Abstract
Cancer chemoprevention strategies are not widely implemented in clinical practice. Targeting biomarkers in patients with elevated risk of developing cancer by means of short-term administration of certain agents may be a strategy to minimize toxicities while maintaining efficacy in clinical trials that can be completed in years rather than decades.
In this issue of Clinical Cancer Research, Takayama and colleagues (1) address two major limitations in cancer chemoprevention clinical trial designs: the selection of surrogate endpoints of cancer and the long-term administration of agents to at-risk populations.
Strong clinical evidence supports colorectal adenoma as a biological intermediate for colorectal cancer (2). In randomized studies, long-term (years) continuous use of COX2-selective inhibitors of nonsteroidal anti-inflammatory drugs (NSAIDs), as well as sulindac, a nonselective NSAID, has been shown to prevent colorectal adenoma in average-risk and genetically high-risk groups (3, 4). The prevention of colorectal adenoma with NSAIDs, along with extensive evidence for the prostanoid pathway in epithelial tumorigenesis, justifies the enthusiasm for considering NSAIDs as a class of drugs with cancer-preventive properties. However, concerns about agent-related toxicity (i.e., gastrointestinal [GI] and cardiovascular) and tolerability with prolonged use bring into question the validity of using NSAIDs in clinical research endeavors.
An estimated 60 million Americans annually are prescribed an NSAID (5, 6), and thanks to the over-the-counter availability of NSAIDs, a large percentage of Americans report regular use of these drugs for more than 30 days. Given the cancer preventive activity of NSAIDs, it is important to clarify agent-specific potency and design studies that will allow iterative testing to find the lowest effective dose and duration. Takayama and colleagues’ study on the use of NSAIDs for eradicating aberrant crypt foci (ACF) is an important example of such a design. This small, double-blinded, placebo-controlled study of 300 mg/d sulindac or 400 mg/d etodolac for 2 months for ACF prevention has a number of notable strengths, including a focus on short-term, discontinuous NSAID use and shorter time to endpoint analysis. To determine the maximally effective, shortest drug duration schedule, the investigators first estimated the effect of 1, 2, 3, and 5 months of 300 mg/d sulindac on ACF in a few subjects. In a larger, placebo-controlled study, they showed that 2 months of sulindac treatment had a significant effect on ACF. Of importance, they also showed that 2 months of daily sulindac followed by no drug was sufficient to reduce the risk of colorectal polyps of any type at 12 months. In contrast, treatment with etodolac (a COX2 inhibitor) for 2 months showed no effect on ACF or polyp formation. Takayma and colleagues postulate that short-duration sulindac eradicates ACF, resulting in fewer total polyps. The lack of COX2 expression in ACF and the off-target (non-COX2) activity of sulindac may explain the differential effect between the agents. These results suggest that short, discontinuous treatment with sulindac may be sufficient to achieve a chemopreventive effect. A better understanding of this finding might allow for more measured use of sulindac in moderate-risk groups to offset the harm associated with long-term use.
The use of surrogate endpoints for colorectal cancer remains controversial. In 2003, Levin (7) expressed concerns about the use of colorectal adenoma, citing the low frequency of conversion to cancer and the possibility that drug effects on lesions with low inherent malignant potential may not be informative for prevention of invasive carcinoma. This criticism has been raised even more strongly regarding the use of ACF, particularly the more common nondysplastic type. In a substudy of patients in the Adenoma Prevention with Celecoxib (APC) trial, neither the presence nor the number of ACF changed with celecoxib treatment, and ACF was not correlated with risk of colorectal adenoma (8). Takayama and colleagues acknowledge the criticism of ACF as a surrogate endpoint for cancer and note the lack of power to assess effects on dysplastic-type ACF. However, they emphasize that the efficacy of sulindac for preventing polyps and colorectal adenoma at 12 months was greater in individuals who showed eradication of ACF with sulindac intervention. This finding lends support to the notion that an ACF lesion is a precursor for colorectal polyps that is eradicated by sulindac but not etodolac therapy.
We believe this study raises two important issues. First, short, discontinuous use of sulindac appears to be as effective in suppressing polyp formation (by eradicating the ACF precursor) as are longer (1–2 years), continuous treatments. This noteworthy observation contrasts with evidence from the APC trial, wherein celecoxib showed no treatment effect for ACF (8). Second, Takayama and colleagues distinguish between preventing adenoma and preventing earlier precursors (ACF). These observations give us an opportunity to discuss trial design modifications that would accelerate answers to questions about agent dose and duration and possibly the differential action of COX2 inhibitors and sulindac.
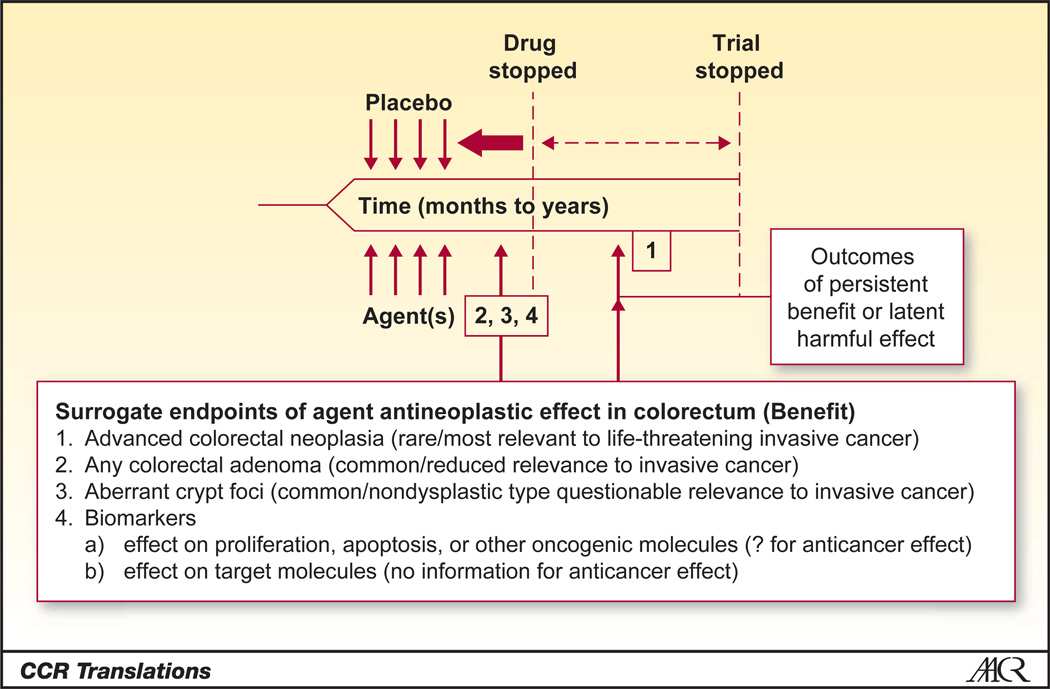
In Figure 1, which was adapted from Levin (7), we address some of the issues raised by Takayama and colleagues’ study. In the classic chemoprevention trial design for colorectal adenoma, patients who have undergone polypectomy (i.e., an at-risk population with clean colon) are recruited, randomized to treatment or placebo, and followed to colonoscopy as dictated clinically by surveillance guidelines. This approach is advantageous because it involves a fairly large population of eligible subjects (persons with adenoma), follows standard-of-care practices (endpoint colonoscopy covered by insurers), and does not introduce additional harm to subjects by nonstandard use of colonoscopy. A disadvantage is that is exposes predominantly average-risk subjects to long interventions, decreasing the benefit-to-risk ratio.
Figure 1.
Fast-tracking the timeline for evaluation of chemoprevention studies of NSAIDs. Integration of the common surrogate endpoints of ACF or any polyp allows design considerations for short interventions with shorter time to follow-up. Truncation of this timeline in an ongoing evaluation of NSAIDs for prevention would allow the formal inclusion of a postintervention evaluation to determine the persistency of effect or emergence of latent harmful effects, aiding in the informed delivery of NSAIDs for chemoprevention. Adapted from Levin, Potential Pitfalls in the Use of Surrogate Endpoints in Colorectal Adenoma Chemoprevention, Journal of the National Cancer Institute, 2003, vol. 95, issue 10, pp. 697–699 by permission of Oxford University Press.
The study by Takayama and colleagues represents an alternative to the classic design, in that it includes planned colonoscopies conducted at shorter intervals. The authors support the ACF endpoint, but one of the strengths of their study is the endoscopy results obtained at 12 months. We support similar designs and suggest consideration of only a large effect size (50% risk reduction) and implementation as a Phase II agent screening approach similar to those conducted in the therapeutic setting (9). This includes loosening the alpha level for significance testing in order to fast-track agent(s) dose/duration combinations to Phase III testing. This approach would result in smaller, faster studies involving reduced exposure to study participants and facilitate identification of the best dose, duration, and drug combinations. The disadvantage of this design is the introduction of a nonstandard endoscopy procedure, which will increase patient risk and study costs.
With regard to distinguishing between preventive and treatment effects, we propose the recruitment of subjects who have a history of polyp and are due for surveillance endoscopy at some near future date (12–18 months) to intervention studies. Many of these patients will have prevalent colorectal adenoma (for which sample size and power can be estimated from published studies) and would be ideal candidates for short-term treatment to test the effect of agents (alone or in combination) on existing adenoma. For this and all intervention studies, we reiterate the cautionary note raised by Levin (7) that posttreatment endpoints should be integrated as part of a formal clinical trial design (see Fig. 1). A partial reduction in growth, as opposed to eradication, could result in altered kinetics such that existing lesions would fall just below the limits of endoscopy detection. This could adversely increase surveillance intervals postintervention in at-risk individuals.
In these short prevention and treatment designs, the completion of primary (effect on a surrogate endpoint) and immediate secondary (toxicities and adenoma rates after cessation of treatment) endpoints could conceivably occur within a 5-year grant cycle. In a recent issue of Cancer Prevention Research, Robert Temple, director of the Office of Medical Policy of the United States Food and Drug Administration’s Center for Drug Evaluation and Research, discussed parallels between the development of drugs for cardiovascular disease and cancer. He noted, "It seems clear that effective cancer prevention drug development will depend on finding clinical features and biomarkers that identify high-risk states.…" (10). He also emphasized the importance of choosing populations in which the drug benefit will clearly exceed the drug risk. Perhaps an increased effort to identify moderate-to-high-risk populations, combined with a focus on determining the minimum necessary dose/duration for drug effect, will extend the application of NSAIDs from the familial high-risk setting to additional patient populations.
Acknowledgment
The authors thank Betsy Wertheim for her help in editing this commentary.
Grant Support
National Institutes of Health (CA095060 and CA123065 to P.A. Thompson and E.W. Gerner).
Footnotes
Disclosure of Potential Conflict of Interest
E.W. Gerner has an ownership interest in Cancer Prevention Pharmaceuticals, Tucson, Arizona. P. Thompson disclosed no potential conflicts of interest.
References
- 1.Takayama T, Nagashima H, Maeda M, Nojiri S, Hirayama M, Nakano Y, et al. Randomized double blind trial of sulindac and etodolac to eradicate aberrant crypt foci and to prevent sporadic colorectal polyps. Clin Cancer Res. 2011;17:3803–3811. doi: 10.1158/1078-0432.CCR-10-2395. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Gill S, Sinicrope FA. Colorectal cancer prevention: is an ounce of prevention worth a pound of cure? Semin Oncol. 2005;32:24–34. doi: 10.1053/j.seminoncol.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 5.Dai C, Stafford RS, Alexander GC. National trends in cyclooxygenase-inhibitor use since market release: nonselective diffusion of a selectively cost-effective innovation. Arch Intern Med. 2005;165:171–177. doi: 10.1001/archinte.165.2.171. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological Association. Wilcox CM, Allison J, Benzuly K, Borum M, Cryer B, et al. Consensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol. 2006;4:1082–1089. doi: 10.1016/j.cgh.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Levin B. Potential pitfalls in the use of surrogate endpoints in colorectal adenoma chemoprevention. J Natl Cancer Inst. 2003;95:697–699. doi: 10.1093/jnci/95.10.697. [DOI] [PubMed] [Google Scholar]
- 8.Cho NL, Redston M, Zauber AG, Carothers AM, Hornick J, Wilton A, et al. Aberrant crypt foci in the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila) 2008;1:21–31. doi: 10.1158/1940-6207.CAPR-07-0011. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 10.Temple R. Cancer chemoprevention—the cardiovascular model. Cancer Prev Res (Phila) 2011;4:307–310. doi: 10.1158/1940-6207.CAPR-11-0049. [DOI] [PubMed] [Google Scholar]