Abstract
Regulation of the ERK pathway is intimately involved in determining whether TCR stimulation is productive or induces anergy. T cells from patients with rheumatoid arthritis (RA) have increased ERK responsiveness which may be relevant for disease pathogenesis. Inflammatory cytokines such as TNF-α did not reproduce the TCR hypersensitivity typical for RA in T cells from healthy individuals. In contrast, priming with the homeostatic cytokines IL-7 and IL-15 amplified ERK phosphorylation to TCR stimulation twofold to threefold. The underlying mechanism involved a priming of the SOS-dependent amplification loop of RAS activation. The sensitization of the TCR signaling pathway has downstream consequences, such as increased proliferation and preferential Th1 differentiation. Importantly, priming with IL-7 or IL-15 enabled T cell responses to autoantigens associated with RA. Production of homeostatic cytokines is induced in lymphopenic conditions, which have been shown to predispose for autoimmunity and which appear to be present in the preclinical stages of RA. We propose that homeostatic cytokines, possibly induced by lymphopenia, decrease the signaling threshold for TCR activation and are thereby partly responsible for autoimmunity in RA.
INTRODUCTION
Despite the unquestionable success of anti-TNF-α therapy to treat rheumatoid arthritis (RA) (1), control of disease activity requires continuous treatment and induction of lasting remission is not accomplished. The current cytokine neutralization therapies, targeting TNF-α and more recently IL-6, function by blocking the effector stage of the disease and thereby predispose for more severe bacterial infections and tuberculosis reactivation (2, 3). Insights into pathogenetic mechanisms upstream of the effector pathways will be necessary to prevent or cure disease.
Characteristic autoimmune phenomena in RA are the production of rheumatoid factor and antibodies against citrullinated epitopes predominantly present on matrix proteins. Autoantibody production is largely dependent on the disease-associated HLA-DRB1*04 alleles and citrullinated T cell epitopes have been identified that are presented in the context of HLA-DR4 molecules (4). However, the pathogenesis of the disease and the role of the adaptive immune response have remained enigmatic (5). We have shown recently that T cells from RA patients exhibit an increased responsiveness of the ERK pathway and that this results in increased sensitivity to TCR stimulation which may permit responses to autoantigens (6). Increased responsiveness was seen for all T cell subsets including naïve and memory CD4 and CD8 T cells raising the possibility of a common exogenous factor conditioning the ERK pathway in RA T cells. The altered signal processing was largely independent of disease activity or treatment with anti-TNF-α agents suggesting that inflammatory cytokines were less likely to be the cause of ERK hyperactivity. Indeed, TNF-α has been shown to impair T cell responses by directly targeting the TCR signaling pathways and by downregulating CD28 expression (7, 8).
In addition to the production of inflammatory cytokines such as TNF-α, IL-6 and IL-1, RA is characterized by an increase in a number of other cytokines both in the inflamed joint and in the periphery, many of which can act on T cells. Of particular interest are homeostatic cytokines (HC) given that T cell homeostasis is profoundly abnormal in RA (9). Production can also be a consequence of inflammation (10). Prototypical HC are IL-7 and IL-15, the functions of which include enhancing T cell survival and proliferation (11, 12). Both cytokines bind to receptors that utilize the common γ chain to signal through JAK3, JAK1 and STAT proteins to induce gene expression and, as such, are sensitive to JAK inhibition. The potential role of HC in the initiation and progression of RA (and other inflammatory diseases) has come to light following the observation of elevated HC levels in patients (13, 14) and further studies showing that IL-7 and IL-15 are able to influence T cell activation and can exacerbate inflammation (14–16). IL-7 can increase Th1 cytokine production by both CD4 and CD8 T cells, particularly in systems utilizing suboptimal antigenic stimulation (17, 18). Recent successes in trials involving the JAK inhibitor Tofacitinib emphasize the relevance of common γ chain cytokines for RA although it cannot be excluded that Tofacitinib functions in RA by inhibiting other JAK STAT pathways (19, 20).
To establish whether chronic or intermittent exposures to increased cytokine concentrations can induce changes in T cell responsiveness, as observed in RA, we examined whether cytokines can condition T cells to respond to subsequent T cell activation with increased ERK phosphorylation and decreased TCR activation thresholds. We show that prior incubation with inflammatory cytokines (TNF-α or IL-6) had little effect on basal or anti-CD3/CD28-induced pERK levels in T cells from healthy individuals. In contrast, the homeostatic cytokines IL-7 and IL-15 conditioned T cells such that they became more responsive to TCR stimulation with low affinity antigen, mimicking the elevated levels of pERK previously observed in T cells from RA patients. This hyperresponsiveness enabled T cell responses to citrullinated autoantigens. We propose that IL-7 or IL-15 priming of the RasGRP/SOS pathway contributes to the increased ERK responsiveness and reduces the threshold above which signaling cascades are initiated, thereby predisposing for autoimmunity.
MATERIALS AND METHODS
Study Population and Cells
T cells were isolated by negative selection using RosetteSep® human T-cell enrichment cocktail (StemCell Technologies, Vancouver, BC) from the peripheral blood of healthy individuals. The protocol was approved by the Emory and Stanford University Institutional Review Boards, and all donors gave written, informed consent.
Antibodies
The following antibodies were used for cell surface or intracellular stains: CD3 APC-Cy7 and V450; CD4 PerCP-Cy5.5 and V500; CD8 PE-Cy7, Alexa Fluor 700 and Qdot605; CD45RA FITC and APC; CD28 PE and APC; CD25 APC; CD69 PE-Cy7; phospho-ERK1/2 Alexa Fluor 647, IL-2 FITC, IL-17 V450, IFN-γ PerCP-Cy5.5 and TNF-α PE-Cy7 (all BD Biosciences, San Jose, CA).
Cytokine conditioning
T cells (2 × 106/ml) were incubated with 10 ng/ml of either IL-1β, TNF-α, IL-7 or IL-15 for 24 h. The cells were washed twice with complete RPMI before TCR stimulation. For dose-response experiments, T cells were incubated with different concentrations of HC starting from 0.0625 pg/ml to 10 ng/ml. For inhibition experiments, 10 μM of LY294002, a PI3K Inhibitor (Invitrogen, Grand Island, NY) and 10 nM of Insolution JAK inhibitor I, a pan JAK inhibitor (EMD Chemicals, Gibbstown, NJ) were added during the conditioning period.
PhosFlow
T cells (0.5 × 106) were stimulated or not with anti-CD3 (1 μg/ml) and subsequent crosslinking (rabbit anti-mouse Ig); fixed in BD Cytofix buffer; permeabilized by BD Perm Buffer II; and stained for CD3, CD4, CD8 and the indicated signaling molecules. Data were acquired on an LSR II or Fortessa flow cytometer and analyzed with FACS DIVA (BD Biosciences) and/or FlowJo (Treestar, Ashland, OR) software.
Western Blot
T cells (3 × 106) were stimulated or not with anti-CD3 (1 μg/ml) and subsequent crosslinking (rabbit anti-mouse Ig); lysed in cell lysis buffer (Cell Signaling, Beverly, MA) adding 1:100 protease inhibitor cocktail (Sigma Aldrich, St Louis, MO) and 1 mM PMSF. The lysates (20 μg) were subjected to SDS-PAGE and immunoblot analysis with a mAb specific for the phosphorylated form of Erk1/2 (Cell Signaling). Membranes were stripped with Restore Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL) before reblotting with an antibody recognizing total Erk1/2 protein (Cell Signaling).
T cell activation and differentiation assays
T cells (1 × 106 cells/ml) purified from blood by RosetteSep® negative selection were conditioned with HC, washed and cultured for another 24 h in a 96-well flat-bottomed plates coated with 1 μg/ml of anti-CD3 and anti-CD28 antibodies in the absence of cytokines. CD25 and CD69 expression was assessed by FACS. For differentiation assays, naïve T cells were purified by FACS sorting based on CD45RA and CD27 expression. Cells were then conditioned with cytokines, washed and stimulated by TCR crosslinking in the absence of cytokines as described above. After seven days of culture, cells were restimulated with 50 ng/ml of PMA and 1 μg/ml of Ionomycin in the presence of Brefeldin A for 5 h and stained for intracellular cytokines per manufacturer’s suggestion (BD Biosciences).
Proliferative assays
CD4 T cells (2.5 × 106/ml) from healthy HLA-DR4+ or HLA-DR4− donors were stimulated for seven days with 1 × 106/ml autologous adherent cells and 25 μg/ml vimentin 65–77 peptide (SAVRARSSVPGVR), VimR70Cit, SAVRACitSSVPGVR) (4) or 20 ug/ml the melanocyte lineage specific glycoprotein gp100 44–59 peptide (WNRQLYPEWTEAQRLD) (21) in the presence of 2.5 μg/ml anti-CD28 and anti-CD49d antibodies. Control cultures were with medium only or 1 ug/ml IE63 overlapping peptide pool of varicella zoster virus (VZV, JPT Peptide Technologies, Berlin, Germany). Proliferative responses were quantified by 3H-thymidine incorporation.
Transfection
T cells were transfected with control siRNA (AllStars Negative Control siRNA from Qiagen, Valencia, CA) or siRNA for SOS-1 and SOS-2 (4 μg each, Santa Cruz Biotechnology, Santa Cruz, CA) using the Amaxa Nucleofector system and the Human T cell Nucleofector kit (Lonza AG, Cologne, Germany). Seventy-two hours following transfection, cell numbers were adjusted, and T cells were stimulated as described.
Active RAS ELISA
Cytokine-conditioned T cells were washed and cell lysates were prepared by adding 1X Mg2+ lysis/wash buffer included in the Ras GTPase Activation ELISA Kit from Millipore (Billerica, MA). Lysates were transferred to a 1.5 ml tube and incubated on ice for 15 min and resuspended thoroughly. After centrifugation at 14000 rpm for 10 min, supernatants were collected and assayed for protein concentration using the Bradford assay. Ras GTPase activity was measured by ELISA using the protocol recommended by the manufacturer (Millipore).
Statistical analysis
Results were compared with one-way ANOVA. A level of p <0.05 was considered significant.
RESULTS
Homeostatic but not inflammatory cytokines prime T cells to respond with increased ERK signaling to TCR stimulation
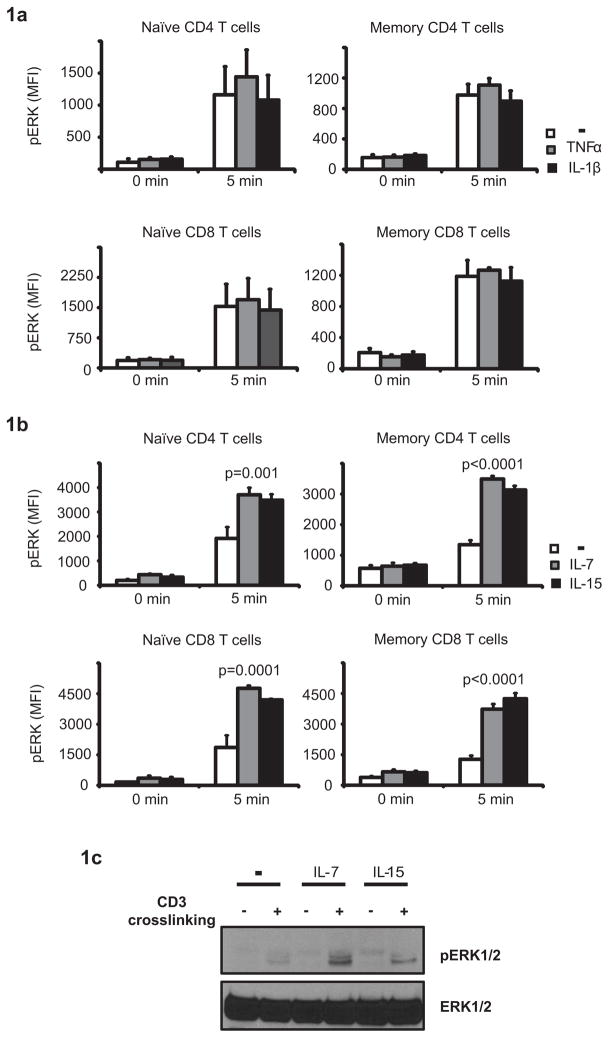
We have recently reported that CD4 and CD8 T cells from rheumatoid arthritis (RA) patients have hyperresponsive ERK activity upon T cell receptor stimulation (6). The overactive ERK cascade was found in all T cell subsets including naïve, memory and effector cells, raising the possibility of an underlying common factor, such as exposure to a cytokine that is elevated in RA and may reset the rheostat controlling ERK activation. Homeostatic cytokines (HC) such as IL-7 and IL-15 are critical for T cell survival, and receptors are expressed on all human T cell subsets. Both cytokines are elevated in RA patients, reflected by increased baseline phosphorylation of STAT5 in RA T cells. In contrast, IL-6, also increased in RA, induces STAT3 preferentially in naïve CD4 T cells suggesting a more restricted expression of the IL-6R (data not shown). T cells also express receptors for inflammatory cytokines such as IL-1β and TNF-α; TNF-α has been implicated in attenuating TCR signals and downregulating CD28 expression in RA T cells (7, 8). To explore whether chronic exposure to one of these cytokines accounts for the recalibration of the ERK pathway in RA, we compared their ability to modify the ERK response to TCR stimulation. T cells from healthy donors were incubated with 10 ng/ml cytokine for 24 h, washed and stimulated by crosslinking the CD3 complex. Activation of the ERK pathway was assessed by PhosFlow or Western blotting. Conditioning T cells with TNF-α and IL-1β had no significant impact on ERK phosphorylation (Fig. 1a). In contrast, conditioning T cells with HC prior to TCR ligation sensitized the ERK pathway (Fig. 1b and c). Sensitizing effects of IL-7 and IL-15 were seen in all T cell subsets. Homeostatic cytokines did not directly induce ERK phosphorylation; short-term incubation with IL-7 or IL-15 did not upregulate pERK (data not shown). Furthermore, basal pERK levels in T cells conditioned with cytokines for 24 h were not significantly altered prior to TCR ligation.
Figure 1. Cytokine-mediated priming of TCR-induced ERK signaling.
T cells were conditioned for 24 h with TNF-α, IL-1β (a), IL-7 or IL-15 (b and c). Cells were washed and stimulated by anti-CD3 crosslinking. Phosphorylation of ERK was analyzed for gated CD28+CD45RA+ CD4 and CD8 naïve and CD28+CD45RA− CD4 and CD8 memory T cells without and 5 min after TCR stimulation. Mean fluorescence intensities of pERK (MFI) are shown as mean ± SD of three to four experiments (b). Phosphorylation of ERK was confirmed using Western blot (c). The experiment shown is representative of three.
Homeostatic cytokines render T cells hyperresponsive to TCR ligation
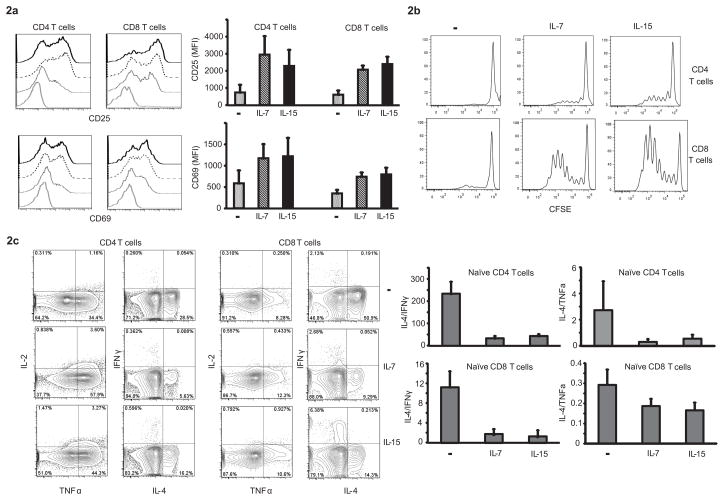
To examine whether the increased ERK response had functional consequences, T cells from healthy donors were exposed to IL-7 and IL-15 and then stimulated by suboptimal crosslinking of CD3 and CD28 in the absence of cytokines. Induction of the activation markers CD25 and CD69 was assessed by flow cytometry. Preincubation with either cytokine enhanced induction of both activation markers to subsequent TCR triggering (Fig. 2a). A similar effect of HC pre-exposure was seen on T cell expansion. CFSE-loaded T cells were cultured in anti-CD3/CD28 coated 96 well plates under suboptimal conditions that were not sufficient for most T cells to enter the cell cycle (Fig. 2b). After priming with HC, a subset of CD4 T cells and even a larger fraction of CD8 T cells proliferated suggesting that pre-exposure to IL-7 or IL-15 increases signaling strength to TCR stimulation.
Figure 2. Functional consequences of cytokine priming.
(a) T cells conditioned with IL-7 or IL-15 were stimulated by crosslinking with anti-CD3/CD28 and assessed for activation markers CD25 and CD69 after 24 h. Representative histograms (left panel) are shown for negative FMO control (gray line), unconditioned (dotted line), IL-7 (dashed line) and IL-15 conditioned T cells (solid line). Results from three experiments are shown as mean ± SD (right panel). (b) T cells loaded with CFSE were conditioned with IL-7 or IL-15. After 24 h, cytokines were washed off and the cells transferred to anti-CD3/anti-CD28 coated plates. On day 7, cells were assessed for CFSE dilution in CD4 and CD8 subsets. Histograms representative of four independent experiments are shown. (c) FACS-sorted naïve T cells (CD27+CD45RA+) conditioned with cytokines for 24 h were washed, transferred to anti-CD3/anti-CD28-coated plates and cultured for 7d under non-polarizing conditions. On day 7, cells were restimulated with ionomycin/PMA, and the frequency of cytokine-producing cells in CD4 and CD8 subsets was assessed. Representative contour density plots (left panel) and ratios of IL-4/IFN-γ and IL-4/TNF-α cytokine producing cells (right panel) are shown as mean ± SD from three donors.
Since high TCR signaling strength is known to bias for Th1 polarization, we determined the impact of HC cytokine priming on subsequent T cell differentiation. Naïve CD27+CD45RA+ T cells were primed with HC for 24 h, washed and cultured in a 96-well plate coated with antibodies against CD3/CD28 for seven days under non-polarizing conditions. The expression of intracellular cytokines IL-2, IL-4, IFN-γ, IL-17 and TNF-α was assessed after 5 h restimulation with PMA and ionomycin. Representative contour plots are shown in Fig. 2c. Pretreatment with IL-7 or IL-15 favored differentiation into TNF-α– and to a lesser extent IFN-γ–producing cells and disfavored production of IL-4. Production of IL-17 was minimal under either condition (data not shown). The cytokine secretion profile suggests that pre-exposure to HC enhances the development of multifunctional T cells defined by IL-2+TNF-α+ phenotype (Fig. 2c, right hand column). As a consequence, ratios of IL-4– to IFN-γ– and IL-4– to TNF-α–producing cells declined (Fig. 2d).
HC primed T cells overcome tolerance against auto-antigens
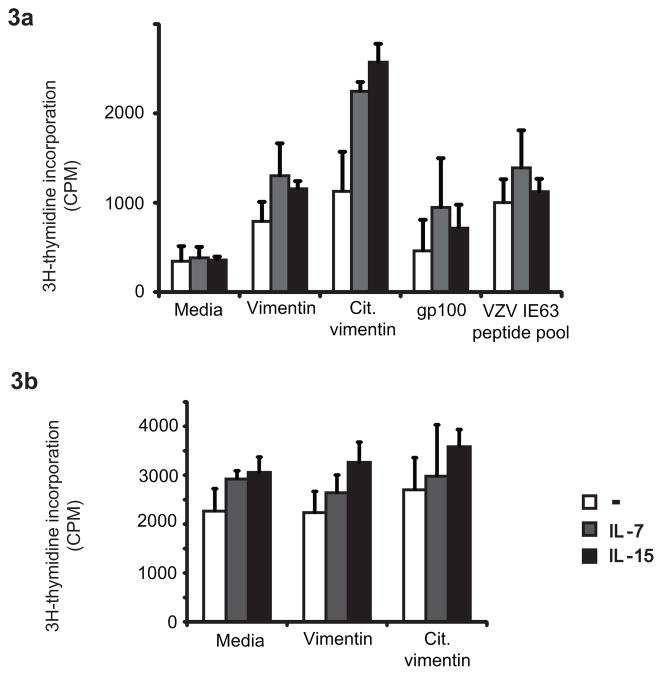
TCR threshold calibration by ERK is instrumental in the discrimination of self from foreign antigens. Thymocytes lose their ability to respond to self-antigen, in part due to the increased expression of DUSP5 and DUSP6 which dephosphorylate ERK (22). An ERK-mediated feedback loop is also important for the distinction between agonistic and antagonistic peptide-induced responses (23). To assess whether conditioning with HC and the associated sensitization of the ERK pathway overcomes the normal non-responsiveness to self-antigens, we examined CD4 T cell responses to citrullinated vimentin and melanocyte gp100. Autoantibody responses against citrullinated proteins, including vimentin and fibrinogen, are serological hallmarks of rheumatoid arthritis and correlated with the disease-associated PTPN22 and HLA-DRB1*04 alleles (24). The glycoprotein gp100 is a differentiation antigen shared between melanoma cells and normal melanocytes (25). Peptides of these self-antigens that bind to HLA-DR4 have been mapped (26). PBMCs from HLA-DRB1*04+ healthy subjects were stimulated with the native and the citrullinated vimentin or the gp100 peptide. An overlapping peptide pool of the VZV IE63 protein to which >99% of the US population has immunity was included as a control. Proliferative responses were assessed by 3H-thymidine incorporation. Unconditioned T cells exhibited low background proliferation to the native vimentin peptide and only slightly higher responses to the citrullinated peptide. In contrast, priming with HC enabled autoreactive T cell responses; preincubation with IL-7 and IL-15 facilitated a strong response to citrullinated vimentin (Fig. 3a). Proliferative responses to the gp100 peptide paralleled those to the native vimentin peptide. Stimulation with the VZV IE63 peptide pool elicited a proliferative response without prior cytokine conditioning consistent with pre-existent memory to these antigens. Of note, peptide concentrations used to examine the VZV response were 2 logs lower than for the self-antigen peptides. Even for these memory responses, priming with IL-7 or IL-15 prior to stimulation tended to increase the response consistent with our observation in Fig. 1 that HC conditioning increases TCR-induced signaling in all T cell subsets including memory T cells. Proliferative responses of PBMC from HLA-DR4− individuals stimulated with native or citrullinated vimentin peptides did not differ from the control cultures with medium alone (Fig. 3b) supporting the notion that the increased responses induced by IL-7 or IL-15 pre-incubation seen in HLA-DR4+ individuals (Fig. 3a) were antigen-specific.
Figure 3. Priming with IL-7 or IL-15 lowers tolerance against self-antigens.
(a) PBMCs from HLA-DR4+ donors were cultured for seven days with native or citrullinated vimentin peptides, melanocyte glycoprotein gp100 peptides or VZV IE63 peptide pool following conditioning with IL-7 or IL-15 for 24 h. 3H-thymidine was added at day 6. (b) PBMCs from non-HLA-DR4+ donor were cultured for seven days with native or citrullinated vimentin peptidesfollowing conditioning with cytokines. Data are shown as mean ± SD counts per minute (cpm) of triplicates wells and are representative of four experiments with PBMC from HLA-DR4+ and one experiment with PBMC from HLA-DR4− individual.
HC priming is transient and involves PI3K pathway activation
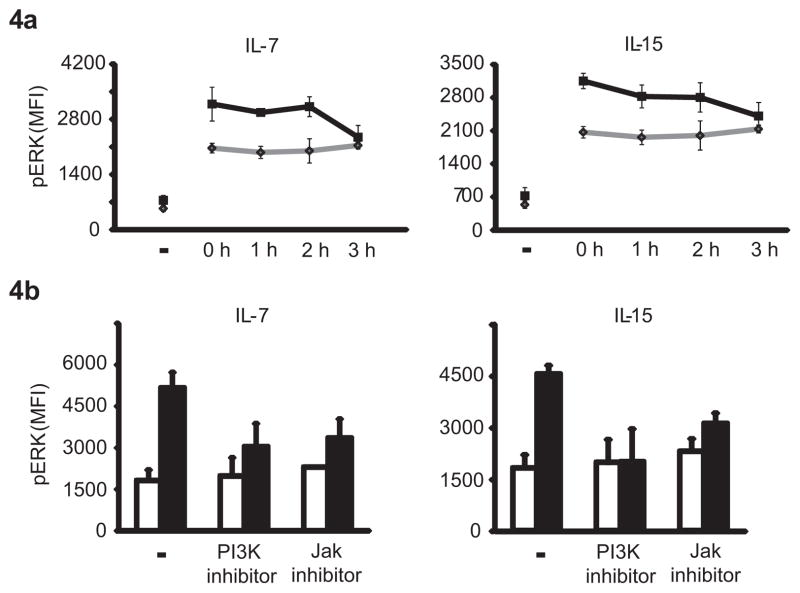
IL-7 and IL-15 mainly function as transcriptional activators through the STAT5 pathway. To gain insights into the mechanisms of TCR pathway sensitization by HC, we first addressed the possibility that HC act by inducing the transcription of a molecule involved in regulating ERK activity. In initial studies, transcriptional inhibitors such as actinomycin D were not able to block the HC effect. Kinetic studies also provided evidence that the HC-mediated effect on TCR signaling did not include transcriptional activation. T cells were incubated with HC for 24 h, cytokines were washed off and T cells were incubated in the absence of cytokines for various time intervals before being assayed for TCR-induced ERK phosphorylation. HC-mediated upregulation of TCR signaling was transient and failed to persist beyond 3 h (Fig. 4a) therefore suggesting that the effect does not involve transcriptional activation by STAT5. Also in support of this interpretation, the common γ chain homeostatic cytokine IL-21, which signals through STAT3 and not STAT5, had the same effect as IL-7 and IL-15. Conditioning with IL-21 sensitized TCR signaling to respond with increased ERK phosphorylation (Supp. Fig. 1)
Figure 4. Cytokine-induced activation is mediated via the PI3K pathway and is short-lived.
(a) T cells conditioned with IL-7 or IL-15 for 24 h were washed and rested for 0–3 h prior to stimulation by crosslinking of CD3. Activation-induced ERK phosphorylation was measured after 5 min using PhosFlow. Results are shown as mean fluorescence intensities (MFIs) of pERK in T cells without (gray diamonds) or with prior cytokine exposure (black squares). (b) T cells were conditioned with IL-7 or IL-15 for 24 h in the presence or absence of 10 μM of the PI3K inhibitor LY294002 or 10 nM of pan JAK inhibitor. Cells were washed, stimulated by crosslinking CD3 and assessed for pERK. Results are shown as mean fluorescence intensities (MFIs) of pERK in T cells without (white bars) and with cytokine exposure (black bars). Data are shown as mean ± SD of T cells from three individuals.
To identify crosstalk between chronic IL-7/IL-15 stimulation and TCR-induced signaling pathways, we analyzed the effect of pharmacological kinase inhibitors on HC priming. Cells were cultured with HC in the absence and presence of inhibitors for 24 h, then washed and stimulated by crosslinking of their CD3 complex for 5 min, at which point pERK was quantified. The pan JAK inhibitor and the PI3K inhibitor LY294002 ablated the effects of HC on ERK phosphorylation (Fig. 4b). These results suggest that HC priming involves JAK-mediated PI3K activation.
HC priming amplifies TCR-induced ERK phosphorylation by activating a SOS-mediated feedback loop
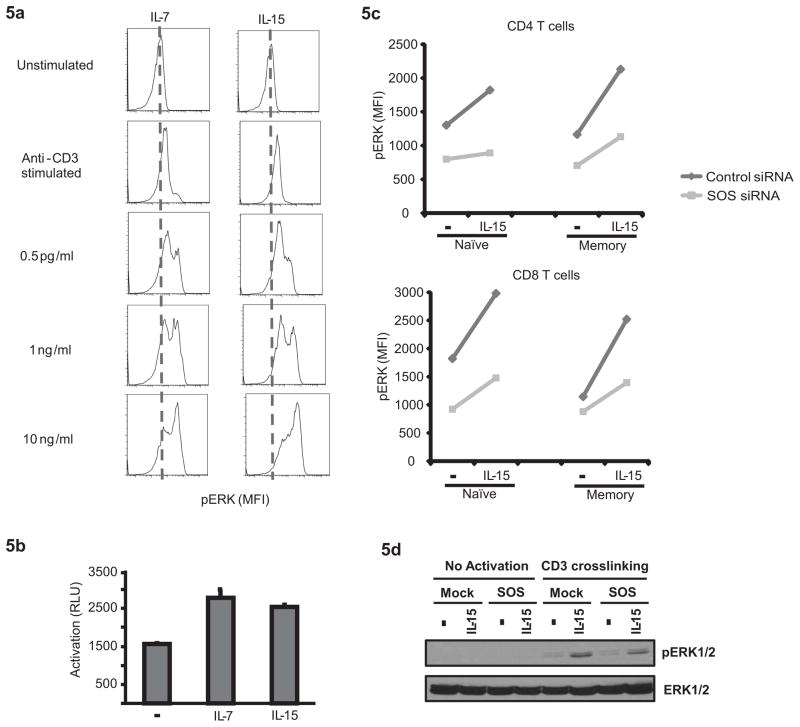
PI3K activation interfaces with the ERK pathway in the activation of RAS through RasGRP. Indeed, chronic HC stimulation resulted in the accumulation of active RAS; an approximate doubling was seen with chronic IL-7 as well as IL-15 exposure as determined with the Millipore active RAS assay (Fig. 5a). TCR-induced ERK phosphorylation is initiated through the activation of RAS by two guanine nucleotide exchange factors (GEFs), Ras-GRP and SOS. While Ras-GRP provides an analog signal resulting in a linear increase in pERK, SOS activates a positive feedback loop on becoming primed through the allosteric binding of active RAS and therefore causes a digital increase of ERK phosphorylation (27). Increased concentrations of active RAS may therefore activate the feedback loop, providing the basis for heteroclitic memory as postulated by Chakraborty and colleagues (28). To examine whether chronic stimulation with IL-7 or IL-15 provides an analog or digital signal in increasing ERK phosphorylation, we examined the relationship between cytokine doses and ERK responses to subsequent TCR stimulation. The results shown in Fig. 5b are consistent with a digital signal. TCR stimulation of cells that were not pretreated with cytokines yielded an increase in pERK in an approximately unimodal distribution. Pretreatment with cytokines in increasing concentrations did not linearly shift this peak, but caused a switch to a status of high ERK phosphorylation. These data are consistent with the model of HC-induced RAS activation priming SOS. To confirm a role of SOS in HC priming, we performed knockdown experiments using siRNA specific for SOS1 and SOS2. T cells were transfected with siRNA, maintained for 72 h under nonproliferating conditions to await the natural degradation of SOS, then stimulated with cytokines for 24 h and subsequently stimulated with anti-CD3 antibodies. Although an effect of HC priming on subsequent TCR-induced ERK responses was seen by PhosFlow for both control siRNA and SOS-specific siRNA transfected cells, it was lower in the T cells with reduced SOS expression (Fig. 5c). Also, Western blot studies showed that SOS-silencing greatly reduced the ERK phosphorylation signal in IL-15–primed cells while only minor differences were seen for the unprimed cells (Fig. 5d).
Figure 5. IL-7 and IL-15 regulate T cell responsiveness by augmenting a positive feedback loop involving SOS.
(a) T cells conditioned with increasing concentrations of IL-7 or IL-15 for 24 h were tested for TCR-induced ERK phosphorylation. Increased cytokine concentrations induced a heteroclitic pERK response consistent with the activation of a feedback loop. (b) Active RAS after cytokine priming was assessed using the Millipore active RAS ELISA kit. Results are shown as mean ± SD of triplicate wells. (c and d) T cells were transfected with siRNA specific for SOS-1 and SOS-2 or control siRNA. Seventy-two hours after transfection, T cells were exposed to IL-15 for 24 h, washed and activated by CD3 crosslinking. Phosphorylation of ERK was measured using PhosFlow (c) and Western blot (d). Each data set is representative of three independent experiments.
DISCUSSION
We have previously shown that RA T cells have heightened TCR sensitivity due to hyperactivity of the ERK pathway, and have proposed that the lowered TCR activation threshold contributes to breach of tolerance and autoimmunity (6). The objective of the current study was to determine whether cytokines known to be elevated in RA are able to calibrate TCR activation threshold settings by modulating ERK responses in T cells from healthy individuals, thus providing a mechanistic model for the signaling changes seen in RA T cells. We found that the homeostatic cytokines IL-7 and IL-15, but not the proinflammatory cytokines IL-1β and TNF-α are able to prime T cells to respond to subsequent TCR engagement with increased ERK activation. As a consequence of cytokine conditioning, T cell responses to weak activation signals are improved and responses to self-antigens such as citrullinated peptides are enabled. Moreover, the increased TCR signal strength skews T cell differentiation.
The negative results with TNF-α were to be expected. TNF-α is known to impair TCR signaling through several mechanisms. It lowers expression of the TCR/CD3 complex, in part due to selective targeting of the TCRζ chain. The TCRζ chain, which contains three immunoreceptor tyrosine based activation motifs (ITAM), serves as an amplification module by coupling signals from the TCR complex to downstream pathways (29). TNF-α also attenuates tyrosine phosphorylation of the protein tyrosine kinase ZAP-70, the transmembrane adaptor protein LAT and PLCγ, likely through the production of reactive oxygen intermediates (29, 30). Chronic TNF-α exposure also acts as a potent downregulator of CD28 expression thereby affecting costimulation and, preferentially, IL-2 production and T cell proliferation (8, 31). In contrast, common γ chain cytokines are able to provide costimulatory function; IL-2 produced in response to TCR and CD28 stimulation provides a critical amplification of the PI3K/Akt signaling module and has been considered as a third signal (32, 33). Costimulatory activity has also been ascribed to IL-7 and IL-15 (34–36). In a normal T cell response, these cytokine-induced signals are not necessary for activation and proliferation, but only for cell survival. However, under limiting costimulatory conditions they provide an essential signal (37) which provides one conceptual framework for the use of a JAK 3 inhibitor in the treatment of RA (19). These cytokine-mediated signals mostly regulate the expansion of effector T cells and the acquisition of effector functions, but they do not appear to contribute to the early stages of T cell activation and they do not enhance memory induction (38).
In contrast to their action on T cell expansion and differentiation, where they deliver a signal subsequent to TCR ligation, we addressed whether HC are able to prime T cell responses as found in vivo under settings of IL-7– or IL-15–mediated T cell stimulation. Increased levels of homeostatic cytokines have been observed in several conditions. Elevated amounts of IL-15 are produced in numerous chronic inflammatory diseases including RA (39). IL-7, induced under conditions of lymphopenia, is likely to elicit homeostatic proliferation in a feedback loop (40–42). In our design, T cells were exposed to cytokines, washed and then subsequently activated through TCR stimulation before they had either proliferated or differentiated. The model, therefore, is different from what is observed with homeostatic proliferation in murine models of extreme lymphopenia. Here, homeostatic proliferation induced by recognition of self-antigens, concomitant with exposure to common γ chain cytokines, drives naïve T cells to differentiate into memory-like cells that have a diminished requirement for CD28 costimulation and display effector functions such as rapid cytokine production upon restimulation (40, 43, 44). The sensitization of T cell responsiveness observed in our study was not dependent on IL-7– or IL-15–induced transcription. Based on our findings, we propose that homeostatic cytokine-derived signals lower the TCR threshold and facilitate the activation and expansion of autoreactive T cells leading to a peripheral selection of repertoire in favor of T cells with TCR that have a higher affinity for self (45, 46). In addition, the increased signal strength favors inflammatory pathway commitments rather than differentiation to regulatory or Th2 lineages.
Our interpretation is consistent with several animal models, where lymphopenia has been shown to be a risk factor for autoimmunity. Lymphopenia is important for the pathogenesis of autoimmune diabetes mellitus in the NOD mouse, as well as the biobreeding rat (47, 48). Disease development in the NOD mouse is dependent on IL-21–mediated homeostatic expansion of islet-specific T cells (47), a common γ chain cytokine that had a similar effect in our studies as IL-7 and IL-15 (Supp. Fig. 1). IL-7 has been implicated in another model by Calzascia et al who demonstrated that β-islet cell self-reactive CD4 T cells required IL-7 to induce overt diabetes mellitus (41).
The model of lymphopenia-associated autoimmunity is relevant for human disease (49). In the immune reconstitution inflammatory syndrome [IRIS], the reconstitution of a functional T cell compartment in HIV patients upon institution of highly active antiretroviral therapy [HAART] causes a systemic inflammatory disease (50). Several lines of indirect evidence also suggest that lymphopenia plays a role in the pathogenesis of rheumatoid arthritis (51). T cells from RA patients have an increased susceptibility to undergo apoptosis that can be attributed to defects in DNA repair mechanisms including telomerase and ATM deficiencies (52). Augmented cell death puts the system under increased homeostatic stress, which could explain why telomeres are age-inappropriately eroded, the T cell repertoire is contracted and T cell receptor excision circles are diminished (53). Our data suggest a possible mechanism for how lymphopenia-induced homeostatic stress might induce autoimmunity and synovial inflammation. One major tolerance defect in RA patients involves the immune response to neoantigens such as the citrullinated matrix proteins vimentin and fibrinogen (54, 55). Autoantibodies to citrullinated peptides frequently emerge long before the onset of clinical disease (56–58). In addition, elevations of serum IL-15 can precede disease onset by many years, possibly due to subclinical lymphopenia (59). In our studies, T cell priming by stimulation with IL-7 or IL-15 was sufficient to enable CD4 T cells from healthy HLA-DR4 individuals to respond to the citrullinated vimentin peptide that has been mapped as an autoantigenic epitope in RA.
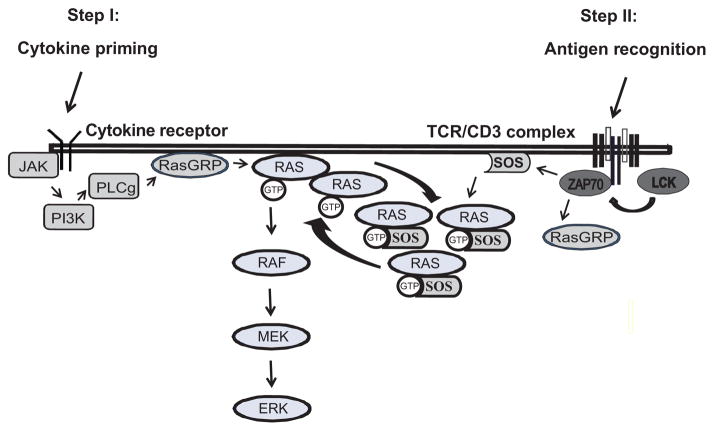
The mechanisms of homeostatic cytokine sensitization has some resemblance to the model proposed for the recognition of agonistic versus antagonistic peptides as developed by Stefanova, Germain and colleagues (60). In this model, ERK activity functions as an important feedback loop to calibrate TCR activation thresholds. Agonistic peptides induce sufficient ERK activity to phosphorylate Lck at Ser59 and thereby interfere with the recruitment of SHP-1. In contrast, antagonistic peptides provide an initial activation signal that is insufficient to activate this positive feedback loop. The ensuing SHP-1 recruitment renders the T cells unresponsive to subsequent antigenic encounters (60). In analogy, IL-7 or IL-15 stimulation for 16 h or longer sensitized the activation of the ERK module upon TCR triggering and increased ERK phosphorylation enough to bypass the TCR signaling threshold and enable the response to citrullinated vimentin (Fig. 6). In support of this model, SHP-1 recruitment to the TCR signaling complex in RA T cells is reduced (6), as one would expect from the studies by Stefanova and colleagues (60). Of particular interest, HC priming as described here appears to be not the only pathway; several mechanisms exist in RA patients that all converge on upregulating the ERK pathway. We have recently found increased transcription of B-RAF and K-RAS in RA T cells, which heightened the ERK response and enabled autoreactive T cell proliferation (61).
Figure 6. IL-7– or IL-15–mediated sensitization of TCR thresholds.
The diagram illustrates proposed mechanisms how IL-7 and IL-15 priming enhance positive feedback loops in TCR signaling resulting in a heightened T cell response that may overcome non-responsiveness to autoantigens.
Our initial studies showed that the cytokine effect was short-lived and did not include transcriptional activation suggesting that IL-7– or IL-15–induced STAT5 phosphorylation was not involved. In cytokine titration experiments, increasing cytokine concentrations had a digital effect on ERK phosphorylation, i.e., at a stipulated cytokine dose ERK phosphorylation switched from an intermediate to a high-level state implicating a positive feedback loop. Such a feedback loop in ERK signaling has been postulated by Roose and colleagues (28, 62). TCR stimulation initiates ERK activation through the RasGRP to activate RAS. Allosteric binding of active RAS then primes SOS to generate additional RAS-GTP molecules. Based on computer simulation, Roose proposed an interesting model that this hysteresis in RAS production is a mechanism to confer short-term memory in T cells, in that T cells remember very recent receptor engagements and that serial triggering of the TCR culminates in suprathreshold signaling. Our data suggest that a similar effect is accomplished by conditioning with homeostatic cytokines, which are able to activate RasGRP through the PI3K – PLCγ axis. Indeed, the cytokine effect on subsequent ERK activation was associated with accumulation of active RAS that is able to bind to RAF, and was abrogated by PI3K inhibition. Moreover, the digital effect on ERK phosphorylation induced by preincubation with IL-7 or IL-15 was sensitive to SOS silencing.
Based on our studies, we propose that transient TCR receptor calibration conferred by increased concentrations of homeostatic cytokines contributes to a greater susceptibility to autoimmunity in lymphopenic hosts. The mechanism may not only be important in the initiation of the disease but also in its maintenance and perpetuation, since ongoing disease activity and progression is dependent on the continuous activation of autoreactive T cells. Interfering with this pathway may, therefore, prevent as well as treat disease consistent with the recently observed therapeutic efficacy of the JAK inhibitor Tofacitinib in rheumatoid arthritis (63). Tofacitinib preferentially inhibits JAK3 and therefore blocks STAT5 as well as PI3K signals downstream of IL-7 and IL-15 stimulation. Our model may not be limited to RA, where there is already evidence for defective T cell homeostasis and for hyperresponsiveness of the ERK pathway; similar mechanisms are likely to be found in other autoimmune diseases.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health, U19 AI 57266 and U19 AI090019 and VA Merit Award BX001669 to JJG and R01 AR42527, R01 EY11916, R01 AI44142 and P01 HL058000 to CMW.
Footnotes
Conflict of interest disclosure
The authors declare no competing financial interests.
References
- 1.Sfikakis P. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]
- 2.Woodrick RS, Ruderman EM. Safety of biologic therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:639–652. doi: 10.1038/nrrheum.2011.145. [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, Filippini G, Skoetz N, Francis D, Lopes LC, Guyatt GH, Schmitt J, La Mantia L, Weberschock T, Roos JF, Siebert H, Hershan S, Lunn MP, Tugwell P, Buchbinder R. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res Ther. 2009;11:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh K, Deshpande P, Pryshchep S, Colmegna I, Liarski V, Weyand C, Goronzy J. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J Immunol. 2009;183:8258–8325. doi: 10.4049/jimmunol.0901784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 9.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 11.Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012;69:1597–1608. doi: 10.1007/s00018-012-0968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprent J, Surh CD. Interleukin 7, maestro of the immune system. Semin Immunol. 2012;24:149–150. doi: 10.1016/j.smim.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Pickens S, Chamberlain N, Volin M, Pope R, Talarico N, Mandelin A, Shahrara S. Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2011;63:2884–2977. doi: 10.1002/art.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 15.Halvorsen E, Strønen E, Hammer H, Goll G, Sollid L, Molberg O. Interleukin-15 induces interleukin-17 production by synovial T cell lines from patients with rheumatoid arthritis. Scand J Immunol. 2011;73:243–252. doi: 10.1111/j.1365-3083.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 16.van Roon J, Glaudemans K, Bijlsma J, Lafeber F. Interleukin 7 stimulates tumour necrosis factor alpha and Th1 cytokine production in joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:113–122. doi: 10.1136/ard.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borger P, Kauffman H, Postma D, Vellenga E. IL-7 differentially modulates the expression of IFN-gamma and IL-4 in activated human T lymphocytes by transcriptional and post-transcriptional mechanisms. J Immunol. 1996;156:1333–1341. [PubMed] [Google Scholar]
- 18.Mehrotra P, Grant A, Siegel J. Synergistic effects of IL-7 and IL-12 on human T cell activation. J Immunol. 1995;154:5093–5195. [PubMed] [Google Scholar]
- 19.Fleischmann R, Cutolo M, Genovese M, Lee E, Kanik K, Sadis S, Connell C, Gruben D, Krishnaswami S, Wallenstein G, Wilkinson B, Zwillich S. Phase 2B dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to DMARDs. Arthritis Rheum. 2011;64:617–629. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 20.Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V, Wallenstein G, Zwillich SH. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–981. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 21.Kierstead L, Ranieri E, Olson W, Brusic V, Sidney J, Sette A, Kasamon Y, Slingluff C, Kirkwood J, Storkus W. gp100/pmel17 and tyrosinase encode multiple epitopes recognized by Th1-type CD4+T cells. Brit J Cancer. 2001;85:1738–1745. doi: 10.1054/bjoc.2001.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell A. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan AW, Thomson W, Martin SG, Carter AM, Erlich HA, Barton A, Hocking L, Reid DM, Harrison P, Wordsworth P, Steer S, Worthington J, Emery P, Wilson AG, Barrett JH. Reevaluation of the interaction between HLA-DRB1 shared epitope alleles, PTPN22, and smoking in determining susceptibility to autoantibody-positive and autoantibody-negative rheumatoid arthritis in a large UK Caucasian population. Arthritis Rheum. 2009;60:2565–2576. doi: 10.1002/art.24752. [DOI] [PubMed] [Google Scholar]
- 25.Adema G, de Boer A, Vogel A, Loenen W, Figdor C. Molecular characterization of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994;269:20126–20133. [PubMed] [Google Scholar]
- 26.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boykevisch S, Zhao C, Sondermann H, Philippidou P, Halegoua S, Kuriyan J, Bar-Sagi D. Regulation of ras signaling dynamics by Sos-mediated positive feedback. Curr Biol. 2006;16:2173–2179. doi: 10.1016/j.cub.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty AK, Das J, Zikherman J, Yang M, Govern CC, Ho M, Weiss A, Roose J. Molecular origin and functional consequences of digital signaling and hysteresis during Ras activation in lymphocytes. Sci Signal. 2009;2:pt2. doi: 10.1126/scisignal.266pt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isomaki P, Panesar M, Annenkov A, Clark JM, Foxwell BM, Chernajovsky Y, Cope AP. Prolonged exposure of T cells to TNF down-regulates TCR zeta and expression of the TCR/CD3 complex at the cell surface. J Immunol. 2001;166:5495–5507. doi: 10.4049/jimmunol.166.9.5495. [DOI] [PubMed] [Google Scholar]
- 30.Gringhuis SI, Papendrecht-van der Voort EA, Leow A, Nivine Levarht EW, Breedveld FC, Verweij CL. Effect of redox balance alterations on cellular localization of LAT and downstream T-cell receptor signaling pathways. Mol Cell Biol. 2002;22:400–411. doi: 10.1128/MCB.22.2.400-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum. 2005;52:2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- 32.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 33.Seder RA, Germain RN, Linsley PS, Paul WE. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon gamma production. J Exp Med. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanegane H, Tosato G. Activation of naive and memory T cells by interleukin-15. Blood. 1996;88:230–235. [PubMed] [Google Scholar]
- 36.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D. The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci U S A. 2004;101:16885–16890. doi: 10.1073/pnas.0407417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calzascia T, Pellegrini M, Lin A, Garza KM, Elford AR, Shahinian A, Ohashi PS, Mak TW. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci U S A. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seddon B, Zamoyska R. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol. 2002;169:3752–3759. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 43.Cho BK, V, Rao P, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goronzy JJ, Weyand CM. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001;22:251–255. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]
- 47.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 48.Wallis RH, Wang K, Dabrowski D, Marandi L, Ning T, Hsieh E, Paterson AD, Mordes JP, Blankenhorn EP, Poussier P. A novel susceptibility locus on rat chromosome 8 affects spontaneous but not experimentally induced type 1 diabetes. Diabetes. 2007;56:1731–1736. doi: 10.2337/db06-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, Tomooka BH, Gregersen PK, Robinson WH. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, van Gorp E, Hyde C, Lau K, Pahau H, Purcell AW, Thomas R. T cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berglin E, Padyukov L, Sundin U, Hallmans G, Stenlund H, Van Venrooij WJ, Klareskog L, Dahlqvist SR. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther. 2004;6:R303–308. doi: 10.1186/ar1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 58.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 59.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH, Holers VM. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62:3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 61.Singh K, Deshpande P, Li G, Yu M, Pryshchep S, Cavanagh M, Weyand CM, Goronzy JJ. K-RAS GTPase- and B-RAF kinase-mediated T-cell tolerance defects in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2012;109:E1629–1637. doi: 10.1073/pnas.1117640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, Chakraborty AK, Roose JP. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–351. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CA, Zwillich SH. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.