Abstract
There is an ever pressing need to develop new drugs for the treatment of cancer. Gallium nitrate, a group IIIa metal salt, inhibits the proliferation of tumor cells in vitro and in vivo and has shown activity against non-Hodgkin’s lymphoma and bladder cancer in clinical trials. Gallium can function as an iron mimetic and perturb iron-dependent proliferation and other iron-related processes in tumor cells. Gallium nitrate lacks cross resistance with conventional chemotherapeutic drugs and is not myelosuppressive; it can be used when other drugs have failed or when the blood count is low. Given the therapeutic potential of gallium, newer generations of gallium compounds are now in various phases of preclinical and clinical development. These compounds hold the promise of greater anti-tumor activity against a broader spectrum of cancers. The development of gallium compounds for cancer treatment and their mechanisms of action will be discussed.
Research into the potential anticancer activity of gallium was stimulated by the discovery that 67Ga, when injected into rodents bearing implanted tumors, localized in high concentration within these tumors. This discovery led the development of the 67Ga scan as a method for detecting tumors in patients and prompted further evaluation of the potential antineoplastic activity of stable gallium salts. In studies conducted at the National Cancer Institute (USA), the antineoplastic activities of gallium, thallium, indium and aluminum salts were investigated in tumor-bearing rodents [1]. Of these metals, gallium nitrate was found to display the greatest in vivo anti-tumor activity and, as a result, it was further evaluated for toxicity in animals and then for both toxicity and antineoplastic activity in humans in Phase I and II clinical trials [2–4]. These studies showed gallium nitrate to have appreciable antineoplastic activity in certain malignancies, as well as to display additional interesting effects in other non-neoplastic conditions [5]. The results of these early studies have subsequently stimulated further research in the field, which has led to the development of newer gallium-based compounds that have the promise of greater antineoplastic activity than the parent compound gallium nitrate. Beginning with gallium nitrate, we will review the different gallium-based metallodrugs that have emerged as cancer therapeutic agents in the preclinical and clinical arenas. Since these gallium compounds have mechanisms of action that are similar in some ways, but different in others, the known mechanisms of antineoplastic activity of these agents will be discussed collectively in the latter part of this review. Important gallium compounds in cancer treatment and the status of their advancement from the laboratory to the clinic are summarized in Table 1.
Table 1.
Gallium compounds in cancer treatment: advancement from the laboratory to the clinic.
| Gallium complex | In vitro studies | Animal studies | Human studies |
|---|---|---|---|
| Gallium nitrate | Cytotoxic against a spectrum of malignant cell lines, especially lymphoma and leukemia cells | Antineoplastic activity demonstrated in rodent tumor models Animal toxicity profile established |
Human-toxicity profile established in clinical Phase I studies. Antineoplastic activity against bladder cancer and non-Hodgkin’s lymphoma demonstrated in numerous Phase II clinical studies. Combination chemotherapy trials conducted |
| Gallium chloride | Cytotoxic against a variety of malignant cell lines at concentrations similar to gallium nitrate | Intraperitoneal administration inhibited the growth of adenocarcinoma CA755 in mice. Low bioavailability with oral administration. Tissue-distribution studies conducted | Clinical pharmacology studies with oral GaCl3 conducted in lung cancer patients. Partial responses to treatment were seen in patients with ovarian cancer |
| G4544 | Not reported | Pharmacokinetic studies conducted | Clinical pharmacology studies conducted |
| Gallium maltolate | IC50 values lower than gallium nitrate. Inhibits the growth of lymphoma cells resistant to gallium nitrate | Pharmacokinetic studies conducted | Clinical pharmacology studies conducted. Anti-tumor activity demonstrated in a patient with hepatocellular carcinoma |
| Tris(8-quinolonato)gallium(III) (KP46) | Cytotoxic in melanoma, ovarian, breast, colon and lung cancer cell lines. IC50 values lower than that reported for gallium nitrate | Tissue distribution studies conducted in mice. Anti-tumor activity demonstrated in vivo | Clinical pharmacology and toxicity studies conducted in clinical Phase I/II trials. Anti-tumor activity noted in renal cancer |
| Pyridine and phenolate ligand complexes of gallium | Cytotoxic to cisplatin-resistant neuroblastoma cells and prostate cancer cell lines. Inhibitors of proteasome activity | Anticancer activity demonstrated against PC-3 prostate cancer xenografts in a nude mouse model | Not reported |
| Gallium tris(salicylate) ethanol | Inhibits the growth of Erlich carcinoma cell lines | Inhibits the growth of Erlich carcinoma cells in ascites in mice | Not reported |
| Gallium thiosemicarbazones | Complexes of gallium with thiosemicarbazones display greater cytotoxicity than either component alone in colon, breast, ovarian and brain tumor cell lines. Cytotoxicity not influenced by p53 mutations | Not reported | Not reported |
| Gallium-pyridoxal isonicotinyl hydrazone | Cytotoxic to solid tumor and lymphoma cell lines, including those resistant to gallium nitrate | Not reported | Not reported |
| Gallium complexes with azole ligands | Cytotoxic to breast, ovarian, cervical and colon cancer cell lines | Not reported | Not reported |
| Bi- and tetra-nuclear gallium(III) complexes with heterocyclic thiolato ligands | Cytotoxic against human and murine solid tumor cell lines, including breast and cervical cancer | Not reported | Not reported |
Similarities between gallium(III) & iron(III)
Gallium(III) shares certain properties with iron(III) with respect to its ionic radius. The octahedral ionic radius is 0.620 Å for Ga3+ and 0.645 Å for high spin Fe3+, respectively; while the tetrahedral ionic radius is 0.47 Å for Ga3+ and 0.49 Å for Fe3+, respectively [6]. The ionization potential and electron affinity values for Ga3+ are 64 eV and 30.71 eV, respectively, while those for high spin Fe3+ are 54.8 eV and 30.65 eV, respectively [6]. As a result of these properties, gallium is able to form complexes with proteins and ligands that bind iron, such as transferrin, the iron-transport protein in blood, and certain organic compounds. The aqueous chemistry of gallium has been summarized by Bernstein [6]. Ga3+ has been shown to bind to the two metal-binding domains of transferrin with binding constants log K1 = 20.3 and log K2 = 19.3 at normal plasma bicarbonate concentrations; these binding constants are comparable to binding constants of log K1 = 22.8 and log K2 = 21.7 reported for the binding of Fe3+ to transferrin [7,8]. These metal–protein interactions are relevant to the development of gallium compounds as therapeutic agents in cancer [9]. At concentrations of up to 50 μM in the blood, gallium binds almost entirely to transferrin while at higher gallium concentrations that exceed transferrin saturation, the percentage of gallium bound to transferrin diminishes and gallium circulates as gallate, [Ga(OH)4−][6]. Despite gallium’s similarities with iron, it differs from iron in that it does not transition from a trivalent to a divalent state and cannot participate in redox reactions.
Gallium nitrate
Gallium nitrate, a simple gallium salt, shown in Figure 1A, can be considered a ‘first-generation’ gallium compound that was investigated for its anticancer activity in humans [2]. This drug is, therefore, the standard against which newer gallium compounds should be compared. In Phase II clinical trials conducted to evaluate the spectrum of its antineoplastic activity, gallium nitrate was administered to patients with a variety of different cancers, including breast, prostate, lung, ovarian, cervical, bladder, renal, melanoma, sarcoma, chronic lymphocytic leukemia, Hodgkin’s lymphoma and non-Hodgkin’s lymphoma. Of these malignancies, gallium nitrate was found to have antineoplastic activity primarily against advanced bladder cancer and non-Hodgkin’s lymphoma [10–12]. In these malignancies, significant responses to gallium nitrate were seen in patients whose tumors had relapsed or failed to respond to conventional chemotherapy. Encouraged by these findings, a limited number of clinical trials ensued in which gallium nitrate was administered in combination with other chemotherapeutic agents (vinblastine, ifosfamide, 5-f luorouracil, mitoguazone, etoposide and hydroxyurea) to patients with bladder cancer or non-Hodgkin’s lymphoma. These trials demonstrated that gallium nitrate could be safely combined with other drugs with a good clinical outcome [10–12]. An important consideration in gallium nitrate-based studies is that, unlike most chemotherapeutic drugs, gallium nitrate does not suppress the white blood cells or platelets and it can, therefore, be used to treat patients with low blood counts or can be combined with other antineoplastic agents without exacerbating their myelosuppressive effects.
Figure 1.
Gallium complexes (A–H).
Gallium nitrate has been administered to patients by two different treatment schedules. In the early clinical trials, patients received this drug at a dose of 700 mg/m2 by rapid intravenous infusion over 15–30 min. Although responses to treatment were noted, this schedule resulted in high peak plasma levels of gallium and toxicity to the kidneys that proved to be dose limiting. Under these conditions, 69 and 91% of the gallium administered were excreted in the urine by 24 and 48 h, respectively [13]. In a second treatment schedule, patients received gallium nitrate by continuous intravenous infusion at a dose of 200–400 mg/m2/day over 5–7 days. Steady-state gallium levels of 0.9–1.9 μg/ml were achieved during the infusion; these levels decreased to 0.45–0.7 μg/ml 4 days after gallium was discontinued. The continuous infusion schedule also allowed patients to receive a greater amount of gallium over time when compared with the brief infusion schedule [14]. Moreover, continuous intravenous infusion gallium nitrate for 5–7 days was better tolerated by patients and it is now the recommended mode of administration for this drug. Alternative treatment schedules for gallium nitrate that are less cumbersome warrant further investigation. It should be noted that, while the anticancer activity of gallium nitrate has been examined in the aforementioned malignancies, its action against a number of other malignancies remains to be investigated. However, while efficacious, the continuous intravenous treatment schedule for gallium nitrate is inconvenient since it requires that patients receive this drug intravenously in the hospital or as an outpatient via a pump device. Unfortunately, the bioavailability of oral gallium nitrate is poor, thus limiting the use of gallium nitrate to the intravenous route.
Gallium nitrate has important effects that are independent of its anti-tumor activity. In the course of its development as an antineoplastic agent, gallium nitrate was found to impact on bone metabolism and to reduce calcium levels in the blood [15]. These observations led to additional trials in patients with cancer and metabolic bone disease that confirmed its ability to inhibit bone resorption and lower pathologically elevated blood calcium levels [15]. As a result, gallium nitrate was approved by the US FDA as a prescription drug for the treatment of malignancy-associated hypercalcemia.
Gallium chloride
Early investigations into the anti-tumor activity of gallium salts demonstrated that gallium chloride, like gallium nitrate, inhibited the proliferation of malignant cell lines in vitro at concentrations that were within the range seen with gallium nitrate. The antineoplastic activity of gallium chloride has been reviewed by Collery et al. [3]. Intraperitoneal administration of gallium chloride to mice bearing adenocarcinoma CA755 produced a decrease in tumor growth [16]. In contrast, daily oral administration of gallium chloride to C3H/HeJ mice bearing C3HBA mammar y adenocarcinoma resulted in significant intratumor concentrations of gallium but cytotoxicity was less than that seen with the parenteral route [17]. Similar to gallium nitrate, the bioavailability of gallium is low following oral administration of gallium chloride. Nonetheless, with prolonged oral administration of gallium chloride to rats, gallium was shown to accumulate in tissues, with the greatest gallium concentrations seen in bone and lung [18]. Lower accumulation of gallium occurred in the spleen, kidneys, liver, adrenals and ovaries, while even lower concentrations of gallium were present in muscle and the brain [18]. In clinical trials, oral gallium chloride at doses of 100–1400 mg/24 h produced partial responses in ovarian cancer but not in lung cancer [3,19]. While the low bioavailability of oral gallium chloride appears to have dampened its further development as an antineoplastic agent, it has stimulated the development of newer gallium compounds with much greater oral bioavailability [3].
G4544
G4544 is an oral tablet formulation of gallium nitrate manufactured using a proprietary drug-delivery technology developed by Emisphere Technologies. The drug is presently in Phase I clinical trials [20]. Preclinical studies in dogs showed that the drug was rapidly absorbed and achieved a mean plasma level of 1800 ng/ml, which was fourfold greater than that achieved with gallium nitrate. Long elimination half-lives of 101 and 338 h were seen in rat and dog, respectively. In a Phase I clinical trial, 30 normal volunteers received a single oral dose of G4544 at doses ranging from 30–150 mg [20]. At the highest dose level, a peak gallium plasma concentration of 485 ng/ml was achieved, which is similar to levels seen with continuous intravenous gallium nitrate. G4544 was tolerated without adverse side effects. These initial Phase I results are encouraging and further trials to examine the efficacy of this agent in skeletal diseases and cancer are planned [20].
Gallium maltolate
Gallium maltolate, tris(3-hydroxy-2-methyl-4H-pyran-4-onato)gallium (Figure 1B) is a gallium compound that has been developed for oral use. It consists of three maltolate ligands bidentately bound to a central gallium atom in a propeller-like arrangement, with one of the ligands disordered in two possible orientations [21]. Maltol, 3-hydroxy-2-methyl-4-pyrone, is a hydroxypyrone with high affinity for a range of metal ions [22]; it has been shown to enhance the gastrointestinal absorption of orally administered iron in iron-deficient and normal subjects [22,23]. Bernstein et al. compared the bioavailability of orally administered or intraduodenal administered (via a feeding tube) gallium maltolate solution with that of oral gallium nitrate solution in male beagle dogs [21]. They showed that the maximum serum concentration of gallium achieved with the administration of 1.5 mg/kg of gallium was 2.2 μg/ml and 1.47 μg/ml for oral and intraduodenal administered gallium maltolate, respectively, and was 0.3 μg/ml for intraduodenal administered gallium nitrate solution. The same group examined the bioavailability of gallium in human subjects following a single oral dose of 100–500 mg of gallium maltolate (equivalent to 15.7–78.4 mg of gallium). The maximum serum concentrations of gallium achieved were 0.115 μg/ml and 0.569 μg/ml with the 100- and 500-mg doses, respectively [21]. Allamneni et al. reported that following oral administration of gallium maltolate to normal subjects, gallium in the circulation is found bound to transferrin, the iron transport protein, with very little of it unbound [24]. These findings are consistent with the earlier studies of Vallahajosula et al., which showed that 67Ga injected intravenously in rabbits binds exclusively to transferrin in circulation [25].
Preclinical investigations of the cytotoxicity of gallium maltolate in various cell lines in vitro have shown that the IC50 value of gallium maltolate (concentration required to inhibit cell growth by 50%) is significantly lower than that of gallium nitrate. In four hepatocellular carcinoma cell lines, the IC50 values of gallium maltolate ranged from 25 to 35 μM while the IC50 value of gallium nitrate ranged from 60 to 250 μM [26]. In another study utilizing a panel of lymphoma cell lines, gallium maltolate was shown to induce apoptosis at earlier time points and at significantly lower concentrations than gallium nitrate [27]. The basis for the enhanced sensitivity of certain cancer cells to gallium maltolate over gallium nitrate remains to be determined; however, it may, in part, be related to more efficient entry of the former compound into cells [27].
In addition to displaying greater cytotoxicity when compared with gallium nitrate, gallium maltolate was shown to block the proliferation of lymphoma cells resistant to growth inhibition by gallium nitrate. In studies utilizing human lymphoma/leukemic CCRF-CEM cells resistant to gallium nitrate, the IC50 of gallium maltolate was 72 μM, whereas the IC50 for gallium nitrate was >400 μM [27].
It is known that mutations of p53 may impact the antineoplastic activity of certain chemotherapeutic drugs. However, the cytotoxicity of gallium maltolate does not appear to be influenced by p53 mutations in B-lymphoma cells. Similar growth–inhibitory activity of gallium maltolate in vitro was seen in p53 wild-type TK-6 cells, p53-null NH-32 cells and p53-mutant WTK-1 cells [27]. In contrast, gallium nitrate did not significantly inhibit the growth of WTK-1 cells [27]. The observation that cells with acquired or inherent resistance to gallium nitrate remain sensitive to the cytotoxicity of gallium maltolate indicates that these gallium compounds do not share cross resistance; it also suggests that tumors that fail to respond to gallium nitrate may respond to gallium maltolate in vivo.
Evidence that gallium maltolate has antineoplastic activit y in humans was recently provided by Bernstein et al. [28]. These investigators reported a patient with advanced hepatocellular carcinoma that had failed to respond to treatment with the drug sorafenib. Avid uptake of 67Ga by the tumor on radiogallium scanning suggested that the hepatic mass would incorporate nonradioactive gallium. Treatment of this patient with 1500 mg/day of oral gallium maltolate resulted in marked clinical improvement of the patient’s symptoms and a significant reduction in the size of the hepatic mass, as measured by imaging studies of the liver [28]. Further studies to evaluate the antineoplastic activity of oral gallium maltolate in hepatoma, lymphoma, bladder cancer and other malignancies are warranted.
Tris(8-quinolonato)gallium(III) (KP46)
Tris(8-quinolonato)gallium(III) (KP46), a gallium complex with an organic ligand 8-quinolinol is currently in clinical trials as an oral gallium compound (Figure 1C). KP46 is reported to have an n-octanol: water partition coefficient, log P value, of 0.88, which represents a hydrophilic/lipophilic balance that should favor adequate membrane permeability with oral administration [29]. The high thermodynamic stability of KP46 (log β3 = 40.7), kinetic stability and the observation that this complex retains chemical stability in solution for hours suggests that, unlike gallium maltolate, orally absorbed KP46 may not rapidly release gallium to transferrin in the circulation, but may do so within an acidic tumor environment [30].
Comparative studies by Collery et al. showed that tris(8-quinolinolato)gallium(III) possesses a tenfold greater growth-inhibitory effect on malignant cells when compared with gallium chloride [31]. The IC50 values of KP46 in a variety of melanoma, ovary, breast, colon and lung cancer cell lines in vitro ranged from 0.85 to 10.4 μM [32], which is much lower than that reported for gallium nitrate by others. In addition, the combination of KP46 and platinum drugs acts synergistically to inhibit the proliferation of ovarian and colon cancer cell lines in vitro [33].
Studies examining the preclinical toxicology and tissue distribution of oral KP46 in mice defined the LD50 dose to be 2870 mg/kg (410 mg Ga3+/kg) and 2370 mg/kg (339 mg Ga3+/kg) for males and females, respectively; a dose of 62.5 mg/kg/day for 14 days was well-tolerated and showed the highest accumulation of gallium in bone, followed by liver, spleen and kidney [34]. Additional studies conducted in animal tumor models confirmed that KP46 produced a reduction in tumor mass in vivo and a lowering of serum calcium levels. The latter finding indicates that in addition to its antineoplastic activity KP46 (like gallium nitrate) may also be beneficial in the treatment of hypercalcemia.
In a Phase I/II clinical trial of oral KP46 in patients with solid tumors (renal, ovarian, stomach and parotid gland), the drug was found to be well-tolerated over a dose range of 30 to 480 mg/m2 daily for 14 days; no significant dose-limiting toxicities were observed [35,36]. Responses to treatment were seen in three out of four patients with renal cancer [35,36]. The treatment of renal cancer is challenging since this disease does not respond to conventional chemotherapy or to gallium nitrate; hence, the initial activity of KP64 in this malignancy is noteworthy and should be further explored.
Gallium thiosemicarbazones
Early studies demonstrated that many α-(N)-heterocyclic carboxaldehyde thiosemicarbazones possessed significant anti-tumor activity and were capable of chelating metals [37]. Saryan et al. showed that the iron complexes of these thiosemicarbazones were three- to six-fold more potent than the free ligand in inhibiting ribonucleotide reductase, the enzyme responsible for the synthesis of deoxyribonucleotides [38]. Kratz et al. reported on the synthesis of gallium(III) complexes of different 2-acet ylpyridine thiosemicarba zones [39]; while Arion et al. later demonstrated the anti-tumor action of gallium complexes with 2-acetylpyridine 4N-dimethylthiosemicarbazone in SW480 (colon adenocarcinoma), SK-B-3 (breast adenocarcinoma) and 41-M (ovarian cancer) cell lines in vitro, thus setting the stage for further development of this gallium complex [40]. Kowol et al. reported on the synthesis and cytotoxicity of gallium complexes with five different 4N-substituted α-N-heterocyclic thiosemicarbazones. One such complex is shown in Figure 1D [41]. Others showed that the coordination of 2-pyridineformamidethiosemicarbazones to gallium greatly increased their ability to induce apoptosis in glioblastoma cell lines in vitro [42]. In these studies, the coordination of these thiosemicarbazones to gallium increased their cytotoxicity by 15–37-fold in RT2 glioblastoma cells with wild-type p53 and by 7–36-fold in T98 glioblastoma cells with mutant p53. It is known that mutations in p53 may affect pathways that determine the sensitivity of cancer cells to chemotherapeutic drugs [43]. Therefore, it is notable that these thiosemicarbazone–gallium complexes were cytotoxic to both wild-type p53- and mutant p53-containing glioblastoma cells. Both RT2 and T98 cells were resistant to the cytotoxicity of gallium nitrate alone. These data indicate that it is the thiosemicarbazone–gallium complex per se and not the individual actions of thiosemicarbazone or gallium, which induces apoptosis in these cells.
Although several thiosemicarbazones have been examined in preclinical investigations, at this time only 3-aminopyridine-2-carboxyaldehyde thiosemicarbazone (3-AP, Triapine®) was advanced to Phase I and II clinical trials several years ago [44], and was recently shown to enhance the response to radiation therapy in cervical cancer [45]. The ability of gallium to potentiate the antiproliferative action of 3-AP was demonstrated in studies that compared the effects of iron and gallium complexes of 3-AP on ribonucleotide reductase and tumor cell proliferation in vitro; these studies showed that the cytotoxicity of this thiosemicarbazone was enhanced by gallium, but weakened by iron [41]. Further preclinical and clinical investigation to explore the antineoplastic activity of the gallium-3AP in vivo would be of interest.
Pyridine & phenolate ligand complexes of gallium
Shakya et al. reported the synthesis of five novel gallium(III) complexes described as [GaIII(LX)2]ClO4, where LX is a negatively charged ligand containing 2-methylpyridine and 2-methylphenolate groups attached to a secondary amine (Figure 1E) [46]. The phenol moiety has substituents (X) that encompass the electron-withdrawing and electron-donating methoxy (complex 1), nitro (complex 2), chloro (complex 3), bromo (complex 4) and iodo groups (complex 5) [46]. Complex 5 is shown in the right panel of Figure 1E. Complexes 2–5 displayed the greatest apoptosis-inducing activity in BE(2)-C neuroblastoma cells in vitro with the IC50 levels for these complexes ranging from 13.3–23.8 μM. In contrast, the IC50 value for complex 1 was 245.4 μM [46]. Chen et al. demonstrated that of these complexes, complex 5 (Figure 1E, right-hand panel) displayed the greatest induction of apoptosis in prostate cancer cell lines in vitro and inhibited the growth of PC-3 prostate cancer cell xenografts in nude mice by 66% [47]. Tumor extracts from mice treated with complex 5 showed an increase in ubiquitinated proteins, an accumulation of p27 (a proteasomal target protein), a decrease in proteasomal chymotrypsin activity, and an induction of apoptosis [47]. The interesting preclinical antineoplastic activity of these gallium complexes, especially complex 5, suggests that they have potential for further development as antineoplastic agents.
Other gallium complexes
Several additional gallium complexes that display antiproliferative activity against malignant cell lines in vitro have been reported. Many of these gallium complexes are in very early stages of their preclinical development and are yet to be advanced to human studies. Some examples of these complexes follow.
Gallium-pyridoxal isonicotinyl hydrazone
Pyridoxal isonicotinyl hydrazone (PIH) and its analogues belong to a class of lipophilic iron chelators that has been shown to inhibit the proliferation of a variety of malignant cells in vitro [48,49]. An interesting property of PIH is that it can also deliver iron to cells to support cell growth and function [50]. Consistent with its ability to facilitate metal uptake, PIH in a complex with gallium (Ga-PIH, Figure 1F) displayed greater cytotoxicity than PIH alone [48,51]. Ga-PIH was also shown to inhibit the growth of CCRF-CEM cells that were resistant to growth inhibition by gallium nitrate [52]. These gallium-resistant cells have a downregulation of transferrin receptor-mediated gallium uptake [53]. The ability of Ga-PIH to inhibit the growth of these gallium-resistant cells suggests that gallium, when coupled with PIH, is able to enter cells by a transferrin-independent route and, thus, induce cell death [52].
Gallium tris(salicylate) ethanol
Sodium salicylate has been shown to inhibit the proliferation of malignant cells in vitro [54]. Ismail et al. reported the synthesis of gallium tris(salicylate)-ethanol and showed that it inhibited the proliferation of Erlich’s ascites carcinoma cells in a dose-dependent fashion [55]. The antineoplastic activity of this gallium complex was examined by measuring the survival of Swiss mice inoculated intraperitoneally with Erlich ascitic carcinoma cells. At 30 days following inoculation of tumor cells, 30% of the mice treated with gallium tris(salicylate) ethanol had survived compared with 0% survivors in the untreated group of mice. However, although treatment with this gallium complex prolonged survival in tumor-bearing mice, the cancer cells were not completely eradicated [55].
Gallium complexes with azole ligands
The synthesis of gallium complexes of 2,1,3-benzothiadiazole, 1,2,3-benzotriazole, and 1-methyl-4,5-diphenylimidazole has been described by Zanias et al. [56]. Compound 1, a Ga(III) complex with 1,2,3-benzotriazole, is shown in Figure 1G. These compounds inhibit the proliferation of breast, ovarian, cervical and colon tumor cell lines in vitro [56].
Bi- & tetranucleargallium(III) complexes with heterocyclic thiolato ligands & dinuclear gallium carboxylate complexes
A variety of gallium complexes with these ligands (structure shown in Figure 1H) have been characterized and reported to display cytotoxicity against solid tumor cell lines in vitro [57,58]. The gallium(III)complex containing the thiolato ligand was shown to bind to fish sperm DNA and display greater cytotoxicity in malignant cell lines than in fibroblast cells [58–60]. The latter property is relevant to their further development as anticancer drugs.
Mechanisms of anti-tumor activity by gallium compounds
Action on cellular iron homeostasis
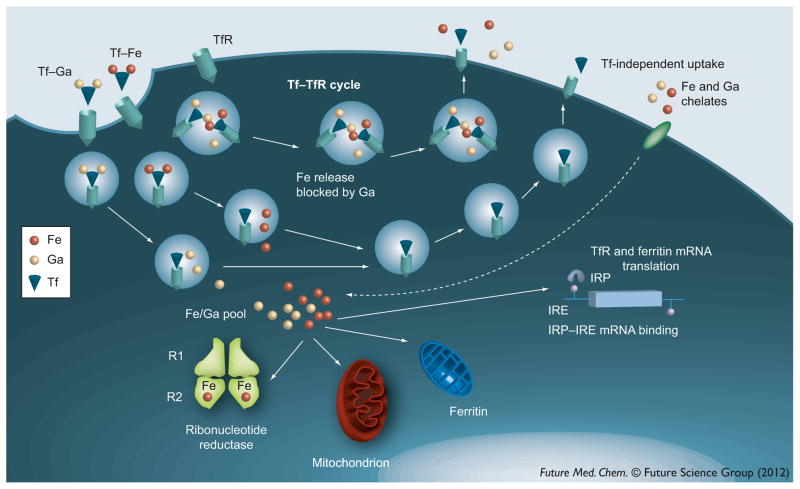
A brief discussion of cellular iron metabolism is relevant to understanding some of gallium’s mechanisms of action. Some of the steps in the interaction of gallium with cellular iron uptake and trafficking are summarized in Figure 2. Iron plays an important role in the function of numerous proteins critical for cell viability and proliferation. It is an essential component of numerous iron–sulfur-containing enzymes of the citric acid cycle and the mitochondrial electron-transport chain. Iron is required for the activity of the R2 subunit of ribonucleotide reductase, the enzyme responsible for deoxyribonucleotide synthesis, a rate-limiting step in DNA synthesis [61]. In the absence of iron, this subunit exists as a non-functional apoR2 protein leading to a block in the synthesis of deoxyribonucleotides [62]. More recent studies have revealed that iron is also needed for the transition of cells through the G1 phase of the cell cycle [63]. While iron deprivation may lead to loss of cell proliferation and viability, excess iron may be equally deleterious as it can serve as a catalyst for the generation of cytotoxic hydroxyl radicals via the Fenton reaction [64]. Hence, iron homeostasis is tightly regulated in the cell through a complex interplay of various proteins responsible for regulating its transport, cellular uptake, intracellular trafficking, storage and efflux from cells [65]. Several studies have demonstrated that cancer cells have a significantly higher requirement for iron than normal cells. Iron is transported in the circulation bound to transferrin and its uptake by cells occurs via transferrin receptor-mediated endocytosis of transferrin–iron complexes. An increase in the number of cell surface transferrin receptors is seen in lymphoma, bladder cancer and breast cancer in vivo [66–68]. In fact, an increased level of transferrin receptor expression has been correlated with an aggressive clinical behavior and adverse outcome in lymphoma, urothelial cancer and other malignancies [66,67,69]. The level of intracellular ferritin, the iron storage protein, is known to be altered in certain malignant cells [68,70]. Recently, it was shown that cancer cells in patients with breast cancer display a decrease in their expression of the iron efflux protein ferroportin. In breast cancer patients, the combined effect of decreased tumor ferroportin and elevated serum hepcidin (a protein that degrades hepcidin) was associated with a poorer clinical outcome than patients who did not display this ferroportin/hepcidin profile [71]. An interpretation of this finding is that a decrease in ferroportin and an increase in transferrin receptor would be expected to elevate intracellular iron status to support aggressive tumor cell growth. Hence, the above clinical findings provide support to the notion that certain tumor cells have a greater requirement for iron than normal cells. It also indicates that treatment strategies directed at iron-dependent tumor growth have potential in cancer therapeutics.
Figure 2. Cellular uptake and interaction of iron and gallium.
Iron(III) and gallium(III) are taken up by cells via transferrin receptor-mediated endocytosis of transferrin–iron (Tf–Fe3+) and transferrin–gallium (Tf–Ga3+). These complexes compete with each other for binding to the cell surface TfR. Theoretically, the endosome can contain Tf-Fe3+ or Tf-Ga3+, or both Tf-Fe3+ and Tf-Ga3+ in variable proportions. Fe3+ and Ga3+ are unloaded from transferrin in an acidic endosome and transferred to an intracellular labile pool containing both iron and gallium. However, gallium may also block the unloading of iron from the endosome, thus resulting in recycling of both metals back to the cell surface to be released to the exterior. In some cells, iron and gallium may enter cells through a transferrin-independent route. Under physiological conditions, the iron in the pool is utilized for ribonucleotide reductase activity, mitochondrial function and other iron-dependent processes. Excess iron(III) is stored in ferritin. Transferrin receptor and ferritin syntheses are regulated by cellular iron status via the interaction of IRP with IREs present on the 5′ or 3′ untranslated regions of the ferritin or transferrin receptor mRNAs, respectively. Gallium can disrupt cellular iron homeostasis and inhibit cell proliferation through action on iron-dependent processes at different levels.
IRE: Iron-response element; IRP: Iron regulatory protein; TfR: Transferrin receptor.
Several studies have suggested that gallium acts as an iron mimetic that can perturb cell proliferation by interposing itself into iron-dependent processes. The mechanisms of action of gallium nitrate and transferrin–gallium can be viewed as occurring in two steps (Figure 2). The first involves the preferential targeting of transferrin–gallium to transferrin receptor 1-expressing tumor cells followed by its uptake via transferrin receptor-mediated endocytosis. Under physiological conditions, approximately one-third of transferrin in the circulation is occupied by iron thus leaving the remainder of transferrin available to bind gallium and to deliver it to cells. The second step involves the action of intracellular gallium on iron-dependent processes resulting in disruption of iron homeostasis and cell death. Gallium, as transferrin–gallium, blocks cellular iron uptake by competitively inhibiting the binding of transferrin–iron to cell surface transferrin receptors and by interfering with the dissociation of iron from transferrin in the endosome [72]. In the latter situation, gallium interferes with endosomal acidification, a step necessary for the release of iron from transferrin [72]. The net result of gallium’s action at this level is cellular iron deprivation [72]; this, in turn, can impact on downstream iron-dependent processes. Iron deprivation has been shown to produce apoptosis [73,74]. Evidence that gallium actually produces cellular iron deficiency in vivo was presented in clinical studies that showed that red blood cells from patients receiving gallium nitrate contained increased levels of zinc protoporhyrin, a marker of iron deficiency [75], and that anemia in patients being treated with gallium chloride may be due to gallium-induced iron deficiency [76].
The effect of gallium on cellular iron homeostasis is, however, not limited to the production of cellular iron deprivation through blockade of iron uptake. Within cells, gallium inhibits DNA synthesis through action on the iron-containing R2 subunit of ribonucleotide reductase [77–79]. Cytoplasmic extracts from cells incubated with gallium contain apoR2 protein that lacks an EPR-detectable tyrosyl free radical signal; this signal can be restored within minutes by the addition of iron salt, thus indicating that gallium blocks the interaction of iron with R2 without decreasing the level of R2 protein [79]. Consistent with inhibition of ribonucleotide reductase activity, cells exposed to gallium display decreased levels of deoxyribonucleotides [77]. Although it could be surmised that the inhibition of R2 subunit activity by gallium results from gallium-induced cellular iron deprivation, other studies show that gallium directly inhibits CDP and ADP reductase activity in a cell-free enzyme assay [78]. Hence, it is likely that ribonucleotide reductase inhibition by gallium occurs through both indirect (cellular iron deprivation) and direct mechanisms.
Actions of gallium unrelated to iron homeostasis
Whereas perturbation of cellular iron homeostasis and inhibition of ribonucleotide reductase are sufficient to inhibit cell proliferation, many of the upstream and downstream events involved in gallium-induced cell death remain to be elucidated. Moreover, it is possible (and likely) that steps independent of iron and iron proteins also contribute to the cytotoxicity of gallium. Early studies into the mechanism of action of gallium showed that it could partially inhibit DNA polymerases, however, the concentration of gallium required to produce this effect could not be correlated with its anti-tumor activity [80]. Inhibition of tyrosine phosphatase activity by gallium nitrate in Jurkat leukemia and HT-29 human colon cancer cell lines has been demonstrated, but since this effect could not be correlated with an inhibition of proliferation its contribution to the antineoplastic activity of gallium is unclear. Competitive inhibition of magnesium-dependent ATPase and inhibition of tubulin formation by gallium has been demonstrated [81–85]; these effects could impact tumor cell growth. Studies into the mechanisms of action of the pyridine and phenolate ligand complexes of gallium have revealed an important target for these compounds: the proteasome. Complexes 2–5 of these gallium compounds (Figure 1E) inhibited proteasome function and induced apoptosis in cells in vitro and in a prostate cancer xenograft in a rat model [47]. In other studies, gallium maltolate was found to inhibit proteasome activity and to synergistically enhance the cytotoxicity of the proteasome inhibitor bortezomib [86]. These mechanisms of action of gallium are important since in recent years the proteasome has emerged as an important target for cancer therapeutics in multiple myeloma and certain lymphomas; these malignancies have an increased sensitivity to proteasome inhibition [87,88]. A report by Niesvizky et al. suggested that patients with multiple myeloma treated with gallium nitrate for bone disease had a better survival than those who did not receive this drug [89]. The action of certain gallium compounds on proteasome function is relevant to the further development of these compounds for the treatment of tumors that are sensitive to proteasome inhibition.
Induction of apoptosis by gallium & cellular adaptation to gallium
Recent investigations conducted in human leukemic/lymphoma cells show that the pathways involved in gallium-induced cell death include the mitochondria [27,90]. Earlier studies had reported an effect of gallium on calcium retention in rat liver mitochondria [91]. Human leukemia/lymphoma cells exposed to gallium nitrate or gallium maltolate display an initial translocation of inositol phosphotidylserine to the cell surface, an early marker of apoptosis. This is followed by a loss of mitochondrial membrane potential leading to the release of cytochrome C from the mitochondria to the cytoplasm and the activation of caspase-3 and morphologic changes of apoptosis (both of which can be inhibited by z-VAD-fmk, a pancaspase inhibitor) [27,90]. In DoHH2 and CCRF-CEM cells exposed to gallium nitrate, the loss of mitochondrial membrane potential is associated with activation of cytoplasmic Bax, a proapoptotic protein. It is known that activated Bax translocates to the mitochondria to induce the loss of mitochondrial membrane potential that precedes apoptosis [92]. Exposure of cells to gallium maltolate results in a loss of mitochondrial membrane potential much more rapidly than gallium nitrate [27]; this may occur without the involvement of Bax [Chitambar CR, Unpublished Data].
The cellular handling of gallium is only partly understood. Although gallium compounds can induce cell death, the initial response of cells to gallium appears to be to mount a cytoprotective response. A study of metal-related genes expressed in CCRF-CEM cells sensitive or resistant to growth inhibition by gallium nitrate revealed a marked upregulation of MT2A and zinc transporter-1 (a zinc efflux protein) genes in gallium-resistant cells [93]. These findings were entirely unexpected since MT2A is a low-molecular weight protein that binds divalent metals. MT is involved in the metabolism of zinc and the sequestration of cadmium (to decrease its toxicity); it is not involved in iron metabolism. An early study by Capel et al. reported a lowering of tissue zinc levels in C57/BL mice treated with gallium nitrate [94], while the more recent studies clearly demonstrate an impact of gallium on zinc metabolism [93]. The extent to which cellular MT levels influence gallium’s cytotoxicity remains to be determined. Upregulation of MT in CCRF-CEM cells by pre-incubation of cells with endogenous zinc sulfate partially diminished the growth-inhibitory effect of gallium; this protection was lost when MT levels returned to baseline [93]. In a panel of lymphoma cell lines, the cytotoxicity of gallium nitrate was shown to correlate with their endogenous level of MT [93]. Although these studies lend support to a role for MT in modulating gallium’s cytotoxicity, it remains to be determined whether this occurs through a direct effect (sequestration of gallium by MT) or an indirect effect mediated by another mechanism that also impacts on MT expression.
Other studies have demonstrated that gallium nitrate produces a shift in intracellular zinc and an increase in MT2A and HO-1 gene expression [95]. The activation of HO-1 expression by gallium involves the p38 MAP kinase pathway and the activation of Nrf2, a zinc finger transcription factor. The increase in both MT2A and HO-1 is possibly explained by the fact that these proteins can be upregulated in response to increased levels of reactive oxygen species (ROS) in gallium-treated cells. In support of this cause-and-effect relationship is the observation that the gallium-induced increase in metallothionein and HO-1 can be abrogated by the antioxidant N-acetyl cysteine [95]. An important question centers on the significance of the gallium-induced increase in MT and HO-1 expression. It is known that both these genes are activated in response to oxidative stress [96,97], and an early event seen within 4 h of exposure of CCRF-CEM cells to gallium nitrate is a decrease in the cellular GSH/GSSG ratio and an increase in intracellular oxidative stress [95]. Hence, gallium-induced increase in cellular ROS precedes the increase in MT and HO-1. These new insights into the cellular response to gallium suggest a model (shown in Figure 3) in which the initial exposure of cells to gallium results in an elevation of intracellular ROS which, in turn, invokes a cytoprotective response (i.e., an increase in MT2A and HO-1). Thus, cell death occurs only when these cytoprotective responses are overwhelmed. How gallium exposure results in ROS production and whether the cytoprotective response to gallium varies among different cell types remains to be determined.
Figure 3. Proposed model for the cytotoxic and cytoprotective events triggered by gallium nitrate.
An early event in the cellular response to gallium(III) is an increase in intracellular ROS of mitochondrial origin. Changes in zinc homeostasis along with ROS generation induce the expression of MT2A. HO-1 gene expression is triggered by ROS via the p38 MAP kinase pathway and activation of transcription factor Nrf2. MT2A and HO-1 are cytoprotective. The iron-dependent R2 subunit of ribonucleotide reductase is inhibited by gallium(III). Apoptosis is triggered through Bax activation and the mitochondrial release of Cyto c leading to caspase-3 activation. Direct action of gallium(III) on the mitochondria is also likely. Cell death ensues when cytoprotective responses are overwhelmed.
ARE: Antioxidant response element; Cyto c: Cytochrome c; MRE: Metal-responsive elements; ROS: Reactive oxygen species; RR: Ribonucleotide reductase.
Future perspective
Advances in the field of gallium research, championed primarily by a relatively small number of investigators, have enhanced our understanding of this interesting metal. However, there remain many areas for future development that warrant additional study to move gallium compounds from the laboratory to the clinic. Some of these areas are discussed below.
Gallium compounds that can target cells independently of transferrin
The binding of gallium to transferrin in the circulation can, theoretically, be a double-edged sword. On one hand, transferrin–gallium complexes have the advantage of directly targeting transferrin receptor-bearing malignant cells. On the other hand, the binding of gallium to transferrin may prevent it from reaching tumor cells that lack transferrin receptors or display low levels of these receptors. In the latter situation, the antineoplastic action of gallium on tumor cells lacking transferrin receptors may require plasma levels of gallium to exceed the metal-binding capacity of transferrin so that gallium’s cytotoxicity can be exerted through transferrin–independent mechanisms. In consideration of these factors, gallium compounds designed to deliver gallium to tumor cells independent of transferrin and its receptor may prove to be more effective against tumors with a low number of transferrin receptors.
Combination therapy with gallium compounds & other antineoplastic agents
Successes in cancer treatment have often stemmed from the use of several drugs in combination. Selecting drugs with synergistic interactions against cancer cells but not against normal cells is key in developing a scientifically based therapeutic strategy. In this regard, gallium compounds can synergistically increase the cytotoxicity of hydroxyurea, fludarabine, IFN-α, gemcitabine, bortezomib, paclitaxel and cisplatin; these drugs are currently in use for the treatment of various malignancies [33,77,86,98–101]. The results of these preclinical studies form a scientific rationale for further studies of gallium-based drug combinations and their evaluation in the clinic.
Biologic markers that predict tumor responsiveness to gallium-based therapy
Cancers are molecularly heterogeneous disorders; tumors that appear to be similar in morphologic appearance can display diverse clinical behavior and response to treatment. Tumor cell resistance to chemotherapeutic agents remains a significant obstacle to success in the treatment of cancer. Hence, the identification of markers (proteins, pathways, or processes) in cancer cells that predict tumor sensitivity to gallium compounds would be a significant advance since it would allow for the selection of patients most likely to benefit treatment with gallium metallodrugs. While reliable markers for tumor sensitivity to gallium treatment have not been established, preclinical studies have provided clues that are worthy of further rigorous investigation. For example, the intensity of cellular 67Ga uptake and cell surface transferrin receptor density would appear to be of value in determining whether gallium might target to a specific tumor. However, a caveat here is that the homing of gallium to a malignant cell does not necessarily mean that the cell will be killed since the mechanisms of gallium-induced cell death depends primarily on its action on intracellular targets. The discovery of elevated MT2A levels in lymphoma CCRF-CEM cells resistant to gallium nitrate begs the question as to whether measurement of metallothionein might have value in identifying gallium-responsive tumors in patients. Clearly, further research is needed to understand the intracellular targets of gallium in malignant cells. It is envisioned that such research will advance our ability to use gallium compounds optimally in the treatment of cancer.
Executive summary.
Gallium compounds in cancer treatment
Gallium compounds are unique metallodrugs with therapeutic potential in cancer and other diseases.
The first-generation gallium nitrate has, in several clinical trials, consistently demonstrated efficacy against bladder cancer and non-Hodgkin’s lymphoma while displaying an acceptable toxicity profile to normal cells.
In preclinical studies, the newer generation gallium compounds hold promise as agents with greater antineoplastic activity against a wider spectrum of cancers than gallium nitrate.
Similarities between iron & gallium chemistry, & gallium’s mechanisms of anti-tumor activity
The unique mechanism of antineoplastic action of gallium that involves its ability to target and disrupt tumor cell iron homeostasis sets it apart from other chemotherapeutic drugs. Gallium shares certain similarities with iron that allow it to function as an iron mimetic, bind to iron transport proteins, and thus inhibit iron-dependent cell function.
At the cell surface, transferrin–gallium complexes interfere with transferrin receptor-mediated uptake of transferrin–iron. Within the cell, gallium can inhibit ribonucleotide reductase and iron-dependent mitochondrial function and induce apoptosis.
Malignant cells have an unusually high requirement for iron and may thus alter their expression of proteins responsible for iron influx, storage and efflux. Hence, targeting tumor iron homeostasis is an attractive strategy for cancer treatment.
Gallium compounds may also inhibit tumor growth through mechanisms unrelated to iron; more recent studies suggest an interaction of gallium with proteins involved in zinc metabolism. In addition, some of the newer gallium compounds have important mechanisms of cytotoxic action such as inhibition of proteasome function in cancer cells.
Advancing the field
Numerous clinical trials have confirmed the clinical efficacy of gallium nitrate in the treatment of lymphoma and bladder cancer. Newer gallium compounds may prove to be more efficacious in other malignancies.
Their development should be vigorously pursued with the goal of advancing them to clinical trials.
Key Terms
- Clinical trials
Drug studies conducted in humans. Phase II clinical trials evaluate the efficacy of a new anticancer drug in different malignancies to establish where it may be most effective
- Metallodrugs
Drugs that contain metals
- Preclinical studies
Studies of a drug conducted in vitro and in animals prior to its use in humans
- Ribonucleotide reductase
An iron-containing enzyme responsible for the synthesis of deoxyribonucleoside diphosphates. This enzyme is an important target for gallium
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
The author’s work discussed in this manuscript was supported by US Public Health Service Grants CA68028 and CA109518. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Hart MM, Adamson RH. Antitumor activity and toxicity of salts of inorganic group IIIa metals. Aluminum, gallium, indium and thallium. Proc Natl Acad Sci USA. 1971;68:1623–1626. doi: 10.1073/pnas.68.7.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster BJ, Clagett-Carr K, Hoth D, Leyland-Jones B. Gallium nitrate. The second metal with clinical activity. Cancer Treat Rep. 1988;70:1311–1319. [PubMed] [Google Scholar]
- 3.Collery P, Keppler B, Madoulet C, Desoize B. Gallium in cancer treatment. Crit Rev Oncol Hematol. 2002;42(3):283–296. doi: 10.1016/s1040-8428(01)00225-6. [DOI] [PubMed] [Google Scholar]
- 4.Chitambar CR. Gallium compounds as antineoplastic agents. Curr Opin Oncol. 2004;16(6):547–552. doi: 10.1097/01.cco.0000142071.22226.d2. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Chitambar CR. Medical applications and toxicities of gallium compounds. Int J Environ Res Public Health. 2010;7(5):2337–2361. doi: 10.3390/ijerph7052337. Reviews the diverse spectrum of clinical and industrial applications and the potential toxicities of gallium compounds beyond their use in cancer treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪▪.Bernstein LR. Mechanisms of therapeutic activity for gallium. Pharmacol Rev. 1998;50:665–682. Provides an important insight into the biochemistry of gallium and its speciation in the blood. [PubMed] [Google Scholar]
- 7.Harris WR, Pecoraro VL. Thermodynamic binding constants for gallium transferrin. Biochemistry. 1983;22:292–299. doi: 10.1021/bi00271a010. [DOI] [PubMed] [Google Scholar]
- 8.Beatty EJ, Cox MC, Frenkiel TA, et al. Ga3+ induced structural changes in human serum transferrin: [1H, 13C] NMR studies of methionine residues in the N-Lobe. In: Collery P, Poiner LA, Littlefield NA, Etienne JC, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 1994. pp. 315–320. [Google Scholar]
- 9.Tajmir-Riahi HA, Nahar S, Diamantoglou S, et al. A comparative study of protein binding to Al(III), Ga(III) and Fe(III) ions. Metal ion binding site and protein conformational variations in aqueous solution. In: Collery P, Poiner LA, Littlefield NA, Etienne JC, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 1994. pp. 321–326. [Google Scholar]
- 10.Einhorn L. Gallium nitrate in the treatment of bladder cancer. Semin Oncol. 2003;30(2 Suppl 5):34–41. doi: 10.1016/s0093-7754(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 11.Straus DJ. Gallium nitrate in the treatment of lymphoma. Semin Oncol. 2003;30(2 Suppl 5):25–33. doi: 10.1016/s0093-7754(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 12.Chitambar CR. Gallium nitrate for the treatment of non-Hodgkin’s lymphoma. Expert Opin Investig Drugs. 2004;13(5):531–541. doi: 10.1517/13543784.13.5.531. [DOI] [PubMed] [Google Scholar]
- 13.Krakoff IH, Newman RA, Goldberg RS. Clinical toxicologic and pharmacologic studies of gallium nitrate. Cancer. 1979;44:1722–1727. doi: 10.1002/1097-0142(197911)44:5<1722::aid-cncr2820440528>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Warrell RP, Jr, Coonley CJ, Straus DJ, Young CW. Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer. 1983;51:1982–1987. doi: 10.1002/1097-0142(19830601)51:11<1982::aid-cncr2820511104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Todd PA, Fitton A. Gallium nitrate. a review of its pharmacological properties and therapeutic potential in cancer-related hypercalcemia. Drugs. 1991;42:261–273. doi: 10.2165/00003495-199142020-00007. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier Y, Liautaud-Roger F, Labbe F, Loirette M, Collery P, Coninx P. Effect of gallium at two phases of the CA 755 tumour growth. Anticancer Res. 1987;7:745–748. [PubMed] [Google Scholar]
- 17.Collery P, Millart H, Pluot M, Anghileri LJ. Effects of gallium chloride oral administration on transplanted C3HBA mammary adenocarcinoma. Ga, Mg, Ca and Fe concentration and anatomopathological characteristics. Anticancer Res. 1986;6(5):1085–1087. [PubMed] [Google Scholar]
- 18.Vistelle R, Collery P, Millart H. In vivo distribution of gallium in healthy rats after oral administration and interactions with iron, magnesium and calcium. Trace Elem Med. 1989;6:27–32. [Google Scholar]
- 19.Collery P, Millart H, Lamiable D, et al. Clinical pharmacology of gallium chloride after oral administration in cancer patients. Anticancer Res. 1989;9(2):353–356. [PubMed] [Google Scholar]
- 20.Novick SC, Julian TN, Majuru S, et al. Initial Phase I clinical and pharmacokinetic assessment of G4544, an oral gallium-containing compound. J Clin Oncol. 2008;26:8592. [Google Scholar]
- 21.Bernstein LR, Tanner T, Godfrey C, Noll B. Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability. Metal-Based Drugs. 2000;7:33–47. doi: 10.1155/MBD.2000.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson KH, Barta CA, Orvig C. Metal complexes of maltol and close analogues in medicinal inorganic chemistry. Chem Soc Rev. 2006;35(6):545–556. doi: 10.1039/b416256k. [DOI] [PubMed] [Google Scholar]
- 23.Maxton DG, Thompson RP, Hider RC. Absorption of iron from ferric hydroxypyranone complexes. Br J Nutr. 1994;71(2):203–207. doi: 10.1079/bjn19940127. [DOI] [PubMed] [Google Scholar]
- 24.Allamneni KP, Burns RB, Gray DJ, Valone FH, Bucalo LR, Sreedharan SP. Gallium maltolate treatment results in transferrin-bound gallium in patient serum. Proc Am Assoc Cancer Res. 2004;45:230, Abstract 1013. [Google Scholar]
- 25.Vallabhajosula SR, Harwig JF, Siemsen JK, Wolf W. Radiogallium localization in tumors:Blood binding and transport and the role of transferrin. J Nucl Med. 1980;21:650–656. [PubMed] [Google Scholar]
- 26.Chua MS, Bernstein LR, Li R, So SK. Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Anticancer Res. 2006;26(3A):1739–1743. [PubMed] [Google Scholar]
- 27▪▪.Chitambar CR, Purpi DP, Woodliff J, Yang M, Wereley JP. Development of gallium compounds for treatment of lymphoma. Gallium maltolate, a novel hydroxypyrone gallium compound induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J Pharmacol Exp Ther. 2007;322:1228–1236. doi: 10.1124/jpet.107.126342. Compares gallium maltolate with gallium nitrate and demonstrates that gallium maltolate inhibits the growth of gallium nitrate-resistant cells and is incorporated into cells more efficiently than gallium nitrate. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Bernstein LR, van der Hoeven JJM, Boer RO. Hepatocellular cancer detection by gallium scan and subsequent treatment by gallium maltolate. rationale and case study. Anti-Cancer Agents Med Chem. 2011;11:585–590. doi: 10.2174/187152011796011046. Important clinical report demonstrating for the first time, the anti-tumor activity of oral gallium maltolate in a patient with progressive hepatocellular cancer. [DOI] [PubMed] [Google Scholar]
- 29.Rudnev AV, Foteeva LS, Kowol C, et al. Preclinical characterization of anticancer gallium(III) complexes. solubility, stability, lipophilicity and binding to serum proteins. J Inorg Biochem. 2006;100(11):1819–1826. doi: 10.1016/j.jinorgbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Timerbaev AR. Advances in developing tris(8-quinolinolato)gallium(III) as an anticancer drug. critical appraisal and prospects. Metallomics. 2009;1(3):193–198. doi: 10.1039/b902861g. Overview of the preclinical and clinical development of KP46. [DOI] [PubMed] [Google Scholar]
- 31.Collery P, Lechenault F, Cazabat A, et al. Inhibitory effects of gallium chloride and tris (8-quinolinolato)gallium(III) on A549 human malignant cell line. Anticancer Res. 2000;20(2A):955–958. [PubMed] [Google Scholar]
- 32.Valiahdi SM, Jakupec MA, Marculescu R, Keppler BK. Tris(8-quinolinolato)gallium(III) exerts strong antiproliferative effects in melanoma cells. In: Alpoim MC, Morais PV, Santos MA, Cristovao L, Centeno JA, Collery P, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 2006. pp. 282–286. [Google Scholar]
- 33.Jakupec MA, Collery P, Keppler BK. Synergistic antiproliferative effects of tris(8-quinolinolato)gallium(III) (KP46) in combination with platinum drugs in ovarian and colon carcinoma cells. In: Collery P, Maymard I, Theophanides T, Khassanova L, Collery T, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 2008. pp. 110–115. [Google Scholar]
- 34▪▪.Collery P, Domingo JL, Keppler BK. Preclinical toxicology and tissue gallium distribution of a novel antitumor gallium compound. Tris(8-quinolinolato) gallium (III) Anticancer Res. 1996;16:687–692. In-depth study of the tissue distribution of gallium after administration of tris (8-quinolinolato)gallium(III) to mice. [PubMed] [Google Scholar]
- 35.Hofheinz RD, Dittrich C, Jakupec MA, et al. Early results from a Phase I study on orally administered tris(8-quinolinolato)gallium(III) (FFC11, KP46) in patients with solid tumors – a CESAR study (Central European Society for Anticancer Drug Research [EWIV]) Int J Clin Pharmacol Ther. 2005;43(12):590–591. doi: 10.5414/cpp43590. [DOI] [PubMed] [Google Scholar]
- 36.Collery P, Jakupec MA, Kynast B, Keppler BK. Preclinical and early clinical development of the oral Gallium complex KP46 (FFC11) In: Alpoim MC, Morais PV, Santos MA, Cristovao L, Centeno JA, Collery P, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 2006. pp. 521–524. [Google Scholar]
- 37.Moore EC, Sartorelli AC. The inhibition of ribonucleotide reductase by α-(N)heterocyclic carboxaldehyde thiosemicarbazones. In: Cory JG, Cory AH, editors. Inhibitors of Ribonucleoside Diphosphate Reductase Activity. Pergamon Press; Oxford, UK: 1989. pp. 203–215. [Google Scholar]
- 38.Saryan LA, Ankel E, Krishnamurti C, Petering DH, Elford H. Comparative cytotoxic and biochemical effects of ligands and metal complexes of α-N-heterocyclic carboxaldehyde thiosemicarbazones. J Med Chem. 1979;22:1218–1221. doi: 10.1021/jm00196a013. [DOI] [PubMed] [Google Scholar]
- 39.Kratz F, Nuber B, Weis J, Keppler BK. Synthesis and characterization of potential antitumor and antiviral gallium(III) complexes of α-(N)-heterocyclic thiosemicarbazones. Synth React Inorg Met-org Chem. 1991;21:1601–1615. [Google Scholar]
- 40.Arion VB, Jakupec MA, Galanski M, Unfried P, Keppler BK. Synthesis, structure, spectroscopic and in vitro antitumour studies of a novel gallium(III) complex with 2-acetylpyridine 4N-dimethylthiosemicarbazone. J Inorg Biochem. 2002;91(1):298–305. doi: 10.1016/s0162-0134(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 41.Kowol CR, Berger R, Eichinger R, et al. Gallium(III) and iron(III) complexes of alpha-N-heterocyclic thiosemicarbazones. synthesis, characterization, cytotoxicity, and interaction with ribonucleotide reductase. J Med Chem. 2007;50(6):1254–1265. doi: 10.1021/jm0612618. [DOI] [PubMed] [Google Scholar]
- 42.Mendes IC, Soares MA, Dos Santos RG, Pinheiro C, Beraldo H. Gallium(III) complexes of 2-pyridineformamide thiosemicarbazones. Cytotoxic activity against malignant glioblastoma. Eur J Med Chem. 2009;44(5):1870–1877. doi: 10.1016/j.ejmech.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Mandinova A, Lee SW. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Sci Transl Med. 2011;3(64):64rv1. doi: 10.1126/scitranslmed.3001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murren J, Modiano M, Clairmont C, et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9(11):4092–4100. [PubMed] [Google Scholar]
- 45.Kunos CA, Waggoner S, von GV, et al. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res. 2010;16(4):1298–1306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shakya R, Peng F, Liu J, Heeg MJ, Verani CN. Synthesis, structure, and anticancer activity of gallium(III) complexes with asymmetric tridentate ligands. growth inhibition and apoptosis induction of cisplatin-resistant neuroblastoma cells. Inorg Chem. 2006;45(16):6263–6268. doi: 10.1021/ic060106g. [DOI] [PubMed] [Google Scholar]
- 47▪▪.Chen D, Frezza M, Shakya R, et al. Inhibition of the proteasome activity by gallium(III) complexes contributes to their antiprostate tumor effects. Cancer Res. 2007;67(19):9258–9265. doi: 10.1158/0008-5472.CAN-07-1813. Describes a new mechanism of action for gallium complexes (inhibition of proteosome activity) and demonstrates anti-tumor activity of these compounds in nude mouse model bearing a prostate cancer xenograft. [DOI] [PubMed] [Google Scholar]
- 48.Richardson DR, Tran EH, Ponka P. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood. 1995;86:4295–4306. [PubMed] [Google Scholar]
- 49.Richardson DR, Milnes K. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: the mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood. 1997;89:3025–3038. [PubMed] [Google Scholar]
- 50.Landschulz W, Thesleff I, Ekblom P. A lipophilic iron chelator can replace transferrin as a stimulator of cell proliferation and differentiation. J Cell Biol. 1984;98:596–601. doi: 10.1083/jcb.98.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knorr GM, Chitambar CR. Gallium-pyridoxal isonicotinoyl hydrazone (Ga-PIH), a novel cytotoxic gallium complex. A comparative study with gallium nitrate. Anticancer Res. 1998;18:1733–1738. [PubMed] [Google Scholar]
- 52.Chitambar CR, Boon P, Wereley JP. Evaluation of transferrin and gallium-pyridoxal isonicotinoyl hydrazone as potential therapeutic agents to overcome lymphoid leukemic cell resistance to gallium nitrate. Clin Cancer Res. 1996;2:1009–1015. [PubMed] [Google Scholar]
- 53.Chitambar CR, Wereley JP. Resistance to the antitumor agent gallium nitrate in human leukemic cells is associated with decreased gallium/iron uptake, increased activity of iron regulatory protein-1, and decreased ferritin production. J Biol Chem. 1997;272:12151–12157. doi: 10.1074/jbc.272.18.12151. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Eu W, Seow-Choen F, Cheah Y. Differential cytostatic effect of sodium salicylate in human colorectal cancers using an individualized histoculture system. Cancer Chemother Pharmacol. 2002;49(6):473–478. doi: 10.1007/s00280-002-0441-7. [DOI] [PubMed] [Google Scholar]
- 55.Ismail DA, Badawi A, Collery P, Zakhary NI, Raouf SA. Antitumor effects of Gallium(III) tris(salicylate)-ethanol on malignant cell lines and tumor bearing Swiss albino mice. In: Alpoim MC, Morais PV, Santos MA, Cristovao AL, Centeno JA, Collery P, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 2006. pp. 351–361. [Google Scholar]
- 56.Zanias S, Papaefstathiou GS, Raptopoulou CP, et al. Synthesis, structure, and antiproliferative activity of three gallium(III) azole complexes. Bioinorg Chem Appl. 2010;2010 doi: 10.1155/2010/168030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaluderovic MR, Gomez-Ruiz S, Gallego B, Hey-Hawkins E, Paschke R, Kaluderovic GN. Anticancer activity of dinuclear gallium(III) carboxylate complexes. Eur J Med Chem. 2010;45(2):519–525. doi: 10.1016/j.ejmech.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 58.Gallego B, Kaluderovic MR, Kommera H, et al. Cytotoxicity, apoptosis and study of the DNA-binding properties of bi- and tetranuclear gallium(III) complexes with heterocyclic thiolato ligands. Invest New Drugs. 2011;29(5):932–944. doi: 10.1007/s10637-010-9449-8. [DOI] [PubMed] [Google Scholar]
- 59.Gallego B, Kaluderovic MR, Kommera H, Hey-Hawkins E, Paschke R, Kaluderovic GN. Novel gallium(III) complexes containing phthaloyl derivatives of neutral amino acids with apoptotic activity in cancer cells. J Organomet Chem. 2009;694(14):2191–2197. [Google Scholar]
- 60.Kaluderovic MR, Kaluderovic GN, Gomez-Ruiz S, et al. Organogallium(III) complexes as apoptosis promoting anticancer agents for head and neck squamous cell carcinoma (HNSCC) cell lines. J Inorg Biochem. 2011;105(2):164–170. doi: 10.1016/j.jinorgbio.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Jordan A, Reichard P. Ribonucleotide reductases. Ann Rev Biochem. 1998;67:71–78. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 62.Nyholm S, Mann GJ, Johansson AG, Bergeron RJ, Gräslund A, Thelander L. Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J Biol Chem. 1993;268:26200–26205. [PubMed] [Google Scholar]
- 63.Yu Y, Kovacevic Z, Richardson DR. Tuning cell cycle regulation with an iron key. Cell Cycle. 2007;6(16):1982–1994. doi: 10.4161/cc.6.16.4603. [DOI] [PubMed] [Google Scholar]
- 64.Lubec G. The hydroxyl radical. From chemistry to human disease. J Invest Med. 1996;44:324–346. [PubMed] [Google Scholar]
- 65.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 66.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues. Their distribution and possible clinical relevance. J Clin Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith NW, Strutton GM, Walsh MD, et al. Transferrin receptor expression in primary superficial human bladder tumours identifies patients who develop recurrences. Br J Urol. 1990;65:339–344. doi: 10.1111/j.1464-410x.1990.tb14752.x. [DOI] [PubMed] [Google Scholar]
- 68.Yang DC, Wang F, Elliott RL, Head JF. Expression of transferrin receptor and ferritin H-chain mRNA are associated with clinical and histopathological prognostic indicators in breast cancer. Anticancer Res. 2001;21(1B):541–549. [PubMed] [Google Scholar]
- 69.Habashy HO, Powe DG, Staka CM, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010;119(2):283–283. doi: 10.1007/s10549-009-0345-x. [DOI] [PubMed] [Google Scholar]
- 70.Weinstein RE, Bond BH, Silberberg BK, Vaughn CB, Subbaiah P, Pieper DR. Tissue ferritin concentration and prognosis in carcinoma of the beast. Breast Cancer Res Treat. 1989;14:349–353. doi: 10.1007/BF01806307. [DOI] [PubMed] [Google Scholar]
- 71.Pinnix ZK, Miller LD, Wang W, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010;2(43):43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chitambar CR, Seligman PA. Effects of different transferrin forms on transferrin receptor expression, iron uptake and cellular proliferation of human leukemic HL60 cells. Mechanisms responsible for the specific cytotoxicity of transferrin–gallium. J Clin Invest. 1986;78:1538–1546. doi: 10.1172/JCI112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haq RU, Wereley JP, Chitambar CR. Induction of apoptosis by iron deprivation in human leukemic CCRF-CEM cells. Exp Hematol. 1995;23:428–432. [PubMed] [Google Scholar]
- 74.Greene BT, Thorburn J, Willingham MC, et al. Activation of caspase pathways during iron chelator-mediated apoptosis. J Biol Chem. 2002;277(28):25568–25575. doi: 10.1074/jbc.M110345200. [DOI] [PubMed] [Google Scholar]
- 75.Seligman PA, Moran PL, Schleicher RB, Crawford ED. Treatment with gallium nitrate. Evidence for interference with iron metabolism in vivo. Am J Hematol. 1992;41:232–240. doi: 10.1002/ajh.2830410403. [DOI] [PubMed] [Google Scholar]
- 76.Collery P, Millart H, Ferrand O, et al. Gallium chloride treatment of cancer patients after oral administration. A pilot study. Chemotherapia. 1985;4:1165–1166. [Google Scholar]
- 77.Chitambar CR, Matthaeus WG, Antholine WE, Graff K, O’Brien WJ. Inhibition of leukemic HL60 cell growth by transferrin–gallium. Effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood. 1988;72:1930–1936. [PubMed] [Google Scholar]
- 78.Chitambar CR, Narasimhan J, Guy J, Sem DS, O’Brien WJ. Inhibition of ribonucleotide reductase by gallium in murine leukemic L1210 cells. Cancer Res. 1991;51:6199–6201. [PubMed] [Google Scholar]
- 79.Narasimhan J, Antholine WE, Chitambar CR. Effect of gallium on the tyrosyl radical of the iron-dependent M2 subunit of ribonucleotide reductase. Biochem Pharmacol. 1992;44:2403–2408. doi: 10.1016/0006-2952(92)90686-d. [DOI] [PubMed] [Google Scholar]
- 80.Berggren MM, Burns LA, Abraham RT, Powis G. Inhibition of protein tyrosine phosphatase by the antitumor agent gallium nitrate. Cancer Res. 1993;53:1862–1866. [PubMed] [Google Scholar]
- 81.Anghileri LJ, Robert J. Radiogallium as a probe for magnesium-binding sites. Magnesium-Bulletin. 1982;2:197–200. [Google Scholar]
- 82.Bara M, Guiet-Bara A, Collery P, Durlach J. Gallium action on ionic transfer through the isolated human amnion. I Effect on the amnion as a whole and interaction between gallium and magnesium. Trace Elements in Medicine. 1985;2:99–102. [Google Scholar]
- 83.Bara M, Guiet-Bara A, Durlach J, Collery P. Comparative effects of a carcinogenic (As) and an anticancer (Ga) metals on the transfer through the human amnion. Relationship with Mg. Ultrastructural and electrophysiological studies. In: Collery P, Poiner LA, Manfait M, Etienne JC, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 1990. pp. 570–572. [Google Scholar]
- 84.Bara M, Guiet-Bara A, Collery P, Durlach J. Gallium action on the ionic transfer through the isolated human amnion. II Effect on cellular and paracellular pathways. Trace Elements in Medicine. 1992;9:117–122. [Google Scholar]
- 85.Perchellet EM, Ladesich JB, Collery P, Perchellet JP. Microtubule-disrupting effects of gallium chloride in vitro. Anticancer Drugs. 1999;10(5):477–488. doi: 10.1097/00001813-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Chitambar CR, Purpi DP. A novel gallium compound synergistically enhances bortezomib-induced apoptosis in mantle cell lymphoma cells. Leuk Res. 2010;34:950–953. doi: 10.1016/j.leukres.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richardson PG, Barlogie B, Berenson J, et al. A Phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 88.O’Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23(4):676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 89.Niesvizky R, Choy CG, Siegel D, Lyons L, Michaeli J. Extended survival in advanced-stage multiple myeloma patients treated with gallium nitrate. Leuk Lymphoma. 2002;43(3):603–605. doi: 10.1080/10428190210321. [DOI] [PubMed] [Google Scholar]
- 90.Chitambar CR, Wereley JP, Matsuyama S. Gallium-induced cell death in lymphoma. role of transferrin receptor cycling, involvement of Bax and the mitochondria, and effects of proteasome inhibition. Mol Cancer Ther. 2006;5(11):2834–2843. doi: 10.1158/1535-7163.MCT-06-0285. [DOI] [PubMed] [Google Scholar]
- 91.Gogvadze V, Zhukova A, Ivanov A, Khassanova L, Khassanova Z, Collery P. The effect of gallium on the calcium retention capacity of rat liver mitochondria. In: Collery P, Corbella J, Domingo JL, Etienne JC, Llobet JM, editors. Metal Ions in Biology and Medicine. John Libbey Eurotext; Paris, France: 1996. pp. 249–252. [Google Scholar]
- 92.Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim Biophys Acta. 2006;1757(9–10):1301–1311. doi: 10.1016/j.bbabio.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 93▪▪.Yang M, Kroft SH, Chitambar CR. Gene expression analysis of gallium-resistant and gallium-sensitive lymphoma cells reveals a role for metal-responsive transcription factor-1, metallothionein-2A, and zinc transporter-1 in modulating the antineoplastic activity of gallium nitrate. Mol Cancer Ther. 2007;6(2):633–643. doi: 10.1158/1535-7163.MCT-06-0557. Demonstrates for the first time that CCRF-CEM cells with acquired resistance to growth inhibition by gallium nitrate display an upregulation of metallothionein-2A expression, and that gallium shares similarities with zinc metabolism. It also shows that metallothionein is variably expressed in non-Hodgkin’s lymphoma. [DOI] [PubMed] [Google Scholar]
- 94.Capel ID, Dorrell HM, Pinnock MH, Jenner M, Williams DC. The influence of zinc status on the anti-Lewis lung tumour activity of cisplatin and gallium. Anticancer Res. 1981;1(5):269–273. [PubMed] [Google Scholar]
- 95▪▪.Yang M, Chitambar CR. Role of oxidative stress in the induction of metallothionein-2A and heme oxygenase-1 gene expression by the antineoplastic agent gallium nitrate in human lymphoma cells. Free Radic Biol Med. 2008;45(6):763–772. doi: 10.1016/j.freeradbiomed.2008.05.031. Provides new insight into the mechanism involved in the induction of metallothionein and heme oxygenase-1 expression by gallium nitrate. It supports the model shown in Figure 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59(1):95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 97.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide. From basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 98.Lundberg JL, Chitambar CR. Interaction of gallium nitrate with fludarabine and iron chelators. Effects on the proliferation of human leukemic HL60 cells. Cancer Res. 1990;50:6466–6470. [PubMed] [Google Scholar]
- 99.Chitambar CR, Wereley JP, Haq RU. Synergistic inhibition of T-lymphoblastic leukemic CCRF-CEM cell growth by gallium and recombinant human α-interferon through action on cellular iron uptake. Cancer Res. 1994;54:3224–3228. [PubMed] [Google Scholar]
- 100.Myette MS, Elford HL, Chitambar CR. Interaction of gallium nitrate with other inhibitors of ribonucleotide reductase. Cancer Lett. 1998;129:199–204. doi: 10.1016/s0304-3835(98)00104-9. [DOI] [PubMed] [Google Scholar]
- 101.Hata Y, Sandler A, Loehrer PJ, Sledge GW, Jr, Weber G. Synergism of taxol and gallium nitrate in human breast carcinoma cells. schedule dependency. Oncol Res. 1994;6(1):19–24. [PubMed] [Google Scholar]