Editor’s note:
Chronic pain affects 1.5 billion people worldwide, an estimated 100 million of whom live in the United States. Yet we currently have no effective treatment options. Fortunately, writes David Borsook, director of the Pain and Imaging Neuroscience Group at Children’s Hospital Boston, Massachusetts General Hospital, and McLean Hospital, research advances have determined some of the ways in which chronic pain changes the brain, and several promising research areas could lead to better treatment approaches. Dr. Borsook recommends steps to facilitate these new treatments, including the establishment of integrated clinical neuroscience centers bridging the gap between bench and bedside.
The medical literature defines chronic pain as pain that has lasted for more than three months. Chronic pain is an epidemic worldwide, with 1.5 billion people feeling its effects. In the United States, about 100 million individuals are estimated to suffer from chronic pain, costing the country billions of dollars in health care and lost work productivity each year.1 While the statistics are staggering, pain research receives less than one percent of the National Institutes of Health (NIH) budget. We do not yet have medications or other treatments that can effectively alleviate chronic pain with speed or efficiency in the majority of patients. Doctors treat patients with a trial-and-error approach, initially prescribing drugs with fewer side effects, reflecting the lack of effective treatment options.
In the past 50 years or so, our main medications have been in four classes: opioids (morphine), nonsteroids (salicylates such as aspirin or ibuprofen), antidepressants (amitriptyline), and antiepileptics (gabapentin); fewer than 10 medications with new mechanisms of action have become available, and only one now in clinical use was designed based on specific mechanisms of action (triptans for migraine). Other drugs, including the anti–nerve growth factor inhibitors (NGF), also target specific mechanisms2 but were on regulatory hold until very recently because of side effects in patients with osteoarthritis. Given these drugs’ significant analgesic effects and new data in other pain conditions,3 the Food and Drug Administration (FDA) allowed trials to proceed.
Worrisome epidemiological trends also contribute to the national pain emergency. Like other Western societies, ours is skewing older, and with increased age come more pain-related problems. In addition, nerve damage unavoidably produced during a surgical operation will cause pain in 15 to 50 percent of patients, a percentage that varies from study to study.4 The medical conditions that can cause pain are manifold. Rates of diabetes are soaring, often leading to diabetic neuropathic pain. Significant numbers of patients with spinal cord trauma have severe chronic pain.5 Millions of individuals with so-called dry eye disease probably suffer from nerve damage to the cornea; surgical interventions such as LASIK (wherein the cornea is shaved to produce improved vision) can also produce corneal nerve damage and chronic pain. Arthritis and arthritis-related pain affect millions. Back pain and headache are the two most common neurological ailments. Nerve damage or neuropathic pain in cancer patients, often side effects of treatment, represent about 20 percent of all cancer-related pains. The elderly are affected more than young, and for the most chronic pain conditions, women are affected more than men. The list goes on, but these examples show how large the problem is, and how chronic pain is part of many diseases across all age groups.
Why are we still in this dire predicament, and what can we do about it? There is now greater focus on pain, particularly with a recent Institute of Medicine report that defined pain as a national public health issue requiring a comprehensive national strategy.1 Defining an optimal strategy and focusing the requisite investment in research and treatment protocols are critical and urgent. We are at a crossroads: Implementing a bold program to conquer pain is a national imperative with worldwide benefits and consequences.
Taking Stock of Current Treatment Approaches
Our understanding of chronic pain has changed dramatically in the past decade or so, but researchers have yet, with the exception of triptans, to use new scientific approaches to successfully develop effective medications. The ideal analgesic for chronic pain should surpass other treatments’ efficacy, have few side effects, be easily available and inexpensive, modify disease, be predictive, and be preventive where possible. But current medications for most chronic pain conditions are relatively ineffective (less than 30 percent in placebo-controlled trials6) and are used because there are few alternatives. In fact, the field is beginning to re-evaluate the use of opioids for chronic pain because long-term benefits are for the most part limited, the drugs can produce chronic changes in the brain, and opioid use can result in addiction.
Alternative pain treatments are either inadequate or are unproven; clinical trials for complementary and alternative medicines have for the most part not shown significant efficacy. New efforts to evaluate the placebo response in the field of pain and analgesia may prove highly useful in our approach to chronic pain patients and may have implications for treatment trials where the placebo response is a major obstacle. Harnessing the placebo response in an effective manner has enormous implications, but the focus should be on replacing inadequate or unproven treatments.
By using a more aggressive translational process, researchers must discover new opportunities to treat pain and to define which therapies now in use work. Procedures with no validity need to be re-evaluated while promising treatments need to be built on so they can be replaced by more effective choices. The use of unproven techniques driven by income generation rather than scientific merit needs to end.
Neuroscience Advances: Chronic Pain Is in the Brain
Most chronic pain conditions produce changes in the brain that contribute to what can be termed the “centralization of pain.” This implies that ongoing pain produces progressive alterations in brain connections, molecular biology, chemistry, and structure, with behavioral consequences. One brain region consistently affected in chronic pain conditions is called the dorsolateral prefrontal lobe, a region in the front of our brains thought to be involved in several higher-order functions, including cognition, motor planning, and working memory. This centralization of pain involves alterations in sensory, emotional, and modulatory circuits, which normally inhibit pain. Thus chronic pain may alter cognition and emotion, leading to increased fear, anxiety, or depression. Previously healthy people, such as wounded warriors, may have an injury that results in a chronic pain syndrome, such as phantom limb pain. The pain could lead to depression, which exacerbates the underlying pain condition. Conversely, a significant number of patients with primary depression and no history of pain may develop a generalized pain syndrome, suggesting changes in brain circuits that result in these clinical manifestations.
Both human and animal studies show that chronic pain changes the brain functionally, structurally, and chemically.7–9 Initial reports point to loss of or decreased volume in gray matter in the brain areas noted above, along with other regions. Chronic pain induces abnormal function in brain circuits, including circuits involved in cognition, autonomic responses, and other more complex integrative behaviors (e.g., fear, anxiety, interoception, reward and aversion). Chronic pain also alters the chemistry of brain regions, generating abnormal levels of excitatory and inhibitory chemicals called neurotransmitters. The ways different parts of the brain “talk” to each other also become abnormal, presumably due to changes in gray matter and its connections through fiber tracts.10 To make matters even more complex, these fiber tracts themselves show abnormal structure in chronic pain. We know less about how to undo these processes in a permanent manner, but early reports suggest that these changes may be reversible in some patients.
Brain imaging research has produced new insights into alterations in brain function. For example, pain changes circuits involved in reward processing. Chronic pain may be considered a reward deficit syndrome,11 which suggests that drugs or treatments that alter the brain’s reward circuitry may be valuable to treating pain.11 In addition, objective measures of brain circuits could provide a fingerprint of specific behaviors (such as sensory, emotional, and cognitive changes), serving as a potential biomarker of disease state and analgesic drug effects. Defining a useful biomarker for chronic pain would allow for objective evaluation of pain treatments, an enormous advance.12, 13
Major Areas of Clinical Research
Scientists are now studying chronic pain treatment from a variety of research angles, several of which I summarize below. Strategies for understanding the neurobiology of pain are front and center to all research in the field. Some topics, such as translational aspects and the molecular biology of pain, are not discussed here but are relevant as major contributors to pain neurobiology and the discovery of new treatment approaches for chronic pain (Figure 2). Here I focus on some aspects related to progress in clinical research.
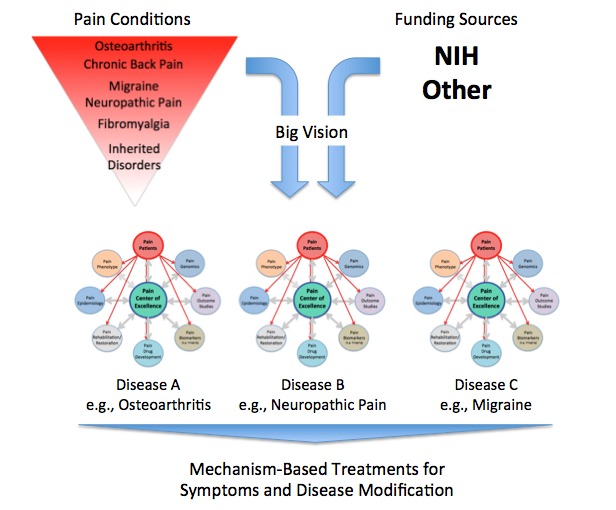
Figure 2.
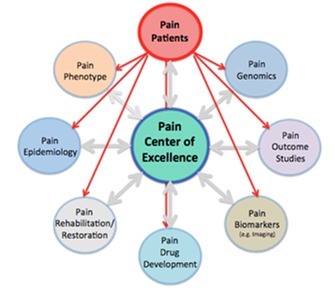
Top: Concept of Centers of Excellence. Piecemeal science suffers from the inability to tackle large problems. Integrated approaches offer a focus on the problem at hand and bring together patients, scientists, and clinicians. The figure depicts the pain patient population as the focus of such a center. Interactions across scientific and clinical disciplines would occur through the center. Multiple processes converge, from clinical and brain imaging phenotype together with genomic information, to evaluation of responders and nonresponders, to current or new treatments.
Bottom: Focus on Targeting Major Chronic Pain Conditions. The figure captures a few salient points: (1) Chronic pain conditions vary in terms of incidence and prevalence. Some conditions are highly prevalent (e.g. osteoarthritis), while others are rare but represent specific gene-based diseases (e.g. hemiplegic migraine). (2) A vision for the development of centers of excellence that focus on a specific chronic pain subtype. These may be constituted through single-site or consortia arrangements; the latter may include academia, government, and industry.
Genetics
We now have a better understanding of genetic risk factors for chronic pain.14–16 Researchers have evaluated four major categories with an immediate clinical impact: epigenetic contributions to chronic pain;17 genes that can predict the outcome of pain following injury or surgery;18 genes that provide information about the efficacy of drugs (so called pharmacogenetics and pharmacogenomics);19, 20 and genes that may define specific pain syndromes, such as migraine.21, 22 One example of the latter is pain sensitivity induced by a gene that controls the sodium channel Nav1.7. Channelopathies have provided a novel model for understanding pain pathophysiology; patients with excessive gene expression have exaggerated pain, and those with a deficit of the gene have congenital insensitivity to pain.23 New treatments may be designed around these discoveries.24, 25
Damaged or Abnormal Pain Systems
Chronic neuropathic pain alters neuronal structure and function along the neural axis—from the peripheral nerve to the spinal cord and higher brain centers (cortical and subcortical regions). The ability to replace neurons through cytotherapeutic technologies such as embryonic stem cells (currently in animal models)26 or gene therapy27 offers exciting new opportunities to the research community.
Neuroinflammation
Some non-neuronal inflammatory systems play an important role in conditions such as chronic neuropathic pain,28, 29 which can produce persistent neuroinflammatory changes in the central nervous system.30 Evaluating ongoing pain in the context of low-grade neuroinflammatory processes in the brain could further define exciting targets for potential therapies.31 Some drugs, such as ketamine, may be beneficial thanks to anti-inflammatory effects.32, 33
Neurorehabilitation
Several neurorehabilitative approaches may allow for the reversal of maladaptive changes in the brain. From mirror therapy and immersive virtual reality34, 35 to processes that can control prosthetic limbs36, 37 to brain stimulation techniques such as transcranial magnetic stimulation (TMS),38 researchers have developed new approaches with potentially useful applications in the treatment of chronic pain. The way in which motor activity or motor cortex stimulation (through TMS or direct stimulation) helps pain is unclear, but it may relate to forced interactions between the brain circuits involved in motor control and those involved in sensory processing. The notion of resetting disrupted or abnormal pain networks is clearly part of disease modification.39 Our understanding of how these and other treatments may modify and improve neural circuits involved in pain is limited, and more randomized clinical trials are warranted.
Applying the Research Process to Treatment: Toward an Ideal Outcome
Chronic pain is an enormous public health challenge that deserves immediate focus and attention. The strategies below could form a cornerstone for integrated planning to conquer the pervasive problem.
First, establishing an independent program or division of pain at the NIH, or more fully integrating pain research across the NIH institutes, could be a powerful engine for change. The current Pain Consortium at the NIH goes a long way to try to integrate pain research, but it lacks essential financial backing that would make it more effective. Pain is a big problem that demands big projects. Public-private partnerships such as the Foundation for the NIH or networks such as the London Pain Consortium, the German Research Network on Neuropathic Pain and the EUROPAIN Innovative Medicines Initiative are the kinds of approaches that we need to set up and embrace in the United States to enhance a focused, deliverable, and integrated research enterprise to conquer pain.
Second, a pain equivalent to the Howard Hughes Medical Institute’s support for biomedical research and science would help move the research field forward. Third, the government should provide incentives for biotechnology companies to invest more heavily in the pain field; this is a major concern at a time when large pharmaceutical companies are pulling out of drug development for brain disorders, including pain, due to perceived risk.
Fourth, we need well-directed philanthropy. Small funding sources are usually adept in taking risks on proof-of-principle research that can leverage applications for larger funding, as the New York–based Mayday Fund, which supports pain research, has demonstrated. Large-scale programs, while more complex to manage and carry out efficiently, can begin to address complex processes such as biological, psychological, and social issues related to chronic pain and its treatment. More efficient, integrated, and better-designed clinical trial approaches, such as ACTION (Analgesic Clinical Trial Innovations, Opportunities, and Networks, a collaboration between academia, the FDA, and the pharmaceutical industry), could decrease the time needed to evaluate new chronic pain treatments.
A layered approach could be a strategy for immediate, intermediate, and long-term progress. For example, significant research investment and focus on surgically induced neuropathic pain could have early benefits. Doctors in the United States perform an estimated 48 million inpatient surgeries each year; these are open laboratories in which to collect and evaluate data and implement clinical trials.40 Our knowledge of the transition from acute to chronic pain is based not on scientific evidence but on an arbitrary timeline; clearly, for conditions such as surgically induced neuropathic pain, the initial surgical trauma (even while under anesthesia) is the initiating event that progresses to chronic neuropathic pain in substantial numbers of patients.4
Another example where the clinical chronic pain problem looms large but is largely unaddressed in terms of effective therapies is osteoarthritis. Trials with the anti–nerve growth factor drug have been very promising, and joint deterioration, an initial side effect, seemed to be linked with concurrent administration of nonsteroidal drugs. Determining differences in patients who respond and patients who do not respond even to current treatments would seem to be a pragmatic approach to research. More complex clinical research requiring larger cohorts—such as to evaluate the genetics of pain, reconstitute neural systems with embryonic stem cells, and modify brain circuits—may require longer-term processes before direct patient applications become possible.
All treatments, including so-called alternative or homeopathic medications, need to be evaluated in clinical trials to filter out what does and does not work. Patients should not be exposed to practices that do not show benefit. Currently, there are no objective measures for chronic pain or analgesic effects, and subjective responses are notoriously variable.41–43 Evaluation of treatments (in the clinic or in clinical trials) is fraught with difficulties because of subjective measures. Researchers need to determine measures that can be used as biomarkers for pain; their development would be a huge boost to clinical treatment and therapeutic trials. In addition, physicians must be trained to have a better understanding of chronic pain as a disease that affects the brain, using clinical insights to drive treatment plans. Integrating basic scientists into the clinic, so that researchers work with clinicians, would be a major step forward in having our brightest minds in pain neurobiology actually understand a patient and her pain-related problem.
When it comes to pain treatments, particularly pharmacological treatments, some populations are neglected. This is particularly true of women, for whom the prevalence of pain is much higher than in men for the majority of chronic pain conditions, and also in children, for whom drugs tested in controlled trials in adults are for the most part simply down-dosed instead of evaluated in trials in pediatric populations. Given the cost of chronic pain to society, it would seem prudent to establish an aggressive agenda whereby private industry and academic institutions have incentives to develop new analgesics in both adults and children in proper clinical trials. Age, genetic, and sex differences related to chronic pain need to be evaluated in appropriately designed clinical trials for new drugs.
Again, the establishment of Clinical Research Centers of Excellence (COEs), set up in a manner similar to the Howard Hughes Institute for Medical Research or the EUROPAIN could have a great impact. Such COEs do not have to be at a single institution but can be distributed to take advantage of talent, patient populations, and research infrastructure. Large data sets from patients and failed trials could be collected to help define issues such as clinical phenotype, genetic markers for disease states, and drug effects. These centers could be the focus for rapid deployment of clinical trials for new treatments.
Collaborative and team initiatives are essential to this effort. The NIH and academics need to adapt to encourage these programs and provide due process for crediting individuals within them. Each of these centers should have an integrated pain research clinic that encourages interaction among basic scientists and research clinicians. We need to start with patients and integrate molecular, genetic, and other processes around them. In order to do this, we need to set up processes for consortia to work effectively and efficiently.
Society depends on pharmaceutical and biotechnology companies, and federal regulatory agencies such as the FDA, to develop and bring treatments to the public domain in a safe and efficient manner. The financial incentive to produce new and effective medications is great: Pain is currently a $100 billion industry, and the successful introduction of more effective medications would be extremely competitive in this marketplace. However, the production of such medications has been difficult. The current approach—reformulating existing drugs—will not change the efficacy of treatments. The reformulation of old products, while financially sound, does not enhance the clinical armamentarium and should be discouraged because the reformulations generally are not more effective than the original products.
Approaching chronic pain as a national emergency would allow for a better future in terms of both treatments for chronic pain, costs to society, and individual well-being. It is time to take an honest look at where we are, get rid of unnecessary and unproven treatments, and advance neuroscience to the patient in the form of better treatments. Pain neuroscience has never been so exciting or well positioned in terms of the potential it offers to change the current impasse. But it will take bold decisions to put into place a vision and plan of action.
Figure 1:

Chronic Pain and the Brain
Multiple biological, psychological, and social factors may contribute to chronic pain. Chronic pain results in functional, morphological, and chemical changes in the brain. These alterations contribute to pain behaviors or the pain phenotype.
Footnotes
Article available online at http://dana.org/news/cerebrum/detail.aspx?id=39160
References
- 1.Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, D.C.: Institute of Medicine of the National Academies; 2011. [PubMed] [Google Scholar]
- 2.Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Current Opinion in Molecular Therapeutics. 2010;12(1):94–106. [PubMed] [Google Scholar]
- 3.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152(10):2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 5.Dijkers M, Bryce T, Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: A systematic review. Journal of Rehabilitation Research and Development. 2009;46(1):13–29. [PubMed] [Google Scholar]
- 6.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA: The Journal of the American Medical Association. 1998;280(21):1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 7.May A. Structural brain imaging: A window into chronic pain. The Neuroscientist. 2011;17(2):209–220. doi: 10.1177/1073858410396220. [DOI] [PubMed] [Google Scholar]
- 8.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. NeuroImage. 2009;47(3):1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One. 2011;6(10):e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: Abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Current Pharmaceutical Design. 2012;18(1):113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: How, where, and what to look for using functional imaging. Discovery Medicine. 2011;11(58):209–219. [PubMed] [Google Scholar]
- 13.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: The need, reality, challenges, and solutions. Discovery Medicine. 2011;11(58):197–207. [PubMed] [Google Scholar]
- 14.Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Woolf CJ. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133(9):2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young EE, Lariviere WR, Belfer I. Genetic basis of pain variability: Recent advances. Journal of Medical Genetics. 2012;49(1):1–9. doi: 10.1136/jmedgenet-2011-100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogil JS. Pain genetics: Past, present and future. Trends in Genetics. 2012 doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Denk F, McMahon SB. Chronic pain: Emerging evidence for the involvement of epigenetics. Neuron. 2012;73(3):435–444. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nature Medicine. 2006;12(11):1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 19.Argoff CE. Clinical implications of opioid pharmacogenetics. Clinical Journal of Pain. 2010;26(Suppl 10):S16–20. doi: 10.1097/AJP.0b013e3181c49e11. [DOI] [PubMed] [Google Scholar]
- 20.Jannetto PJ, Bratanow NC. Pain management in the 21st century: Utilization of pharmacogenomics and therapeutic drug monitoring. Expert Opinion on Drug Metabolism and Toxicology. 2011;7(6):745–752. doi: 10.1517/17425255.2011.565051. [DOI] [PubMed] [Google Scholar]
- 21.de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM. Molecular genetics of migraine. Human Genetics. 2009;126(1):115–132. doi: 10.1007/s00439-009-0684-z. [DOI] [PubMed] [Google Scholar]
- 22.Lafreniere RG, Cader MZ, Poulin JF, Andres-Enguix I, Simoneau M, Gupta N, Rouleau GA. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nature Medicine. 2010;16(10):1157–1160. doi: 10.1038/nm.2216. [DOI] [PubMed] [Google Scholar]
- 23.Fischer TZ, Waxman SG. Familial pain syndromes from mutations of the NaV1.7 sodium channel. Annals of the New York Academy of Sciences. 2010;1184:196–207. doi: 10.1111/j.1749-6632.2009.05110.x. [DOI] [PubMed] [Google Scholar]
- 24.Heinzmann S, McMahon SB. New molecules for the treatment of pain. Current Opinion in Supportive and Palliative Care. 2011;5(2):111–1115. doi: 10.1097/SPC.0b013e328345bb7e. [DOI] [PubMed] [Google Scholar]
- 25.Lotsch J, Geisslinger G. Pharmacogenetics of new analgesics. British Journal of Pharmacology. 2011;163(3):447–460. doi: 10.1111/j.1476-5381.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexanian AR, Svendsen CN, Crowe MJ, Kurpad SN. Transplantation of human glial-restricted neural precursors into injured spinal cord promotes functional and sensory recovery without causing allodynia. Cytotherapy. 2011;13(1):61–68. doi: 10.3109/14653249.2010.510504. [DOI] [PubMed] [Google Scholar]
- 27.Jain KK. Gene therapy for pain. Expert Opinion on Biological Therapy. 2008;8(12):1855–1866. doi: 10.1517/14712590802496977. [DOI] [PubMed] [Google Scholar]
- 28.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nature Medicine. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skaper SD, Giusti P, Facci L. Microglia and mast cells: Two tracks on the road to neuroinflammation. FASEB Journal. 2012 doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- 30.Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport. 2001;12(16):3439–3442. doi: 10.1097/00001756-200111160-00012. [DOI] [PubMed] [Google Scholar]
- 31.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of Neuroimmunology. 2010;229(1–2):26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Bhutta AT, Schmitz ML, Swearingen C, James LP, Wardbegnoche WL, Lindquist DM, Anand KJ. Ketamine as a neuroprotective and anti-inflammatory agent in children undergoing surgery on cardiopulmonary bypass: A pilot randomized, double-blind, placebo-controlled trial. Pediatric Critical Care Medicine. 2011 doi: 10.1097/PCC.0b013e31822f18f9. [DOI] [PubMed] [Google Scholar]
- 33.Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: State of the art. Acta Anaesthesiologica Belgica. 2011;62(1):47–58. [PubMed] [Google Scholar]
- 34.Lamont K, Chin M, Kogan M. Mirror box therapy: Seeing is believing. Explore. 2011;7(6):369–372. doi: 10.1016/j.explore.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Ramachandran VS. Plasticity and functional recovery in neurology. Clinical Medicine. 2005;5(4):368–373. doi: 10.7861/clinmedicine.5-4-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wodlinger B, Durand DM. Peripheral nerve signal recording and processing for artificial limb control. Conference Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2010; 2010. pp. 6206–6209. [DOI] [PubMed] [Google Scholar]
- 37.Hogan N, Krebs HI. Physically interactive robotic technology for neuromotor rehabilitation. Progress in Brain Research. 2011;192:59–68. doi: 10.1016/B978-0-444-53355-5.00004-X. [DOI] [PubMed] [Google Scholar]
- 38.Plow EB, Pascual-Leone A, Machado A. Brain stimulation in the treatment of chronic neuropathic and non-cancerous pain. Journal of Pain. 2012 doi: 10.1016/j.jpain.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flor H, Diers M. Sensorimotor training and cortical reorganization. NeuroRehabilitation. 2009;25(1):19–27. doi: 10.3233/NRE-2009-0496. [DOI] [PubMed] [Google Scholar]
- 40.National Center for Health Statistics. FASTSTATS - Inpatient Surgery. 2012. Retrieved April 25, 2012, from http://www.cdc.gov/nchs/fastats/insurg.htm.
- 41.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: Intrasubject variability and correlation with a numeric scale. Anesthesia and Analgesia. 1998;86(1):102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen CS, Staud R, Price DD. Individual differences in pain sensitivity: Measurement, causation, and consequences. Journal of Pain. 2009;10(3):231–237. doi: 10.1016/j.jpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Coghill RC. Individual differences in the subjective experience of pain: New insights into mechanisms and models. Headache. 2010;50(9):1531–1535. doi: 10.1111/j.1526-4610.2010.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


