were photographed at 0h and at 24h. The area between the migration fronts was determined using ImagePro Plus 5.1 (Media Cybernetics) and the percent migration at 24h was calculated.
University of Michigan Oral Cavity/Oropharyngeal Cancer Organ Preservation Trial
This randomized clinical trial of stage III/IV HNSCC compared patients treated concurrently with chemotherapy and radiation versus surgery and radiation, after induction chemotherapy30. A tissue microarray (TMA) constructed from pre-treatment specimens from this clinical trial was used for immunhistochemical studies after Institutional Review Board approval.
Immunohistochemistry
Immunodetection in tissue sections was performed as described24 with affinity purified anti-IL-6 (R&D; 25 µg/ml) or affinity purified TTP antibody (Abcam; 3 µg/ml) or the corresponding IgG controls at the same concentrations.
Data analysis
Statistical analysis of in vitro assays was performed using a Student’s t-test or one-way ANOVA. A p-value of < 0.05 was considered to be statistically significant. For analysis of TMA data, interpretation and scoring were performed by a board certified pathologist, as described23,28. The covariates of interest were T stage and N stage, which were analyzed as ordinal data. The outcomes of interest were overall survival, disease-specific survival, time to indication of surgery at primary site, and time to recurrence or second primary tumors. The Spearman correlation coefficient was used to evaluate univariate associations between markers and numerical and ordinal variables of interest. The Cox proportional hazards model was used to relate time-to-event outcomes to marker levels and other numerical and ordinal covariates. Statistical analyses of TMA data were done using Statistical Analysis System version 9.0 (SAS). A two-tailed p-value of 0.05 was considered to be statistically significant.
Results
TTP is downregulated in HNSCC
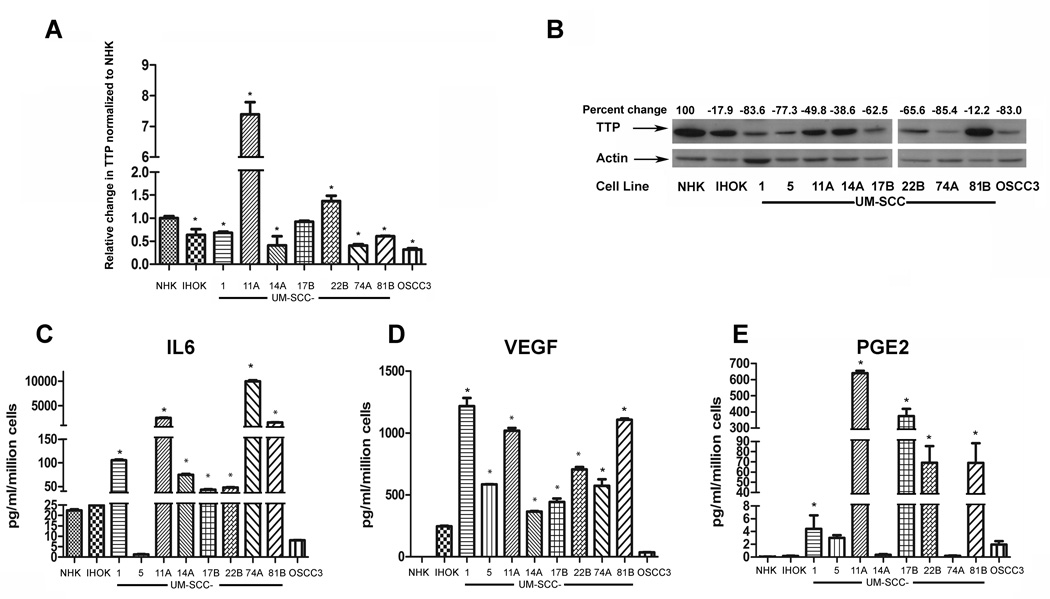
TTP expression was evaluated by Q-RT-PCR and normalized to GAPDH. The TTP transcript was lower in 6 HNSCC cell lines compared to NHK (Fig. 1A) (p<0.05, Students t-test). UM-SCC-11A and UM-SCC-22B had significantly more TTP mRNA than NHK (p<0.05, Students t-test). Whole-cell lysates from 9 HNSCC cell lines, and from 2 non-malignant cell lines, normal human keratinocytes (NHK) and immortalized keratinocytes (HOK16B, designated IHOK) were immunoblotted with anti-TTP antibody. As shown in Fig. 1B (immunoblot and densitometric analysis), TTP expression is higher in NHK and IHOK compared to HNSCC cell lines when normalized to actin as a loading control. OSCC3 expressed the least amount of TTP compared to NHK (83% reduction) while UM-SCC-81B showed the least reduction in TTP expression compared to NHK. There was an overall decrease in TTP expression by 62% (SD +/− 24.6) in HNSCC cell lines compared to NHK.
Figure 1. TTP is downregulated in HNSCC cell lines.
A) Total RNA was isolated from normal human keratinocytes (NHK), HOK16B immortalized keratinocytes (IHOK) and HNSCC cell lines. cDNAs were prepared and Q-RT-PCR was performed using SyBr-Green. B) Whole cell lysates from NHK, IHOK and HNSCC cell lines were electrophoresed and blotted with TTP antibody. Actin was used as a loading control. TTP expression was quantified by densitometry, normalized to actin and then to NHK and expressed as percent change. C, D and E) Conditioned media from NHK, IHOK and HNSCC cell lines, was collected at 24 hours. (*p<0.001 compared to NHK). ELISA experiments were performed in triplicate with similar results.
IL-6, VEGF, and PGE2 were quantified by ELISA in conditioned media from normal and IHOK and HNSCC cells, to correlate TTP expression with cytokine secretion. In 7 of the 11 HNSCC cell lines, IL-6 secretion is significantly upregulated (*p<0.001, one-way ANOVA for all cell lines noted) in HNSCC cell lines compared to NHK. VEGF and PGE2, the other two cytokines that were evaluated, showed a similar trend. The increase of VEGF secretion was significant in 8 out of 9 HNSCC cell lines cell lines (*p<0.001, one-way ANOVA). The increase of PGE2 secretion was significant in 5 out of 9 HNSCC cell lines cell lines (p<0.001, one-way ANOVA). Of the cell lines evaluated, NHK secreted the least VEGF and PGE2 (Fig. 1D and Fig. 1E, respectively). IHOK showed increased VEGF and PGE2 secretion compared to normal cells, but less than that observed in HNSCC cell lines. UM-SCC-81B, secreted high levels of all three cytokines evaluated, despite high TTP expression in these cell lines (Fig. 1B). However, in most cell lines (>65%) TTP expression was inversely correlated with cytokine secretion.
Overexpression of TTP reduces cytokine secretion in HNSCC
Three HNSCC cell lines with about 50% reduced endogenous TTP expression compared to NHK (Fig. 1B) were used for overexpression studies. To verify TTP overexpression, whole-cell lysates were generated from HNSCC cell lines transduced with serotype-5 adenovirus (Ad-5) that contained either TTP or β-gal (control) and were immunoblotted for TTP. As shown in Fig. 2A, TTP was overexpressed in UM-SCC-11A, UM-SCC-17B, and OSCC3 transduced with Ad5-TTP compared to cells transduced with control vector.
Figure 2. Overexpression of TTP in HNSCC cell lines decreases cytokine secretion.
UM-SCC-11A, UM-SCC-17B and OSCC3 cells (A, B and C respectively) were transduced with adenovirus-5 containing β-gal (control) or TTP. Cell lysates and conditioned media were harvested. Cell lysates were electrophoresed and immunoblotted with TTP antibody. Conditioned media was used to quantify IL-6, VEGF, and PGE2 secretion by ELISA. All experiments were performed in triplicate (p<0.001, Standard error bars are present in all graphs).
IL-1β is secreted by inflammatory cells adjacent to a tumor and activates the p38 MAPK pathway within tumor cells31. MK2, the downstream target of p38 MAPK, inactivates TTP by phosphorylation32,33. Therefore, we investigated the effect of TTP on cytokine secretion in the presence and absence of IL-1β. In mock-transduced cells, IL-1β increased cytokine secretion in all three cell lines, consistent with inactivation of residual TTP. However, overexpression of TTP led to a significant reduction of IL-6, VEGF, and PGE2 secretion in all three cell lines (Fig. 2B, 2C, and 2D) (*p<0.001) when compared to cells transfected with control vector, even in the presence of IL-1β.
In a complementary approach, TTP expression was downregulated by RNAi in three HNSCC cell lines representing a spectrum of TTP expression. Knockdown of TTP, verified by immunoblot analysis, led to increased secretion of IL-6, VEGF, and PGE2 (Fig. 3). In UM-SCC-1, siTTP mediated a significant increase in all three cytokines evaluated (Fig. 3A; p<0.05). IL-6 secretion was increased more than PGE2 secretion in UM-SCC-22B (p<0.05). VEGF secretion showed a similar trend (Fig. 3B). In UM-SCC-81B cells, which strongly express TTP (Fig. 1A), siTTP also induced a significant increase in secretion of IL-6, VEGF, and PGE2. (Fig. 3C) (p<0.05). Collectively, overexpression and knockdown data suggest that TTP inversely regulates IL-6, VEGF, and PGE2 secretion in HNSCC.
Figure 3. TTP knockdown induces cytokine secretion in HNSCC cells.
UM-SCC-1 (A), UM-SCC-22B (B) and UM-SCC-81B (C) were transfected with siRNA against TTP or non-target siRNA (nt). Whole cell lysates were electrophoresed and blotted with TTP antibody. Actin was used as loading control. Conditioned media was assayed in triplicate for IL-6, VEGF, and PGE2 by ELISA. All experiments were performed in triplicate. Data are representative of the mean and standard deviation of three replicates within an experiment. (*p<0.05).
TTP regulates cytokine mRNA stability in HNSCC
In order to determine if TTP targets cytokine mRNA for degradation in HNSCC, cells were treated with actinomycin D, which inhibits DNA polymerase. Stable cell lines with shTTP or shVSVG in UM-SCC-1 and UM-SCC-22B were generated. Knockdown of TTP, confirmed by immunoblot analysis (Fig 4A), increased cytokine mRNA stability. For UM-SCC-1, mRNA transcripts of IL-6 and COX-2 in shTTP transduced cells remained between 90–100% stable over a 3 hour period. In contrast, in cells transduced with shVSVG, mRNA transcripts degraded to nearly 50% at 3 hours (Fig. 4B) (p<0.05). Similarly, in UM-SCC-22B, IL-6 and COX-2 mRNAs were more stable in cells transduced with shTTP (~100% after three hours) than control (shVSVG) cells, in which IL-6 and COX-2 mRNA were degraded in 3 hours (Fig. 4C) (p <0.05).
Figure 4. TTP knockdown increases cytokine mRNA half-life.
A) Whole cell lysates from UM-SCC-1 and -22B stably expressing shSVSG or shTTP were immunoblotted. B) and C) RNA was purified, cDNAs were prepared and Q-RT-PCR was performed using SyBr-Green. Ct values for IL-6 and COX-2 were normalized to GAPDH and expressed as a percentage of time zero. Data are representative of the mean and standard deviation of three individual experiments (*p<0.05, **p<0.001).
Loss of TTP enhances invasion and migration
In HNSCC, invasion and migration facilitate tumor progression. In order to determine the functional significance of loss of TTP, invasion and migration assays were performed with UM-SCC-1 after shRNA-mediated knockdown of TTP (see Fig. 4A). Loss of TTP in UM-SCC-1 increased invasion nearly 300% compared to control cells transduced with empty vector (Fig. 5A). Migration assays show that suppression of TTP increases cell migration. In UM-SCC-1, the percent area migrated, normalized to time zero, was 75% in shTTP cells whereas control cells migrated 15% (Fig. 5B). Similar results for invasion and migration were observed in UM-SCC-22B (not shown).
Figure 5. TTP knockdown increases HNSCC invasion and migration.
UM-SCC-1 cells were stably transduced with shTTP or shVSVG. A) Invasion. Transduced cells were plated in serum free media on Matrigel coated inserts or control inserts. Percent invasion was calculated as described in Methods. B) Migration. UM-SCC-1 cells were treated with 25 mmol of Mitomycin C (Sigma). Media was removed and a scratch was made. Immediately following the scratch, complete media DMEM with 25 mmol of Mitomycin C was added and the cells were photographed at 0h and at 24h. Data are representative of two independent experiments with three replicates within an experiment (*p<0.02).
Increased IL-6 secretion is correlated with human HNSCC progression
Immunohistochemical studies were performed on normal epithelial and HNSCC tumor tissue and a TMA of pre-treatment tissue specimens from HNSCC. Normal epithelial tissue shows strong TTP staining in the basal third of the epithelium (Fig. 6A, left panel, arrows) whereas the invasive epithelial islands in the HNSCC tissue do not show strong TTP staining (Fig. 6A, right panel, inset). IgG controls were negative (not shown). Fig. 6B (upper and lower panels) shows high IL-6 and low TTP staining in the same HNSCC core. IgG controls were appropriately negative (not shown). Low TTP and high IL-6 intensity are correlated with poor disease-specific survival (DSS), (p=0.043) (Fig. 6B). Additionally, tissue microarray findings revealed that high IL-6 intensity is prognostic for bad patient outcomes, specifically, poor DSS (p=0.023) (Fig. 6C), tumor recurrence or second primary tumor (p=0.014) (Fig. 6D), and poor overall survival (p=0.019, not shown). Thus, these findings based on a small sample size, show that IL-6 and TTP expression are inversely correlated and IL-6 is associated with poor patient outcome.
Figure 6. IL-6 intensity is prognostic for disease specific survival and tumor recurrence in HNSCC.
A) Immunohistochemistry was performed on tissue sections of normal human epithelium (left panel, bar = 100 µm) and human HNSCC (right panel, bar = 1000 µm) with TTP antibody. Arrows show staining in the basal third of normal epithelium. The inset image is a higher magnification of the outlined box showing invasive epithelium (bar = 60 µm). Arrow shows invasive epithelium. B) Immunohistochemistry was performed on tissue sections of a human HNSCC TMA with the IL-6 and TTP antibodies. The slides were counterstained with hematoxylin. The top and lower panels represent high IL-6 and low TTP staining, respectively, in the same HNSCC tissue (bars = 100 µm). C) Low TTP and high IL-6 are correlated for poor disease specific survival. Patient groups: red line, low TTP, low IL-6 (n = 9); yellow line, low TTP, high IL-6, (n = 3); blue line, high TTP, low IL-6 (n = 11); green line, high TTP, high IL-6 (n = 10). Events (drop in the graph lines) were deaths due to HNSCC. Subjects who did not experience the events, such as deaths from unrelated causes, were censored. D and E) High IL-6 intensity is prognostic for poor disease specific survival (D) and for tumor recurrence and second primary tumor (E). Patient groups, red line, no IL-6 (n=6), yellow line, low IL-6 (n=19), blue line, medium IL-6 (n=14), green line, high IL-6 (n=3). IL-6 intensity is also prognostic for poor overall survival (not shown).
Discussion
Changes in oncogenic mRNA stability can alter the production of inflammatory cytokines. For example, upregulation of HuR, an RNA-BP that stabilizes mRNA, enhances COX-2 and VEGF production34,35. This increase in cytokine production is detrimental because tumor-derived cytokines facilitate oncogenic phenotypes such as invasion, proliferation, and survival, thereby promoting tumor growth1,3,7,13,36. Consistent with this notion, increased COX-2 production in colon cancer increases tumor invasion8. In the present study, we show that downregulation of TTP in HNSCC stabilizes transcripts and promotes secretion of multiple cytokines, including IL-6, VEGF and COX2 and increases invasion and migration. Furthermore, IL-6 and TTP expression are inversely correlated in human HNSCC tissue. High IL-6 expression is predictive of poor disease specific survival, tumor recurrence and second primary tumors. Based on a study in a small group of patients, HNSCC tumors with high IL-6 staining intensity have a much poorer prognosis than those patients with tumors expressing IL-6 at low levels.
HNSCC is a disease with a poor prognosis with 5-year survival rates of about 50%37. HNSCCs secrete multiple growth, inflammatory and angiogenic factors, including VEGF, IL-6, IL-8, and granulocyte macrophage colony stimulating factor, which promote tumor progression3,4,5. IL-6 promotes tumor growth in mice, by promoting survival and proliferation of HNSCC cells2. Other cytokines, such as VEGF, promote angiogenesis and metastasis37,38.
The critical role of cytokines in progression of many cancers makes them an attractive treatment target3,4,36,40. In a murine model, IL-6 monoclonal antibodies decreased the size of prostate cancer xenografts significantly39. However, targeting individual cytokines is unlikely to be of therapeutic benefit in humans. For example, results from a clinical trial in multiple myeloma with antibodies against IL-6, showed that there is no improvement in clinical outcome for these patients40. This suggests that cytokines secreted can compensate for one another and therefore limits the effect of an inhibitor specific for each cytokine. Targeting a common regulatory mechanism for multiple cytokines such as IL-6, VEGF, and PGE2 in HNSCC, may decrease tumor progression and improve response to treatment.
Cytokine mRNA expression is tightly regulated in resting cells through continuously active mRNA decay mechanisms. Induction of mRNA decay via inflammatory signals facilitates rapid changes in the cellular production of cytokines through alterations in binding of RNA-BP41. RNA-BPs regulate multiple cytokines by inducing decay or inhibiting translation. RNA-BPs may bind to multiple AREs, possibly contributing to competition for binding. At least 20 different proteins that can bind to ARE segments have been identified, including TTP, human antigen related protein (HuR), butyrate response factor-1 and 2 (BRF-1 and 2), ARE/poly(U)-binding/degradation factor (AUF-1) and translational silencer TIA-1 (TIA-1 and TIAR). However, only a subset of RNA-BPs has been shown to influence the stability or translational efficiency of target mRNAs.
One of the most well studied RNA-BPs is TTP, which targets mRNA for rapid degradation by binding to the AREs in the 3’UTR42. Studies with knockout mice suggest that TTP is crucial in regulating TNF-α and IL-643. Since TTP regulates mRNAs of multiple inflammatory cytokines by increasing turnover and inducing decay, loss of TTP may lead to increased production of multiple cytokines, which may be associated with tumor progression. In some cancers, such as colon cancer, loss of TTP contributes to tumor growth by increasing COX-2, VEGF or MMP-1 production12,13,44,45. However, these studies did not evaluate how TTP overexpression influences the production of multiple cytokines. In the present study, we show that knockdown of TTP in HNSCC stabilizes the transcripts of multiple cytokines and enhances invasion and migration.
Due to the host-tumor microenvironment, inflammatory mediators, such as IL-1β produced by tumor-adjacent inflammatory cells may activate the p38 MAPK pathway within the tumor cells31. Therefore, in this study we investigated the effect of TTP on cytokine secretion in the presence or absence of IL-1β. However, TTP overexpression decreased the secretion of cytokines regardless of stimulation with IL-1β. Although the absolute value of the reduction i.e. pg/ml/ million cells was greater with the addition of IL-1β, the fold decrease in cytokine secretion after TTP overexpression is greater in unstimulated than in IL-1β treated cells, consistent with inactivation of part of the overexpressed TTP by IL-1β.
TTP expression was reduced in all HNSCC cell lines compared to NHK. We observed that there was a 62% (SD +/− 24.6) reduction in TTP expression in HNSCC compared to NHK. However, reduced TTP expression did not correlate 100% to increased cytokine secretion in HNSCC cells, which may be due to phosphorylation-mediated inactivation of TTP. MK2, the downstream target of MAPK p38, phosphorylates TTP at serine sites 52 and 178 and renders the protein inactive by inducing binding of TTP to protein 14-3-332,33. We observed that TTP expression was inversely correlated with cytokine secretion except for UM-SCC-81B, which strongly expresses TTP but secretes high levels of cytokines. This may be due to TTP phosphorylation because active p38, which inactivates TTP by phosphorylation is upregulated in HNSCC. We were unable to immunoprecipitate TTP using available antibodies, but the p38 MAPK is active in this cell line. Collectively, these data suggest that loss or inactivation of TTP contributes to production of multiple cytokines in HNSCC. Since p38 can be constitutively active in HNSCC15, our studies suggest that inhibition of p38 in HNSCC would decrease cytokine secretion by preventing inactivation of TTP.
We also observed that TTP knockdown increased the secretion of multiple cytokines, but the effects on individual cytokines varied. This suggests that other pathways or multiple RNA-BPs are involved in the regulation of cytokines and the final effect on secretion may be determined by a balance among these factors. For instance, TTP overexpression in mouse embryonic fibroblasts isolated from TTP knockout mice decreased VEGF but increased proliferation, whereas TTP overexpression in HeLA cells decreased cellular proliferation12, suggesting that other proteins may influence proliferation in different cell types. These studies underscore the importance of delineating how cytokines are regulated in the context of a particular cell or tissue.
IL-6 is a pleiotropic cytokine that functions during inflammation, immunity, bone metabolism, neural development, reproduction, and hematopoiesis46. Although epithelial malignancies may have elevated IL-6, the presence of tumor-derived IL-6 in HNSCC has not been investigated in the context of tumor progression47. Identification of biomarkers that are prognostic of poor survival will allow the selection of tumors for more aggressive treatment. Our findings, from a small group of patients, suggest that HNSCCs with low TTP and high IL-6 have poorer DSS than HNSCCs with high TTP and low IL-6. Similarly, in breast cancer, downregulation of TTP is a negative prognostic indicator associated with increased tumor grade and mortality12. However, TTP expression may not be the actual prognostic indicator in HNSCC. Our findings with IL-6, independent of TTP expression, support this notion. HNSCCs with high IL-6 expression have poor outcomes, including poor DSS, tumor recurrence, and second primary tumors. Consistent with these observations, high serum IL-6 in HNSCC patients is correlated with poor overall survival and an increase in IL-6 during treatment is associated with tumor recurrence37,48. Furthermore, high serum IL-6 is associated with resistance to radiation therapy49.
In HNSCC we demonstrate that loss of TTP increases invasion and cell migration. Similarly, in breast cancer, invasion is decreased when TTP expression is re-established45. The increased invasion and migration may be due to increased cytokine production or TTP may directly regulate proteins responsible for migration in HNSCC42. TTP stabilizes multiple transcripts that affect invasion and tumor progression, including; VEGF, COX-2, uPA, MMP-1 and IL-813,44,45,50.
Given the importance of cytokines in invasion and proliferation, both of which contribute to HNSCC progression, disruption of cytokine secretion is an attractive treatment strategy. However, targeting cancers with a cocktail of inhibitors is impractical and, as mentioned earlier, targeting individual cytokines, such as monoclonal antibodies against IL-6 in multiple myeloma, is ineffective in improving patient survival36. Therefore, upregulation of a protein, such as TTP that inhibits multiple cytokines, is an attractive treatment strategy, particularly if it can be combined with p38 inhibitors, since p38, which inactivates TTP, is constitutively active in HNSCC15. However, due to the importance of cytokines in the immune response, the potential for systemic immunosuppression as a side effect of p38 inhibitors would be a challenge particularly since it may promote tumor progression51,52. This may be overcome by strategies that specifically target the treatment to tumor cells. Future studies will explore the mechanisms by which TTP-mediated cytokine secretion regulates tumor progression and the feasibility of targeting this protein to control tumor growth.
Acknowledgments
Financial support: This work was supported by National Institute of Dental and Craniofacial Research grants R01-DE018512, K02-DE019513, and R21-DE017977, project and developmental grants from the National Cancer Institute P50-CA97248 (University of Michigan Head and Neck SPORE) (N.J. D’Silva), R01-DE018290, 5R21 DE017966 (KL Kirkwood), T32-DE007057-33 (Tissue Engineering and Regeneration Training Grant) and 5F32 DE213052.
Footnotes
The authors report no conflict of interest with this manuscript.
References
- 1.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong G, Loukinova E, Smith CW, Chen Z, Van Waes C. Genes differentially expressed with malignant transformation and metastatic tumor progression of murine squamous cell carcinoma. J Cell Biochem Suppl. 1997;28–29:90–100. [PubMed] [Google Scholar]
- 3.Kanazawa T, Nishino H, Hasegawa M, et al. Interleukin-6 directly influences proliferation and invasion potential of head and neck cancer cells. Eur Arch Otorhinolaryngol. 2007;264:815–821. doi: 10.1007/s00405-007-0264-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 5.Pries R, Nitsch S, Wollenberg B. Role of cytokines in head and neck squamous cell carcinoma. Expert Rev Anticancer Ther. 2006;6:1195–1203. doi: 10.1586/14737140.6.9.1195. [DOI] [PubMed] [Google Scholar]
- 6.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev. 2006;17:141–146. doi: 10.1016/j.cytogfr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26:1393–1399. [PubMed] [Google Scholar]
- 8.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 10.Audic Y, Hartley RS. Post-transcriptional regulation in cancer. Biol Cell. 2004;96:479–498. doi: 10.1016/j.biolcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Carrick DM, Blackshear PJ. Comparative expression of tristetraprolin (TTP) family member transcripts in normal human tissues and cancer cell lines. Arch Biochem Biophys. 2007;462:278–285. doi: 10.1016/j.abb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Brennan SE, Kuwano Y, Alkharouf N, Blackshear PJ, Gorospe M, Wilson GM. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69:5168–5176. doi: 10.1158/0008-5472.CAN-08-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–1679. doi: 10.1053/j.gastro.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 15.Riebe C, Pries R, Kemkers A, Wollenberg B. Increased cytokine secretion in head and neck cancer upon p38 mitogen-activated protein kinase activation. Int J Mol Med. 2007;20:883–887. [PubMed] [Google Scholar]
- 16.Nystrom ML, McCulloch D, Weinreb PH, Violette SM, Speight PM, Marshall JF, et al. Cyclooxygenase-2 inhibition suppresses alphavbeta6 integrin-dependent oral squamous carcinoma invasion. Cancer Res. 2006;66:10833–10842. doi: 10.1158/0008-5472.CAN-06-1640. [DOI] [PubMed] [Google Scholar]
- 17.Peters WH, Lacko M, Te Morsche RH, Voogd AC, Oude Ophuis MB, Manni JJ. COX-2 polymorphisms and the risk for head and neck cancer in white patients. Head Neck. 2009;31:938–943. doi: 10.1002/hed.21058. [DOI] [PubMed] [Google Scholar]
- 18.Sappayatosok K, Maneerat Y, Swasdison S, Viriyavejakul P, Dhanuthai K, Zwang J, et al. Expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in Oral Squamous Cell Carcinoma (OSCC), relationship with angiogenesis and their clinico-pathological correlation. Med Oral Patol Oral Cir Bucal. 2009;14:E319–E324. [PubMed] [Google Scholar]
- 19.Kurihara Y, Hatori M, Ando Y, Ito D, Toyoshima T, Tanaka M, et al. Inhibition of cyclooxygenase-2 suppresses the invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 production and activation. Clin Exp Metastasis. 2009;26:425–432. doi: 10.1007/s10585-009-9241-3. [DOI] [PubMed] [Google Scholar]
- 20.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol. 2008;35:286–297. doi: 10.1053/j.seminoncol.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul WE. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989;57:521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 22.Sok JC, Coppelli FM, Thomas SM, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 23.Mitra RS, Goto M, Lee JS, Maldonado D, Taylor JM, Pan Q, et al. Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer Res. 2008;68:3959–3969. doi: 10.1158/0008-5472.CAN-07-2755. [DOI] [PubMed] [Google Scholar]
- 24.Mitra RS, Zhang Z, Henson BS, Kurnit DM, Carey TE, D'Silva NJ. Rap1A and rap1B ras-family proteins are prominently expressed in the nucleus of squamous carcinomas: nuclear translocation of GTP-bound active form. Oncogene. 2003;22:6243–6256. doi: 10.1038/sj.onc.1206534. [DOI] [PubMed] [Google Scholar]
- 25.Park NH, Min BM, Li SL, Huang MZ, Cherick HM, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991;12:1627–1631. doi: 10.1093/carcin/12.9.1627. [DOI] [PubMed] [Google Scholar]
- 26.Patil CS, Liu M, Zhao W, Coatney DD, Li F, VanTubergen EA, et al. Targeting mRNA stability arrests inflammatory bone loss. Mol Ther. 2008;16:1657–1664. doi: 10.1038/mt.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henson BS, Neubig RR, Jang I, Ogawa T, Zhang Z, Carey TE, et al. Galanin receptor 1 has anti-proliferative effects in oral squamous cell carcinoma. J Biol Chem. 2005;280:22564–22571. doi: 10.1074/jbc.M414589200. [DOI] [PubMed] [Google Scholar]
- 28.Goto M, Mitra RS, Liu M, Lee J, Henson BS, Carey T, et al. Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent transcription and invasion in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2010;16:65–76. doi: 10.1158/1078-0432.CCR-09-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillenwater AM, Zhong M, Lotan R. Histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis through both mitochondrial and Fas (Cd95) signaling in head and neck squamous carcinoma cells. Mol Cancer Ther. 2007;6:2967–2975. doi: 10.1158/1535-7163.MCT-04-0344. [DOI] [PubMed] [Google Scholar]
- 30.Wolf GT, Urba S, Hazuka M. Induction chemotherapy for organ preservation in advanced squamous cell carcinoma of the oral cavity and oropharynx. Recent Results Cancer Res. 1994;134:133–143. doi: 10.1007/978-3-642-84971-8_15. [DOI] [PubMed] [Google Scholar]
- 31.Ng DC, Long CS, Bogoyevitch MA. A role for the extracellular signal-regulated kinase and p38 mitogen-activated protein kinases in interleukin-1 beta-stimulated delayed signal tranducer and activator of transcription 3 activation, atrial natriuretic factor expression, and cardiac myocyte morphology. J Biol Chem. 2001;276:29490–29498. doi: 10.1074/jbc.M100699200. [DOI] [PubMed] [Google Scholar]
- 32.Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, et al. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- 33.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. Embo J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brosens LA, Keller JJ, Pohjola L, Haglund C, Morsink FH, Iacobuzio-Donahue C, et al. Increased expression of cytoplasmic HuR in familial adenomatous polyposis. Cancer Biol Ther. 2008;7:424–427. doi: 10.4161/cbt.7.3.5417. [DOI] [PubMed] [Google Scholar]
- 35.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 36.Hideshima T, Akiyama M, Hayashi T, Richardson P, Schlossman R, Chauhan D, et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood. 2003;101:703–705. doi: 10.1182/blood-2002-06-1874. [DOI] [PubMed] [Google Scholar]
- 37.Druzgal CH, Chen Z, Yeh NT, Thomas GR, Ondrey FG, Duffey DC, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2005;27:771–784. doi: 10.1002/hed.20246. [DOI] [PubMed] [Google Scholar]
- 38.Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999;59:1592–1598. [PubMed] [Google Scholar]
- 39.Smith PC, Keller ET. Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate. 2001;48:47–53. doi: 10.1002/pros.1080. [DOI] [PubMed] [Google Scholar]
- 40.Bataille R, Barlogie B, Lu ZY, Rossi JF, Lavabre-Bertrand T, Beck T, et al. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood. 1995;86:685–691. [PubMed] [Google Scholar]
- 41.Anderson P. Intrinsic mRNA stability helps compose the inflammatory symphony. Nat Immunol. 2009;10:233–234. doi: 10.1038/ni0309-233. [DOI] [PubMed] [Google Scholar]
- 42.Stoecklin G, Stoeckle P, Lu M, Muehlemann O, Moroni C. Cellular mutants define a common mRNA degradation pathway targeting cytokine AU-rich elements. Rna. 2001;7:1578–1588. [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor GA, Carballo E, Lee DM, Lai WS, et al. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 44.Lee HH, Son YJ, Lee WH, Park YW, Chae SW, Cho WJ, et al. Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int J Cancer. 2009 doi: 10.1002/ijc.24847. [DOI] [PubMed] [Google Scholar]
- 45.Al-Souhibani N, Al-Ahmadi W, Hesketh JE, Blackshear PJ, Khabar KS. The RNA-binding zinc-finger protein tristetraprolin regulates AU-rich mRNAs involved in breast cancer-related processes. Oncogene. 2010;29:4205–4215. doi: 10.1038/onc.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller ET, Wanagat J, Ershler WB. Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci. 1996;1:d340–d357. doi: 10.2741/a136. [DOI] [PubMed] [Google Scholar]
- 47.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 48.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 49.De Schutter H, Landuyt W, Verbeken E, Goethals L, Hermans R, Nuyts S. The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy +/- chemotherapy. BMC Cancer. 2005;5:42. doi: 10.1186/1471-2407-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suswam E, Li Y, Zhang X, Gillespie GY, Li X, Shacka JJ, et al. Tristetraprolin down-regulates interleukin-8 and vascular endothelial growth factor in malignant glioma cells. Cancer Res. 2008;68:674–682. doi: 10.1158/0008-5472.CAN-07-2751. [DOI] [PubMed] [Google Scholar]
- 51.Basu A, Datta D, Zurakowski D, Pal S. Altered VEGF mRNA stability following treatments with immunosuppressive agents: implications for cancer development. J Biol Chem. 285:25196–25202. doi: 10.1074/jbc.M110.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fantini MC, Becker C, Kiesslich R, Neurath MF. Drug insight: novel small molecules and drugs for immunosuppression. Nat Clin Pract Gastroenterol Hepatol. 2006;3:633–644. doi: 10.1038/ncpgasthep0611. [DOI] [PubMed] [Google Scholar]