Abstract
Objective
The effect of national quality initiatives aiming at limiting lower extremity amputations in diabetic patients remains uncertain. We therefore explored trends in amputation among Medicare diabetic patients with a focus on those at highest risk.
Methods
The Diabetes Analytical File, an enhanced sample of all diabetic patients from the Medicare 5% sample, was used to study the national incidence of amputation in diabetic patients. Within a cohort of ~5 million diabetic patients between 1999 and 2006, we compared the incidence of amputation in high-risk (end-stage renal disease or more than three comorbidities) and low-risk groups and by race.
Results
Between 1999 and 2006, 23,976 amputations were performed, comprising 11,558 in high-risk and 12,418 in low-risk patients. The amputation rate declined over time from 4.8/1000 in 1999 to 4.4/1000 in 2006 (P < .001). High-risk patients represented a growing proportion of all amputations (33% in 1999, 50% in 2006; P < .001) despite representing 4% of diabetic patients in 1999 and 10% in 2006 (P < .001). The incidence of amputation was 29.6/1000 in the high-risk group vs 2.7/1000 in low-risk patients (P < .001). African Americans had higher rates of amputation in high-risk and low-risk groups.
Conclusions
High-risk patients represent a minority of Medicare diabetic patients but account for 50% of all amputations, and this effect is magnified in African Americans. Future quality improvement efforts should focus on high-risk patients and African Americans.
Diabetes is a growing epidemic in the Medicare population.1 Among the numerous complications associated with poorly controlled diabetes, lower extremity amputations represent one of the most morbid adverse outcomes.2 Accordingly, several payer- and provider-based initiatives have published management guidelines aimed at preventing amputation as well as other adverse sequelae of diabetes.3–6 Specifically, Healthy People 2010, an ongoing health improvement effort maintained by the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), provides numerous recommendations toward improving the care of diabetic patients. On the basis of their estimates of the economic and societal effect of amputations secondary to diabetes, the CDC and NIH set forth a specific goal of decreasing the number of amputations among diabetic individuals by 55% during a 10-year period.4
Despite such ambitious goals, the real-world effectiveness of national guidelines and quality improvement initiatives to decrease the incidence of amputations among diabetic patients remains uncertain, because initiatives similar to Healthy People 2010 instituted in other countries have not resulted in convincing improvement in amputation. For example, England established similar recommendations and goals as Healthy People 2010 in 1999. An analysis of a large administrative database of ~50,000 nontraumatic amputations in England demonstrated an increase in the burden of amputations on the diabetic population after initiation of management recommendations.7 In The Netherlands, data on amputation rates after initiation of diabetes quality improvement initiatives also demonstrated little effect.8
Within the U.S., although many have lauded attempts to improve diabetic care with quality improvement initiatives, whether they have resulted in any real effect on amputations among diabetic individuals remains unknown. Therefore, to assess the effect of Healthy People 2010 on the incidence of lower extremity amputations among diabetic individuals, we analyzed trends in the incidence of lower extremity amputation among the Medicare diabetic population in recent years.
METHODS
Data set description
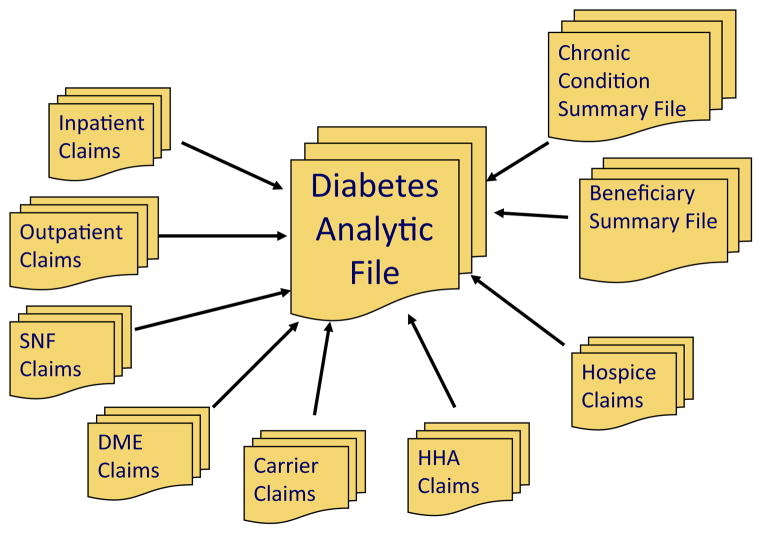
We used the Diabetes Analytic File (DAF), an enhanced administrative data source, for our analysis. The Centers for Medicare and Medicaid Services (CMS) created the Chronic Condition Warehouse DAF to compile data on diabetic patients within the Medicare system. The DAF contains a wide array of beneficiary data from fee-for-service and non–fee-for-service participants as well as institutional and noninstitutional claims (Fig 1).9 All beneficiaries from the Medicare 5% sample are entered into the database if they have a diagnosis of diabetes according to a validated algorithm.9,10 In brief, the diagnostic algorithm requires patients to have at least one inpatient or two outpatient claims for diabetes during a 2-year period according to International Classification of Disease-9th Clinical Modification diagnostic codes 250.00 –93, 357.2, 362.01 –02, and 366.41.
Fig 1.
Input files contributing to the Diabetes Analytical File (DAF). DME, Durable medical equipment; HHA, home health agencies; SNF, skilled nursing facility.
The enhanced 5% DAF differs from the traditional 5% sample because the enhanced 5% DAF keeps a patient in the database over subsequent years. In the traditional 5% sample, patients may be present in Medicare claims in 1 year but be dropped from the data set in another year because of insufficient claims. In contrast, a patient who reaches the criteria for inclusion into the DAF is never dropped from the database. This expanded database permits more robust longitudinal analysis of elderly diabetic patients, regardless of their longitudinal connection to Medicare.
Cohort formation
Our cohort consisted of beneficiaries aged ≥50 years who underwent lower extremity amputation between 1999 and 2006 (the most recent years available). Basic demographic information was gathered, including age, sex, and race. As coded in the data set, lower extremity amputations were defined as major and minor amputations, including toe, below-knee, and above-knee amputations. The database lists lower extremity amputation as a single categoric variable for a given year, which includes all major and minor amputations (International Classification of Disease-9th Clinical Modification procedural codes 84.10-17). Beneficiary comorbidities are defined by the CMS Chronic Condition convention.9 Co-morbidities included in our analysis were stroke, congestive heart failure, myocardial infarction, ischemic heart disease, and chronic kidney disease.
Because the risk of amputation in diabetic patients varies according to patient-level comorbidities, beneficiaries were divided into low-risk and high-risk cohorts, using previously published definitions of high-risk and low-risk populations.11 The high-risk cohort was defined as those with end-stage renal disease (ESRD) or three or more comorbidities (including diabetes), or both; whereas the low-risk cohort had three or fewer comorbidities and did not have a diagnosis of ESRD.
Analysis
Our main outcome measure was the amputation rate between 1999 and 2006. The Healthy People 2010 initiative was implemented in 2000. Healthy People 2010 used data from a variety of sources, including National Hospital Discharge Survey, CDC, and National Health Interview Survey, to track amputation outcomes. Accordingly, we examined secular trends in the amputation rate during this period to assess the association between the implementation of Healthy People 2010 and population-based amputation rates. Dichotomous variables were analyzed using χ2 and continuous variables were analyzed using t-tests and analysis of variance. Data were analyzed using STATA 10 software (StataCorp, College Station, Tex).
RESULTS
Patient characteristics
Overall, we studied 4,943,398 Medicare patients (mean age, 74 years) between 1999 and 2006 (Table I) Women comprised 59.7% of all diabetic patients, and 12.7% of the diabetic patients were African American. Overall, 8.2% of diabetic patients were high-risk, defined as having a diagnosis of ESRD or more than three comorbidities.
Table I.
Demographic differences between Medicare diabetic patients by amputation status
| Variablea | Amputationb | No amputationb |
|---|---|---|
| Total number | 23,976 (0.5) | 4,919,422 |
| Age | ||
| Mean years | 73.4 | 74.0 |
| 50–64 | 4,571 (19) | 509,932 (10) |
| 65–74 | 7,958 (33) | 2,077,317 (42) |
| 75–84 | 7,961 (33) | 1,732,289 (35) |
| >84 | 3,486 (15) | 599,884 (12) |
| Female | 11,543 (48) | 2,939,357 (59) |
| Race | ||
| White | 16,317 (68) | 3,954,265 (80) |
| Black | 6,013 (25) | 627,877 (13) |
| ESRD | 4,917 (21) | 86,525 (2) |
| >3 comorbidities | 10,292 (43) | 348,711 (7) |
| Overall deaths during study period | 6,339 (26) | 260,628 (5) |
ESRD, End-stage renal disease.
Data are shown as number (%) or as indicated.
P <.001 calculated from χ2 for all comparisons.
Incidence of amputation
There were 23,976 amputations over the course of the period studied, with an overall amputation rate of 4.9/1000 diabetic patients (Table I). Amputees were slightly younger than those who did not undergo amputation (mean age, 73.4 vs 74; P < .001). Among diabetic patients undergoing amputation, there was an equal distribution of men and women (48% vs 52%). As expected, diabetic patients who underwent amputation were more likely to have ESRD, multiple comorbidities, and to die than diabetic patients who did not undergo amputation (Table I).
Differences over time
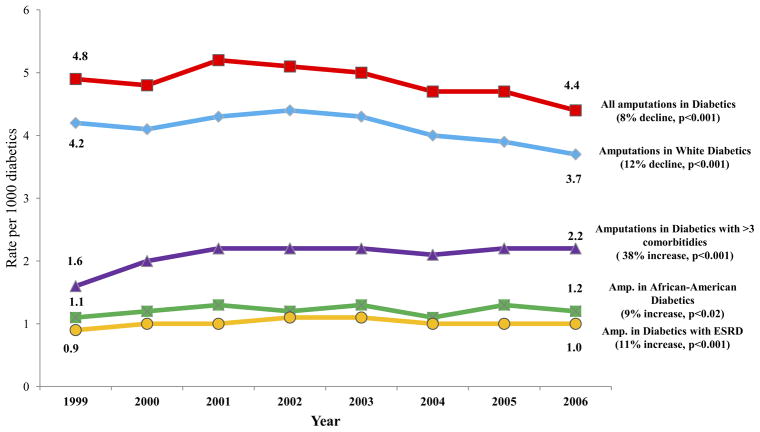
As outlined on its Web site (www.healthypeople.gov/2010/), Healthy People 2010 established a goal of reducing amputation among diabetic patients by 5.5% per year in the 10-year period between its inception and 2010. However, during the early part of this study period, the decline in amputation rate was much less dramatic. Between 2000 and 2006, the overall rate of amputation among all diabetic individuals declined by 0.6% per year (4.8/1000 in 2000 to 4.4/2006; P < .001; Table II; Fig 2). Rather than the goal proposed by Healthy People 2010 of a 38.5% decline, an 8% decline was achieved during the 8-year period.
Table II.
Trends in characteristics of diabetic patients undergoing amputation over time, overall and by risk strata
| Variablea | Diabetic patients undergoing amputations
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All
|
High-riskb
|
Low-riskc
|
|||||||
| 1999 | 2006 | Pd | 1999 | 2006 | Pd | 1999 | 2006 | Pd | |
| Total number | 3039 | 2549 | 1209 | 1389 | 1830 | 1160 | |||
| Age | |||||||||
| Mean, years | 73.5 | 73.0 | <.001 | 71.7 | 72.2 | .4 | 75.3 | 73.9 | <.001 |
| 50–64 | 16 | 22 | <.001 | 24 | 24 | .3 | 12 | 19 | <.001 |
| 65–74 | 35 | 31 | .001 | 35 | 30 | .01 | 34 | 32 | .03 |
| 75–84 | 34 | 33 | .02 | 30 | 33 | .3 | 37 | 32 | <.001 |
| >84 | 15 | 14 | .8 | 10 | 12 | .4 | 17 | 17 | .7 |
| Female | 51 | 46 | <.001 | 51 | 46 | <.001 | 51 | 46 | <.001 |
| Race | |||||||||
| White | 23 | 26 | .021 | 26 | 30 | .01 | 22 | 22 | .6 |
| Black | 69 | 67 | .009 | 66 | 63 | .02 | 72 | 72 | .9 |
| ESRD | 17 | 23 | <.001 | 45 | 43 | .2 | … | … | … |
| >3 comorbidities | 33 | 50 | <.001 | 82 | 92 | <.001 | … | … | … |
| Overall deaths during study period | 25 | 27 | .1 | 36 | 34 | .04 | 17 | 18 | .3 |
ESRD, End-stage renal disease.
Data are shown as percentage, unless otherwise indicated.
≤3 comorbidities (including diabetes), and did not have a diagnosis of ESRD.
>3 comorbidities (including diabetes) or ESRD, or both.
P calculated from χ2 comparison.
Fig 2.
Temporal trends of amputations in diabetic individuals (above-knee, below-knee, and minor), by race and comorbidity status. ESRD, End-stage renal disease.
During this interval, we noted two changes in our population of Medicare patients with diabetes: First, diabetic patients undergoing amputation became younger. The mean age of amputees decreased from 73.9 to 73.0 years (P < .001). The decrease in mean age occurred because the proportion of amputees who were aged 50 to 64 years increased over time (16.4% to 21.9%; P < .001), and the proportion in the group aged 65 to 74 years decreased over time (34.8% to 30.8%; P < .001). The second change was an increase in the proportion of high-risk amputees over time. High-risk patients represented a growing proportion of all amputations (33% in 1999, 50% in 2006; P < .001), despite representing a minority of all diabetic individuals (4% in 1999 to 10% in 2006; P <.001).
Other smaller changes were evident over time as well. Sex distribution changed significantly, with a smaller proportion of amputations occurring in women (51% in 1999, 46% in 2006; P < .001). Although the amputation rate among all African American beneficiaries decreased (9.2/1000 in 1999 to 8.8/1000 in 2006; P = .021) the rate of amputation in black patients remained significantly higher than the rate of amputation in white beneficiaries (4.2/1000 in 1999 and 3.7/1000 in 2006).
Differences in amputations between high-risk and low-risk groups
Among all diabetic patients undergoing amputation, 11,558 (48.2%) were high-risk, and 12,418 (51.8%) were low-risk (Table III). Although representing almost half of all amputations, high-risk patients represented only 7.3% of the entire population with diabetes (Table I). Among high-risk amputees, 42.5% had ESRD, and 89.1% had more than three co-morbidities. The high-risk cohort had a significantly higher amputation rate than the low-risk cohort (28.6/1000 vs 3.0./1000 diabetic individuals; P < .001). Furthermore, compared with the low-risk amputee cohort, high-risk amputees were younger (mean age, 72.1 vs 74.6 years; P < .001), were more likely to be women (48.6% vs 47.8%; P < .001), and a greater proportion were African American (64% vs 22%; P < .001).
Table III.
Characteristics among diabetic patients undergoing amputation by risk strata
| Variablea | High-riskb | Low-riskc |
|---|---|---|
| Total number | 11,558 (48.2) | 12,418 (51.8) |
| Mean age, years | 72.1 | 74.6 |
| 50–64 | 2646 (22.9) | 1925 (15.5) |
| 65–74 | 3907 (33.8) | 4051 (32.6) |
| 75–84 | 3724 (32.2) | 4237 (34.1) |
| >84 | 1281 (11.1) | 2205 (17.8) |
| Female | 5611 (48.6) | 5932 (47.8) |
| Race | ||
| White | 3285 (28.4) | 2728 (22.0) |
| Black | 7397 (64.0) | 8920 (71.8) |
| ESRD | 4917 (42.5) | … |
| >3 comorbidities | 10,292 (89.1) | … |
| Overall deaths during study period | 4175 (36.1) | 2164 (17.4) |
ESRD, End-stage renal disease.
Data are shown as number (%), unless otherwise indicated.
≤3 comorbidities (including diabetes), and did not have a diagnosis of ESRD. P <.001 calculated from χ2 for all comparisons between groups.
>3 comorbidities (including diabetes) or ESRD, or both. P <.001 calculated from χ2 for all comparisons between groups.
Effect of race and risk
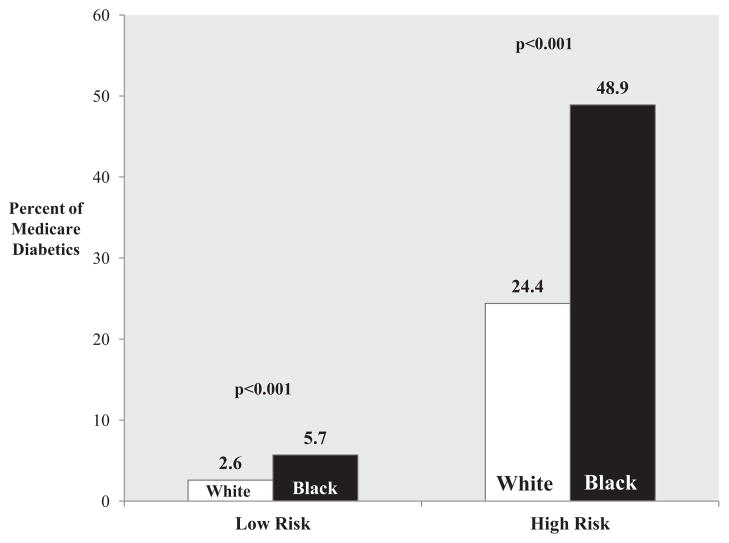
Overall, the proportion of African Americans was 25.1% in the amputee cohort vs 12.6% in nonamputee cohort (P < .001). In both high-risk and low-risk diabetic cohorts, African Americans had higher rates of amputations. The overall rate of amputations was 48.9/1000 among high-risk diabetic blacks vs 24.4/1000 among high-risk diabetic whites (P < .001). A similar difference existed in the low-risk cohorts, with an amputation rate of 5.7/1000 in blacks and 2.6/1000 in whites (P < .001; Fig 3).
Fig 3.
Amputation rate among Medicare diabetic patients by risk category and race.
DISCUSSION
This study sought to determine the effect of a national diabetes quality initiative, Healthy People 2010, on amputation rates among Medicare diabetics. Our analysis of an enhanced 5% sample of Medicare diabetic patients revealed a modest decrease, 8%, in the amputation rate over the course of the period studied, with most of the decline occurring among white patients with diabetes. However, amputation rates were significantly higher among African Americans and high-risk patients such as those with ESRD or multiple comorbidities. Although the average age of amputees declined slightly, among high-risk patients, amputees tended to be older than their low-risk counterparts. Furthermore, over the course of the study, amputees had a higher comorbidity burden. In 2000, Healthy People 2010 established a goal of decreasing the incidence of amputations among diabetic individuals from a baseline of 4.1/1000 in 2000 to 1.8/1000 in 2010, a 56% decline.4 Our data suggest that at the current rate of improvement, the rate of decline in the rate of amputations will be insufficient to achieve this goal. Some may question if the estimates from our enhanced Medicare data set (a 5% sample of all Medicare diabetics) are generalizable to the national population of diabetic individuals; however, our estimates align closely with those of the CDC and NIH, including Healthy People 2020, a recently published revision of Healthy People 2010, in which the estimated 2010 amputation rate among diabetic individuals was 4/1000.5,12
Given these modest improvements in amputation rates among diabetic individuals, should Healthy People 2010 be declared a success or a failure? Although the rate of decline was not as large as anticipated, the rate of amputation among individuals with diabetes fell by 8%, and most of this decline was among white diabetic patients. Racial disparity in diabetes care is not a new discovery.13 In the setting of establishing preventive measures, our findings highlight the need to focus specific efforts on traditional underserved populations. Furthermore, the population of amputees became more ill over the course of the study, with a doubling of the number of amputees with more than three comorbidities.
Before deciding whether this represents success or failure, one might consider these results in the context of similar quality improvement efforts in limiting amputations among diabetic individuals. Similar to Healthy People 2010, previously performed diabetes quality improvement initiatives have also been associated with a variable degree of success at reducing amputation rates. For example, analysis of U.S. Veterans Administration data, well-known for its broad implementation of quality improvement measures, demonstrated a decline in amputation rate from 7/1000 patients to 4.6/1000 patients between 2000 and 2004.14
Second, analysis of all amputations among insulin-dependent diabetic patients in the United Kingdom between 1996 and 2005 demonstrated an 11% decline.15 Therefore, although Healthy People 2010 did not reach the ultimate goal of 1.8/1000 diabetic patients, the overall outcomes (amputation rates and magnitude of change in amputation rate over time) in Healthy People 2010 were similar to the outcomes observed in other quality improvement forums.
Our analysis suggests that to achieve progressive and meaningful reduction in amputations among patients with diabetes, quality initiatives need to focus on high-risk groups and African Americans. Limiting amputation risk in these groups will be difficult, however. Diabetic patients undergoing amputation are now younger, with more burden of comorbid illness. Disparities in care between African American and white patients have a multitude of complex associations involving several domains, including socioeconomic status and access to care.16 Regardless of these challenges, our data suggest that quality improvement efforts need to further investigate and target their efforts on these patients to maximize the reduction in amputation.
Our study has several limitations. First, because our data set is based on administrative data, our ability to perform risk adjustment by the severity of diabetes is limited. For instance, quantitative assessment of hemoglobin A1c, insulin-dependence, and attempts at lower extremity revascularization for those with underlying peripheral vascular disease were not available and would have provided further insight into the character and severity of disease.
Second, operation-specific details are lacking in the database. Amputation was coded as a binomial variable, without taking into account the level at which the extremity was amputated, and certainly, an above-knee amputation incurs markedly different disability than a minor forefoot or toe amputation. However, even minor amputations are important in this context because any tissue loss in diabetic patients is strongly associated with a further risk of future amputation.17
Third, an important limitation of the applicability of our results to the general diabetic population is that not all diabetic patients receive medical care in the Medicare system. The effect this subset of patients has on the population-based assessment of amputation rates was not captured in our analysis.
Lastly, because we used the last version of the enhanced Medicare data set, the most recent data within the database are from 2006. Thus, analysis and inferences on more recent trends will necessitate a more recent composition of enhanced Medicare data.
CONCLUSIONS
Amputation rates have modestly declined in Medicare diabetic patients during a period of growing awareness and propagation of diabetes preventive care. However, significant disparity remains among African Americans and those with a greater comorbidity burden. Future quality improvement initiatives should focus on these groups to have maximum preventive effect.
Footnotes
Author conflict of interest: none.
Presented at the 2011 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Ill, June 16–18, 2011.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: JG, PG, JC, FB
Analysis and interpretation: JG, PG, JC, FB
Data collection: JG, PG
Writing the article: JG, PG
Critical revision of the article: JG, PG, JC, FB
Final approval of the article: JG, PG, JC, FB
Statistical analysis: JG, PG
Obtained funding: Not applicable
Overall responsibility: JG
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Feinglass J, Pearce WH, Martin GJ, Gibbs J, Cowper D, Sorensen M, et al. Postoperative and late survival outcomes after major amputation: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program. Surgery. 2001;130:21–9. doi: 10.1067/msy.2001.115359. [DOI] [PubMed] [Google Scholar]
- 3.The Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions [version 3.1] Rockville, MD: AHRQ; 2007. [Google Scholar]
- 4.U.S. Department of Health and Human Services. [Accessed December 1, 2010];Healthy People 2010. Available at: http://www.healthypeople.gov/2010/
- 5.U.S. Department of Health and Human Services. [Accessed December 1, 2010];Healthy People 2020. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=8.
- 6.World Health Organization. [Accessed December 1, 2010];Working to ensure quality care for persons with diabetes. Available at: http://www.who.int/diabetesactiononline/en/index.html.
- 7.The Health and Social Care Information Centre. Quality and outcomes framework achievement data 2009/10. National Institute for Health and Clinical Excellence; [Accessed December 1, 2010]. Available at: http://www.ic.nhs.uk/webfiles/QOF/2009-10/QOF_Achievement_Prevalence_Bulletin_2009-10_v1.0.pdf. [Google Scholar]
- 8.van Houtum WH, Rauwerda JA, Ruwaard D, Schaper NC, Bakker K. Reduction in diabetes-related lower-extremity amputations in The Netherlands: 1991–2000. Diabetes Care. 2004;27:1042–6. doi: 10.2337/diacare.27.5.1042. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed December 1, 2010];CMS Chronic Condition Data Warehouse User Manual. Available at: http://www.ccwdata.org/analytic-guidance/index.htm.
- 10. [Accessed April 10, 2010];CMS Chronic Condition Data Warehouse Condition Categories 10/2010. Available at: http://www.ccwdata.org/chronic-conditions/index.htm.
- 11.Tseng CL, Rajan M, Miller DR, Hawley G, Crystal S, Xie M, et al. Use of administrative data to risk adjust amputation rates in a national cohort of Medicare-enrolled veterans with diabetes. Med Care. 2005;43:88–92. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. [Accessed April 1, 2010];Healthy People 2010: Final Review. Available at: http://www.cdc.gov/nchs/data/hpdata2010/hp2010_final_review.pdf.
- 13.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;41:1221–32. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- 14.Tseng CL, Rajan M, Miller DR, Lafrance JP, Pogach L. Trends in initial lower extremity amputation rates among veterans health administration health care system users from 2000 to 2004. Diabetes Care. 2011;34:1157–63. doi: 10.2337/dc10-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vamos EP, Bottle A, Majeed A, Millett C. Trends in lower extremity amputations in people with and without diabetes in England, 1996 –2005. Diabetes Res Clin Pract. 2010;87:275–82. doi: 10.1016/j.diabres.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Brown AF, Gregg EW, Stevens MR, Karter AJ, Weinberger M, Safford MM, et al. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2005;28:2864–70. doi: 10.2337/diacare.28.12.2864. [DOI] [PubMed] [Google Scholar]
- 17.Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48:1464–71. doi: 10.1016/j.jvs.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]