Abstract
Background
An n-6 essential fatty acid, arachidonic acid (ARA) is converted into prostaglandin E2, which is involved in tumour extension. However, it is unclear whether dietary ARA intake leads to cancer in humans. We thus systematically evaluated available observational studies on the relationship between ARA exposure and the risk of colorectal, skin, breast, prostate, lung, and stomach cancers.
Methods
We searched the PubMed database for articles published up to May 17, 2010. 126 potentially relevant articles from the initial search and 49,670 bibliographies were scrutinised to identify eligible publications by using predefined inclusion criteria. A comprehensive literature search yielded 52 eligible articles, and their reporting quality and methodological quality was assessed. Information on the strength of the association between ARA exposure and cancer risk, the dose-response relationship, and methodological limitations was collected and evaluated with respect to consistency and study design.
Results
For colorectal, skin, breast, and prostate cancer, 17, 3, 18, and 16 studies, respectively, were identified. We could not obtain eligible reports for lung and stomach cancer. Studies used cohort (n = 4), nested case-control (n = 12), case-control (n = 26), and cross-sectional (n = 12) designs. The number of subjects (n = 15 - 88,795), ARA exposure assessment method (dietary intake or biomarker), cancer diagnosis and patient recruitment procedure (histological diagnosis, cancer registries, or self-reported information) varied among studies. The relationship between ARA exposure and colorectal cancer was inconsistent based on ARA exposure assessment methodology (dietary intake or biomarker). Conversely, there was no strong positive association or dose-response relationship for breast or prostate cancer. There were limited numbers of studies on skin cancer to draw any conclusions from the results.
Conclusions
The available epidemiologic evidence is weak because of the limited number of studies and their methodological limitations, but nonetheless, the results suggest that ARA exposure is not associated with increased breast and prostate cancer risk. Further evidence from well-designed observational studies is required to confirm or refute the association between ARA exposure and risk of cancer.
Background
Cancer remains an important health problem worldwide. It is estimated that 58.8 million people died of all causes in 2004 [1]. Deaths from cancer represented around one-eighth of these deaths, although many people who died had cancer even though it was not the direct cause of death. By 2030, it is projected that there will be approximately 26 million new cancer cases and 17 million cancer deaths per year [2]. Given these considerations, the prevention of cancer is a major public health issue around the world.
It is well established that dietary and other lifestyle factors play an important role in cancer control. In terms of dietary factors, earlier studies suggested a relationship between fat intake and the risk of several types of cancer. Prospective cohort studies found no association between fat intake and breast cancer, but a randomised trial organised within the Women’s Health Initiative trial suggested a 9% reduction of borderline significance in breast cancer occurrence with decreased fat intake [3-5]. Analysis of the information in the Multiethnic Cohort Study found that intake of different types of fat indicated no association with overall prostate cancer risk or with non-localised or high-grade prostate cancer [6]. A prospective cohort study and a clinical trial failed to find evidence for an association between fat intake and colorectal cancer [7,8]. A dietary intervention study demonstrated that a reduction in fat intake reduces the risk of skin cancer [9,10], but the evidence from observational studies [11,12] has been controversial. Japan is a high-risk area for stomach and lung cancer, but no association with fat intake and these types of cancer has been suggested [2].
Essential fatty acids, namely n-3 and n-6 fatty acids, are involved in many important biological functions [13-16]. They play a structural role in cell membranes, influencing their fluidity and membrane enzyme activities; in addition, some are the precursors of prostaglandins and other lipid mediators. Arachidonic acid (ARA) is an n-6 essential fatty acid and also a major constituent of biomembranes. It is released from membranes by phospholipase A2 and converted into various lipid mediators that exert many physiological actions [17-19]. Many studies have shown that lipid mediators derived from ARA, particularly prostaglandin E2 (PGE2), are associated with various diseases, which is mainly based on the fact that cyclooxygenase (COX) inhibitors are effective against those conditions [20-24]. PGE2 is regarded as enhancing tumour extension as well, but it has been suggested that some other ARA mediators inhibit tumour growth [21-25]. In animal models, ARA administration did not affect tumour extension [26,27]. Some observational studies also suggested no relationship between ARA exposure and cancer risk [28,29]. However, there are the inconsistent observational studies that ARA exposure was positively correlated with the risk of colorectal cancer [30,31]. ARA is one of the major polyunsaturated fatty acid, and this inconsistency is not negligible.
No systematic review or meta-analysis has been conducted to evaluate the long-term effects of ARA intake and blood or tissue ARA composition on the risk of colorectal, skin, breast, prostate, lung, and stomach cancers in free-living populations. The objective of this study was to systematically evaluate available observational studies on the relationship between ARA intake and blood or tissue composition of ARA and the risk of these types of cancer.
Methods
Search strategy
The PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) was searched for observational studies on the relationship between dietary or blood ARA levels with cancer risk that were published up to May 17, 2010. To identify target articles effectively, the strategy for the PubMed search was as follows: keywords for outcome and study types were adopted as commonly used terms representing cancer and study design, whereas terms for exposure were selected from specific words that stand for “arachidonic acid” (see Additional file 1). The initial PubMed search yielded 126 potentially relevant articles.
Study selection
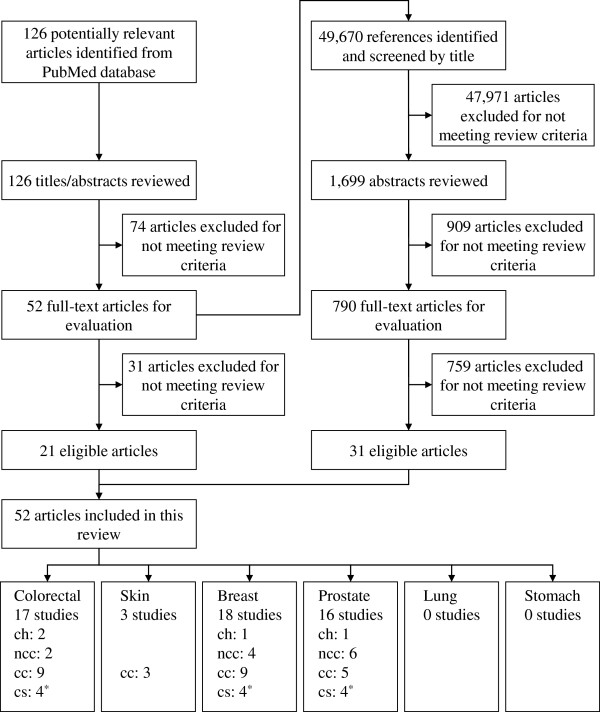
Inclusion criteria were English articles that reported original data on the relationship between ARA exposure (intake or blood level) and target cancer risk in free-living adults. Eligible study designs were cohort, case-control, or cross-sectional studies, and target types of cancer were colorectal, skin, breast, prostate, lung, or stomach cancer. Also included were studies investigating tissue ARA levels and target cancer risk. The study selection process is presented in Figure 1. We omitted reports in which titles or abstracts indicated that: (1) they were not human studies; (2) they were limited to special populations such as people with unusual eating habits; (3) they were intervention studies; or (4) they were not about the target cancers and fatty acids (not fat). We then evaluated the full text of the passed articles. Titles and abstracts of 126 identified publications from the PubMed database were checked and reviewed against the predefined inclusion criteria, and afterward, the full text of 52 articles were similarly assessed for eligibility by three authors (SK, CH, and HT, not independently). The 49,670 bibliographies of these full-text articles were scrutinised to identify additional eligible publications. One article on breast cancer was excluded because an inaccuracy of ARA assessment was clearly reported, although this article met the inclusion criteria described above [32]. Finally, 52 eligible articles were included in this review: 21 and 31 articles were obtained from primary PubMed searches and bibliographies, respectively.
Figure 1.
Flow diagram for literature search and study selection.CH Cohort study, NCC Nested case-control study, CC Case-control study, CS Cross-sectional study. *One article that includes data on colorectal, breast and prostate cancer is counted as three studies.
Quality assessment and data extraction
Quality assessment was conducted based on the reporting quality and methodological quality of each study. The reporting quality shows whether the necessary information for observational studies is well indicated. It is the number of fulfilled items from the Strengthening the Reporting of Observational Studies in Epidemiology Statement (STROBE) checklist and varied 0 to 34 [33]. The reporting quality of included observational studies was assessed individually by two reviewers (CH and HT) and then confirmed by another two authors (SK and MS). The methodological quality, a level of suitability of methods used in a study, was assessed by two authors (SK and MS) qualitatively from the following methodological aspects used in the article: subject selection, ARA exposure assessment, diagnosis or recruitment procedure of cancer patients, methods for controlling confounders, and statistical analysis.
For each eligible article, the following information was tabulated: authors and year of publication, study settings and design, subject characteristics (such as age, sex, and number), matching strategy (if applicable), ARA exposure assessment used (as well as information about validity or precision), outcome assessment, adjusted confounders, reporting quality score from the STROBE checklist, and main findings from the fully adjusted model. Case-control studies were classified into two groups based on whether they reported temporal study settings information between exposure and outcome assessment: “case-control study (temporal relationship among exposure and outcome is demonstrated)” was defined as articles in which ARA exposure preceded the occurrence of cancer, whereas “case-control study (temporal relationship among exposure and outcome is unclear)” did not describe sufficient temporal information about exposure and outcome assessment.
Our qualitative definition of the study quality was as below: the reporting quality score under 13 or the insufficient temporal information, low; the other studies were qualitatively divided into high/medium/low according to their strength and weakness. A meta-analysis was not conducted because of the heterogeneity among studies, particularly subject characteristics and exposure/outcome assessment, and the insufficient number of studies with high methodological quality suitable for a meta-analysis. Therefore, qualitative assessment of ARA intake and cancer risk is presented in this review.
Results
For colorectal, skin, breast, and prostate cancer, 52 eligible articles were selected from potentially related reports and were included in the present systematic review (Figure 1); the number of each was 17, 3, 18, and 16 studies, respectively. In contrast, we could not identify eligible reports for lung and stomach cancer.
Colorectal cancer
Major characteristics are shown in Table 1[28,30,31,34-47]. Five reports did not provide sufficient information about the methodology of outcome measurement. Some cohort and case-control studies were adjusted for well-known potential confounders, such as family history, body weight and smoking, and specific factors for colorectal cancer, such as body mass index (BMI), physical activity, alcohol drinking and total energy. No confounding factors were adjusted for in eight articles.
Table 1.
Summary of observational studies on the association between ARA and risk of colorectal cancer
| References | Study | Subjects | Exposure Assessment | Colorectal cancer assessment (diagnosis) | Adjustment for potential confounders | Assessment of reporting quality * |
Main findings |
||
|---|---|---|---|---|---|---|---|---|---|
| Intergroup comparison | P or Ptrend | ||||||||
|
Study design: cohort study | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Murff et al. 2009 [30] |
SWHS, China, 1996-2007, prospective cohort design (7-year biennial follow-up, follow-up rate = 96.7%) |
73,243 women aged 40-70, no prior history of cancer |
SWHS's FFQ, 77 items, previously validated against 24 x 24-HDR |
Self-reported physician diagnosis, combined with annual record linkage with the Shanghai Cancer Registry and Shanghai Vital Statistics database |
Age at baseline, total energy intake, smoking status, alcohol intake, physical activity, energy-adjusted total red meat consumption, menopausal status, use of HRT, multivitamin, aspirin, total n-3 PUFA intake, n-6 to n-3 PUFA ratio |
18 |
Dietary ARA intake, g/day, quintile, median |
RR (95%CI) |
Ptrend |
| Q1: 0.02 |
1.00 |
0.03 |
|||||||
| Q2: 0.03 |
1.20 (0.87-1.64) |
||||||||
| Q3: 0.05 |
1.44 (1.05-1.98) |
||||||||
| Q4: 0.06 |
1.61 (1.17-2.23) |
||||||||
| Q5: 0.09 |
1.39 (0.97-1.99) |
||||||||
| Lin et al. 2004 [28] |
WHS, USA, 1993–2003, prospective cohort design nested randomized, double-blind, placebo-controlled 2 × 2 factorial aspirin and vitamin A trial (average 8.7 years follow-up) |
37,547 female health professionals aged ≥45, free of heart disease and cancer except NMSC |
FFQ, 131 items, validated against 2 x 7-day WR |
Self-reported physician diagnosis, reviewed and confirmed medical diagnoses |
Age, treatment assignment, BMI, family history of CRC, colorectal polyps, physical activity, smoking status, alcohol intake, use of HRT, total energy intake |
15 |
Dietary ARA intake, %energy, quintile, median |
RR (95%CI) |
Ptrend |
| Q1: 0.04 |
1.00 |
0.55 |
|||||||
| Q2: 0.06 |
0.86 (0.57-1.32) |
||||||||
| Q3: 0.07 |
0.84 (0.55-1.28) |
||||||||
| Q4: 0.09 |
0.73 (0.47-1.14) |
||||||||
| Q5: 0.12 |
0.90 (0.59-1.36) |
||||||||
|
Study design: nested case-control study | |||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Hall et al. 2007 [34] |
PHS, USA, 1982-1995, nested case-control design within a randomized, double-blind, placebo-controlled factorial aspirin and beta-carotene trial (average 5 and 7 years follow-up) |
178 CRC patients, 282 controls, male physicians without history of cancer aged 40-84 years at baseline, 1 case matched with 1-2 controls by age, smoking status |
Whole blood fatty acids, GC analysis blinded to case-control status at a time, precision indicated |
Self-report, combined with review of medical records |
None |
23 |
ARA composition%, geometric mean(95%CI) Case: |
ARA composition%, geometric mean(95%CI) Control: |
P |
| 9.77(9.57-9.99) |
9.93(9.77-10.10) |
Not significant |
|||||||
| Kojima et al. 2005 [35] |
JACC Study, Japan, 1988-1997, nested case-control design (7 years follow-up) |
169 primary CRC patients, 481 controls without previous history of cancer, aged 40-79 years at baseline, 1 case matched with 2-3 controls by age, sex, resident area |
Serum fatty acids, GC analysis blinded to case-control status, precision not indicated |
Population-based cancer registries, supplemented by death certificates |
Age at completing final education, family history of CRC, BMI, smoking status, alcohol intake, intake of green leafy vegetables, physical activity |
23 |
ARA composition, weight % of total serum lipids, quartile |
OR (95%CI) |
P trend |
| Men: |
Men: |
Men: |
|||||||
| Q1: <3.71 |
1.00 |
0.99 |
|||||||
| Q2: 3.71-4.619 |
1.24 (0.55-2.78) |
||||||||
| Q3: 4.62-5.269 |
0.79 (0.32-1.96) |
||||||||
| Q4: ≥5.27 |
1.16 (0.49-2.75) |
||||||||
| Women: |
Women: |
Women: |
|||||||
| Q1: <4.20 |
1.00 |
0.40 |
|||||||
| Q2: 4.20-4.879 |
0.67 (0.31-1.46) |
||||||||
| Q3: 4.88-5.634 |
0.49 (0.22-1.10) |
||||||||
| Q4: ≥5.635 |
0.65 (0.30-1.44) |
||||||||
|
Study design: case-control study (temporal relationship among exposure and outcome is demonstrated) | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Theodoratou et al. 2007 [36] |
Survey, UK, 1999-2006, case-control design |
1,455 primary CRC patients aged 16-79, 1,455 controls (eligibility criteria not shown), matched by age, sex, resident area |
Scotish FFQ, 150 items, validated against 4-day WR, (response rate = case 82%, control 97%) |
Not shown |
Family history of CRC, total energy intake, total fiber intake, alcohol intake, NSAIDs use, smoking status, BMI, physical activity, total fatty acid intake |
20 |
Dietary ARA intake, mg/day, quartile |
OR (95%CI) |
Ptrend |
| Q1: 0-5.82 |
1.00 |
0.163 |
|||||||
| Q2: 5.83-8.40 |
1.09 (0.87-1.37) |
||||||||
| Q3: 8.41-11.34 |
0.79 (0.63-1.01) |
||||||||
| Q4:≥11.35 |
0.93 (0.72-1.19) |
||||||||
| Nkondjock et al. 2003 [31] |
Survey, Canada, 1989-1993, case-control design |
402 CRC patients aged 35-79, 688 controls, matched by age, language, place of residence |
FFQ, 132 items, validated against 7-day Food Record |
Histological diagnosis |
Age, BMI, family history of CRC, marital status, physical activity |
20 |
Dietary ARA intake, g/day, quartile |
OR (95% CI) |
Ptrend |
| Q1:<0.06 |
1.00 |
0.001 |
|||||||
| Q2:0.06-0.09 |
1.24 (0.84-1.84) |
||||||||
| Q3:0.10-0.14 |
1.64 (1.12-2.40) |
||||||||
| Q4:>0.14 |
2.11 (1.47-3.06) |
||||||||
| Slattery et al. 1997 [37] |
Survey, USA, 1991-1994 |
1993 CRC patients aged 30-79, 2410 controls without history of CRC (population characteristic partially not shown), matched by age, sex, resident state |
CARDIA Diet History Questionnaire, validated against 7 x 24-HDR |
Cancer registries |
Total energy intake, age at selection, BMI, family history of CRC, physical activity, dietary cholesterol, calcium, fiber, NSAIDs use |
19 |
Dietary ARA intake, g/MJ, quintile |
OR (95%CI) |
Ptrend |
| Men: |
Men: |
Men: |
|||||||
| Q1:<0.17 |
1.00 |
Not shown |
|||||||
| Q2:0.17-0.22 |
1.25 (0.95-1.65) |
||||||||
| Q3:0.23-0.26 |
1.08 (0.81-1.44) |
||||||||
| Q4:0.27-0.33 |
1.37 (1.03-1.83) |
||||||||
| Q5:>0.33 |
1.17 (0.85-1.61) |
||||||||
| Women: |
Women: |
Women: |
|||||||
| Q1:<0.039 |
1.00 |
Not shown |
|||||||
| Q2:0.039-0.051 |
0.99 (0.73-1.33) |
||||||||
| Q3:0.052-0.063 |
1.15 (0.86-1.55) |
||||||||
| Q4:0.064-0.077 |
0.98 (0.72-1.35) |
||||||||
| Q5:>0.077 |
0.98 (0.70-1.37) |
||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Kuriki et al. 2006 [38] |
Survey, Japan, 2002-2004, case-control design |
74 CRC patients, 221 controls, aged 20-80 without history of cancer or current diseases, 1 case matched with 3 controls by age, sex, season of blood collection |
Erythrocyte phospholipids, GC analysis blinded to case-control status, precision indicated |
Histological diagnosis |
BMI, habitual exercise, alcohol intake, smoking status, green-yellow vegetable intake, family history of CRC |
22 |
ARA composition, mol%, tertile |
OR (95% CI) |
Ptrend |
| T1: <8.625 |
1.00 |
<0.05 |
|||||||
| T2: 8.625-10.178 |
0.91 (0.48-1.73) |
||||||||
| T3: >10.178 |
0.42 (0.18-0.95) |
||||||||
|
Study design: case-control study (temporal relationship among exposure and outcome is unclear) | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Busstra et al. 2003 [39] |
Survey, Netherlands, 1995-1998, case-control design |
52 CRC patients, 57 controls, aged under 75 without history of CRC, colon resection, polyposis coli, inflammatory bowel disease, included subjects with familial HNPCC, matching not indicated |
FFQ developed for the Dutch cohorts of the EPIC study, 178 items, validated against 12 x 24-HDR |
Histological diagnosis |
Age, total energy intake, sex, familial background of HNPCC |
13 |
Dietary ARA intake, g/day, tertile |
OR (95% CI) |
Ptrend |
| T1: <0.02 |
1.0 |
0.37 |
|||||||
| T2: 0.02-0.04 |
1.3 (0.4-3.9) |
||||||||
| T3: ≥0.04 |
0.6 (0.2-1.8) |
||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Ghadimi et al. 2008 [40] |
Survey, Japan, 1997-2003, case-control design |
203 CRA patients, 179 controls (negative faecal occult blood test), matching not indicated |
Serum fatty acids (fasting blood), GC analysis, precision indicated |
Histological diagnosis |
Age, BMI, family history of CRA or CRC, history of diabetes, smoking status, alcohol intake, physical activity, season of data collection |
18 |
ARA concentration, mg/dl, quartile |
OR (95%CI) |
Ptrend |
| Men: |
Men: |
Men: |
|||||||
| Q1:<17.40 |
1.00 |
0.104 |
|||||||
| Q2:17.40-19.90 |
0.60 (0.21-1.68) |
Women: |
|||||||
| Q3:19.91-22.50 |
0.58 (0.21-1.60) |
0.001 |
|||||||
| Q4:>22.50 |
0.52 (0.19-1.42) |
||||||||
| Women: |
Women: |
Women: |
|||||||
| Q1:<18.05 |
1.00 |
0.001 |
|||||||
| Q2:18.05-20.50 |
0.49 (0.19-1.24) |
||||||||
| Q3:20.51-22.38 |
0.11 (0.28-0.45) |
||||||||
| Q4:>22.38 |
0.11 (0.03-0.43) |
||||||||
| Baró et al. 1998 [41] |
Survey, Spain |
17 CRC patients aged 35-82, 12 controls aged 33-81 with no malignant diseases, matched by age, resident area |
Plasma and erythrocyte fatty acids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
12 |
Plasma ARA concentration, mg/dl, mean(SEM) |
Plasma ARA concentration, mg/dl, mean(SEM) |
P |
| Case: |
Control: |
Plasma: |
|||||||
| 18.59(1.31) |
21.31(1.22) |
Not significant |
|||||||
| Erythrocyte ARA composition%, mean(SEM) |
Erythrocyte ARA composition%, mean(SEM) |
Erythrocyte: |
|||||||
| Case: |
Control: |
Not significant |
|||||||
| 14.61(0.24) |
13.50(0.40) |
||||||||
| Neoptolemos et al. 1990 [42] |
Survey, UK |
32 CRC patients, 42 controls admitted for elective operations for benign without DM, metabolic disorders, blood transfusions, matched by age, sex, admittance period |
Erythrocyte phospholipids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
13 |
ARA composition%, median(range) |
ARA composition%, median(range) |
P |
| Case: |
Control: |
|
|||||||
| 20.7(12.8-48.9) |
18.0(0.0-47.3) |
Not significant |
|||||||
| Neoptolemos et al. 1988 [43] |
Survey, UK |
49 CRC patients aged 49-92, 49 controls with benign diaseases aged 48-90, matched by age, sex |
Erythrocyte phospholipids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
12 |
ARA composition%, median(range) |
ARA composition%, median(range) |
P |
| Case: |
Control: |
|
|||||||
| 21.8 (15.3-28.4) |
23.5 (13.8-32.8) |
0.043 |
|||||||
|
Exposure assessment: tissue ARA level | |||||||||
| Busstra et al. 2003 [39] |
Survey, Netherlands, 1995-1998, case-control design |
52 CRC patients, 57 controls, aged under 75 without history of CRC, colon resection, polyposis coli, inflammatory bowel disease, included subjects with familial HNPCC, matching not indicated |
Buttock adipose tissue fatty acids, GC analysis, precision not indicated |
Histological diagnosis |
Age, total energy intake, sex, familial background of HNPCC |
13 |
ARA composition mass%, tertile |
OR(95%CI) |
Ptrend |
| T1: <0.35 |
1.0 |
0.42 |
|||||||
| T2: 0.35-0.45 |
2.6 (0.7-8.5) |
||||||||
| T3: ≥0.45 |
1.7 (0.5-5.8) |
||||||||
|
Study design: cross-sectional study | |||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Almendingen et al. 2006 [44] |
Survey, Norway |
38 FAP patients aged 24-70 (all colectomized), 160 healthy controls aged 21-66 |
Serum phospholipids (fasting blood), GC analysis, precision indicated |
Diagnosis by endoscopy and histology |
None |
17 |
ARA composition weight%, mean(SD) |
ARA composition weight%, mean(SD) |
P |
| Case: |
Control: |
|
|||||||
| 10.96(1.85) |
7.26(1.51) |
≤0.0001 |
|||||||
| Fernández-Bañares et al. 1996 [45] |
Survey, Spain |
22 colonic cancer patients, 27 colonic adenoma patients, 12 controls with benign diseases, no significant differences in sex and age |
Plasma phospholipids (fasting blood), GC analysis, precision not indicated |
Total fibreoptic colonoscopy |
None |
13 |
ARA composition%, mean(SEM) Carcinoma: |
ARA composition%, mean(SEM) Controls: |
P |
| 9.38(0.37) |
10.2(0.32) |
Not significant |
|||||||
| Adenoma: |
|||||||||
| 9.95(0.49) | |||||||||
| Hietanen et al. 1994 [46] |
Survey, UK, cross-sectional design |
20 colon cancer patients aged 38-84, controls, matched by age, sex, smoking status |
Erythrocyte phospholipids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
8 |
ARA concentration, mg/dl, mean(SD) |
ARA concentration, mg/dl, mean(SD) |
P |
| Case: |
Control: |
|
|||||||
| 18.5(0.6) |
20.2(0.5) |
<0.05 |
|||||||
|
Exposure assessment: tissue ARA level | |||||||||
| Fernández-Bañares et al. 1996 [45] |
Survey, Spain |
15 colonic cancer patients, 21 colonic adenoma patients, 8 controls with benign diseases |
Normal colon mucosa fatty acids, GC analysis, precision not indicated |
Total fibreoptic colonoscopy |
None |
13 |
ARA composition%, mean(SEM) Carcinoma: |
ARA composition%, mean (SEM) Controls: |
P |
| 10.9(0.57) |
11.4 (0.88) |
Not significant |
|||||||
| Adenoma: |
|
||||||||
| 12.3(0.55) |
|
||||||||
| Berry et al. 1986 [47] | Survey, Israel, 1982-1985 | 155 consecutive colonoscopies (53 carcinoma, 34 benign neoplastic polyps, 68 controls) | Buttock adipose tissue fatty acids, GC analysis, precision indicated | Histological diagnosis | None | 13 | ARA composition%, mean (SD) Carcinoma: |
ARA composition%, mean (SD) Controls: |
P |
| 0.54 (0.2) |
0.55 (0.2) |
Not significant | |||||||
| Benign neoplastic polyps: |
|||||||||
| 0.52 (0.2) | |||||||||
24-HDR 24-h dietary recall, ARA Arachidonic acid, BMI Body mass index, CRA Colorectal adenoma, CRC Colorectal cancer, DM Diabetes mellitus, FAP Familial adenomatous polyposis, FFQ Food frequency questionnaire, GC Gas chromatography, HNPCC Hereditary non-polyposis colorectal cancer, HRT Hormone replacement therapy, JACC Japan Collaborative Cohort, NMSC Nonmelanoma skin cancer, NSAIDs Nonsteroidal antiinflammatory drugs, OR Odds ratio, PHS Physician's health study, RR Relative risk, SWHS Shanghai Women's Health Study, UK United Kingdom, USA United States of America, WHS Women's Health Study, WR Weighed dietary record.
*Result of the critical evaluation carried out using the STROBE tool.
Dietary ARA intake was estimated in two cohort studies and four case-control studies. Median dietary ARA intake ranged widely from 0.008 to 0.15 g/day, or from 0.04% to 0.07% of energy. Two articles reported a significant increase in colorectal cancer risk. Muff et al. indicated that colorectal cancer risk was significantly increased in the third and fourth quintiles of ARA intake, and that the overall trend was significant (P for trend = 0.03). Nkondjock et al. reported significantly increased colorectal cancer risk in the third and fourth quartiles and significance in the overall trend (P for trend = 0.001).
In seven case-control studies and three cross-sectional studies, the exposure was indicated as blood ARA levels. The precision of blood analysis was mentioned in only four reports, and blinded fatty acid measurement was conducted in only three reports. Five articles showed a significant trend of decreasing colorectal cancer risk or a significant difference of blood ARA levels in cancer subjects. Kuriki et al. found that colorectal cancer risk was significantly decreased in the highest tertile of erythrocyte ARA levels, and that the overall trend was significant (P for trend < 0.05). The remaining four reports, Ghadimi et al., Hietanen et al., Neoptolemos et al. (1988), and Almending et al., were a case-control study with little temporal information between exposure and outcome or a cross-sectional study.
One case-control study with little temporal information between exposure and outcome and two cross-sectional studies investigated tissue ARA levels. The precision of tissue analysis was mentioned in only one article, and none reported masking of disease status. Their reporting quality was generally low.
Skin cancer
Only three articles were included in the present systematic review. Major characteristics are shown in Table 2[48-50]. Their exposure assessment and subjects’ characteristics were too diverse to be compared to each other.
Table 2.
Summary of observational studies on the association between ARA and risk of skin cancer
| References | Study | Subjects | Exposure assessment | Skin cancer assessment (diagnosis) | Adjustment for potential confounders | Assessment of reporting quality * |
Main findings |
||
|---|---|---|---|---|---|---|---|---|---|
| Intergroup comparison | P or Ptrend | ||||||||
|
Study design: case-control study (temporal relationship among exposure and outcome is unclear) | |||||||||
| |
|
|
|
|
|
|
|
|
|
|
Exposure assessment: dietary intake | |||||||||
| |
|
|
|
|
|
|
|
|
|
| Hakim et al. 2000 [48] |
Survey, USA, case-control design |
301 nonmetastatic skin SCC patients aged ≥30, 267 population-baseed controls with no prior history of skin cancer, matched by age, sex |
24-HDR of 4 days, validated |
Histopathologically diagnosed skin SCC selected from Southeastern Arizona Skin Cancer Registry |
Age, sex, total energy intake, history of diagnosed actinic keratosis, tanning ability, freckles on arms, use of sunscreen |
22 |
Dietary ARA intake, g/day, tertile |
OR (95% CI) |
Ptrend |
| T1: ≤0.1 |
1.00 |
0.16 |
|||||||
| T2: 0.11-0.20 |
0.86 (0.57-1.29) |
||||||||
| T3: >0.20 |
0.70 (0.46-1.08) |
||||||||
| |
|
|
|
|
|
|
|
|
|
|
Exposure assessment: blood ARA level | |||||||||
| |
|
|
|
|
|
|
|
|
|
| Harris et al. 2005 [49] |
Survey, USA, case-control design |
336 nonmetastatic skin SCC patients aged ≥30, 321 controls with no prior history of skin cancer, matched by age, sex, race |
Erythrocyte fatty acids (fasting blood), GC analysis, precision indicated |
Histopathologically diagnosed skin SCC selected from Southeastern Arizona Skin Cancer Registry |
Age, sex, lab, tanning ability, freckles on arms, exclusion of 94 controls with history of prior actinic keratosis |
25 |
ARA composition weight%, quartile |
OR (95% CI) |
Ptrend |
| Q1 |
1.00 |
Not shown |
|||||||
| Q2 |
1.61 (0.92-2.80) |
||||||||
| Q3 |
1.40 (0.79-2.49) |
||||||||
| Q4 |
2.38 (1.37-4.12) |
||||||||
|
Exposure assessment: tissue ARA level | |||||||||
| Mackie et al. 1987 [50] | Survey, Australia, 1984-1985 | 100 primary melanoma patients, 100 controls with no history of malignant skin tumor, matched by age, sex, race | Subcutaneous adipose tissue triglyceride, GC analysis blinded to case-control status, precision not indicated | Selected from Sydney Melanoma Unit | None | 10 | ARA composition%, mean |
ARA composition%, mean |
P |
| Case: |
Control: |
|
|||||||
| 0.41 | 0.28 | <0.001 | |||||||
24-HDR: 24-h dietary recall, ARA Arachidonic, GC Gas chromatography, OR Odds Ratio, SCC Squamous cell caricinoma, USA United States of America.
*Result of the critical evaluation carried out using the STROBE tool.
Breast cancer
Major characteristics are shown in Table 3[29,46,51-66]. Five articles did not provide sufficient information about the methodology of outcome measurement. In addition to general confounding factors, specific factors for breast cancer, such as reproductive factors and history of benign breast disease, were considered in some articles; however, no confounding factors were investigated in eight articles.
Table 3.
Summary of observational studies on the association between ARA and risk of breast cancer
| References | Study | Subjects | Exposure Assessment | Breast cancer assessment (diagnosis) | Adjustment for potential confounders | Assessment of reporting quality * |
Main findings |
||
|---|---|---|---|---|---|---|---|---|---|
| Intergroup comparison | P or Ptrend | ||||||||
|
Study design: cohort study | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Holmes et al. 1999 [51] |
NHS, USA, 1976- 1994, prospective cohort design (14 year biennial follow-up, follow-up rate = 95%) |
88,795 female nurses aged 30-55, no prior history of cancer other than nonmelanoma skin cancer |
Semiquantitative FFQ, 131 items, validated against 2 x 7-day WR |
Self-reported physician diagnosis, deaths identified by family member of participants, postal services and National Death Index, supplemented by medical record |
Total energy intake, age, energy-adjusted vitamin A intake, alcohol intake, time period, height, parity, age at first birth, weight change, BMI, age at menopause, menopausal status, use of HRT, family history, benign breast disease, age at menarche |
19 |
%energy increment of dietary ARA intake per day 0.03 |
RR(95% CI) |
P |
| 1.05(1.00-1.10) |
Not shown |
||||||||
|
Study design: nested case-control study | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Voorrips et al. 2002 [52] |
NLCS, Netherlands, 1986-1992 (6.3 years follow-up), case-cohort design |
941 breast cancer patients from entire cohort, 1,598 subcohort members (selection criteria not shown), aged 55-69 at baseline, no prior history of cancer other than nonmelanoma skin cancer, matching not indicated |
Semiquantitative FFQ, 150 items, validated against 3 x 3-day DR |
All regional cancer registries and Dutch national database of pathology reports |
Age, history of benign breast disease, maternal breast cancer, breast cancer in one or more sisters, age at menarche, age at menopause, oral contraceptive use, parity, age at first birth, Quetelet index, educational level, alcohol intake, smoking status, total energy intake, total energy-adjusted fat intake |
19 |
Dietary ARA intake, g/day, quintile, median |
RR(95%CI) |
Ptrend |
| Q1: 0.05 |
1.00 |
0.93 |
|||||||
| Q2: 0.07 |
0.80(0.59-1.07) |
||||||||
| Q3: 0.09 |
0.84(0.63-1.13) |
||||||||
| Q4: 0.11 |
0.80(0.59-1.08) |
||||||||
| Q5: 0.15 |
0.99(0.73-1.34) |
||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Saadatian-Elahi et al. 2002 [29] |
NYUWHS, USA, 1985-1995 (average 4.3 years follow-up), nested case-control design |
197 breast cancer patients, 197 controls (free of cancer), aged 34-65, matched by age, menopausal status, date of blood sampling, number of blood samplings, day of menstrual cycle |
Serum phospholipids, GC analysis, precision indicated |
Self-reported physician diagnosis, combined with tumor registries, mortality databases and review of clinical and pathological documents |
Family history, age at first full-term birth, total cholesterol, history of treatment for benign breast conditions |
19 |
ARA composition%, quartile |
OR(95% CI) |
P for the overall categorial variable: |
| Q1 |
1.00 |
0.80 |
|||||||
| Q2 |
0.79(0.43-1.46) |
|
|||||||
| Q3 |
0.99(0.55-1.81) |
Ptrend with the score variable |
|||||||
| Q4 |
0.81(0.45-1.47) |
0.66 |
|||||||
| Pala et al. 2001 [53] |
ORDET study, Italy, 1987-1995 (average 5.5 years follow-up) |
71 breast cancer patients, 141 controls (free of cancer), 1 case matched with 2 controls by age, menopausal status at recruitment, daylight-saving period at blood sampling, recruitment center and date |
Erythrocyte phospholipids (fasting blood), GC analysis blinded to case-control status, precision indicated |
Lombardy Cancer Registry |
None (BMI, WHR, age at menarche, age at first birth, age at menopause, months of lactation, parity and educational level were investigated) |
23 |
ARA composition%, tertile |
OR(95%CI) |
Ptrend |
| T1: <16.67 |
1.00 |
0.42 |
|||||||
| T2: ≥16.67- |
1.76(0.88-3.53) |
||||||||
| <17.94 |
1.40(0.64-3.10) |
||||||||
| T3: ≥17.94 |
|
||||||||
| |
|
|
|
|
|
|
|
|
|
| Chajès et al. 1999 [54] |
Three ongoing cohort studies in Sweden, VIP(1986- 1997), northern Sweden component of the WHO MONICA(1986, 1990 and 1994), MSP(1995-1997), nested case-control design |
196 breast cancer patients (VIP 103, MONICA 9, MSP 84), 388 controls (VIP 214, MONICA 6, MSP 168), 1 case matched with 2 controls by age, age of blood sample, sampling center |
Serum phospholipids (for VIP and MONICA fasting blood, for MSP very little fasting blood), GC analysis, precision indicated |
Regional cancer registry, National Cancer Registry, follow-up for vital status (death) or losses to follow-up determined through local and national population registries |
Age at menarche, parity, age at first full-term pregnancy, use of hormones, menopausal status |
19 |
ARA composition%, quartile |
OR(95%CI) |
Ptrend |
| Q1 |
1.00 |
0.091 |
|||||||
| Q2 |
0.49(0.24-0.99) |
||||||||
| Q3 |
0.48(0.22-1.04) |
||||||||
| Q4 |
0.51(0.24-1.09) |
||||||||
|
Study design: case-control study (temporal relationship among exposure and outcome is demonstrated) | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Nkondjock et al. 2003 [55] |
Survey, Canada, 1989-1993, case-control design |
414 primary breast cancer patients aged 35-79, 688 controls (eligibility criteria not shown), population-based, matched by age, language, place of residence |
French version FFQ, >200 items, validated against 7-day FD |
Histological diagnosis |
Age at first full-term pregnancy, smoking status, family history of breast cancer, history of benign breast disease, marital status, number of full-term pregnancies, total energy intake |
20 |
Dietary ARA intake, g/day, quartile |
OR(95%CI) |
Ptrend |
| Q1 |
1.00 |
0.723 |
|||||||
| Q2 |
0.65(0.44-0.97) |
||||||||
| Q3 |
1.01(0.70-1.53) |
||||||||
| Q4 |
0.86(0.58-1.30) |
||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Vatten et al. 1993 [56] |
Janus Serum Bank, Norway, 1973-1991, case-control design |
87 breast cancer patients, 235 controls with no prior history of cancer, matched by age, date of blood sampling |
Serum phospholipid, GC analysis blinded to case-control state, precision indicated |
National cancer registry linked to Janus Serum Bank donor information |
None |
20 |
ARA concentration, mg/l, mean(SD) 78(30) |
ARA concentration, mg/l, mean(SD) 79(29) |
P |
| Not significant | |||||||||
|
Exposure assessment: tissue ARA level | |||||||||
| London et al. 1993 [57] |
Survey, USA, 1986-1988, case-control design |
Postmenopausal women, 380 breast cancer patients, 573 controls with breast abnormality (free of breast cancer), matching not indicated |
Buttock adipose tissue fatty acids, GC analysis, precision indicated |
Physician diagnosis (detail not shown) |
Age, alcohol intake, age at first birth, parity, family history of breast cancer, age at menopause, age at menarche, history of benign breast disease, weight |
19 |
ARA composition%, quintile |
OR(95% CI) |
Ptrend |
| Q1 |
1.0 |
0.60 |
|||||||
| Q2 |
0.8(0.5-1.2) |
||||||||
| Q3 |
0.9(0.6-1.5) |
||||||||
| Q4 |
1.0(0.6-1.6) |
||||||||
| Q5 |
1.0(0.6-1.6) |
||||||||
|
Study design: case-control study (temporal relationship among exposure and outcome is unclear) | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Zhu et al. 1995 [58] |
Survey, Finland, 1990-1992 |
17 premenopausal, 32 postmenopausal primary breast cancer patients, 34 premenopausal, 16 postmenopausal controls with benigh breast disease (eligibility criteria not shown), matching not indicated |
Semiquantitative FFQ, 110 items, validated against 14-day DR |
Histological diagnosis |
Age, total energy intake |
13 |
Dietary ARA intake, mg/day, mean(SD) |
Dietary ARA intake, mg/day, mean(SD) |
P |
| Premenopausal case: |
Premenopausal control: |
Premenopausal: |
|||||||
| 58(27) |
163(323) |
Not significant |
|||||||
| Postmenopausal case: |
Postmenopausal control: |
Postmenopausal: |
|||||||
| 90(191) |
62(26) |
Not significant |
|||||||
|
Exposure assessment: blood ARA level | |||||||||
| Aro et al. 2000 [59] |
Kuopio Breast Cancer Study, Finland, 1992-1995, case-control design |
195 primary breast cancer patients aged 25-75, 208 controls drawn randomly from the National Population Register, matched by age, long-term area of residence |
Serum fatty acids (fasting blood), GC analysis, precision indicated |
Histological diagnosis |
Age, area, age at menarche, age at first full-term pregnancy, use of oral contraceptives, use of HRT, family history of breast cancer, history of benign breast disease, educational level, alcohol intake, smoking status, physical activity, WHR, BMI |
15 |
ARA composition%, quintile, median |
OR(95% CI) |
Ptrend |
| Postmenopausal: |
Postmenopausal: |
Postmenopausal: |
|||||||
| Q1: 3.84 |
1.0 |
Signifncant |
|||||||
| Q2: 4.89 |
1.1(0.4-2.8) |
||||||||
| Q3: 5.46 |
2.0(0.8-4.8) |
||||||||
| Q4: 6.04 |
2.4(1.0-5.9) |
||||||||
| Q5: 7.15 |
3.1(1.3-7.8) |
||||||||
| ARA composition%, mean(SD) |
ARA composition%, mean(SD) |
P |
|||||||
| Premenopausal case: |
Premenopausal control: |
Premenopausal: |
|||||||
| 5.68(1.01) |
5.49(1.16) |
Not significant |
|||||||
| |
|
|
|
|
|
|
|
|
|
| Zaridze et al. 1990 [60] |
Survey, now-defunct Union of Soviet Socialist Republics, case-control design |
25 premenopausal, 21 postmenopausal primary breast cancer patients, 20 premenopausal, 33 postmenopausal neighborhood controls (eligibility criteria not shown), matching not indicated |
Erythrocyte phospholipids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
11 |
ARA concentration, μg/mg phospholipids, bisectional, (Summer-Autumn/Winter-Spring) |
RR(95%CI) |
P |
| Premenopausal: |
Premenopausal: |
Premenopausal: |
|||||||
| ≤11.70/9.89 vs |
0.33(0.08-1.35) |
0.122 |
|||||||
| >11.70/9.89 | |||||||||
| Postmenopausal: |
Postmenopausal: |
Postmenopausal: |
|||||||
| ≤11.70/9.89 vs |
0.23(0.07-0.78) |
0.018 |
|||||||
| >11.70/9.89 | |||||||||
|
Exposure assessment: tissue ARA level | |||||||||
| Bagga et al. 2002 [61] |
Survey, USA, 1995-1996, case-control design |
73 breast cancer patients, 73 controls undergoing reduction mammoplasty for mastomegaly, matching not indicated |
Breast adipose tissue fatty acids, GC analysis, precision not indicated |
Not shown |
None |
15 |
ARA concentration, μmol/g total fatty acid, mean(SEM) |
ARA concentration, μmol/g total fatty acid, mean(SEM) |
P |
| Case: |
Control: |
0.27 |
|||||||
| 15.03(1.20) |
13.13(1.25) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Maillard et al. 2002 [62] |
Survey, France, 1992-1996, case-control design |
241 patients with non-metastatic invasive breast carcinoma, 88 controls with benign breast diseases, matching not indicated |
Breast adipose tissue triglycerides, GC analysis blinded to case-control status, precision indicated |
Not shown |
Age at diagnosis, height, BMI, menopausal status, BMI-menopausal status interaction |
16 |
ARA composition%, tertile |
OR(95% CI) |
Ptrend |
| T1 |
1.00 |
0.32 |
|||||||
| T2 |
0.87(0.41-1.84) |
||||||||
| T3 |
0.98(0.42-2.29) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Zhu et al. 1995 [58] |
Survey, Finland, 1990-1992 |
26 premenopausal, 47 postmenopausal primary breast cancer patients, 35 premenopausal, 20 postmenopausal controls with benign breast disease (eligibility criteria not shown), matching not indicated |
Breast adipose tissue triglycerides and phospholipids, GC analysis, precision not indicated |
Histological diagnosis |
Age |
13 |
Triglyceride ARA composition mol%, mean(SD) |
Triglyceride ARA composition mol%, mean(SD) |
P |
| Premenopausal case: |
Premenopausal control: |
Triglyceride |
|||||||
| 0.33(0.18) |
0.33(0.27) |
Premenopausal: |
|||||||
| Postmenopausal case: |
Postmenopausal control: |
Not significant |
|||||||
| 0.33(0.18) |
0.55(0.62) |
Postmenopausal: |
|||||||
| Phospholipid ARA composition mol%, mean(SD), Premenopausal case: |
Phospholipid ARA composition mol%, mean(SD), Premenopausal control: |
<0.01 |
|||||||
| 9.67(2.56) |
9.58(2.17) |
Phospholipid |
|||||||
| Postmenopausal case: |
Postmenopausal control: |
Premenopausal: |
|||||||
| 9.64(2.26) |
10.95(3.26) |
Not significant |
|||||||
| Postmenopausal: | |||||||||
| Not significant | |||||||||
| |
|
|
|
|
|
|
|
|
|
| Petrek et al. 1994 [63] |
Survey, USA, 1987-1989, case-control design |
154 invasive breast cancer patients, 125 controls at average risk of breast cancer, matching not indicated |
Breast adipose tissue fatty acids, GC analysis, precision not indicated |
Histological diagnosis |
None |
7 |
ARA composition weight%, mean(SD) |
ARA composition weight%, mean(SD) |
P |
| Case: |
Control: |
Not significant |
|||||||
| 0.40(0.15) |
0.39(0.16) |
||||||||
|
Study design: cross-sectional study | |||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Williams et al. 1993 [64] |
Survey, UK |
12 malignant breast disease patients, 10 benign breast disease patients, 22 normal controls |
Erythrocyte PIs and PCs (fasting blood), GC analysis, precision not indicated |
Histological diagnosis |
None |
8 |
ARA composition%, only shown as figure: |
P |
|
| Erythrocyte PIs: not significant |
PCs: |
||||||||
| Erythrocyte PCs: significantly higher in control compared with benign and malignant group |
Malignant/Control: |
||||||||
| <0.02 | |||||||||
| Benign/Control: | |||||||||
| <0.02 | |||||||||
| |
|
|
|
|
|
|
|
|
|
| Hietanen et al. 1994 [46] |
Survey, UK, cross-sectional design |
20 breast cancer patients aged 37-85, controls matched by age, sex, smoking status |
Erythrocyte phospholipids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
10 |
ARA composition%, mean(SD) |
ARA composition%, mean(SD) |
P |
| Case: |
Control: |
Not significant |
|||||||
| 17.5(0.8) |
18.5(1.5) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Punnonen et al. 1989 [65] |
Survey, Finland |
6 breast cancer patients, 9 normal controls |
Erythrocyte phospholipids, GC analysis, precision not indicated |
Histological diagnosis |
None |
5 |
ARA composition%, mean(SEM) |
ARA conposition%, mean(SEM) |
P |
| Case: |
Control: |
Not significant |
|||||||
| 12.1(1.5) |
13.3(0.9) |
||||||||
|
Exposure assessment: tissue ARA level | |||||||||
| Williams et al. 1993 [64] |
Survey, UK |
12 malignant breast disease patients, 10 benign breast disease patients, 6 normal controls |
Breast tissue PIs and PCs, GC analysis, precision not indicated |
Histological diagnosis |
None |
8 |
ARA composition%, only shown as figure: |
P |
|
| Breast tissue PIs: not significant |
PCs: |
||||||||
| Breast tissue PCs: significantly higher in control compared with benign and malignant group |
Malignant/Control: |
||||||||
| <0.02 | |||||||||
| Benign/Control: | |||||||||
| <0.02 | |||||||||
| Eid et al. 1988 [66] | Survey, Israel | 85 sequential patients (37 carcinoma, 27 fibroadenoma, 21 others) | Breast adipose tissue fatty acids, GC analysis, precision indicated | Not shown | None | 8 | ARA composition%, mean(SD) |
ARA composition, mean(SD) |
P |
| Carcinoma: |
Others: |
Not significant | |||||||
| 0.62(0.05) |
0.46(0.04) | ||||||||
| Fibroadenoma: | |||||||||
| 0.78(0.18) | |||||||||
ARA Arachidonic acid, BMI Body mass index, DR Diet record, FD Food record, FFQ Food frequency questionnaire, GC Gas chromatography, HRT Hormone replacement therapy, MONICA multinational study for Monitoring of Trends and Cardiovascular Disease study, MSP Mammary-Screening Project, NHS Nurses' Health study, NLCS Netherlands Cohort Study on Diet and Cancer, NYUWHS New York University Women's Health Study, OR Odds ratio, ORDET study: the Hormones and Diet in the Etiology of Breast Cancer Risk study, PC Phosphatidyl-choline, PI Phosphatidyl-inositol, RR Relative risk, UK United Kingdom, USA United States of America, VIP Västerbotten Intervention Project, WHR Waist-to-hip ratio, WR Weighed dietary record.
*Result of the critical evaluation carried out using the STROBE tool.
Dietary ARA intake was estimated in one cohort study and three case-control studies. These four showed no significant change in breast cancer risk except in the second quartile of ARA intake in the report by Nkondjock et al.
Six case-control studies and three cross-sectional studies investigated blood ARA levels. The precision of blood analysis was reported in only five articles, and blinded fatty acid measurement was conducted in only two articles. Three articles indicated significant differences in breast cancer risk; however, they were a case-control study with little temporal information between exposure and outcome or a cross-sectional study. Aro et al. reported significantly increased breast cancer risk in the highest quintile of serum ARA in post-menopausal women. The reporting quality of the remaining two articles, those by Zaridze et al. and Williams et al., was quite low.
Five case-control studies and two cross-sectional studies examined tissue ARA levels. The precision of tissue analysis was mentioned in only three articles, and only in one report fatty acids measurement was performed in a blinded fashion. A significant change in breast cancer risk or a significant difference in tissue ARA level was not found, except for breast tissue triglyceride ARA levels in a report by Zhu et al. and breast tissue phosphatidylcholine ARA levels in a report by Williams et al.
Prostate cancer
Major characteristics are shown in Table 4[46,67-81]. Four articles did not provide sufficient information about the methodology of outcome measurement. As well as well-known confounding factors, specific factors for prostate cancer, for instance BMI, physical activity, and total energy, were considered in some articles; however, no confounding factors were adjusted for in seven articles.
Table 4.
Summary of observational studies on the association between ARA and risk of prostate cancer
| References | Study | Subjects | Exposure Assessment | Prostate cancer assessment (diagnosis) | Adjustment for potential confounders | Assessment of reporting quality * |
Main findings |
||
|---|---|---|---|---|---|---|---|---|---|
| Intergroup comparison | P or Ptrend | ||||||||
|
Study design: cohort study | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Leitzmann et al. 2004 [67] |
HPFS, USA, 1986-2000, prospective cohort design (14 years follow-up) |
47,866 health professionals aged 40-65, no prior history of cancer |
Semiquantitative FFQ, 131 items, validated against 2 x 1-week DR |
Self-reported physician diagnosis supplemented by medical record and pathology report |
Age, time period, race, family history of prostate cancer, history of type 2 DM and vasectomy, BMI, height, smoking status, physical activity, total energy intake, % of energy from protein intake, monounsaturated fat intake, saturated fat intake and trans unsaturated fat intake, calcium intake, supplemental vitamin E and lycopene |
21 |
Dietary ARA intake, %energy, quintile |
RR(95% CI) |
Ptrend |
| Q1: <0.028 |
1.00 |
0.44 |
|||||||
| Q2: 0.028-0.035 |
1.06(0.94-1.19) |
||||||||
| Q3: 0.036-0.041 |
1.04(0.92-1.18) |
||||||||
| Q4: 0.042-0.049 |
1.02(0.89-1.16) |
||||||||
| Q5: >0.049 |
1.08(0.94-1.25) |
||||||||
|
Study design: nested case-control study | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Männistö et al. 2003 [68] |
ATBC study, Finland, 1985-1993, nested case-control design (5-8 years follow-up) |
198 prostate cancer patients, 198 controls (free of prostate cancer) matched by age, trial supplementation group |
Self-administered dietary questionnaire, 276 items, validated against 12 x 2-day DR |
Finnish Cancer Registry and Register of Causes of Death |
Resident area, educational level, BMI, alcohol intake, smoking period |
23 |
Dietary ARA intake, g/day, median |
OR(95%CI) |
Ptrend |
| Q1: 0.04 |
1.00 |
0.23 |
|||||||
| Q2: 0.06 |
0.89(0.52-1.54) |
||||||||
| Q3: 0.07 |
1.10(0.64-1.90) |
||||||||
| Q4: 0.10 |
1.31(0.77-2.21) |
||||||||
| |
|
|
|||||||
| Schuurman et al. 1999 [69] |
NLCS, Netherlands, 1986-1992 (6.3 years follow-up), case-cohort design |
642 primary prostate cancer patients from entire cohort, 1,525 subcohort members (selection criteria not shown) aged 55-69 at baseline, without prevalent cancer other than skin cancer, matching not indicated |
Semiquantitative FFQ, 150 items, validated against 3 x 3-day DR |
All regional cancer registries and Dutch national database of pathology reports |
Age, family history of prostate carcinoma, socioeconomic status, total energy intake, total energy-adjusted fat intake |
23 |
Dietary ARA intake, g/day, quintile, median |
RR(95%CI) |
Ptrend |
| Q1: 0.06 |
1.00 |
0.30 |
|||||||
| Q2: 0.09 |
1.21(0.88-1.66) |
||||||||
| Q3: 0.11 |
1.37(1.00-1.87) |
||||||||
| Q4: 0.13 |
1.11(0.80-1.54) |
||||||||
| Q5: 0.17 |
1.20(0.87-1.66) |
||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Crowe et al. 2008 [70] |
EPIC study, Denmark, Germany, Greece, Italy, Netherlands, Spain, Sweden, UK, 1992-2000, nested case-cohort design |
962 prostate cancer patients, 1,061 controls without prevalent cancer other than NMSC, 1 case matched with 1-2 control(s) by study center, age, time of blood sampling, time between blood sampling and last consumption of food or drink |
Plasma phospholipids, GC analysis, precision indicated |
Regional or national cancer registries or combination of health insurance records, cancer and pathology registries and self-report |
BMI, smoking status, alcohol intake, educational level, marital status, physical activity |
26 |
ARA composition mol%, quintile |
RR(95%CI) |
Ptrend |
| Q1: 4.40–7.93 |
1.00 |
0.419 |
|||||||
| Q2: 7.93–8.89 |
1.28(0.96-1.70) |
||||||||
| Q3: 8.90–9.86 |
1.17(0.88-1.56) |
||||||||
| Q4: 9.86–10.98 |
0.81(0.60-1.10) |
||||||||
| Q5: 10.99–19.14 |
0.91(0.65-1.25) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Chavarro et al. 2007 [71] |
PHS, USA, 1982-1995, nested case-control design within a randomized, double-blind, placebo-controlled factorial aspirin and beta-carotene trial (13 years follow-up) |
476 prostate cancer patients, 476 controls, male physicians without history of cancer except NMSC, 1 case matched with 1 control by age, smoking status, with consideration for trial intervention |
Whole blood fatty acids, GC analysis blinded to case-control status, precision indicated |
Self-report, combined with review of hospital records and pathology reports |
Age, smoking status, length of follow-up |
22 |
ARA concentration (%,), quintile, median |
OR(95%CI) |
Ptrend |
| Q1: 7.9 |
1.00 |
0.98 |
|||||||
| Q2: 9.3 |
1.22(0.82-1.81) |
||||||||
| Q3: 10.1 |
1.05(0.70-1.57) |
||||||||
| Q4: 10.9 |
0.98(0.66-1.46) |
||||||||
| Q5: 12.3 |
1.09(0.72-1.64) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Männistö et al. 2003 [68] |
ATBC study, Finland, 1985-1993, nested case-control design (5-8 years follow-up) |
198 prostate cancer patients, 198 controls (free of prostate cancer) matched by age, trial supplementation group |
Serum cholesterol ester fatty acids, GC analysis, precision indicated |
Finnish Cancer Registry and Register of Causes of Death |
Resident area, educational level, BMI, alcohol intake, smoking period |
23 |
ARA composition %, quartile, median |
OR(95%CI) |
Ptrend |
| Q1: 3.96 |
1.00 |
0.34 |
|||||||
| Q2: 4.55 |
1.05(0.60-1.84) |
||||||||
| Q3: 5.09 |
0.94(0.54-1.64) |
||||||||
| Q4: 5.89 |
1.39(0.79-2.44) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Harvei et al. 1997 [72] |
Janus serum bank, Norway, 1973-1994, nested case-control design |
141 prostate cancer patients, 282 controls (eligibility criteria not shown), 1 case matched with 2 controls by age, date of blood sampling, resident area |
Serum phospholipids, GC analysis, blinded to case-control status, precision not indicated |
Cancer Registry and Statistics Norway |
None |
14 |
ARA concentration mg/l, quartile, upper limit |
OR(95%CI) |
Ptrend |
| Q1: 4.86 |
1.0 |
0.6 |
|||||||
| Q2: 5.68 |
1.1(0.6-1.9) |
||||||||
| Q3: 6.68 |
1.2(0.7-2.1) |
||||||||
| Q4: >6.68 |
0.8(0.4-1.5) |
||||||||
| Gann et al. 1994 [73] |
PHS, USA, 1982-1988, nested case-control design within a randomized, double-blind, placebo-controlled factorial aspirin and beta-carotene trial (6 years follow-up) |
120 prostate cancer patients, 120 controls, male physicians without history of cancer except NMSC, 1 case matched with 1 control by age, smoking status without regard to trial intervention |
Plasma cholesterol ester fatty acids, GC analysis blinded to case-control status, precision indicated |
Self-report, combined with review of medical records |
None |
19 |
ARA composition of plasma cholesterol estel %, quartile |
OR |
Ptrend |
| Q1 |
1.00 |
0.76 |
|||||||
| Q2 |
1.81 |
||||||||
| Q3 |
1.00 |
||||||||
| Q4 |
1.36(vs Q1 95% CI: 0.63-2.90) |
||||||||
|
Study design: case-control study (temporal relationship among exposure and outcome is unclear) | |||||||||
|
Exposure assessment: dietary intake | |||||||||
| Hodge et al. 2004 [74] |
Survey, Australia, 1994-1997, case-control design |
858 prostate cancer patients aged <70, 905 controls matched by age |
Melbourne FFQ, 121 items, validated against 2 x 4-day WFR |
Not shown |
Age at selection, study center, calendar year, family history of prostate cancer, country of birth, socioeconomic status |
18 |
Dietary ARA intake, g/day, quintile |
OR(95%CI) |
Ptrend |
| Q1: <0.028 |
1.0 |
0.6 |
|||||||
| Q2: 0.028-0.036 |
1.2(0.8-1.6) |
||||||||
| Q3: 0.037-0.046 |
1.2(0.8-1.6) |
||||||||
| Q4: 0.047-0.059 |
1.0(0.7-1.3) |
||||||||
| Q5: ≥0.06 |
1.0(0.7-1.4) |
||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Ukori et al. 2010 [75] |
Survey, USA and Nigeria, case-control design |
48 African American and 66 Nigerian prostate cancer patients, 96 African American and 226 Nigerian controls, aged ≥40, without any cancer history other than skin cancer, matching not indicated |
Plasma fatty acids (fasting blood), GC analysis, precision not indicated |
Abnormal DRE and/or abnormal PSA (>4ng/ml) with histological diagnosis |
Age, educational level, family history of prostate cancer, WHR |
14 |
ARA concentration μg/ml, quartile American African: |
OR(95%CI) |
Ptrend |
| Q1 vs Q4 |
American African: |
American African: |
|||||||
| Nigerian: |
0.3(0.08-1.11) |
||||||||
| Q1 vs Q4 |
Nigerian: |
<0.05 |
|||||||
| 0.75(0.32-1.74) |
Nigerian: |
||||||||
| Not significant | |||||||||
| Ukori et al. 2009 [76] |
Survey, Nigeria, case-control design |
66 prostate cancer patients, 226 controls, aged ≥40, matching not indicated (same population as Nigerian participants of Ukori et al. 2010) |
Plasma fatty acids (fasting blood), GC analysis, precision not indicated |
Abnormal DRE and/or abnormal PSA (>4ng/ml) with histological diagnosis |
Age, educational level, family history of prostate cancer, WHR |
11 |
ARA concentration μg/ml, quartile |
OR(95%CI) |
Ptrend |
| Q1 |
1.00 |
0.06 |
|||||||
| Q2 |
2.59(0.85-7.86) |
||||||||
| Q3 |
1.93(0.73-5.14) |
||||||||
| Q4 |
0.75(0.32-1.74) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Newcomer et al. 2001 [77] |
Survey, USA, case-control design |
67 prostate cancer patients, 156 population-based controls, 1 case matched with about 2 controls by age distribution |
Erythrocyte fatty acids, GC analysis blinded to case-control status, precision indicated |
Not shown |
Age |
23 |
ARA composition weight%, quartile |
OR(95%CI) |
Ptrend |
| Q1: ≤13.25 |
1.0 |
0.88 |
|||||||
| Q2: 13.26-14.12 |
1.6(0.7-3.7) |
||||||||
| Q3: 14.13-14.90 |
1.6(0.7-3.5) |
||||||||
| Q4: ≥14.91 |
0.9(0.4-2.3) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Yang et al. 1999 [78] |
Survey, Korea |
19 prostate cancer patients, 24 benign prostatic hyperplasia patients, 21 normal controls, matched by age, demographics |
Serum fatty acids, GC-MS analysis, precision not indicated |
Not shown |
None |
4 |
ARA composition%, mean (SD) |
ARA composition%, mean(SD) |
P |
| Cancer: |
Normal control: |
Not significant |
|||||||
| 0.77(0.31) |
1.15(0.45) |
||||||||
| Benign: | |||||||||
| 0.95(0.16) | |||||||||
|
Study design: cross-sectional study | |||||||||
|
Exposure assessment: blood ARA level | |||||||||
| Faas et al. 2003 [79] |
Survey, USA, 1995-1998 |
Prostate cancer patients, benign prostate disease patients |
Erythrocyte and plasma phospholipids, GC analysis, precision not indicated |
Pathology reports |
None |
10 |
Erythrocyte ARA composition%, mean(SEM) |
Erythrocyte ARA composition%, mean(SEM) |
P |
| Malignant: |
Benign: |
Erythrocyte: |
|||||||
| 16.33(0.28) |
16.68(0.25) |
Not significant |
|||||||
| Plasma ARA composition%, mean(SEM) |
Plasma ARA composition%, mean(SEM) |
Plasma: |
|||||||
| Malignant: |
Benign: |
Not significant |
|||||||
| 12.60(0.27) |
13.03(0.29) |
||||||||
| Hietanen et al. 1994 [46] |
Survey, UK, cross-sectional design |
10 prostate cancer patients aged 64-85, controls, matched by age, sex, smoking status |
Erythrocyte phospholipids (fasting blood), GC analysis, precision not indicated |
Not shown |
None |
8 |
ARA composition%, mean(SD) |
ARA composition%, mean(SD) |
P |
| Case: |
Control: |
Not significant |
|||||||
| 17.8(1.3) |
18.6(1.3) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Chaudry et al. 1991 [80] |
Survey, UK |
20 patients admitted for prostatic surgery (10 malignant, 10 benign) |
Plasma phospholipids (fasting blood), GC analysis, precision not indicated |
Histological diagnosis |
None |
6 |
ARA composition%, median(IQR) |
ARA composition%, median(IQR) |
P |
| Malignant: |
Benign: |
Not significant |
|||||||
| 8.93(1.84) |
8.78(2.03) |
||||||||
|
Exposure assessment: tissue ARA level | |||||||||
| Faas et al. 2003 [79] |
Survey, USA, 1995-1998 |
Prostate cancer patients, benign prostate disease patients |
Prostate tissue phospholipids, GC analysis, precision not indicated |
Pathology reports |
None |
10 |
ARA composition%, mean(SEM) |
ARA composition%, mean(SEM) |
P |
| Malignant: |
Benign: |
<0.001 |
|||||||
| 15.20(0.33) |
16.99(0.29) |
||||||||
| |
|
|
|
|
|
|
|
|
|
| Mamalakis et al. 2002 [81] |
Survey, Greece, 1997-1999 |
36 prostate cancer patients, 35 benign prostate hyperplasia patients |
Gluteal adipose tissue and prostate tissue fatty acids, GC analysis, precision not indicated |
DRE, serum PSA, transrectal ultrasound, prostate biopsy |
None |
12 |
Gluteal adipose tissue ARA composition%, mean(SD) |
Gluteal adipose tissue ARA composition%, mean(SD) |
P |
| Malignant: |
Benign: |
Gluteal adipose tissue: |
|||||||
| 0.28(0.12) |
0.25(0.14) |
Not significant |
|||||||
| Prostate tissue ARA composition%, mean(SD) |
Prostate tissue ARA composition%, mean(SD) |
|
|||||||
| Malignant: |
Benign: |
Prostate tissue: |
|||||||
| 5.99(3.65) |
10.71(2.69) |
<0.001 |
|||||||
| Chaudry et al. 1991 [80] | Survey, UK | 20 patients admitted for prostatic surgery (10 malignant, 10 benign) | Prostate tissue phospholipids, GC analysis, precision not indicated | Histological diagnosis | None | 6 | ARA composition%, median(IQR) |
ARA composition%, median(IQR) |
P |
| Malignant: |
Benign: |
|
|||||||
| 11.33(4.12) | 15.55(2.54) | 0.002 | |||||||
ARA Arachidonic acid, ATBC Study: Alpha-tocopherol. Beta-carotene cancer prevention study, BMI Body mass index, DM Diabetes mellitus, DR Diet record, DRE Digital rectal examination, EPIC European prospective investigation into cancer and nutrition, FFQ Food frequency questionnaire, GC Gas chromatography, HPFS Health professionals follow-up study, IQR Interquartile range, NLCS Netherlands cohort study on diet and cancer, NMSC Non-melanoma skin cancer, OR Odds ratio, PHS Physician's health study, PSA Serum level of prostate specific antigen, RR Relative risk, UK United Kingdom, USA United States of America, USDA United states Department of Agriculture, WFR Weighed food record, WHR Waist-to-hip ratio.
*Result of the critical evaluation carried out using the STROBE tool.
One cohort study and three case-control studies examined dietary ARA intake. They showed no significant change in prostate cancer risk according to increased ARA intake.
Blood ARA levels were estimated in nine case-control studies and three cross-sectional studies. The precision of blood analysis was mentioned in only five articles, and masking of disease status was conducted in only four. Ukori et al. (2010) reported that prostate cancer risk of African-Americans decreased in the fourth quartile of blood ARA level, and that the overall trend was significant (P for trend < 0.05). A significant change in prostate cancer risk or a significant difference in blood ARA levels was not found in the other 11 articles.
Three cross-sectional studies examined tissue ARA levels. All of them reported significant decreases of tissue ARA levels in cancer subjects; however, their reporting quality was generally quite low. None of them mentioned the precision of tissue analysis and masking of groups.
Discussion
In the present review, we systematically reviewed observational studies investigating the association between ARA and cancer of six organs in free-living populations. Fifty-two eligible articles were obtained from our search strategy, and 31 out of the 52 articles were identified from hand searches for references (Figure 1). Thus, reference searching serves an important role in comprehensive literature searches. This pointed out the characteristics of the reporting style of the observational studies for ARA and cancer risk.
Among the 31 eligible articles from reference searches, 22 were not recognised by our PubMed search formula due to keywords related to “exposure”, three were not recognised due to keywords related to “study types”, and six were not recognised due to both. For “exposure” terms, 26 articles could be identified by the addition of the search term “fatty”. The remaining two articles related to the term “exposure” reported fatty acid compositions of tissues only. In the case of “study type” terms, none of the nine articles used a general study design word (i.e., cohort, case-control, or cross-sectional), although the STROBE statement recommends that authors should indicate the study design with a commonly used term in the title or abstract. These reporting characteristics made it difficult to effectively search for observational studies with a focus on individual fatty acids such as ARA. We therefore adopted the search strategy described above.
The findings from articles for colorectal cancer differ depending on the methodology of ARA exposure assessment. A positive dose-response relationship between dietary ARA intake and colorectal cancer was indicated in two reports [30,31], whereas four articles [38,40,43,46] indicated a negative association or significant ARA decrease with blood ARA levels, and no article reported a positive relationship between colorectal cancer risk and tissue ARA level. These inconsistent results seem to indicate that there is little firm evidence that ARA correlates with the risk of cancer.
There were limited numbers of studies on skin cancer, and they varied in the assessment method used for ARA exposure and the target cancer. It is therefore impossible to draw any conclusions from the results.
Among studies for breast and prostate cancer, a strong positive association and a clear dose-response relationship between increased cancer risk and ARA exposure were not observed, although the results were replicated in different settings using different methods. This suggests that ARA exposure is not associated with increased breast and prostate cancer risk.
We suppose that the contradictory findings mentioned above were caused by four main factors. First, methodologies for estimating dietary ARA intake have not been developed sufficiently. Most adults with mixed diets consume approximately 50 to 250 mg of ARA per day from foodstuffs [82-84], whereas some articles on colorectal and prostate cancer have reported lower values [30,36,39,68,74]. Various validated questionnaires were used in articles which assessed dietary ARA intake, but the validation was not conducted for ARA specifically; total fat, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, linoleic acid, or eicosapentaenoic acid intake was assessed, but ARA was not. Since the validity of the estimation of dietary ARA intake is sometimes not enough [82], it should be considered whether exposure assessment is conducted with an appropriate method.
Second, assessment of ARA biomarkers such as blood or tissue ARA levels was diverse; assessed blood fractions included erythrocyte, serum, plasma, or total blood. Tissue sampling was conducted from adipose tissue of buttock or malignant target cancer tissue (i.e., colon, skin, breast, and prostate). Individual biomarkers of fatty acids represent intakes for different time periods [85]. Serum or plasma levels of ARA are considered to reflect dietary intake over a few days, whereas erythrocyte and tissue ARA composition serve as more long-term biomarkers. Overall, habitual dietary ARA intake could not be assessed sufficiently in articles that measured ARA composition of serum or plasma, and this might be one of the causes of inconsistent results among eligible articles.
Third, methodologies of participant selection used in the reports could lead to selection bias. A nested case-control study from a physicians’ health study (aspirin and beta-carotene intervention study) did not consider trial intervention through the participant selection procedure [73]. Also, reporting of participant selection was important; among the total of 52 articles, 28 did not sufficiently report the eligibility criteria, and the sources and methods of participant selection, and 31 did not fully describe the numbers of individuals at each stage of the study and reasons for nonparticipation. This makes it difficult to estimate the effect of selection bias, and therefore, the relationship between ARA intake and cancer risk could not be determined.
Fourth, publication bias based on findings of a significant association could exist, especially in breast and prostate cancers. We evaluated publication bias qualitatively, not using any statistical tests. Most of the significant results were found in the studies with low reporting quality. There is a possibility of publication bias. The results of the studies with low reporting quality may tend to be significant by chance, due to the lack of appropriate design. This suggests that publication bias may affect our review result on breast and prostate cancers, but the effect should be small, because we did not give importance to these studies with low reporting quality.
The biological plausibility of the relationship between ARA intake and cancer risk is still being debated. Previous clinical studies with aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) have suggested that the cyclooxygenase metabolites of ARA may be associated with risk for colorectal, breast, and prostate cancers [21-24]. Many observational studies, however, have failed to find any association between ARA intake, or its level in blood or tissue, and cancer risk. These controversial findings may be explained by the following three reasons. First, ARA levels of blood or tissue may not always represent dietary intake. Garland et al. and Kobayashi et al. reported that correlations between dietary estimates and the ARA contents of adipose tissue or serum phospholipids were very low [82,86]. Second, the increment of blood or tissue ARA levels may not be connected with the amount of ARA metabolites. Our previous study, in which the supplementation of 240 or 720 mg of ARA per day in healthy elderly persons for four weeks was conducted, investigated plasma PGE2 and urinary PGE2 metabolites [87]. Their concentrations did not differ significantly with regard to ARA supplementation or time points, although plasma ARA compositions increased dose-dependently. Third, ARA metabolites that are produced by pathways other than the cyclooxygenase pathway may decrease cancer risk. LXA4 is an anti-inflammatory mediator produced by the lipoxygenase pathway and is regarded to be a suppressor of tumour growth based on anti-angiogenic properties [25,88]. Aspirin or NSAIDs may not only inhibit the production of cyclooxygenase metabolites, but also divert ARA into the lipoxygenase pathway. However, it is unclear whether LXA4 contributes to reduced cancer risk in humans.
In the present study, we reviewed all bibliographies of full-text articles for potential inclusion because reference searches serve an important role in comprehensive literature searches. 49,670 articles were listed, and 99.9% of them were not eligible. We considered that this large exclusion resulted from the many articles in which ARA was not described at all; therefore, we tried another reference search from the bibliographies of articles after their exclusion. Fifty full-text articles from the PubMed database and 146 from the reference search mentioned ARA. A total of 13,657 articles were listed in their bibliographies and their number was reduced to a third; however, we could select 30 eligible articles out of 31 articles that were selected from all full-text searching. The one article remaining that was not selected was a report on skin cancer [50]. This might have resulted from the smaller number of articles identified on PubMed for skin cancer (10 reports) than for colorectal, breast, or prostate cancers (48, 31, or 41 reports). This suggests that reference searches from bibliographies of articles including ARA are more efficient when enough articles are identified from the PubMed database.
This systematic review has limitations. First, studies for inclusion could not be selected independently by two or more reviewers. Our inclusion/exclusion criteria were clear and there were few differences which depended on who was in charge; however that may have introduced a potential selection bias. Second, our search was restricted to English publications and articles from the PubMed database. Furthermore, articles that investigated tissue levels of ARA as an exposure assessment could not be identified comprehensively. We did not set the search terms for ARA levels of tissue before the PubMed search, and identified the articles in the reference search. Third, the search term “fatty” or “fatty acid” was not used in the PubMed search. It led to the efficient search but may cause the possibility that the review may not be completed. Fourth, quality assessment of observational studies is difficult because of the heterogeneity of study designs and methods. The reporting quality was quantitatively expressible using the STROBE checklist; in contrast, the methodological quality could not be quantified and was qualitatively estimated by two independent reviewers. This may have seriously influenced the results and conclusions of the present review.
Note that there are insufficient studies to draw any firm conclusions about the relationship between ARA and cancer risk. Further evidence from well-designed observational studies is required.
Conclusions
In conclusion, we systematically identified articles that investigated the association between dietary ARA intake or its biomarkers and the risk of colorectal, skin, breast, prostate, lung, or stomach cancer, and only a limited number of observational studies were found (17, 3, 18, 16, 0, and 0 studies were found on colorectal, skin, breast, prostate, lung, or stomach cancer, respectively). Furthermore, most studies had one or more critical limitations, such as the obscurity of temporal information about exposure and outcome, the methodology of ARA exposure assessment, and inadequate treatment of potential confounding factors. These studies did not sufficiently demonstrate any relationships between ARA exposure and cancer risk; however, they seem to suggest that ARA exposure was not related to increased breast or prostate cancer risk because strong positive associations and clear dose-response relationships were not observed. Findings concerning the association between ARA exposure and colorectal cancer were inconsistent between studies. Thus, further evidence from well-designed observational studies is required to confirm or refute the association between ARA exposure and cancer risk.
Competing interests
This study was supported in part by a grant from Suntory Wellness Limited, Japan. MS, SK, CH, HS, HK, and HS are employees of Suntory Wellness Limited. HO has no competing interests. SS has consultancy relationships with Suntory Wellness Limited.
Authors' contributions
SK conducted database searches. SK, CH, and HT made decisions on the inclusion and exclusion of the articles. MS and SK conducted quality and bias assessments, contributed to interpretation of findings, wrote the manuscript, and incorporated changes suggested by others. HK, HS, HO, and SS helped to interpret the findings and refined the manuscript. All authors have read and approved the final manuscript
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
PubMed search terms and strategies.
Contributor Information
Mai Sakai, Email: Mai_Sakai@suntory.co.jp.
Saki Kakutani, Email: Saki_Kakutani@suntory.co.jp.
Chika Horikawa, Email: Chika_Horikawa@suntory.co.jp.
Hisanori Tokuda, Email: Hisanori_Tokuda@suntory.co.jp.
Hiroshi Kawashima, Email: Hiroshi_Kawashima@suntory.co.jp.
Hiroshi Shibata, Email: Hiroshi_Shibata@suntory.co.jp.
Hitomi Okubo, Email: okubo@m.u-tokyo.ac.jp.
Satoshi Sasaki, Email: stssasak@m.u-tokyo.ac.jp.
References
- World Health Organization. The global burden of disease: 2004 update. Geneva: WHO; 2008. [Google Scholar]
- International Agency for Research on Cancer. World Cancer Report 2008. Lyon: WHO; 2008. [Google Scholar]
- Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Anderson GL. The women’s health initiative: lessons learned. Annu Rev Publ Health. 2008;29:131–150. doi: 10.1146/annurev.publhealth.29.020907.090947. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Cancer: causes, occurrence and control. Lyon: John Wiley & Sons, Ltd; 1990. [Google Scholar]
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007;121:1339–1345. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, Anderson GL, Assaf AR, Bassford T, Bowen D, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Harrigan RC, Hays J, Heber D, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Kotchen JM, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lewis CE, Manson JE, Margolis KL, Mossavar-Rahmani Y, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Stefanick ML, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Whitlock E. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- Jaax S, Scott LW, Wolf JE Jr, Thornby JI, Black HS. General guidelines for a low-fat diet effective in the management and prevention of nonmelanoma skin cancer. Nutr Canc. 1997;27:150–156. doi: 10.1080/01635589709514517. [DOI] [PubMed] [Google Scholar]
- Black HS, Thornby JI, Wolf JE Jr, Goldberg LH, Herd JA, Rosen T, Bruce S, Tschen JA, Scott LW, Jaax S, Foreyt JP, Reusser B. Evidence that a low-fat diet reduces the occurrence of non-melanoma skin cancer. Int J Cancer. 1995;62:165–169. doi: 10.1002/ijc.2910620210. [DOI] [PubMed] [Google Scholar]
- Davies TW, Treasure FP, Welch AA, Day NE. Diet and basal cell skin cancer: results from the EPIC-Norfolk cohort. Br J Dermatol. 2002;146:1017–1022. doi: 10.1046/j.1365-2133.2002.04763.x. [DOI] [PubMed] [Google Scholar]
- Mackie BS, Johnson AR, Mackie LE, Fogerty AC, Ferris M, Baxter RI. Dietary polyunsaturated fats and malignant melanoma. Med J Aust. 1980;1:159–163. doi: 10.5694/j.1326-5377.1980.tb134733.x. [DOI] [PubMed] [Google Scholar]
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(Suppl 1):343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr. 2004;23:281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Helliwell RJA, Adams LF, Mitchell MD. Prostaglandin synthase: recent developments and a novel hypothesis. Prostag Leukot Essent Fatty Acids. 2004;70:101–113. doi: 10.1016/j.plefa.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Kuliczkowski W, Witkowski A, Polonski L, Watala C, Filipiak K, Budaj A, Golanski J, Sitkiewicz D, Pregowski J, Gorski J, Zembala M, Opolski G, Huber K, Arnesen H, Kristensen SD, De Caterina R. Interindividual variability in the response to oral antiplatelet drugs: a position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2009;30:426–435. doi: 10.1093/eurheartj/ehn562. [DOI] [PubMed] [Google Scholar]
- Rostom A, Dubé C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. US Preventive Services Task Force. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- Dubé C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. US Preventive Services Task Force. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–375. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- Takkouche B, Regueira-Méndez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Canc Inst. 2008;100:1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–99. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DWP, Rovati GE, Shimizu T, Yokomizo T, Brink C. The Lipoxin Receptor ALX: Potent Ligand-Specific and Stereoselective Action in Vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Petrik MB, McEntee MF, Chiu CH, Whelan J. Antagonism of Arachidonic Acid Is Linked to the Antitumorigenic Effect of Dietary Eicosapentaenoic Acid in ApcMin/1 Mice. J Nutr. 2000;130:1153–1158. doi: 10.1093/jn/130.5.1153. [DOI] [PubMed] [Google Scholar]
- Imai N, Kawabe M, Tamano S, Doi Y, Nakashima H, Suguro M, Numano T, Hara T, Hagiwara A, Furukawa F, Kaneda Y, Tateishi N, Fujii W, Kawashima H, Shibata H, Sakakibara Y. Arachidonate-enriched triglyceride oil does not promote tumor development in a rat medium-term multi-organ carcinogenesis model. Food Chem Toxicol. 2012;50:2780–2791. doi: 10.1016/j.fct.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol. 2004;160:1011–1022. doi: 10.1093/aje/kwh319. [DOI] [PubMed] [Google Scholar]
- Saadatian-Elahi M, Toniolo P, Ferrari P, Goudable J, Akhmedkhanov A, Zeleniuch-Jacquotte A, Riboli E. Serum fatty acids and risk of breast cancer in a nested case-control study of the New York University Women's Health Study. Canc Epidemiol Biomarkers Prev. 2002;11:1353–1360. [PubMed] [Google Scholar]
- Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao YT, Zheng W. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Canc Epidemiol Biomarkers Prev. 2009;18:2283–2291. doi: 10.1158/1055-9965.EPI-08-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkondjock A, Shatenstein B, Maisonneuve P, Ghadirian P. Assessment of risk associated with specific fatty acids and colorectal cancer among French-Canadians in Montreal: a case-control study. Int J Epidemiol. 2003;32:200–209. doi: 10.1093/ije/dyg048. [DOI] [PubMed] [Google Scholar]
- Caleffi M, Ashraf J, Rowe PH, Thomas BS, Fentiman IS. A comparison of the fatty acid profiles of subcutaneous fat from women with breast cancer, benign breast disease and normal controls. Anticancer Res. 1987;7:1305–1307. [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Campos H, Li H, Sesso HD, Stampfer MJ, Willett WC, Ma J. Blood levels of long-chain polyunsaturated fatty acids, aspirin, and the risk of colorectal cancer. Canc Epidemiol Biomarkers Prev. 2007;16:314–321. doi: 10.1158/1055-9965.EPI-06-0346. [DOI] [PubMed] [Google Scholar]
- Kojima M, Wakai K, Tokudome S, Suzuki K, Tamakoshi K, Watanabe Y, Kawado M, Hashimoto S, Hayakawa N, Ozasa K, Toyoshima H, Suzuki S, Ito Y, Tamakoshi A. JACC Study Group. Serum levels of polyunsaturated fatty acids and risk of colorectal cancer: a prospective study. Am J Epidemiol. 2005;161:462–471. doi: 10.1093/aje/kwi066. [DOI] [PubMed] [Google Scholar]
- Theodoratou E, McNeill G, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary fatty acids and colorectal cancer: a case-control study. Am J Epidemiol. 2007;166:181–195. doi: 10.1093/aje/kwm063. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Potter JD, Duncan DM, Berry TD. Dietary fats and colon cancer: assessment of risk associated with specific fatty acids. Int J Cancer. 1997;73:670–677. doi: 10.1002/(SICI)1097-0215(19971127)73:5<670::AID-IJC10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kuriki K, Wakai K, Hirose K, Matsuo K, Ito H, Suzuki T, Saito T, Kanemitsu Y, Hirai T, Kato T, Tatematsu M, Tajima K. Risk of colorectal cancer is linked to erythrocyte compositions of fatty acids as biomarkers for dietary intakes of fish, fat, and fatty acids. Canc Epidemiol Biomarkers Prev. 2006;15:1791–1798. doi: 10.1158/1055-9965.EPI-06-0180. [DOI] [PubMed] [Google Scholar]
- Busstra MC, Siezen CL, Grubben MJ, van Kranen HJ, Nagengast FM, van't Veer P. Tissue levels of fish fatty acids and risk of colorectal adenomas: a case-control study (Netherlands) Canc Causes Contr. 2003;14:269–276. doi: 10.1023/A:1023650201730. [DOI] [PubMed] [Google Scholar]
- Ghadimi R, Kuriki K, Tsuge S, Takeda E, Imaeda N, Suzuki S, Sawai A, Takekuma K, Hosono A, Tokudome Y, Goto C, Esfandiary I, Nomura H, Tokudome S. Serum concentrations of fatty acids and colorectal adenoma risk: a case-control study in Japan. Asian Pac J Canc Prev. 2008;9:111–118. [PubMed] [Google Scholar]
- Baró L, Hermoso JC, Núñez MC, Jiménez-Rios JA, Gil A. Abnormalities in plasma and red blood cell fatty acid profiles of patients with colorectal cancer. Br J Cancer. 1998;77:1978–1983. doi: 10.1038/bjc.1998.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos JP, Thomas BS. Erythrocyte membrane stearic acid: oleic acid ratios in colorectal cancer using tube capillary column gas liquid chromatography. Ann Clin Biochem. 1990;27:38–43. doi: 10.1177/000456329002700108. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Clayton H, Heagerty AM, Nicholson MJ, Johnson B, Mason J, Manson K, James RF, Bell PR. Dietary fat in relation to fatty acid composition of red cells and adipose tissue in colorectal cancer. Br J Cancer. 1988;58:575–579. doi: 10.1038/bjc.1988.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendingen K, Høstmark AT, Fausa O, Mosdøl A, Aabakken L, Vatn MH. Familial adenomatous polyposis patients have high levels of arachidonic acid and docosahexaenoic acid and low levels of linoleic acid and alpha-linolenic acid in serum phospholipids. Int J Cancer. 2006;120:632–637. doi: 10.1002/ijc.22337. [DOI] [PubMed] [Google Scholar]
- Fernández-Bañares F, Esteve M, Navarro E, Cabré E, Boix J, Abad-Lacruz A, Klaassen J, Planas R, Humbert P, Pastor C, Gassull MA. Changes of the mucosal n3 and n6 fatty acid status occur early in the colorectal adenoma-carcinoma sequence. Gut. 1996;38:254–259. doi: 10.1136/gut.38.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen E, Bartsch H, Béréziat JC, Camus AM, McClinton S, Eremin O, Davidson L, Boyle P. Diet and oxidative stress in breast, colon and prostate cancer patients: a case-control study. Eur J Clin Nutr. 1994;48:575–586. [PubMed] [Google Scholar]
- Berry EM, Zimmerman J, Peser M, Ligumsky M. Dietary fat, adipose tissue composition, and the development of carcinoma of the colon. J Natl Canc Inst. 1986;77:93–97. [PubMed] [Google Scholar]
- Hakim IA, Harris RB, Ritenbaugh C. Fat intake and risk of squamous cell carcinoma of the skin. Nutr Canc. 2000;36:155–162. doi: 10.1207/S15327914NC3602_3. [DOI] [PubMed] [Google Scholar]
- Harris RB, Foote JA, Hakim IA, Bronson DL, Alberts DS. Fatty acid composition of red blood cell membranes and risk of squamous cell carcinoma of the skin. Canc Epidemiol Biomarkers Prev. 2005;14:906–912. doi: 10.1158/1055-9965.EPI-04-0670. [DOI] [PubMed] [Google Scholar]
- Mackie BS, Mackie LE, Curtin LD, Bourne DJ. Melanoma and dietary lipids. Nutr Canc. 1987;9:219–226. doi: 10.1080/01635588709513930. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson SE, Speizer FE, Rosner B, Willett WC. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA. 1999;281:914–920. doi: 10.1001/jama.281.10.914. [DOI] [PubMed] [Google Scholar]
- Voorrips LE, Brants HA, Kardinaal AF, Hiddink GJ, van den Brandt PA, Goldbohm RA. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: the Netherlands Cohort Study on Diet and Cancer. Am J Clin Nutr. 2002;76:873–882. doi: 10.1093/ajcn/76.4.873. [DOI] [PubMed] [Google Scholar]
- Pala V, Krogh V, Muti P, Chajès V, Riboli E, Micheli A, Saadatian M, Sieri S, Berrino F. Erythrocyte membrane fatty acids and subsequent breast cancer: a prospective Italian study. J Natl Canc Inst. 2001;93:1088–1095. doi: 10.1093/jnci/93.14.1088. [DOI] [PubMed] [Google Scholar]
- Chajès V, Hultén K, Van Kappel AL, Winkvist A, Kaaks R, Hallmans G, Lenner P, Riboli E. Fatty-acid composition in serum phospholipids and risk of breast cancer: an incident case-control study in Sweden. Int J Cancer. 1999;83:585–590. doi: 10.1002/(SICI)1097-0215(19991126)83:5<585::AID-IJC2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nkondjock A, Shatenstein B, Ghadirian P. A case-control study of breast cancer and dietary intake of individual fatty acids and antioxidants in Montreal, Canada. Breast. 2003;12:131–138. doi: 10.1016/s0960-9776(02)00284-9. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Bjerve KS, Andersen A, Jellum E. Polyunsaturated fatty acids in serum phospholipids and risk of breast cancer: a case-control study from the Janus serum bank in Norway. Eur J Cancer. 1993;29A:532–538. doi: 10.1016/s0959-8049(05)80146-7. [DOI] [PubMed] [Google Scholar]
- London SJ, Sacks FM, Stampfer MJ, Henderson IC, Maclure M, Tomita A, Wood WC, Remine S, Robert NJ, Dmochowski JR, Willett WC. Fatty acid composition of the subcutaneous adipose tissue and risk of proliferative benign breast disease and breast cancer. J Natl Canc Inst. 1993;85:785–793. doi: 10.1093/jnci/85.10.785. [DOI] [PubMed] [Google Scholar]
- Zhu ZR, Ågren J, Männistö S, Pietinen P, Eskelinen M, Syrjänen K, Uusitupa M. Fatty acid composition of breast adipose tissue in breast cancer patients and in patients with benign breast disease. Nutr Canc. 1995;24:151–160. doi: 10.1080/01635589509514403. [DOI] [PubMed] [Google Scholar]
- Aro A, Männistö S, Salminen I, Ovaskainen ML, Kataja V, Uusitupa M. Nutr Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Cancer. 2000;38:151–157. doi: 10.1207/S15327914NC382_2. [DOI] [PubMed] [Google Scholar]
- Zaridze DG, Chevchenko VE, Levtshuk AA, Lifanova YE, Maximovitch DM. Fatty acid composition of phospholipids in erythrocyte membranes and risk of breast cancer. Int J Cancer. 1990;45:807–810. doi: 10.1002/ijc.2910450502. [DOI] [PubMed] [Google Scholar]
- Bagga D, Anders KH, Wang HJ, Glaspy JA. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Canc. 2002;42:180–185. doi: 10.1207/S15327914NC422_5. [DOI] [PubMed] [Google Scholar]
- Maillard V, Bougnoux P, Ferrari P, Jourdan ML, Pinault M, Lavillonnière F, Body G, Le Floch O, Chajès V. N-3 and n-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int J Cancer. 2002;98:78–83. doi: 10.1002/ijc.10130. [DOI] [PubMed] [Google Scholar]
- Petrek JA, Hudgins LC, Levine B, Ho M, Hirsch J. Breast cancer risk and fatty acids in the breast and abdominal adipose tissues. J Natl Canc Inst. 1994;86:53–56. doi: 10.1093/jnci/86.1.53. [DOI] [PubMed] [Google Scholar]
- Williams CM, Maunder K. Fatty acid compositions of inositol and choline phospholipids of breast tumours and normal breast tissue. Eur J Clin Nutr. 1993;47:260–267. [PubMed] [Google Scholar]
- Punnonen K, Hietanen E, Auvinen O, Punnonen R. Phospholipids and fatty acids in breast cancer tissue. J Cancer Res Clin Oncol. 1989;115:575–578. doi: 10.1007/BF00391361. [DOI] [PubMed] [Google Scholar]
- Eid A, Berry EM. The relationship between dietary fat, adipose tissue composition, and neoplasms of the breast. Nutr Canc. 1988;11:173–177. doi: 10.1080/01635588809513984. [DOI] [PubMed] [Google Scholar]
- Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- Männistö S, Pietinen P, Virtanen MJ, Salminen I, Albanes D, Giovannucci E, Virtamo J. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Canc Epidemiol Biomarkers Prev. 2003;12:1422–1428. [PubMed] [Google Scholar]
- Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–1027. doi: 10.1002/(SICI)1097-0142(19990915)86:6<1019::AID-CNCR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Crowe FL, Allen NE, Appleby PN, Overvad K, Aardestrup IV, Johnsen NF, Tjønneland A, Linseisen J, Kaaks R, Boeing H, Kröger J, Trichopoulou A, Zavitsanou A, Trichopoulos D, Sacerdote C, Palli D, Tumino R, Agnoli C, Kiemeney LA, Bueno-de-Mesquita HB, Chirlaque MD, Ardanaz E, Larrañaga N, Quirós JR, Sánchez MJ, González CA, Stattin P, Hallmans G, Bingham S, Khaw KT, Rinaldi S, Slimani N, Jenab M, Riboli E, Key TJ. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1353–1363. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J. A prospective study of polyunsatutated fatty acid levels in blood and prostate cancer risk. Canc Epidemiol Biomarkers Prev. 2007;16:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–551. doi: 10.1002/(SICI)1097-0215(19970516)71:4<545::AID-IJC7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Canc Inst. 1994;86:281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- Hodge AM, English DR, McCredie MR, Severi G, Boyle P, Hopper JL, Giles GG. Foods, nutrients and prostate cancer. Canc Causes Contr. 2004;15:11–20. doi: 10.1023/B:CACO.0000016568.25127.10. [DOI] [PubMed] [Google Scholar]
- Ukoli FA, Fowke JH, Akumabor P, Oguike T, Taher KA, Murff HJ, Amaefuna ER, Kittles R, Ahaghotu C, Osime U, Beech DJ. The association of plasma fatty acids with prostate cancer risk in African Americans and Africans. J Health Care Poor Underserved. 2010;21(Suppl 1):127–147. doi: 10.1353/hpu.0.0242. [DOI] [PubMed] [Google Scholar]
- Ukoli FA, Akumabor PN, Oguike TC, Dent LL, Beech D, Osime U. The association of plasma fatty acids with prostate cancer risk in Nigerians. Ethn Dis. 2009;19:454–461. [PubMed] [Google Scholar]
- Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–268. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Lee SH, Hong SJ, Chung BC. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem. 1999;32:405–409. doi: 10.1016/S0009-9120(99)00036-3. [DOI] [PubMed] [Google Scholar]
- Faas FH, Dang AQ, White J, Schaefer RF, Johnson DE. Decreased prostatic arachidonic acid in human prostatic carcinoma. BJU Int. 2003;92:551–554. doi: 10.1046/j.1464-410X.2003.04387.x. [DOI] [PubMed] [Google Scholar]
- Chaudry A, McClinton S, Moffat LE, Wahle KW. Essential fatty acid distribution in the plasma and tissue phospholipids of patients with benign and malignant prostatic disease. Br J Cancer. 1991;64:1157–1160. doi: 10.1038/bjc.1991.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamalakis G, Kafatos A, Kalogeropoulos N, Andrikopoulos N, Daskalopulos G, Kranidis A. Prostate cancer vs hyperplasia: relationships with prostatic and adipose tissue fatty acid composition. Prostag Leukot Essent Fatty Acids. 2002;66:467–477. doi: 10.1054/plef.2002.0384. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Sasaki S, Kawabata T, Hasegawa K, Tsugane S. JPHC. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess fatty acid intake: comparison with dietary records and serum phospholipid level. J Epidemiol. 2003;13(Suppl 1):S64–S81. doi: 10.2188/jea.13.1sup_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmadfa I, Kornsteiner M. Fats and Fatty Acid Requirements for Adults. Ann Nutr Metab. 2009;55:56–75. doi: 10.1159/000228996. [DOI] [PubMed] [Google Scholar]
- Kawabata T, Hirota S, Hirayama T, Adachi N, Hagiwara C, Iwama N, Kamachi K, Araki E, Kawashima H, Kiso Y. Age-related changes of dietary intake and blood eicosapentaenoic acid, docosahexaenoic acid, and arachidonic acid levels in Japanese men and women. Prostag Leukot Essent Fatty Acids. 2011;84:131–137. doi: 10.1016/j.plefa.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Arab L. Biomarkers of Fat and Fatty Acid Intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- Kakutani S, Ishikura Y, Tateishi N, Horikawa C, Tokuda H, Kontani M, Kawashima H, Sakakibara Y, Kiso Y, Shibata H, Morita I. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids Health Dis. 2011;10:241. doi: 10.1186/1476-511X-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hao H, He S, Cai L, Li Y, Hu S, Ye D, Hoidal J, Wu P, Chen X. Lipoxin A4 and Its Analogue Suppress the Tumor Growth of Transplanted H22 in Mice: The Role of Antiangiogenesis. Mol Cancer Ther. 2011;9:2164–2174. doi: 10.1158/1535-7163.MCT-10-0173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PubMed search terms and strategies.