Abstract
Introduction
Although carotid artery stenosis and coronary artery disease often coexist, many debate which patients are best served by combined concurrent revascularization (carotid endarterectomy [CEA]/coronary artery bypass graft [CABG]). We studied the use of CEA/CABG in New England and compared indications and outcomes, including stratification by risk, symptoms, and performing center.
Methods
Using data from the Vascular Study Group of New England from 2003 to 2009, we studied all patients who underwent combined CEA/CABG across six centers in New England. Our main outcome measure was in-hospital stroke or death. We compared outcomes between all patients undergoing combined CEA/CABG to a baseline CEA risk group comprised of patients undergoing isolated CEA at non-CEA/CABG centers. Further, we compared in-hospital stroke and death rates between high and low neurologic risk patients, defining high neurologic risk patients as those who had at least one of the following clinical or anatomic features: (1) symptomatic carotid disease, (2) bilateral carotid stenosis > 70%, (3) ipsilateral stenosis >70% and contralateral occlusion, or (4) ipsilateral or bilateral occlusion.
Results
Overall, compared to patients undergoing isolated CEA at non-CEA/CABG centers (n = 1563), patients undergoing CEA/CABG (n =109) were more likely to have diabetes (44% vs 29%; P =.001), creatinine >1.8 mg/dL (11% vs 5%; P =.007), and congestive heart failure (23% vs 10%; P < .001). Patients undergoing CEA/CABG were also more likely to take preoperative beta-blockers (94% vs 75%; P < .001) and less likely to take preoperative clopidogrel (7% vs 25%; P < .001). Patients undergoing CEA/CABG had higher rates of contralateral carotid occlusion (13% vs 5%; P = .001) and were more likely to undergo an urgent/emergent procedure (30% vs 15%; P < .001). The risk of complications was higher in CEA/CABG compared to isolated CEA, including increased risk of stroke (5.5% vs 1.2%; P < .001), death (5.5% vs 0.3%; P < .001), and return to the operating room for any reason (7.6% vs 1.2%; P <.001). Of 109 patients undergoing CEA/CABG, 61 (56%) were low neurologic risk and 48 (44%) were high neurologic risk but showed no demonstrable difference in stroke (4.9% vs 6.3%; P =.76), death, (4.9 vs 6.3%; P =.76), or return to the operating room (10.2% vs 4.3%; P = .25).
Conclusions
Although practice patterns in the use of CEA/CABG vary across our region, the risk of complications with CEA/CABG remains significantly higher than in isolated CEA. Future work to improve patient selection in CEA/CABG is needed to improve perioperative results with combined coronary and carotid revascularization.
Combined carotid endarterectomy and coronary artery bypass graft (CEA/CABG) surgery has been performed to treat ischemic heart disease while simultaneously reducing the risk of perioperative stroke attributable to carotid artery stenosis. Many suggest that the ideal patient for this combined procedure is one who has symptomatic carotid artery stenosis and an indication for CABG such as unstable angina or left main coronary artery disease (CAD).1–3 In this patient population, the role of combined CEA/CABG is well-established, and most agree that a combined approach is indicated.1–6
However, it is unclear how commonly patients who are selected for CEA/CABG have symptomatic carotid disease. A recent review of the Nationwide Inpatient Sample (NIS) showed that as many as 96% of patients undergoing CEA/CABG in the United States have asymptomatic carotid artery stenosis.7 In another national review of Medicare patients in 10 states, 56% of patients undergoing CEA/CABG had asymptomatic carotid artery stenosis.8 Therefore, it is unclear how frequently combined CEA/CABG is being offered to patients with asymptomatic carotid stenosis and the extent to which this differs across our region.
Using a large cohort compiled by the Vascular Study Group of New England (VSGNE), we hope to better define the patient population that is undergoing CEA/CABG in our region and analyze in-hospital outcomes of the procedure.
METHODS
Establishing a patient cohort
Patients undergoing CEA at 12 community and academic centers who take part in the VSGNE were selected from the group’s prospectively maintained database from the years 2003 to 2009. Patients were excluded from this study if they had a history of an ipsilateral CEA or if they underwent a concurrent surgical procedure other than CABG. Details regarding the VSGNE have previously been published9 and are also available online at http://www.vsgne.com.
Patient characteristics were determined across groups using existing data elements in the VSGNE. Patients were considered to have symptomatic carotid disease if they had any recorded history of ipsilateral or contralateral transient ischemic attack or stroke. In order to capture all patients who had symptoms potentially related to carotid artery disease, patients with vertebrobasilar symptoms and those recorded as “nonspecific” were also considered symptomatic. Because the VSGNE does not provide operative details on the cardiac portion of CEA/CABG, we attempted to gain further cardiac-specific details from the Northern New England Cardiovascular Disease Study Group, a voluntary research consortium composed of 10 institutions that perform coronary revascularization (http://www.nnecdsg.org). Data availability was limited to cardiac-specific preoperative and operative details from the largest center that performed CEA/CABG during the study period.
Categorizing centers and establishing risk strata
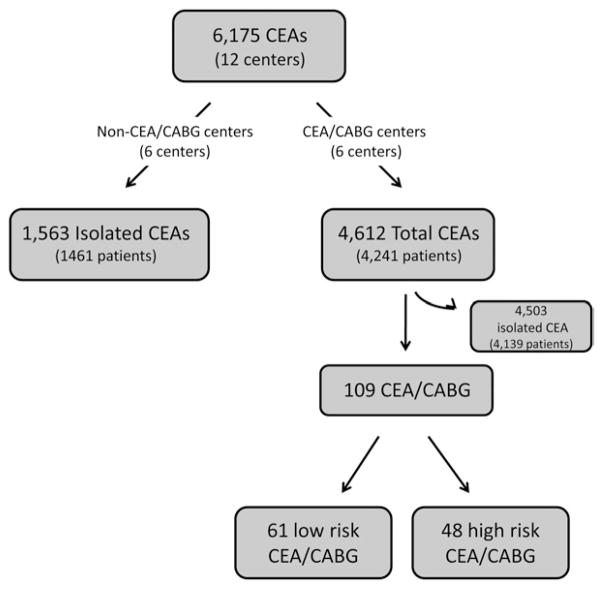
In order to determine the regional practice patterns in the performance of CEA/CABG, we categorized the centers in our region in two ways, as outlined in Fig 1: centers that performed no CEA/CABG during the study period (n = 6) and centers that performed CEA/CABG during the study period (n = 6).
Fig 1.
Flow diagram depicting categorization of patients undergoing carotid endarterectomy (CEA). CABG, Coronary artery bypass graft.
We further subdivided all patients who underwent CEA/CABG into a high neurologic risk group (“high risk”) and a low neurologic risk group (“low risk”) to determine the effect of patient risk on outcome. Although symptomatic carotid artery disease is a known risk factor for stroke during cardiac surgery, several studies have suggested that stroke risk also increases if any of the following anatomic features are present: (1) bilateral carotid stenosis >70%, (2) unilateral carotid stenosis >70% and contralateral occlusion, or (3) ipsilateral or bilateral occlusion.10,11 Based on these criteria, we classified patients undergoing CEA/CABG as high neurologic risk if they had symptomatic carotid artery disease or any of the anatomic features described. Patients were classified as low risk if they did not meet any of these criteria.
Main outcome measure
Our main outcome measure was the occurrence of in-hospital stroke or death, measured using the variable for this outcome as defined in our data-set. Strokes were defined as either disabling or nondisabling, according to previously published definitions from the VSGNE.12 Both types of stroke (disabling and nondisabling) were aggregated for this analysis.
Our secondary outcomes were in-hospital length of stay and need for return to the operating room (OR). Reasons for return to the OR were either for neurologic symptoms or bleeding. Specific details regarding reoperation for CABG-related complications are not available from data elements in the VSGNE.
Analysis
First, we analyzed patient characteristics and outcomes in all patients undergoing CEA/CABG. There were potentially three groups of patients that would serve as appropriate comparisons to CEA/CABG in our cohort: (1) patients who underwent isolated CEA (6066 patients), (2) patients who underwent isolated CEA at centers that also perform CEA/CABG (4503 patients), or (3) patients who underwent isolated CEA at centers that do not perform CEA/CABG (1563 patients). We selected the third group as our “baseline carotid risk group” because we believe this group best represents the baseline risk of CEA. Regardless, rates of stroke (1.4%, 1.4%, and 1.2%, respectively), death (0.2%, 0.2%, and 0.3%, respectively), and stroke or death (1.4%, 1.5%, and 1.3%, respectively) were similar across all three potential comparison groups. Next, to examine any effect secondary to differences in volume, we compared outcomes across centers that performed >5 CEA/CABG during the study period. Consequently, all centers excluded using this cutoff performed <1 CEA/CABG/year. In order to examine any effect secondary to case mix, we compared outcomes according to our predefined neurologic risk classification.
Means were compared with Fisher exact test or χ2 test where appropriate. A P value of < .05 was considered statistically significant. Data were compiled and analyzed using Stata version 11.2 (StataCorp, College Station, Tex).
RESULTS
Patient characteristics of CEA/CABG compared to isolated CEA
Across our region, from 2003 to 2009, 6175 patients underwent CEA at one of 12 centers in New England who participated in the VSGNE. Of these, 109 CEA/CABGs were performed. These patients comprised our “CEA/CABG group.” At the center level, six centers performed no CEA/CABG. During the study period, these centers performed isolated CEAs on 1563 patients. These patients comprised the “isolated CEA” group (baseline carotid risk group).
We compared the patient characteristics and operative details of the CEA/CABG group to the isolated CEA group, the details of which can be found in Table I. Patients undergoing CEA/CABG had more severe ipsilateral stenosis when compared to those undergoing isolated CEA, as 96% of patients undergoing CEA/CABG had a critical (>70%) carotid stenosis, compared to 86% in the isolated CEA group (P = .002). Patients undergoing CEA/CABG had more severe contralateral carotid artery disease as well: they were found to have higher rates of contralateral carotid occlusion (13% vs 5%; P = .001) and a trend toward higher rates of critical (>70%) carotid stenosis (17% vs 12%; P = .12).
Table I.
Preoperative characteristics and operative details for patients undergoing CEA in baseline risk group and patients undergoing CEA/CABG
| Variable | Isolated CEA in non-CEA/CABG centers | CEA/CABG | P value | Of 109 total CEA/CABG:
|
||
|---|---|---|---|---|---|---|
| Low-risk group | High-risk group | P value | ||||
| Number of patients | 1563 | 109 | 61 | 48 | ||
| Male gender | 922 (59%) | 75 (69%) | .04 | 40 (66%) | 35 (73%) | .41 |
| Right side | 781 (50%) | 51 (47%) | .52 | 29 (48%) | 22 (46%) | .86 |
| White race | 1532 (98%) | 108 (99%) | .43 | 60 (98%) | 48 (100%) | .37 |
| Mean age | 70.2 (69.7–70.7) | 69.0 (67.1–70.8) | .2 | 69.2 (66.6–71.8) | 68.7 (66.0–71.5) | .81 |
| Age | ||||||
| <40 years | 2 (<1%) | 0 (0%) | .71 | 0 (0%) | 0 (0%) | — |
| Age 40–49 years | 40 (3%) | 5 (5%) | .21 | 3 (5%) | 2 (4%) | .85 |
| Age 50–59 years | 190 (12%) | 12 (11%) | .72 | 8 (13%) | 4 (8%) | .43 |
| Age 60–69 years | 462 (30%) | 38 (35%) | .24 | 19 (31%) | 19 (40%) | .36 |
| Age 70–79 years | 604 (39%) | 40 (37%) | .69 | 23 (38%) | 17 (36%) | .81 |
| Age 80–89 years | 249 (16%) | 13 (12%) | .27 | 7 (11%) | 6 (13%) | .87 |
| Age >90 years | 16 (1%) | 1 (1%) | .92 | 1 (2%) | 0 (0%) | .37 |
| Smoking (prior or current) | 1190 (76%) | 87 (80%) | .68 | 44 (72%) | 43 (90%) | .02 |
| Diabetes | 455 (29%) | 48 (44%) | .001 | 28 (46%) | 20 (42%) | .66 |
| Creatinine ≥1.8 mg/dL | 77 (5%) | 12 (11%) | .007 | 6 (10%) | 6 (13%) | .66 |
| Dialysis | 10 (1%) | 0 (0%) | .61 | 0 (0%) | 0 (0%) | — |
| Hypertension | 1368 (88%) | 97 (89%) | .69 | 55 (90%) | 42 (88%) | .66 |
| Prior CABG or coronary intervention | 394 (25%) | 29 (27%) | .76 | 19 (31%) | 10 (21%) | .23 |
| Congestive heart failure | 155 (10%) | 25 (23%) | .00002 | 10 (16%) | 15 (31%) | .07 |
| Prior radiation therapy | 21 (1%) | 1 (1%) | .7 | 1 (1.6%) | 0 (0%) | .37 |
| Preoperative medication regimen | ||||||
| Aspirin | 1430 (92%) | 103 (95%) | .56 | 58 (95%) | 45 (94%) | .76 |
| Clopidogrel | 386 (25%) | 8 (7%) | .0002 | 3 (5%) | 5 (10%) | .27 |
| Aspirin and clopidogrel | 333 (21%) | 7 (6%) | .0002 | 3 (5%) | 4 (8%) | .47 |
| Statin use | 1169 (75%) | 85 (78%) | .69 | 47 (77%) | 28 (79%) | .79 |
| Beta blockers | 1173 (75%) | 102 (94%) | .00001 | 56 (92%) | 46 (96%) | .39 |
| Symptom status | ||||||
| Asymptomatic | 966 (62%) | 89 (82%) | .00003 | 61 (100%) | 28 (58%) | 2 × 10−8 |
| Any symptom | 597 (38%) | 20 (18%) | .00003 | 0 (0%) | 20 (42%) | 2 × 10−8 |
| Ipsilateral symptoms (TIA or stroke) | 363 (23%) | 12 (11%) | .003 | 0 (0%) | 12 (25%) | .00003 |
| Contralateral symptoms (TIA or stroke) | 116 (7%) | 4 (4%) | .14 | 0 (0%) | 4 (8%) | .22 |
| Vertebrobasilar symptoms (TIA or stroke) | 57 (4%) | 1 (1%) | .13 | 0 (0%) | 1 (2%) | .26 |
| Nonspecific | 172 (11%) | 7 (6%) | .13 | 0 (0%) | 7 (15%) | .002 |
| Ipsilateral degree of stenosis | ||||||
| <50% stenosis | 23 (1%) | 0 (0%) | .2 | 0 (0%) | 0 (0%) | — |
| >50% stenosis | 1511 (97%) | 108 (99%) | .17 | 61 (100%) | 47 (98%) | .26 |
| >60% stenosis | 1450 (93%) | 108 (99%) | .011 | 61 (100%) | 47 (98%) | .26 |
| >70% stenosis | 1341 (86%) | 105 (96%) | .002 | 60 (98%) | 45 (94%) | .2 |
| >80% stenosis | 1002 (64%) | 69 (63%) | .87 | 33 (54%) | 36 (75%) | .03 |
| Occluded | 22 (1%) | 1 (1%) | .67 | 0 (0%) | 1 (2%) | .26 |
| Contralateral degree of stenosis | ||||||
| <50% stenosis | 989 (63%) | 41 (38%) | 1 × 10−7 | 33 (54%) | 8 (17%) | .00006 |
| <50% stenosis | 451 (29%) | 52 (48%) | .00003 | 26 (43%) | 26 (54%) | .23 |
| >60% stenosis | 282 (18%) | 35 (32%) | .0003 | 13 (21%) | 22 (46%) | .006 |
| >70% stenosis | 181 (12%) | 18 (17%) | .12 | 0 (0%) | 18 (34%) | 2 × 10−7 |
| >80% stenosis | 69 (4%) | 7 (6%) | .33 | 0 (0%) | 7 (15%) | .002 |
| Occluded | 82 (5%) | 14 (13%) | .001 | 0 (0%) | 14 (29%) | 6 × 10−6 |
| Operative characteristics | ||||||
| Urgency | ||||||
| Elective | 1321 (85%) | 76 (70%) | .00004 | 49 (80%) | 27 (56%) | .007 |
| Urgent or emergent | 239 (15%) | 33 (30%) | .00004 | 12 (20%) | 21 (44%) | .007 |
| Local/regional anesthesia | 55 (4%) | 4 (4%) | .98 | |||
| General anesthesia | 1505 (96%) | 105 (96%) | .98 | 59 (97%) | 46 (96%) | .81 |
| Conventional CEA | 1519 (97%) | 88 (81%) | 7 × 10−19 | 47 (77%) | 41 (85%) | .27 |
| Conventional CEA with primary closure | 35 (2%) | 16 (15%) | 3 × 10−13 | 8 (13%) | 8 (17%) | .6 |
| Conventional CEA with patch | 1484 (95%) | 72 (66%) | 2 × 10−30 | 39 (64%) | 33 (69%) | .6 |
| Eversion endarterectomy | 41 (3%) | 21 (19%) | 7 × 10−19 | 14 (23%) | 7 (15%) | .27 |
| Routine shunting | 1416 (91%) | 12 (11%) | 4 × 10−103 | 6 (10%) | 6 (13%) | .66 |
| Shunting for neurologic changes (indication) | 12 (1%) | 9 (8%) | 1 × 10−11 | 4 (7%) | 5 (10%) | .47 |
| Dextran use | 21 (1%) | 18 (17%) | 4 × 10−24 | 14 (23%) | 4 (8%) | .04 |
| Protamine use | 986 (63%) | 88 (81%) | .0002 | 48 (79%) | 40 (83%) | .54 |
CABG, Coronary artery bypass graft; CEA, carotid endarterectomy; TIA, transient ischemic attack.
Despite the higher comorbidity burden, patients undergoing CEA/CABG were significantly less likely to be symptomatic from their carotid stenosis at the time of surgery when compared with patients undergoing isolated CEA (18% vs 38%; P = .00003). Patients undergoing CEA/CABG were also less likely to have symptoms ipsilateral to their carotid artery disease (11% vs 23%; P = .003).
Operative characteristics of CEA/CABG compared to isolated CEA
At the time of surgery, CEA/CABG was more likely to be classified as urgent/emergent when compared to isolated CEA (30% vs 15%; P = .00004; Table I). In CEA/CABG, eversion endarterectomy was performed more commonly (19% vs 3%; P = 7 × 10−19). Routine shunting was performed less commonly in CEA/CABG (11% vs 91%; P = 4 × 10−103), whereas shunting for neurologic change was performed more commonly (8% vs 1%; P = 1 × 10−11). Dextran was used more commonly in CEA/CABG (17% vs 1%; P = 4 × 10−24) as was protamine (81% vs 63%; P = .0002).
Cardiac details were available for isolated CABGs and CEA/CABGs from the largest center in our dataset. At this center, 2451 CABGs were performed during the study period. The rates of stroke, death, and stroke/death after CABG at this center were 1%, 2.2%, and 3%, respectively. Regarding CEA/CABGs at this center, most patients underwent treatment of three-vessel CAD (58.6%) in comparison to two-vessel (34.5%) and one-vessel CAD (6.9%). A majority of patients had three, four, or five coronary anastomoses (25.3%, 37.9%, and 20.7%, respectively). Off-pump surgery was used in 44% of cases, and among those undergoing on-pump surgery, pump time averaged 90.4 minutes (SD 58.9 minutes), and clamp time averaged 63.5 minutes (SD 25.8 minutes). Intraoperative or postoperative intra-aortic balloon pump support was used in fewer than 5% of cases. Fewer than 3% of patients required inotropes >48 hours after surgery.
Outcomes of CEA/CABG compared to isolated CEA
When compared to patients undergoing isolated CEA, patients undergoing CEA/CABG were more likely to suffer postoperative stroke (5.5% vs 1.2%; P = .0003) and death (5.5% vs 0.3%; P = 6 × 10−12; Table II). Of patients undergoing CEA/CABG, 9.2% suffered either postoperative stroke or death before discharge compared to 1.3% in patients undergoing isolated CEA (P = 2 × 10−12).
Table II.
Outcomes of isolated CEA vs CEA/CABG
| Isolated CEA in Non-CEA/CABG centers | CEA/CABG (overall) | P value | |
|---|---|---|---|
| Number of patients | 1563 | 109 | |
| Stroke | 19 (1.2%) | 6 (5.5%) | .0003 |
| Death | 4 (0.3%) | 6 (5.5%) | 6 × 10−12 |
| Stroke/death | 20 (1.3%) | 10 (9.2%) | 2 × 10−12 |
| Return to OR | 19 (1.2%) | 8 (7.6%) | 6 × 10−7 |
| Length of stay (days) | 2.2 (2.0–2.3) | 11.7 (10.0–13.5) | 7 × 10−95 |
CABG, Coronary artery bypass graft; CEA, carotid endarterectomy; OR, operating room.
Our secondary outcome measures were in-hospital length of stay and return to the OR. Patients undergoing CEA/CABG were more likely to return to the OR when compared to isolated CEA (7.6% vs 1.2%; P = 6 × 10−7; Table II). Of the 19 patients in the baseline carotid risk group who returned to the OR, 10 returned for bleeding complications, five returned for neurologic alteration, and the remaining patients had no available indication for reoperation. Of the eight patients in the CEA/CABG group who returned to the OR, only two patients had reasons for reoperation recorded (one bleeding indication, one neurologic indication); of the remaining six patients, it is unknown how many required reoperation for CABG-related complications. Patients undergoing CEA/CABG had a mean length of stay of 11.7 days (95% confidence interval [CI], 10.0–13.5) which was significantly longer than the 2.2-day mean length of stay for isolated CEA (95% CI, 2.0–2.3; P = 7 × 10−95).
Due to the differences in operative techniques between the isolated CEA group and the CEA/CABG group, we performed further analyses to determine if any of these factors contributed to the differences in outcomes. In the CEA/CABG group, we found no statistically significant differences in the rates of stroke, death, or combined stroke or death when accounting for differences in technique (including eversion vs conventional, selective shunting, use of a patch, and use of dextran).
Comparison of patient outcomes stratified by symptom status, center, and by risk category
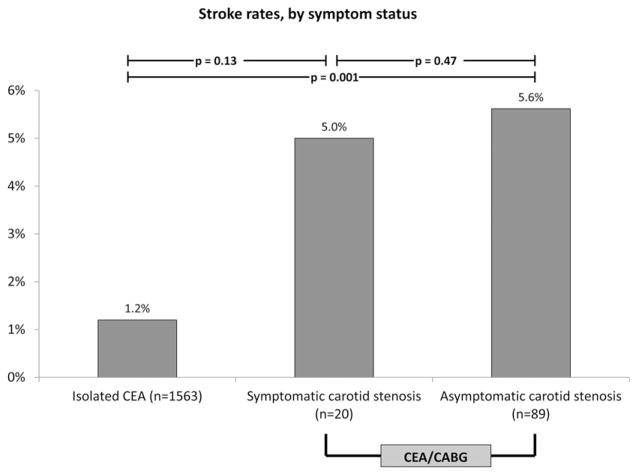
Compared to a stroke rate of 1.2% in the isolated CEA group, patients with asymptomatic CAD undergoing CEA/CABG had a stroke rate of 5.6% (5 of 89 patients) and patients with symptomatic CAD had a stroke rate of 5% (one of 20 patients; P = .91; Fig 2). Combined stroke/death rates were also higher in patients with asymptomatic or symptomatic carotid disease (10% and 5%, respectively; P = .47) when compared to the baseline stroke/death rate of 1.3%. Differences in outcomes between isolated CEA and asymptomatic CEA/CABG groups were statistically significant (P = .001), although the symptomatic group could not be demonstrated to be significantly different than the other two groups, likely due to low incidence of events.
Fig 2.
Rates of perioperative stroke in patients undergoing isolated carotid endarterectomy (CEA) in baseline risk group compared to rates of stroke in patients undergoing CEA/coronary artery bypass graft (CABG), stratified by symptom status.
Of the six centers that performed at least one CEA/CABG, only three performed more than five CEA/CABG procedures during the study period. At these centers, the proportion of patients who had symptomatic carotid disease varied, from 12% to 26%. Specifically, one center selected a relatively low proportion of patients with symptomatic carotid disease (12%), while the other two centers selected symptomatic patients twice as often (25% and 26%). Despite the differences in the proportion of symptomatic patients, there were no statistically significant differences in stroke or death rates between centers. Further, there was also variation in center-level performance of CEA/CABG in relation to total CEA. At one center, patients selected for CEA/CABG represented 14% of the total CEA volume at that center, whereas at other centers, CEA/CABGs represented <3% of all CEAs performed at that center.
Finally, when we stratified the 109 patients who underwent CEA/CABG by preoperative neurologic risk, we found that 44% (48 of 109 patients) were high risk and 56% (61 of 109 patients) were low risk (Fig 1). Patients in the high-risk group were more likely to have a history of smoking (90% vs 72%; P = .02) and were more likely to have their procedure classified as urgent or emergent (44% vs 20%; P = .007). Differences in anatomy and symptom status between high-risk and low-risk groups are summarized in Table I.
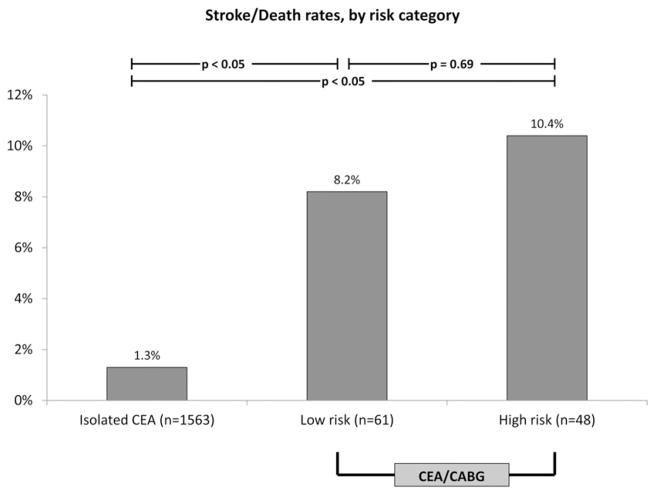
Both low-risk and high-risk patients undergoing CEA/CABG had a significantly higher rate of stroke and death compared to patients undergoing isolated CEA. However, the risk of combined stroke or death was not significantly different between the low-risk and high-risk groups (8.2% vs 10.4%; P = .69; Table III; Fig 3). There were no statistically significant differences in rates of return to the OR (10.2% vs 4.3%; P = .25) or in-hospital length of stay (12.7 days vs 10.4 days; P = .09; Table III), potentially due to our small sample size of 109 patients.
Table III.
Outcomes of CEA/CABG in low-risk vs high-risk patients
| Low neurologic risk CEA/CABG | High neurologic risk CEA/CABG | P value | |
|---|---|---|---|
| Number of patients | 61 | 48 | |
| Stroke | 3 (4.9%) | 3 (6.3%) | .76 |
| Death | 3 (4.9%) | 3 (6.3%) | .76 |
| Stroke/death | 5 (8.2%) | 5 (10.4%) | .69 |
| Return to OR | 6 (10.2%) | 2 (4.3%) | .25 |
| Length of stay (days) | 12.7 (9.9–15.5) | 10.4 (8.8–12.1) | .2 |
CABG, Coronary artery bypass graft; CEA, carotid endarterectomy; OR, operating room.
Fig 3.
Rates of perioperative stroke in patients undergoing isolated carotid endarterectomy (CEA) in baseline risk group compared to rates of stroke in patients undergoing CEA/coronary artery bypass graft (CABG), stratified by risk category. Exact P value for isolated CEA vs low-risk category is .00002. Exact P value for isolated CEA vs high risk category is 4.5 × 10−7.
DISCUSSION
In our region, CEA/CABG is performed uncommonly, comprising <2% of all CEAs from 2003 to 2009. We found that when compared to patients undergoing isolated CEA, patients undergoing CEA/CABG were more likely to be men and more likely to have a higher burden of comorbid conditions. With regard to the severity of carotid artery stenosis, patients undergoing CEA/CABG had more advanced ipsilateral and contralateral disease burden. However, compared to isolated CEA in our region, where approximately half of patients undergo surgery for asymptomatic disease, over three-quarters of patients undergoing CEA/CABG have asymptomatic carotid stenosis. Further, compared to patients undergoing isolated CEA, patients undergoing CEA/CABG had significantly higher rates of stroke, death, and return to the OR as well as a longer mean length of stay. Finally, across the centers performing CEA/CABG in our region, patient selection and outcomes varied widely, indicating the potential for standardization in patient selection and improvement in outcomes.
Large clinical series suggest that the risk of stroke associated with isolated CABG in the absence of carotid artery stenosis is approximately 1.3% to 2%.10,13 Historically, this risk was thought to be much higher in the presence of >50% carotid stenosis or occlusion.11 Despite the fact that many early reports did not take into account neurologic symptom status, contralateral disease, or the presence of occlusion, a causal relationship between the presence of CAD and perioperative stroke has been assumed. Accordingly, CEA/CABG emerged as a way to treat patients with concurrent CAD who are found to have carotid artery stenosis, often on preoperative screening, while minimizing perioperative stroke risk.
In actual practice, the utilization and outcomes of CEA/CABG have varied. For example, a study utilizing the NIS reported that between 2000 and 2004, 26,197 staged or combined CEA/CABGs were performed in the United States with a periprocedural stroke rate of 3.9%.7 A second study, also utilizing the NIS, examined cases of combined CEA/CABG only when the procedures were coded on the same day and showed the same stroke rate (3.9%).6 These results are consistent with our findings, wherein postoperative stroke occurred in 5.5% of all patients who underwent CEA/CABG. However, other studies have reported outcomes that differ, varying between a stroke rate of 1.1% at a single institution14 and a stroke rate of 12% in the Medicare population.8 Key studies are summarized in Table IV and demonstrate this variation.
Table IV.
Key studies in CEA/CABG
| Authors | Year | Years studied | No. (total) | Symptomatic patients (% of total) | Asymptomatic patients (% of total) | Perioperative stroke rate % | Perioperative mortality rate % | Combined stroke/death rate % | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Illuminati et al25 | 2011 | 2004–2009 | 185 | 0 (0) | 185 (100) | 0 | 1.0 | 1.0 | Randomized trial, 90-day stroke rate 1.0% |
| Gopaldas et al6 | 2011 | 1998–2007 | 16,639 | NA | NA | 3.9 | 4.5 | 7.7 | Nationwide Inpatient Sample |
| Dick et al21 | 2011 | 1996–2006 | 134 | NA | NA | 4.0 | 1.0 | NA | Single institution |
| Timaran et al7 | 2008 | 2000–2004 | 26,197 | 938 (4) | 25,259 (96) | 3.9 | 5.4 | 8.6 | Nationwide Inpatient Sample |
| Kougias et al22 | 2007 | 1981–2003 | 277 | 29 (10) | 248 (90) | 2.8 | 3.6 | NA | Single institution |
| Byrne et al14 | 2006 | 1980–2005 | 702 | 0 (0) | 702 (100) | 1.1 | 3.1 | 4.3 | Single institution, 100% asymptomatic |
| Brown et al8 | 2003 | 1995–1996 | 226 | 100 (44) | 126 (56) | 12.0 | 6.6 | 17.7 | Medicare population |
| Mackey et al16 | 1996 | 1984–1994 | 100 | 57 (57) | 43 (43) | 9 | 8 | 15 | Single institution |
| D’Agostino et al15 | 1996 | 1990–1995 | 28 | 28 (100) | 0 (0) | 18.0 | NA | NA | Single institution, 100% symptomatic |
CABG, Coronary artery bypass graft; CEA, carotid endarterectomy; NA, not applicable.
Why might stroke/death rates vary so widely across different studies of CEA/CABG? We believe that at least two important reasons exist for this variation. First, variation in carotid symptom status is evident across each of the studies we examined (Table IV). Rates of perioperative stroke are prohibitively high in patients with symptomatic CAD who undergo CABG without carotid intervention. In one study of patients with symptomatic unilateral carotid artery stenosis undergoing isolated cardiac surgery, the stroke rate was 18% (five of 28 patients).15 This risk is mitigated somewhat by performing CEA/CABG, although the stroke rate in symptomatic patients still may be as high as 14.2% nationally.7 Similarly high rates of 9% to 12% perioperative stroke rates have been reported in a series where a high proportion of symptomatic patients (57% and 44%, respectively) underwent CEA/CABG.8,16 Alternatively, in studies where a much smaller proportion of patients are symptomatic, perioperative stroke occurs less frequently. Ninety-six percent of patients undergoing CEA/CABG nationally are asymptomatic and incur a stroke rate of 3.9%.7 Additionally, a single center reported a CEA/CABG perioperative stroke rate of 1.1% in a cohort of 100% asymptomatic patients.14 In our cohort of 109 patients undergoing CEA/CABG, 18% had symptomatic carotid artery stenosis. We were unable to demonstrate any difference in outcome between the symptomatic and asymptomatic groups. Notably, patients with asymptomatic carotid disease had a 5.6% stroke rate, higher than would be expected from available literature.11
The second reason underlying variation in CEA/CABG outcomes lies in structural characteristics of the hospitals (and surgeons) that perform these procedures. Large studies have shown that high-volume CABG centers have improved mortality rates when compared to low-volume centers.17,18 Similarly, mortality and neurologic complication rates after CEA are lower in high-volume CEA centers when compared to low-volume centers.19,20 It follows that centers in which a larger volume of CEA/CABG are performed should be able to demonstrate lower rates of stroke and death, and this has been shown in high-volume single-center series14,21,22 (Table IV). In our region, three centers performed CEA/CABG with regularity. Although there was a trend toward lower complication rates with increasing volume, this was not significant and likely was also limited by sample size.
Patients and surgeons should consider the established indications for CEA/CABG, as well as the underlying patient-level risks when considering whether or not to perform CEA/CABG. Most societal recommendations agree that in patients who have symptomatic carotid artery stenosis and an indication for CABG, combined CEA/CABG is currently the best option, especially when performed at a large-volume center. However, for patients with asymptomatic carotid artery stenosis, the theoretic reduction in perioperative stroke attributable to carotid artery stenosis may, or may not, justify the increased risk of stroke or death seen in patients undergoing combined CEA/CABG. In these cases, an individualized approach should be adopted until broader guidelines can be established. Many options exist for these patients, including proceeding simply with CABG alone. Recent small studies have shown that CABG can be performed safely on selected groups of patients with asymptomatic carotid artery stenosis without carotid intervention.23,24 Our data support efforts in this regard, given the relatively high rate of stroke or death after combined CEA/CABG, even in low-risk patients.
Others have argued that the risk of cardiac surgery in the presence of untreated carotid artery stenosis is prohibitively high. A recent randomized trial25 found that in patients with unilateral asymptomatic carotid artery stenosis >70% who underwent CABG without CEA, the peri-operative stroke rate was 3.3% but increased to 7.7% when followed up to 90 days after surgery, significantly higher than the 1% stroke rate seen in patients undergoing CEA/CABG. The question of late strokes after cardiac surgery deserves more attention in the future because this phenomenon has not previously been reported.
Our study has several limitations. First, given that the data in the VSGNE dataset is collected under the auspices of quality improvement in vascular surgery, we collected relatively little information in regard to CABG-related operative details or cardiac risk factors for stroke. It has been reported that off-pump CABG can be performed with lower complications than on-pump CABG when performed in conjunction with CEA,5,6,26 which may be partly attributable to the known increased incidence of epiaortic atherosclerosis in patients with severe carotid disease or carotid occlusion.27 A second limitation is that there was not a large enough number of patients who underwent CEA/CABG during the period studied to illustrate differences in outcomes by symptom status, by center, or by risk category. Future efforts in this regard will address this question as sample size accumulates in our dataset, especially as the use of regional quality improvement efforts in vascular surgery expands nationally.9 Third, we were only able to provide meaningful analysis for in-hospital outcomes. Additional adverse events may have accrued in the early postoperative period that we did not capture. Furthermore, we were only able to analyze patients who actually underwent CEA or CEA/CABG instead of being able to look at a population of patients by their disease processes: concurrent carotid artery stenosis and CAD. In the current era, it is likely that some of these patients were treated with nonconcomitant revascularization, especially in centers with experience in carotid artery stenting. Nationwide, patients who have carotid artery stents and CABG during the same hospitalization have been shown to have a fivefold increased rate of postoperative stroke.7 Finally, the longer operative time associated with combined CEA/CABG may predispose patients to increased risk of bleeding complications when compared to isolated CEA, although information regarding operative time in CEA/CABG was not available in our database.
In conclusion, our study demonstrates that within our region, CEA/CABG is infrequently performed and is associated with a higher risk of in-hospital stroke or death when compared to isolated CEA. Further, the use of CEA/CABG varies across the centers in our region, although patients with asymptomatic carotid artery stenosis make up a majority of those that undergo CEA/CABG at all centers. Especially in the setting of asymptomatic carotid disease, patients and surgeons should consider the risks and benefits associated with CEA/CABG before undergoing a combined procedure.
Acknowledgments
Dr Goodney, is partially funded by National Institutes of Health grant 1K08HL05676-01 and Society for Vascular Surgery (SVS) Foundation grant.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: DJ, DS, MC, YB, BW, DL, JC, PG
Analysis and interpretation: DJ, DS, YB, BW, DL, JC, PG
Data collection: DJ, DL, PG
Writing the article: DJ, PG
Critical revision of the article: DJ, DS, MC, YB, BW, JC, PG
Final approval of the article: DJ, DS, MC, YB, BW, DL, JC, PG
Statistical analysis: DJ, DL, PG
Obtained funding: PG
Overall responsibility: PG
References
- 1.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) J Am Coll Cardiol. 2004;44:e213–310. doi: 10.1016/j.jacc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Naylor AR. Synchronous cardiac and carotid revascularisation: the devil is in the detail. Eur J Vasc Endovasc Surg. 2010;40:303–8. doi: 10.1016/j.ejvs.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Kolh P, Wijns W, Danchin N, Di Mario C, et al. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI) Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2010;38 (Suppl):S1–S52. doi: 10.1016/j.ejcts.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Castaldo J, Van der Heyden J, Plokker HW. Is carotid artery disease responsible for perioperative strokes after coronary artery bypass surgery? J Vasc Surg. 2010;52:1716–21. doi: 10.1016/j.jvs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Carter R. Off-pump concomitant coronary revascularization and carotid endarterectomy. J Cardiovasc Surg (Torino) 2009;50:83–91. [PubMed] [Google Scholar]
- 6.Gopaldas RR, Chu D, Dao TK, Huh J, LeMaire SA, Lin P, et al. Staged versus synchronous carotid endarterectomy and coronary artery bypass grafting: analysis of 10-year nationwide outcomes. Ann Thorac Surg. 2011;91:1323–9. doi: 10.1016/j.athoracsur.2011.02.053. discussion 1329. [DOI] [PubMed] [Google Scholar]
- 7.Timaran CH, Rosero EB, Smith ST, Valentine RJ, Modrall JG, Clagett GP. Trends and outcomes of concurrent carotid revascularization and coronary bypass. J Vasc Surg. 2008;48:355–60. doi: 10.1016/j.jvs.2008.03.031. discussion 360-1. [DOI] [PubMed] [Google Scholar]
- 8.Brown KR, Kresowik TF, Chin MH, Kresowik RA, Grund SL, Hendel ME. Multistate population-based outcomes of combined carotid endarterectomy and coronary artery bypass. J Vasc Surg. 2003;37:32–9. doi: 10.1067/mva.2003.60. [DOI] [PubMed] [Google Scholar]
- 9.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW, et al. A regional registry for quality assurance and improvement: the vascular study group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion 1101-2. [DOI] [PubMed] [Google Scholar]
- 10.Venkatachalam S, Shishehbor MH. Management of carotid disease in patients undergoing coronary artery bypass surgery: is it time to change our approach? Curr Opin Cardiol. 2011;26:480–7. doi: 10.1097/HCO.0b013e32834a7035. [DOI] [PubMed] [Google Scholar]
- 11.Naylor AR, Bown MJ. Stroke after cardiac surgery and its association with asymptomatic carotid disease: an updated systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2011;41:607–24. doi: 10.1016/j.ejvs.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Goodney PP, Likosky DS, Cronenwett JL Vascular Study Group of Northern New England. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–45. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Naylor AR, Mehta Z, Rothwell PM, Bell PR. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg. 2002;23:283–94. doi: 10.1053/ejvs.2002.1609. [DOI] [PubMed] [Google Scholar]
- 14.Byrne J, Darling RC, 3rd, Roddy SP, Mehta M, Paty PS, Kreienberg PB, et al. Combined carotid endarterectomy and coronary artery bypass grafting in patients with asymptomatic high-grade stenoses: an analysis of 758 procedures. J Vasc Surg. 2006;44:67–72. doi: 10.1016/j.jvs.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino RS, Svensson LG, Neumann DJ, Balkhy HH, Williamson WA, Shahian DM. Screening carotid ultrasonography and risk factors for stroke in coronary artery surgery patients. Ann Thorac Surg. 1996;62:1714–23. doi: 10.1016/s0003-4975(96)00885-5. [DOI] [PubMed] [Google Scholar]
- 16.Mackey WC, Khabbaz K, Bojar R, O’Donnell TF., Jr Simultaneous carotid endarterectomy and coronary bypass: perioperative risk and long-term survival. J Vasc Surg. 1996;24:58–64. doi: 10.1016/s0741-5214(96)70145-3. [DOI] [PubMed] [Google Scholar]
- 17.Shahian DM, O’Brien SM, Normand SL, Peterson ED, Edwards FH. Association of hospital coronary artery bypass volume with processes of care, mortality, morbidity, and the Society of Thoracic Surgeons composite quality score. J Thorac Cardiovasc Surg. 2010;139:273–82. doi: 10.1016/j.jtcvs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Showstack JA, Rosenfeld KE, Garnick DW, Luft HS, Schaffarzick RW, Fowles J. Association of volume with outcome of coronary artery bypass graft surgery. Scheduled vs nonscheduled operations. JAMA. 1987;257:785–9. [PubMed] [Google Scholar]
- 19.Perler BA, Dardik A, Burleyson GP, Gordon TA, Williams GM. Influence of age and hospital volume on the results of carotid endarterectomy: a statewide analysis of 9918 cases. J Vasc Surg. 1998;27:25–31. doi: 10.1016/s0741-5214(98)70288-5. discussion 31-3. [DOI] [PubMed] [Google Scholar]
- 20.Holt PJ, Poloniecki JD, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between hospital volume and outcome following carotid endarterectomy. Eur J Vasc Endovasc Surg. 2007;33:645–51. doi: 10.1016/j.ejvs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Dick AM, Brothers T, Robison JG, Elliott BM, Kratz JM, Toole JM, et al. Combined carotid endarterectomy and coronary artery bypass grafting versus coronary artery bypass grafting alone: a retrospective review of outcomes at our institution. Vasc Endovascular Surg. 2011;45:130–4. doi: 10.1177/1538574410393752. [DOI] [PubMed] [Google Scholar]
- 22.Kougias P, Kappa JR, Sewell DH, Feit RA, Michalik RE, Imam M, et al. Simultaneous carotid endarterectomy and coronary artery bypass grafting: results in specific patient groups. Ann Vasc Surg. 2007;21:408–14. doi: 10.1016/j.avsg.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh J, Murray D, Khwaja N, Murphy MO, Walker MG. The influence of asymptomatic significant carotid disease on mortality and morbidity in patients undergoing coronary artery bypass surgery. Eur J Vasc Endovasc Surg. 2005;29:88–90. doi: 10.1016/j.ejvs.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Baiou D, Karageorge A, Spyt T, Naylor AR. Patients undergoing cardiac surgery with asymptomatic unilateral carotid stenoses have a low risk of peri-operative stroke. Eur J Vasc Endovasc Surg. 2009;38:556–9. doi: 10.1016/j.ejvs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Illuminati G, Ricco JB, Caliò F, Pacilè MA, Miraldi F, Frati G, et al. Short-term results of a randomized trial examining timing of carotid endarterectomy in patients with severe asymptomatic unilateral carotid stenosis undergoing coronary artery bypass grafting. J Vasc Surg. 2011;54:993–8. doi: 10.1016/j.jvs.2011.03.284. discussion 998-9. [DOI] [PubMed] [Google Scholar]
- 26.Fareed KR, Rothwell PM, Mehta Z, Naylor AR. Synchronous carotid endarterectomy and off-pump coronary bypass: an updated, systematic review of early outcomes. Eur J Vasc Endovasc Surg. 2009;37:375–8. doi: 10.1016/j.ejvs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Baribeau YR, Westbrook BM, Charlesworth DC, Maloney CT. Increased incidence of proximal aortic atherosclerotic disease in patients with internal carotid occlusion. Heart Surg Forum. 2002;6:55–9. doi: 10.1532/hsf.604. Submitted Jan 3, 2012; accepted Feb 9, 2012. [DOI] [PubMed] [Google Scholar]