Abstract
Agonists activating β2-adrenoceptors (β2ARs) on airway smooth muscle (ASM) are the drug of choice for rescue from acute bronchoconstriction in patients with both asthma and chronic obstructive pulmonary disease (COPD). Moreover, the use of long-acting β-agonists combined with inhaled corticosteroids constitutes an important maintenance therapy for these diseases. β-Agonists are effective bronchodilators due primarily to their ability to antagonize ASM contraction. The presumed cellular mechanism of action involves the generation of intracellular cAMP, which in turn can activate the effector molecules cAMP-dependent protein kinase (PKA) and Epac. Other agents such as prostaglandin E2 and phosphodiesterase inhibitors that also increase intracellular cAMP levels in ASM, can also antagonize ASM contraction, and inhibit other ASM functions including proliferation and migration. Therefore, β2ARs and cAMP are key players in combating the pathophysiology of airway narrowing and remodeling. However, limitations of β-agonist therapy due to drug tachyphylaxis related to β2AR desensitization, and recent findings regarding the manner in which β2ARs and cAMP signal, have raised new and interesting questions about these well-studied molecules. In this review we discuss current concepts regarding β2ARs and cAMP in the regulation of ASM cell functions and their therapeutic roles in asthma and COPD.
Keywords: asthma, airway smooth muscle, β-adrenoceptor, cAMP, COPD, protein kinase A, Epac
INTRODUCTION
3′-5′-Cyclic adenosine monophosphate (cAMP) is the archetypal second messenger whose specific biochemical and signaling properties in numerous cell types have been intensively researched for more than half a century. The resulting wealth of data clearly highlights the importance of cAMP in diverse physiological and pathophysiological processes via its modulation of diverse cellular events. cAMP plays a key role in the functions of many airway cells including controlling ciliary beat frequency (critical for mucus clearance) in airway epithelial cells [1] and suppressing the pro-inflammatory activity of various immune and inflammatory cells. However it is arguably the inhibitory effect of increased cytosolic cAMP levels on the contraction of airway smooth muscle (ASM) which constitutes the most profound cAMP-mediated effect in the lung.
As discussed in greater detail below, cAMP-mediated pathways are most commonly initiated following the binding of specific ligands to G protein-coupled receptors (GPCRs) of the Gs family. Perhaps the most exhaustively studied GPCR is the β2-adrenoceptor (β2AR) and it is specifically the β2ARs on ASM cells which, due to their ability to rapidly promote bronchorelaxation, constitute the frontline target for asthma therapy. In addition to driving this clinically critical effect, cAMP also modulates many other aspects of ASM function including proliferation, migration, secretion of inflammatory mediators, and deposition of extracellular matrix (ECM). Beyond these acute effects, cAMP also appears to influence the “phenotype” of ASM cells; i.e., properties relating to their capacity to function as a contractile cell or one more capable of proliferation and secretory/immune-modulatory functions.
Whilst the molecular events occurring between β2AR activation and bronchorelaxation were thought to be well understood, several recent studies have introduced layers of complexity which have the potential to profoundly alter our understanding of the β2AR signaling pathway and how asthma is treated clinically.
In this review we aim to explore some of the myriad ways in which cAMP regulates ASM function with an emphasis placed on novel or controversial observations. A brief overview of cAMP regulation will be given encompassing modulation via GPCRs, phosphodiesterases (PDEs), and two direct downstream effectors of cAMP namely protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac). We shall then consider the ways in which diverse ASM functions are mediated via cAMP and, where possible, address whether these effects are PKA- or Epac-driven. Finally we will consider the therapeutic implications both in terms of future targets and problems with current therapy and ask “What Next?”
1. ROLE OF GS-COUPLED RECEPTORS AND PDES IN cAMP ELEVATION
cAMP generation in most cells typically occurs through a classical GPCR transmembrane signaling paradigm. A specific subfamily of GPCRs couple to Gα subunits of the Gs subfamily of heterotrimeric G proteins, which in turn activate the enzyme adenylyl cyclase which hydrolyzes ATP to cAMP [2]. In ASM, numerous Gs-coupled receptors have been identified, including the EP2 and EP4 prostanoid (PGE2) (discussed in detail below), the A2b adenosine, and the IP (PGI2) receptors [2]. However, the most well-recognized and studied is the β2AR, and (inhaled) agonists of the β2AR (β-agonists) are the drug of choice for relief from acute asthmatic attacks, based on their ability to prevent and reverse ASM contraction (bronchoconstriction). Moreover, long-acting β-agonists (LABAs) are now widely used in maintenance therapy for asthma as well as COPD [3]. Although LABA monotherapy is available for treatment of COPD, a combination of LABAs with inhaled corticosteroids (ICS) is recommended for the regular use of LABA in controlling asthma. Despite concerns (discussed below) regarding the loss of therapeutic benefit with prolonged use, and possible mortality effects, β-agonists along with corticosteroids remain at the cornerstone of asthma therapy.
Subsequent to the Gs-coupled receptor-induced accumulation of cAMP, the principal process by which intracellular cAMP levels are reduced is via their hydrolysis – a function performed by a subset of members of the PDE family. The cAMP-elevating role of PDE inhibitors has been exploited for centuries in the treatment of asthma and COPD therapy and, despite possessing a narrow therapeutic window, PDE inhibitors remain in clinical use and a subject of intense interest today [4-6]. PDEs which preferentially hydrolyze the intracellular cyclic nucleotides, cAMP or cGMP, are grouped into 11 families [7]. The presence of PDE1-5 classes has been found in human ASM cells [8, 9]. Among those classes, PDE3 and PDE4 are the two major cAMP-hydrolyzing enzymes [8] and inhibitors of PDE3 and PDE4 cause relaxation of human ASM [9]. Rabe et al. demonstrated that the selective PDE3 inhibitor SKF94120 was more potent in inhibiting contraction of human bronchi than a selective PDE4 inhibitor rolipram [9]. However, the PDE4D subfamily, particularly the PDE4D5 isoform, appears to play a pivotal role in controlling cAMP degradation in human ASM cells [10]. Recently, Trian and colleagues reported that human ASM cells from asthmatics exhibit increased PDE4D expression which is associated with impaired β2AR-stimulated cAMP production [11]. This work invites consideration as to how the effect of current therapies could be suboptimal when administered against a background of upregulated PDE activity.
2. cAMP EFFECTORS: PKA AND EPAC
Historically, cAMP has been believed to mediate its action on ASM contractile state via activation of the effector PKA. The phosphorylation of numerous targets in ASM by PKA (shown in the context of β2AR activation in Fig. 1) has been proposed to cause either reduced intracellular Ca2+ concentrations ([Ca2+]i) or reduced Ca2+ sensitivity in ASM [12-15] with both effects leading to impaired ability to promote myosin light chain (MLC) phosphorylation which enables ASM to contract. In addition, heat shock protein 20 (HSP20) has recently been identified as another potentially important PKA target capable of inhibiting ASM contraction via an ill-defined mechanism that appears independent of MLC regulation [16, 17].
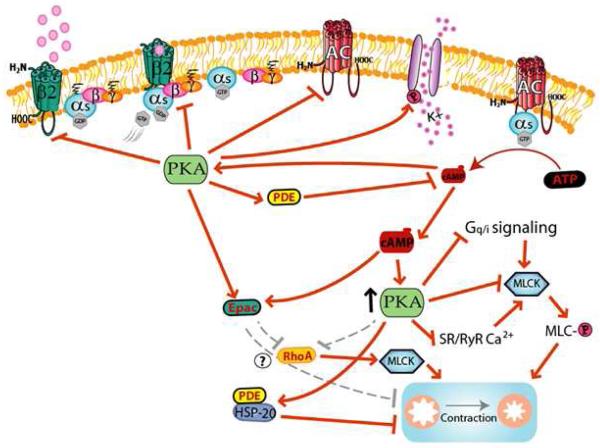
Figure 1. Proposed model of signaling by the β2 AR, cAMP, PKA, and Epac involved in regulation of airway smooth muscle contraction.
Signaling events elicited by agonist activation of the β2AR in ASM promote numerous regulatory effects that inhibit pro-contractile signaling in ASM, as well as events that feedback inhibits β2AR signaling. See text for details.
It is important to note however that although for years now PKA has been assumed to be the critical effector through which cAMP causes ASM relaxation, the role of PKA has never been directly demonstrated, in part due to difficulties in demonstrating that inhibition of PKA inhibits the proposed mechanisms and reverses the effect of cAMP on ASM contraction. Yan et al. demonstrated that inhibitory effects of β-agonists and PGE2 on cell growth of human ASM cells are PKA-dependent using an effective molecular strategy for inhibiting PKA [18]. Using the same approach, Goncharova et al. showed that PKA was required for the inhibition of human ASM cell migration by albuterol a short-acting β2-agonist [19]. To date, no studies have provided direct evidence of the role of PKA in mediating the relaxant effect of β-agonists or other cAMP-generation agents on ASM. Indeed, Spicuzza et al. provided intriguing evidence to the contrary, and suggested a cAMP-dependent yet PKA-independent mechanism by which β-agonists antagonize ASM contraction [20]. Of note, cAMP can cross-activate cGMP-dependent protein kinase (PKG) in smooth muscle cells [21] and Torphy et al. demonstrated that cAMP activates both PKG and PKA in canine trachealis [22]. However, the capacity of cAMP-mediated PKG activation to promote bronchodilatation is unclear, given cGMP-mobilizing agents such as nitroglycerin and zaprinast appear to be much less potent in inhibiting ASM contraction than β-agonists [8, 9, 23].
Recently, an alternative mechanism has emerged that challenges the dogma of PKA as the important cAMP effector in ASM. In 1998, Epac, a Rap1 guanine nucleotide exchange factors (GEF), were found as another downstream effector of cAMP action [24]. Epac that can be activated by either PKA or by cAMP in a PKA-independent manner, has been proposed as sufficient to cause ASM relaxation and to inhibit cell proliferation in the absence of PKA activation [25-32]. In an h-TERT ASM cell line, expression of both Epac isoforms, Epac1 and Epac2, was observed, and Epac1 and Epac2 exhibited different cellular localization but cooperated each other [33]. The main Epac effector is GTPase Rap1 and proposed downstream pathways involve mitogen-activated protein kinases, transcriptional factors, and integrin signaling [25, 26, 33]. Roscioni et al. recently demonstrated that Epac activation using an Epac-selective cAMP analog decreased contraction, MLC phosphorylation, and RhoA activities in response to methacholine in human and guinea pig tracheal smooth muscle tissues [31]. Similarly, Zieba et al. demonstrated the sufficiency of an Epac-selective cAMP analogue to relax permeabilized mouse bronchi [32]. Collectively, these studies suggest our long-held assumptions regarding β2AR and cAMP effector mechanisms in ASM need to be revisited, and set the stage for future work defining the relative importance of PKA versus Epac in mediating the functional effects of β-agonist and cAMP in ASM.
3. MECHANISMS OF β2AR-MEDIATED ASM RELAXATION
The stimulation of ASM with β-agonists has been shown to inhibit many signaling events that are activated by contractile stimuli to promote ASM contraction. Although the majority of these pro-contractile signaling events ultimately converge at and regulate the phosphorylation status of MLC20, the molecules involved are diverse with the main contributors being Gq-coupled receptors, phospholipase C (PLC), plasma membrane and sarcoplasmic reticulum (SR) ion channels and pumps, RhoA, Ca2+-calmodulin and MLC kinase (MLCK). As each of these effectors is inextricably linked to aspects of Ca2+ signaling in ASM cells, we will discuss them in terms of the different stages of intracellular Ca2+ signaling in which they are pertinent.
3.1. Pro-contractile signaling
Although roles for specific Gi-coupled receptors have recently been implicated in cooperative crosstalk with Gq-coupled receptors [34, 35], it is ligands binding to GPCRs of the Gq-subfamily which are frequently the initiators of contraction in ASM with the most widely recognized contractile Gq-coupled receptors being the M3 muscarinic acetylcholine, histamine H1, bradykinin B2, cysteinyl leukotriene 1, and endothelin-A/B receptors [2]. Following the agonist binding, a conformational change in the receptor occurs which enables coupling to and activation of Gαq, subsequent activation of PLC and production of inositol 1,4,5-trisphosphate (IP3), which binds to IP3 receptors on specialized intracellular stores to promote Ca2+ release from these stores. This flux combines with Ca2+ release from ryanodine-sensitive stores and influx through plasma membrane Ca2+ channels to elevate [Ca2+]i to levels that stimulate MLCK, leading to MLC20 phosphorylation, myosin ATPase activity, cross bridge cycling and sarcomere shortening (reviewed in [36, 37]). Moreover, Ca2+ “sensitization” mechanisms are also invoked, via RhoA/Rho-kinase, that augment the effect of Ca2+ via regulation of MLC20 phosphorylation/dephosphorylation dynamics. Both the mechanisms of Ca2+ mobilization and Ca2+ sensitization can be regulated by β-agonists/cAMP.
3.2. Inhibition of Gq-coupled receptor transmembrane signaling
PKA can regulate Gq-dependent contractile signaling at multiple junctures. PKA-mediated phosphorylation and inhibition of Gq-coupled receptors, Gαq, or PLC resulting in reductions in agonist-induced phosphoinositide (PI) hydrolysis and Ca2+ mobilization has been demonstrated in numerous cell systems. In ASM, β-agonists and other cAMP-generating agents have been shown to inhibit both PI hydrolysis and Ca2+ mobilization, yet mechanistic detail and a demonstrated role of PKA or Epac are lacking. Both methacholine and histamine-induced Ca2+ mobilization has been observed to be inhibited following exposure to isoproterenol in bovine tracheal smooth muscle [38], whilst in guinea-pig tracheal smooth muscle tissue isoproterenol, forskolin and db-cAMP have been demonstrated to inhibit methacholine-induced contraction via both Ca2+ mobilization and Ca2+ sensitization [15]. When PI production is specifically assessed, different routes of Gαq-mediated stimulation impart different outcomes with histamine- but not methacholine-induced PI production inhibited by elevators of cAMP [38, 39]. Conversely, in human cultured ASM cells, a small increase in histamine-induced PI hydrolysis was reported following exposure to salmeterol potentially reflecting the somewhat modified ASM phenotype observed following repeated cell culture [40].
Recently, another novel mechanism by which the β2AR inhibits human ASM contraction mediated by Gq-coupled receptors was proposed by Holden et al [41]. They found that the expression of regulator G-protein signaling 2 (RGS2) which is a member of GTPase-activating proteins (GAPs) and specific for Gq is transcriptionally upregulated by LABAs in human ASM cells. Moreover, RGS2 expression was associated with a decrease of intracellular Ca2+ concentrations elicited by Gq-agonists such as histamine and methacholine [41]. These findings demonstrated the involvement of RGS2 in the β2AR–mediated ASM relaxation of Gq–mediated signaling. Importantly, RGS2 expression was enhanced by LABA treatment, and further enhanced by combined (LABA plus corticosteroid) treatment. These data suggest an important mechanism underlying the efficacy of ICS/LABA combination therapy as a long-term management of asthma and COPD [3].
3.3. Inhibition of Ca2+ influx channels by β-agonist and cAMP
It is known that cAMP modulates regulation of intracellular Ca2+ homeostasis, one of the principal drivers of ASM responses. The ASM cell has multiple Ca2+ influx pathways and channels, including voltage-dependent Ca2+ channels, receptor-operated Ca2+ entry (ROC), store-operated Ca2+ entry (SOC), stretch-activated Ca2+ entry, and the reverse mode of Na+/Ca2+ exchanger [14, 42-47]. Blockade of voltage-dependent Ca2+ channels has failed to improve asthma symptoms and inhibit ASM contraction induced by contractile agonists and inflammatory mediators [44, 46, 48]. Therefore, it is now considered that the major Ca2+ influx pathways in ASM cells are ROC, possibly TRPC family channels, and SOC which is mediated by the SR Ca2+ sensor STIM1 and plasma-membrane Ca2+ channel Orai1 [44, 45, 47, 49].
Ay et al. have shown that cAMP modulates these Ca2+ entry pathways in ASM cells [50]. For example, induction of SOC by store depletion with sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) antagonist, cyclopiazonic acid in the presence of β-agonist or cAMP synthetic analogue confirms inhibition of Ni2+- and La3+- sensitive Ca2+ channels that constitute SOC. This inhibition was attenuated by a pharmacological PKA inhibitor, suggesting a cAMP-PKA mediated effect. Interestingly, the effects mediated by cAMP also appear to activate protein kinase G to indirectly inhibit SOC [50].
3.4. Regulation of Ca2+ oscillations by β-agonist and cAMP
Intracellular Ca2+ homeostasis involves ion channels and transporters and some of these mediate Ca2+ influx or efflux [14, 44, 51]. These events are important in the regulation of ASM contraction and occur in a dynamic process that oscillates between Ca2+ entry/release and Ca2+ removal in the ASM cell. Bai and Sanderson demonstrated that cAMP-elevating agents forskolin, isoproterenol, and 8-bromo-cAMP decreased contractility, frequency of Ca2+ oscillations and methacholine-induced Ca2+ release from the SR in murine lung slices [52]. In addition, they observed diminished sensitivity of the IP3 receptor to IP3, and this was reversed using caged-IP3 [52]. This outcome fits with the proposed structure-function of the IP3 receptor, as the IP3 receptor has at least two PKA binding sites in the modulatory domain exposed to the cytosolic side [53].
Further exploratory studies to elucidate the effects of cAMP on Ca2+ oscillations have also shown that oscillation frequency induced by BAYK8644 via voltage-gated Ca2+ channel activation was attenuated by β-agonist in porcine ASM cells [54]. Whilst these studies continue to shed more light on cAMP regulatory effects, similar studies by Janssen et al. using bovine and porcine bronchial smooth muscle found instead that cAMP- or isoproterenol-mediated relaxation remained unaffected in the presence of L-type Ca2+ channel blocker, nifedipine [55].
Other studies suggest the effects of β-agonists or cAMP raising agents on Ca2+ oscillations and relaxation are in fact occurring through cAMP-dependent activation of large-conductance Ca2+-activated K+ (BKCa) channels. These studies build on original observations from the Kotlikoff lab noting the ability of β-agonist, cAMP, and PKA to regulate gating of BKCa channels in ASM [56, 57]. In a recent paper by Zhou and colleagues, BKCa currents could be stimulated by PKA provided serine site (S695) in the regulator of K+ conductance (RCK) domains had not been phosphorylated by protein kinase C [58]. A current kinetics study by Ishikawa and colleagues revealed low dose isoproterenol and forskolin both mediate a modest Ca2+ inward current, however a synthetic cAMP analogue elicited the opposite effect [59]. This finding implies adenylyl cyclase-dependent cAMP synthesis may regulate Ca2+ homeostasis at low doses through small Ca2+ inward currents via L-type Ca2+ channels, but at high doses attenuates the amplitude of inward Ca2+ currents. However, since voltage-dependent Ca2+ channel inhibitors are not effective for asthma therapy or bronchodilation, the contribution of BKCa and L-type Ca2+ channels to β2AR-mediated ASM relaxation is likely minimal.
3.5. Modulation of Ca2+ removal system by β-agonist and cAMP
Ca2+ removal from the cytosol constitutes a major event during Ca2+ homeostasis. Recent findings indicate cAMP raising agents or β-agonist also modulate Ca2+ removal. The SERCA transporter sequesters Ca2+ back into SR stores after Ca2+ release, and this event is predominantly responsible for the removal of intracellular Ca2+. cAMP-elevating including β-agonists indirectly modulate Ca2+ homeostasis through phospholamban, which inhibits SERCA activity despite phospholamban expression being relatively low in ASM cells are [60]. Notably, a previous work reported ASM cells from patients with asthma to already have decreased levels of SERCA2, the predominant isoform in ASM cells [61]. Unpublished reports by Ojo et al. suggest that chronic stimulation of ASM cells with short and long acting β-agonists reduces SERCA2 protein expression [62]. Similar effects of LABAs have also been reported on SERCA2 expression in cardiac myocytes [63]. Collectively, these studies suggest that SERCA may play an important role in ASM Ca2+ homeostasis, and SERCA expression or activity may be regulated by β-agonist therapy.
3.6. Reduced Ca2+ sensitivity by β-agonist and cAMP
ASM contraction is also regulated by modulation of Ca2+ sensitivity or Ca2+-independent mechanisms [64]. Contraction of ASM occurs in a phasic manner followed by a tonic contractile response. The phasic response is dependent on Ca2+ release from the SR, and the tonic or sustained response relies on Ca2+ entry as well as Ca2+ sensitivity via inactivation of MLC phosphatase (MLCP) by RhoA/Rho-kinase [48, 65-68]. Several studies have shown that cAMP-elevating agents reduce ASM Ca2+ sensitivity. Oguma et al. simultaneously measured intracellular Ca2+ concentrations and isometric tension using guinea pig tracheal smooth muscle tissue strips and showed that relaxation induced by isoproterenol or forskolin was indeed not fully Ca2+ dependent [15]. This observation is in agreement with similar work by Janssen and colleagues, showing isoproterenol-induced MLCP activity [55]. Although MLCP activity was not investigated by Ise et al., the differential response of theophylline further supports the regulation of Ca2+ sensitivity by cAMP-elevating agents. Using porcine ASM, they showed an attenuated tension but minimal change to intracellular Ca2+ transient for high KCl evoked contractions. Yet in carbachol treated ASM strips both tension and Ca2+ transients were attenuated in the absence of extracellular Ca2+ and store release [69]. Janssen et al. indicated these effects only applied to non-human tissue and were not reproducible in human ASM cells [55]. However, investigations using α-toxin permeabilized rabbit and porcine tracheal smooth muscle tissue strips showing attenuation of Ca2+-induced contractions in the presence of theophylline and/or cAMP analogue further supports a role for cAMP or PKA in Ca2+ sensitivity [12, 69]. Although MLCP phosphorylation by Rho-kinase is the main mechanism of Ca2+ sensitivity in ASM contraction, whether the RhoA/Rho-kinase inactivation is involved in the mechanisms of reduced Ca2+ sensitivity by cAMP is controversial [12, 15, 31].
Protein kinase C (PKC)-dependent phosphorylation of the 17-kD myosin phosphatase inhibitor protein (CPI-17) [70] is another pathway reported to lead to increased Ca2+ sensitivity in ASM contraction [71]. Morin et al. demonstrated that siRNA-induced knockdown of CPI-17 resulted in an inhibition of contraction of human bronchial smooth muscle by decreasing Ca2+ sensitivity [71].
Another possible mechanism of reduced sensitivity to Ca2+ by β-agonists is myosin-independent effects on actin polymerization. Actin dynamics are essential for maintaining contractile force of smooth muscle. Previous studies have implicated the cAMP-PKA pathway in inhibiting the actin cytoskeleton in ASM cells [17, 72]. Komalavias et al. showed that PKA-induced HSP20 phosphorylation decreased phosphorylation of cofilin and disrupted actin polymerization, leading to ASM relaxation [17].
4. PGE2-MEDIATED MODULATION OF ASM FUNCTION
Prostaglandins are mediators derived from arachidonic acid by cyclooxygenase activation [73] with PGE2 being the most abundant and, arguably the most physiologically relevant in the lung [74, 75]. In ASM cells PGE2-mediates anti-proliferative, anti-migratory and pro-relaxant effects mainly via cAMP-dependent pathways in a similar fashion to other cAMP-increasing agents [18, 26, 27, 76-85].
The biological effects of PGE2 are mediated via four distinct prostanoid EP receptor subtypes, EP1, EP2, EP3, and EP4 receptors, from the GPCR superfamily [73, 86]. Recent studies have explored the concept of EP receptor targeting as a means of improving the therapeutic potential of E prostanoids and the expression of mRNA for EP2, EP3, and EP4 receptors has been identified in human ASM cells [80, 82, 87]. Burgess et al. demonstrated that ASM cells isolated from patients with asthma had more expression of EP3 and EP2 receptors than those from control subjects [82]. Interestingly, the anti-mitogenic effects of PGE2 were enhanced in patients with asthma compared with control subjects [82].
There are no previous reports demonstrating functional expression of EP1 receptor in human ASM cells [18, 80, 88]. Indeed, either EP1 receptor agonist SC19220 or ONO-DI-004, did not affect proliferation of human ASM cells [18, 80]. In contrast, EP1 receptor activation increases ASM tone in guinea pigs [89] and reduces the broncho-dilatory function of β-agonists receptor in mouse ASM cells [90]. Thus the role of the EP1 receptor in regulating ASM cell functions appear to be species-specific [87, 89-92].
Stimulation of Gs-coupled EP2 and EP4 receptors increases intracellular cAMP levels via adenylyl cyclase activation, whereas EP3 receptor inhibits adenylyl cyclase [86]. The EP2 receptor has been reported as the functionally dominant EP receptor isoform in the mechanisms of relaxation by PGE2 in human ASM [91]. However, a role for the EP4 receptor in ASM relaxation has also been reported. Data from Buckley et al. suggest a component of PGE2-mediated relaxation of human and rat airways is mediated by the EP4 receptor, while the EP2 receptor-selective agonists AH13205 failed to relax contracted human ASM tissue [93]. In addition, Mori et al. using subtype-selective agonists/antagonists suggested that both EP2- and EP4- receptors appear important in mediating the anti-proliferative effects of PGE2 on fetal bovine serum (FBS)-induced human ASM cell growth [80]. Whilst EP3-mediated signals were observed to induce increased [Ca2+]i concentrations, the specific EP3 receptor agonist ONO-AE-248 did not affect ASM cell growth. Yan et al. also reported EP2-mediated PGE2 signaling to be anti-proliferative, whereas the EP3-selective agonist sulprostone was pro-mitogenic in human ASM cells [18].
Previous findings highlight the potential therapeutically advantageous effects of EP2 and EP4 agonists on airway remodeling and add to the intriguing literature examining EP receptor subtype function in ASM [18, 88, 93-96]. Although phase 1 clinical trials previously conducted on the EP2 agonist AH13205 [97] were disappointing as this compound did not elicit bronchorelaxation and exhibited side effects including cough and airway irritation, research on prostanoid EP receptors specifically EP2 and EP4 receptors may still lead to novel therapeutic strategies for the treatment of asthma and COPD.
5. cAMP-MEDIATED MODULATION OF ASM SYNTHETIC AND MITOGENIC FUNCTIONS
5.1. Proliferation and migration
Elevators of cAMP including β-agonists, PDE inhibitors, forskolin, and PGE2 have been observed to inhibit ASM cell proliferation and migration [76, 83, 84, 98-103]. This is of particular clinical relevance, especially in chronic asthma where an increase in ASM mass is a key feature of airway remodeling [104-107]. This increase in ASM mass can be attributed to altered proliferation, migration, or both. Anti-mitogenic effects of cAMP involve multiple mechanisms, including inhibition of ERK1/2 and phosphoinositide 3′-kinase (PI3K), via PKA activation and Epac in ASM cells [27, 100, 105, 108, 109] (also see Section 2 in this review). Roscioni et al. demonstrated that activation of PKA and Epac prevents PDGF-induced ASM cell proliferation and cell cycle progression [108]. It has been shown that IL-1β and TNF-α inhibit mitogen-stimulated ASM cell proliferation although they have mitogenic effects by themselves [110, 111]. Following IL-1β and TNF-α receptor stimulation, activation of cAMP/PKA pathway occurs via endogenous cyclooxygenase activation and PGE2 production, leading to reduced ASM DNA synthesis and cell proliferation [95, 110, 112].
ASM cell migration is enhanced by the activation of specific GPCRs and receptor tyrosine kinases such as PDGF receptors that trigger remodeling of the cytoskeleton and change focal adhesion dynamics [107]. Recently, Goncharova et al. demonstrated that β-agonists and PGE2 inhibit PDGF-induced human ASM cell migration associated with vasodilator-stimulated phosphoprotein (VASP) phosphorylation via PKA activation [19]. Since VASP belongs to a conserved family of actin-regulatory proteins [113], inhibition of the actin cytoskeleton may be a mechanism by which ASM migration is inhibited by cAMP/PKA. The role and sufficiency of Epac as a cAMP effector in regulating ASM cell migration is uncertain. Glucocorticoids have been observed to enhance the inhibitory effects of β-agonists and PGE2 on human ASM cell migration [76]. Again, this in vitro finding may identify a cooperative effect underlying the utility of LABA/ICS combination therapy for asthma and COPD.
5.2. Synthesis of cytokines and chemokines
ASM cells have the ability to produce inflammatory cytokines and chemokines. Therefore, the ASM cell not only contracts but also functions as an immune-modulator in the airway. cAMP-elevating agents can modulate these synthetic properties of ASM cells [114, 115]. TNF-α-induced expression of eotaxin and RANTES is inhibited by cAMP-mobilizing agents including β-agonists, PDE inhibitors, and PGE2 [85, 116, 117]. In contrast, it has been reported that cAMP upregulates the synthesis and release of cytokines and chemokines such as IL-6, IL-8, and GM-CSF [85, 116, 117]. In IL-1β-stimulated human ASM cells, Kaur et al. demonstrated that enhanced IL-8 release evoked by IL-1β was significantly reduced by PGE2, β2-agonists and forskolin via a PKA-dependent mechanism [79]. Thus cAMP and β-agonists can have both pro- and anti-inflammatory effects on ASM cell function. Importantly, the combination of β-agonist and corticosteroid was observed to be more effective in inhibiting TNF-α-induced IL-8 release in human ASM cells although β-agonist itself increased IL-8 production [117].
5.3. cAMP-mediated ASM phenotype switching
In addition to measuring altered human ASM cell functions in vitro, much interest lies in the mechanisms of human ASM phenotype switching, i.e. identifying and quantifying human ASM cells with increased “proliferative” or “synthetic” rather than “contractile” phenotypes [118-120]. The phenotype is regulated by environmental factors such as cell-cell interactions, ECM, cytokines, growth factors and mechanical forces. It is known that smooth muscle specific proteins including smooth muscle myosin heavy chain (smMHC), SM22α, α-actin, and calponin are markers for the contractile phenotype and differentiation in ASM cells [120]. It has been shown that serum response factor (SRF) is a crucial transcriptional factor of smooth muscle cell differentiation including ASM cells [121, 122]. In vascular smooth muscle cells, PKA negatively regulates SRF and inhibits differentiation [123, 124]. Although the effects of PKA and Epac on SRF activation have yet to be studied in ASM cells, the observations by Roscioni et al. appear to contrast with those from studies of vascular smooth muscle cells [108, 123-125]. Roscioni and colleagues investigated the relative requirement for both PKA and Epac in proliferative/contractile phenotypic switching in human tracheal smooth muscle strips. Using selective activators of each, both Epac and PKA were able to prevent the phenotype altering to a PDGF-induced proliferative one. Collectively, these studies suggest a hitherto unrealised extended therapeutic benefit of asthma therapy utilising PKA- and Epac-mediated signaling.
6. THERAPEUTIC IMPLICATIONS: NOVEL TARGETS VERSUS CURRENT THERAPIES
6.1. Problematic features of β2AR-agonist/cAMP signaling
As mentioned in the Introduction, despite the reported clinical benefits of β-agonists in asthma therapy, regular use of β-agonist may in fact promote harmful effects in the longer term [77, 126]. Recent studies have drawn considerable attention to limitations of such therapy. Chronic or repeated exposure to β-agonist results in loss of its relaxing effects against ASM contraction, in at least a significant subpopulation of asthmatics [127]. Numerous studies suggest that the β2AR dysfunction is brought about by inflammatory cytokines and lipid mediators [81, 96, 128-130]. β2AR desensitization has been presumed to underlie the reduction of β-agonist therapeutic effects associated with both airway inflammation and chronic β-agonist use. One of the main mechanisms of desensitization to β-agonists is mediated by phosphorylation of β2AR. Benovic et al.found that β2AR of the hamster lung is phosphorylated by PKA [131]. In the asthmatic lung, products of inflammation such as PGE2 represent powerful agents capable of stimulating PKA activity in ASM. In cultured ASM cells, either direct application of PGE2 or induction of autocrine PGE2 following treatment with the cytokines IL-1β and TNF-α [132], results in heterologous desensitization of human β2AR signaling [96, 133] that is reversed in cells expressing the PKA inhibitory peptide PKI. Moreover, chronic treatment of murine tracheal rings with the cytokines IL-1β and TNF-α attenuates the relaxant effect of β-agonist, suggested a functional heterologous β2AR desensitization [96].
It is well recognized that the agonist-specific desensitization of many GPCRs is mediated by G protein coupled receptor kinases (GRKs) in many cell types. β2AR phosphorylation by GRKs induces receptor binding to β-arrestin, creating steric inhibition between the β2AR and Gαs [134, 135], effectively “arresting” signaling. Using HEK-293 cells transfected by mutated β2AR lacking PKA and/or GRK phosphorylation sites on ASM, Wang et al. demonstrated that isoproterenol-induced binding to β-arrestin-2 to the β2AR was regulated by GRK rather than PKA [136]. GRK inhibition using either Gβγ sequestrants or GRK2/3 siRNA has demonstrated the role of GRK2/3 in agonist-specific (homologous) desensitization of the β2AR in human ASM cells [88], whereas siRNA targeting β-arrestin1/2 augments β2AR signaling in human ASM, and genetic ablation of β-arrestin-2 increases both β2AR signaling and the ability of β-agonist to relax contracted murine ASM ex vivo and in vivo. Thus, GRK/arrestin-dependent mechanisms play a clear role in the biochemical and functional desensitization of the β2AR in ASM.
6.2. Future directions: what next?
To date, attempts to co-opt or exploit critical elements of β2AR signaling have not translated into alternative treatments for asthma, and β-agonists along with corticosteroids remain the most frequently used asthma drugs. Whether by design or coincidence, salmeterol has in cell-based studies exhibited resistance to agonist-induced β2AR desensitization, raising the possibility that the intrinsic activity of an agonist may influence mechanisms of homologous desensitization [137, 138]. However, this issue remains unclear as is the question as to whether functional tachyphylaxis is different in those who use different inhaled β-agonists, be they LABAs such as salmeterol, indacaterol, and formoterol, or short acting β-agonists such as albuterol.
However, as we continue to advance our understanding of β2AR signaling and regulation, and the mechanisms it employs to regulate ASM contraction and other pathological features of asthma, we can envision “better” β-agonists or new drugs that similarly mediate their beneficial effects. For example, β-agonists with biased signaling properties [77] that enable avoidance/minimization of β2AR desensitization, or adjuncts that target β2AR desensitization mechanisms such as GRK inhibitors. Alternatively, drugs that effect similar bronchorelaxant mechanisms as those mediated by β-agonists yet are not subject to such profound negative feedback or exhibit tachyphylaxis. Such drugs might possibly target specific EP receptor isoforms, specific PDE isoforms or their key localized activities, or include other agents capable of mediating critical PKA or Epac activities. Such alternative agents might be necessary if we are ever to improve our protection from or treatment of asthma, in order to avoid potentially deleterious effects of β2AR signaling that may be permissible for asthma to develop [139, 140] and possibly contribute to increased mortality [77, 126, 141]. Clearly however, such therapeutic advances will rely on additional basic science in the form of β2AR molecular pharmacology, cell biology and biochemistry providing insight into mechanisms mediating antagonism of ASM contraction, and complementary physiology linking such signaling to ASM function.
ACKNOWLEDGEMENTS
Sources of support for Charlotte Billington’s research are Medical Research Council UK grants G1000861 G0701390 and G0400910. Work in Raymond Penn’s lab is supported by HL58506, HL093103, and HL108071. Satoru Ito was supported by Grants-in-Aid for Scientific Research C (#22890837) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Oluwaseun O Ojo is supported by the Canadian Institute of Health Research (CIHR) Integrated and Mentored Pulmonary and Cardiovascular Training (IMPACT). The authors also like to thank Astra-Zeneca for sponsoring to attend seventh International Young Investigators’ Symposium on Smooth Muscle in Winnipeg, Canada, May 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Salathe M. Effects of β-agonists on airway epithelial cells. J Allergy Clin Immunol. 2002;110:S275–81. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- [2].Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- [3].Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol. 2008;153:1090–104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–15. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Diamant Z, Spina D. PDE4-inhibitors: a novel, targeted therapy for obstructive airways disease. Pulm Pharmacol Ther. 2011;24:353–60. doi: 10.1016/j.pupt.2010.12.011. [DOI] [PubMed] [Google Scholar]
- [6].Page C, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol. 2012 doi: 10.1016/j.coph.2012.02.016. [DOI] [PubMed] [Google Scholar]
- [7].Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–48. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- [8].Torphy TJ, Undem BJ, Cieslinski LB, Luttmann MA, Reeves ML, Hay DW. Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscle. J Pharmacol Exp Ther. 1993;265:1213–23. [PubMed] [Google Scholar]
- [9].Rabe KF, Tenor H, Dent G, Schudt C, Liebig S, Magnussen H. Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterization. Am J Physiol. 1993;264:L458–64. doi: 10.1152/ajplung.1993.264.5.L458. [DOI] [PubMed] [Google Scholar]
- [10].Billington CK, Le Jeune IR, Young KW, Hall IP. A major functional role for phosphodiesterase 4D5 in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:1–7. doi: 10.1165/rcmb.2007-0171OC. [DOI] [PubMed] [Google Scholar]
- [11].Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, et al. β2-Agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PLoS One. 2011;6:e20000. doi: 10.1371/journal.pone.0020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Endou K, Iizuka K, Yoshii A, Tsukagoshi H, Ishizuka T, Dobashi K, et al. 8-Bromo-cAMP decreases the Ca2+ sensitivity of airway smooth muscle contraction through a mechanism distinct from inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L641–8. doi: 10.1152/ajplung.00287.2003. [DOI] [PubMed] [Google Scholar]
- [13].Ito Y, Takagi K, Tomita T. Relaxant actions of isoprenaline on guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1995;116:2738–42. doi: 10.1111/j.1476-5381.1995.tb17235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca2+ homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax. 2010;65:547–52. doi: 10.1136/thx.2009.129296. [DOI] [PubMed] [Google Scholar]
- [15].Oguma T, Kume H, Ito S, Takeda N, Honjo H, Kodama I, et al. Involvement of reduced sensitivity to Ca2+ in β-adrenergic action on airway smooth muscle. Clin Exp Allergy. 2006;36:183–91. doi: 10.1111/j.1365-2222.2006.02412.x. [DOI] [PubMed] [Google Scholar]
- [16].Ba M, Singer CA, Tyagi M, Brophy C, Baker JE, Cremo C, et al. HSP20 phosphorylation and airway smooth muscle relaxation. Cell Health Cytoskelet. 2009;2009:27–42. doi: 10.2147/chc.s5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Komalavilas P, Penn RB, Flynn CR, Thresher J, Lopes LB, Furnish EJ, et al. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L69–78. doi: 10.1152/ajplung.00235.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, et al. Anti-mitogenic effects of beta-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J. 2011;25:389–97. doi: 10.1096/fj.10-164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, Panettieri RA., Jr. β2-Adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am J Respir Cell Mol Biol. 2012;46:48–54. doi: 10.1165/rcmb.2011-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spicuzza L, Belvisi MG, Birrell MA, Barnes PJ, Hele DJ, Giembycz MA. Evidence that the anti-spasmogenic effect of the β-adrenoceptor agonist, isoprenaline, on guinea-pig trachealis is not mediated by cyclic AMP-dependent protein kinase. Br J Pharmacol. 2001;133:1201–12. doi: 10.1038/sj.bjp.0704213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem. 1992;267:1015–9. [PubMed] [Google Scholar]
- [22].Torphy TJ, Freese WB, Rinard GA, Brunton LL, Mayer SE. Cyclic nucleotide-dependent protein kinases in airway smooth muscle. J Biol Chem. 1982;257:11609–16. [PubMed] [Google Scholar]
- [23].Gruetter CA, Childers CE, Bosserman MK, Lemke SM, Ball JG, Valentovic MA. Comparison of relaxation induced by glyceryl trinitrate, isosorbide dinitrate, and sodium nitroprusside in bovine airways. The American review of respiratory disease. 1989;139:1192–7. doi: 10.1164/ajrccm/139.5.1192. [DOI] [PubMed] [Google Scholar]
- [24].de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- [25].Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–75. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- [26].Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–84. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kassel KM, Wyatt TA, Panettieri RA, Jr., Toews ML. Inhibition of human airway smooth muscle cell proliferation by β2-adrenergic receptors and cAMP is PKA independent: evidence for EPAC involvement. Am J Physiol Lung Cell Mol Physiol. 2008;294:L131–8. doi: 10.1152/ajplung.00381.2007. [DOI] [PubMed] [Google Scholar]
- [28].Billington CK, Hall IP. Novel cyclic AMP signalling paradigms: therapeutic implications for airway disease. Br J Pharmacol. 2012;166:401–10. doi: 10.1111/j.1476-5381.2011.01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giembycz MA, Newton R. Beyond the dogma: novel β2-adrenoceptor signalling in the airways. Eur Respir J. 2006;27:1286–306. doi: 10.1183/09031936.06.00112605. [DOI] [PubMed] [Google Scholar]
- [30].Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roscioni SS, Maarsingh H, Elzinga CR, Schuur J, Menzen M, Halayko AJ, et al. Epac as a novel effector of airway smooth muscle relaxation. J Cell Mol Med. 2011;15:1551–63. doi: 10.1111/j.1582-4934.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, et al. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem. 2011;286:16681–92. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roscioni SS, Kistemaker LE, Menzen MH, Elzinga CR, Gosens R, Halayko AJ, et al. PKA and Epac cooperate to augment bradykinin-induced interleukin-8 release from human airway smooth muscle cells. Respir Res. 2009;10:88. doi: 10.1186/1465-9921-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA, Jr., Emala CW. Gi-coupled γ-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol. 2011;45:1232–8. doi: 10.1165/rcmb.2011-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gallos G, Gleason NR, Virag L, Zhang Y, Mizuta K, Whittington RA, et al. Endogenous γ-aminobutyric acid modulates tonic guinea pig airway tone and propofol-induced airway smooth muscle relaxation. Anesthesiology. 2009;110:748–58. doi: 10.1097/aln.0b013e31819c44e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2007;29:834–60. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal. 2006;18:2105–20. doi: 10.1016/j.cellsig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [38].Hoiting BH, Meurs H, Schuiling M, Kuipers R, Elzinga CR, Zaagsma J. Modulation of agonist-induced phosphoinositide metabolism, Ca2+ signalling and contraction of airway smooth muscle by cyclic AMP-dependent mechanisms. Br J Pharmacol. 1996;117:419–26. doi: 10.1111/j.1476-5381.1996.tb15207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Madison JM, Brown JK. Differential inhibitory effects of forskolin, isoproterenol, and dibutyryl cyclic adenosine monophosphate on phosphoinositide hydrolysis in canine tracheal smooth muscle. J Clin Invest. 1988;82:1462–5. doi: 10.1172/JCI113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith N, Browning CA, Duroudier N, Stewart C, Peel S, Swan C, et al. Salmeterol and cytokines modulate inositol-phosphate signalling in human airway smooth muscle cells via regulation at the receptor locus. Respir Res. 2007;8:68. doi: 10.1186/1465-9921-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Holden NS, Bell MJ, Rider CF, King EM, Gaunt DD, Leigh R, et al. beta2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci U S A. 2011;108:19713–8. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ito S, Kume H, Naruse K, Kondo M, Takeda N, Iwata S, et al. A novel Ca2+ influx pathway activated by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:407–13. doi: 10.1165/rcmb.2007-0259OC. [DOI] [PubMed] [Google Scholar]
- [43].Ito S, Kume H, Yamaki K, Katoh H, Honjo H, Kodama I, et al. Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by Rho-kinase in tracheal smooth muscle. Am J Respir Cell Mol Biol. 2002;26:491–8. doi: 10.1165/ajrcmb.26.4.4701. [DOI] [PubMed] [Google Scholar]
- [44].Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther. 2009;22:388–97. doi: 10.1016/j.pupt.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol. 2004;30:145–54. doi: 10.1165/rcmb.2003-0134OC. [DOI] [PubMed] [Google Scholar]
- [46].Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991;435:123–44. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–9. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ito S, Kume H, Honjo H, Katoh H, Kodama I, Yamaki K, et al. Possible involvement of Rho kinase in Ca2+ sensitization and mobilization by MCh in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1218–24. doi: 10.1152/ajplung.2001.280.6.L1218. [DOI] [PubMed] [Google Scholar]
- [49].Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ay B, Iyanoye A, Sieck GC, Prakash YS, Pabelick CM. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L278–83. doi: 10.1152/ajplung.00188.2005. [DOI] [PubMed] [Google Scholar]
- [51].Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol. 2002;282:L1161–78. doi: 10.1152/ajplung.00452.2001. [DOI] [PubMed] [Google Scholar]
- [52].Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol. 2006;291:L208–21. doi: 10.1152/ajplung.00494.2005. [DOI] [PubMed] [Google Scholar]
- [53].Yoshida Y, Imai S. Structure and function of inositol 1,4,5-trisphosphate receptor. Jpn J Pharmacol. 1997;74:125–37. doi: 10.1254/jjp.74.125. [DOI] [PubMed] [Google Scholar]
- [54].Prakash YS, van der Heijden HF, Kannan MS, Sieck GC. Effects of salbutamol on intracellular calcium oscillations in porcine airway smooth muscle. J Appl Physiol. 1997;82:1836–43. doi: 10.1152/jappl.1997.82.6.1836. [DOI] [PubMed] [Google Scholar]
- [55].Janssen LJ, Tazzeo T, Zuo J. Enhanced myosin phosphatase and Ca2+-uptake mediate adrenergic relaxation of airway smooth muscle. Am J Respir Cell Mol Biol. 2004;30:548–54. doi: 10.1165/rcmb.2003-0212OC. [DOI] [PubMed] [Google Scholar]
- [56].Wang ZW, Kotlikoff MI. Activation of KCa channels in airway smooth muscle cells by endogenous protein kinase A. Am J Physiol. 1996;271:L100–5. doi: 10.1152/ajplung.1996.271.1.L100. [DOI] [PubMed] [Google Scholar]
- [57].Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. β-Adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–9. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, et al. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci U S A. 2010;107:8005–10. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ishikawa T, Hume JR, Keef KD. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res. 1993;73:1128–37. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- [60].Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L26–34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci U S A. 2009;106:10775–80. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ojo OO, Lee TH, Ward JPT. The long acting β2 agonist formoterol and inflammatory cytokines suppress SERCA-2 expression in human airway smooth muscle. Am J Respir Crit Care Med. 2010:A5302. [Google Scholar]
- [63].Ryall JG, Schertzer JD, Murphy KT, Allen AM, Lynch GS. Chronic β2-adrenoceptor stimulation impairs cardiac relaxation via reduced SR Ca2+-ATPase protein and activity. Am J Physiol Heart Circ Physiol. 2008;294:H2587–95. doi: 10.1152/ajpheart.00985.2007. [DOI] [PubMed] [Google Scholar]
- [64].Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc. 2008;5:23–31. doi: 10.1513/pats.200704-050VS. [DOI] [PubMed] [Google Scholar]
- [65].Ressmeyer AR, Bai Y, Delmotte P, Uy KF, Thistlethwaite P, Fraire A, et al. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am J Respir Cell Mol Biol. 2010;43:179–91. doi: 10.1165/rcmb.2009-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Delmotte P, Sanderson MJ. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;42:373–81. doi: 10.1165/rcmb.2008-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schaafsma D, Gosens R, Bos IS, Meurs H, Zaagsma J, Nelemans SA. Allergic sensitization enhances the contribution of Rho-kinase to airway smooth muscle contraction. Br J Pharmacol. 2004;143:477–84. doi: 10.1038/sj.bjp.0705903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Penn RB, Benovic JL. Regulation of heterotrimeric G protein signaling in airway smooth muscle. Proc Am Thorac Soc. 2008;5:47–57. doi: 10.1513/pats.200705-054VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ise S, Nishimura J, Hirano K, Hara N, Kanaide H. Theophylline attenuates Ca2+ sensitivity and modulates BK channels in porcine tracheal smooth muscle. Br J Pharmacol. 2003;140:939–47. doi: 10.1038/sj.bjp.0705508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997;410:356–60. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- [71].Morin C, Sirois M, Echave V, Rousseau E. CPI-17 silencing-reduced responsiveness in control and TNF-alpha-treated human bronchi. Am J Respir Cell Mol Biol. 2008;39:638–43. doi: 10.1165/rcmb.2008-0177RC. [DOI] [PubMed] [Google Scholar]
- [72].Hirshman CA, Zhu D, Panettieri RA, Emala CW. Actin depolymerization via the beta-adrenoceptor in airway smooth muscle cells: a novel PKA-independent pathway. Am J Physiol Cell Physiol. 2001;281:C1468–76. doi: 10.1152/ajpcell.2001.281.5.C1468. [DOI] [PubMed] [Google Scholar]
- [73].Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–6. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [74].Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- [75].Aso H, Ito S, Mori A, Morioka M, Suganuma N, Kondo M, et al. Prostaglandin E2 enhances interleukin-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L266–73. doi: 10.1152/ajplung.00248.2011. [DOI] [PubMed] [Google Scholar]
- [76].Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, et al. Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol. 2003;29:19–27. doi: 10.1165/rcmb.2002-0254OC. [DOI] [PubMed] [Google Scholar]
- [77].Walker JK, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding β2-adrenoceptor ligands in the treatment of asthma. Br J Pharmacol. 2011;163:18–28. doi: 10.1111/j.1476-5381.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Florio C, Martin JG, Styhler A, Heisler S. Antiproliferative effect of prostaglandin E2 in cultured guinea pig tracheal smooth muscle cells. Am J Physiol. 1994;266:L131–7. doi: 10.1152/ajplung.1994.266.2.L131. [DOI] [PubMed] [Google Scholar]
- [79].Kaur M, Holden NS, Wilson SM, Sukkar MB, Chung KF, Barnes PJ, et al. Effect of β2-adrenoceptor agonists and other cAMP-elevating agents on inflammatory gene expression in human ASM cells: a role for protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2008;295:L505–L14. doi: 10.1152/ajplung.00046.2008. [DOI] [PubMed] [Google Scholar]
- [80].Mori A, Ito S, Morioka M, Aso H, Kondo M, Sokabe M, et al. Effects of specific prostanoid EP receptor agonists on cell proliferation and intracellular Ca2+ concentrations in human airway smooth muscle cells. Eur J Pharmacol. 2011;659:72–8. doi: 10.1016/j.ejphar.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [81].Kume H, Ito S, Ito Y, Yamaki K. Role of lysophosphatidylcholine in the desensitization of β-adrenergic receptors by Ca2+ sensitization in tracheal smooth muscle. Am J Respir Cell Mol Biol. 2001;25:291–8. doi: 10.1165/ajrcmb.25.3.4364. [DOI] [PubMed] [Google Scholar]
- [82].Burgess JK, Ge Q, Boustany S, Black JL, Johnson PR. Increased sensitivity of asthmatic airway smooth muscle cells to prostaglandin E2 might be mediated by increased numbers of E-prostanoid receptors. J Allergy Clin Immunol. 2004;113:876–81. doi: 10.1016/j.jaci.2004.02.029. [DOI] [PubMed] [Google Scholar]
- [83].Stewart AG, Harris T, Fernandes DJ, Schachte LC, Koutsoubos V, Guida E, et al. β2-Adrenergic receptor agonists and cAMP arrest human cultured airway smooth muscle cells in the G1 phase of the cell cycle: role of proteasome degradation of cyclin D1. Mol Pharmacol. 1999;56:1079–86. doi: 10.1124/mol.56.5.1079. [DOI] [PubMed] [Google Scholar]
- [84].Tomlinson PR, Wilson JW, Stewart AG. Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: relationship to elevated cAMP levels. Biochem Pharmacol. 1995;49:1809–19. doi: 10.1016/0006-2952(94)00532-q. [DOI] [PubMed] [Google Scholar]
- [85].Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DW, Torphy TJ, et al. Tumor necrosis factor-α-induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells. Modulation by cyclic adenosine monophosphate. Am J Respir Cell Mol Biol. 2000;23:794–802. doi: 10.1165/ajrcmb.23.6.4184. [DOI] [PubMed] [Google Scholar]
- [86].Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- [87].Clarke DL, Belvisi MG, Smith SJ, Hardaker E, Yacoub MH, Meja KK, et al. Prostanoid receptor expression by human airway smooth muscle cells and regulation of the secretion of granulocyte colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2005;288:L238–50. doi: 10.1152/ajplung.00313.2004. [DOI] [PubMed] [Google Scholar]
- [88].Kong KC, Gandhi U, Martin TJ, Anz CB, Yan H, Misior AM, et al. Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry. 2008;47:9279–88. doi: 10.1021/bi801056w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ndukwu IM, White SR, Leff AR, Mitchell RW. EP1 receptor blockade attenuates both spontaneous tone and PGE2-elicited contraction in guinea pig trachealis. Am J Physiol. 1997;273:L626–33. doi: 10.1152/ajplung.1997.273.3.L626. [DOI] [PubMed] [Google Scholar]
- [90].McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate β2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–9. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Norel X, Walch L, Labat C, Gascard JP, Dulmet E, Brink C. Prostanoid T IP receptors involved in the relaxation of human bronchial preparations. Br J Pharmacol. 1999;126:867–72. doi: 10.1038/sj.bjp.0702392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, et al. Receptors and J R pathways mediating the effects of prostaglandin E2 on airway tone. Am Physiol Cell Mol Physiol. 2003;284:L599–606. doi: 10.1152/ajplung.00324.2002. [DOI] [PubMed] [Google Scholar]
- [93].Buckley J, Birrell MA, Maher SA, Nials AT, Clarke DL, Belvisi MG. EP4 Lung receptor as a new target for bronchodilator therapy. Thorax. 2011;66:1029–35. doi: 10.1136/thx.2010.158568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, et al. PGE2 receptor (EP4) agonists: Potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther. 2012;25:115–8. doi: 10.1016/j.pupt.2011.12.012. [DOI] [PubMed] [Google Scholar]
- [95].Misior AM, Yan H, Pascual RM, Deshpande DA, Panettieri RA, Penn RB. Mitogenic effects of cytokines on smooth muscle are critically dependent protein kinase A are unmasked MANUS on and by steroids and cyclooxygenase inhibitors. Mol Pharmacol. 2008;73:566–74. doi: 10.1124/mol.107.040519. [DOI] [PubMed] [Google Scholar]
- [96].Guo M, Pascual RM, Wang S, Fontana MF, Valancius CA, Panettieri RA., Jr. Cytokines regulate β-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and et EP2 receptor-dependent. Biochemistry. 2005;44:13771–82. doi: 10.1021/bi051255y. al. [DOI] [PubMed] [Google Scholar]
- [97].Nials AT, Coleman RA, Johnson M, Magnussen H, Rabe KF, Vardey CJ. Effects of beta-adrenoceptor agonists in human bronchial smooth muscle. Br J Pharmacol. 1993;110:1112–6. doi: 10.1111/j.1476-5381.1993.tb13929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Carlin SM, Roth M, Black JL. Urokinase potentiates PDGF-induced chemotaxis of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1020–6. [Google Scholar]
- [99].Parameswaran K, Cox G, Radford K, Janssen LJ, Sehmi R, O’Byrne PM. Cysteinyl leukotrienes C promote human airway smooth muscle migration. Am J Respir Crit Care Med. 2002;166:738–42. doi: 10.1164/rccm.200204-291OC. [DOI] [PubMed] [Google Scholar]
- [100].Hirakawa M, Karashima Y, Watanabe M, Kimura C, Ito Y, Oike M. Protein kinase A inhibits lysophosphatidic acid-induced migration of airway smooth muscle cells. J Pharmacol Exp Ther. 2007;321:1102–8. doi: 10.1124/jpet.106.118042. [DOI] [PubMed] [Google Scholar]
- [101].Roth M, Johnson PR, Rudiger JJ, King GG, Ge Q, Burgess JK, et al. Interaction between glucocorticoids and β2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–9. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- [102].Billington CK, Joseph SK, Swan C, Scott MG, Jobson TM, Hall IP. Modulation of human airway smooth muscle proliferation by type 3 phosphodiesterase inhibition. Am J Physiol. 1999;276:L412–9. doi: 10.1152/ajplung.1999.276.3.L412. [DOI] [PubMed] [Google Scholar]
- [103].Lazaar AL, Panettieri RA., Jr. Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005;116:488–95. doi: 10.1016/j.jaci.2005.06.030. quiz 96. [DOI] [PubMed] [Google Scholar]
- [104].Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–7. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- [105].Hirst SJ, Martin JG, Bonacci JV, Chan V, Fixman ED, Hamid QA, et al. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S2–17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- [106].Madison JM. Migration of airway smooth muscle cells. Am J Respir Cell Mol Biol. 2003;29:8–11. doi: 10.1165/rcmb.F272. [DOI] [PubMed] [Google Scholar]
- [107].Gerthoffer WT. Migration of airway smooth muscle cells. Proc Am Thorac Soc. 2008;5:97–105. doi: 10.1513/pats.200704-051VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Roscioni SS, Prins AG, Elzinga CR, Menzen MH, Dekkers BG, Halayko AJ, et al. Protein kinase A and the exchange protein directly activated by cAMP (Epac) modulate phenotype plasticity in human airway smooth muscle. Br J Pharmacol. 2011;164:958–69. doi: 10.1111/j.1476-5381.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gosens R, Roscioni SS, Dekkers BG, Pera T, Schmidt M, Schaafsma D, et al. Pharmacology of airway smooth muscle proliferation. European journal of pharmacology. 2008;585:385–97. doi: 10.1016/j.ejphar.2008.01.055. [DOI] [PubMed] [Google Scholar]
- [110].Belvisi MG, Saunders M, Yacoub M, Mitchell JA. Expression of cyclo-oxygenase-2 in human airway smooth muscle is associated with profound C reductions in cell growth. Br J Pharmacol. 1998;125:1102–8. doi: 10.1038/sj.bjp.0702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pascual RM, Billington CK, Hall IP, Panettieri RA, Jr., Fish JE, Peters SP, et al. Mechanisms of cytokine effects on G protein-coupled receptor-mediated signaling in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1425–35. doi: 10.1152/ajplung.2001.281.6.L1425. [DOI] [PubMed] [Google Scholar]
- [112].Pascual RM, Carr EM, Seeds MC, Guo M, Panettieri RA, Jr., Peters SP, et al. Regulatory features of interleukin-1beta-mediated prostaglandin E2 synthesis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L501–8. doi: 10.1152/ajplung.00420.2005. [DOI] [PubMed] [Google Scholar]
- [113].Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton MANUS and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- [114].Howarth PH, Knox AJ, Amrani Y, Tliba O, Panettieri RA, Jr., Johnson M. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol. 2004;114:S32–50. doi: 10.1016/j.jaci.2004.04.041. [DOI] [PubMed] [Google Scholar]
- [115].Tliba O, Panettieri RA., Jr. Noncontractile functions of airway smooth muscle cells in asthma. Annual review of physiology. 2009;71:509–35. doi: 10.1146/annurev.physiol.010908.163227. [DOI] [PubMed] [Google Scholar]
- [116].Lazzeri N, Belvisi MG, Patel HJ, Yacoub MH, Chung KF, Mitchell JA. Effects of prostaglandin E2 and cAMP drugs on airway TE elevating GM-CSF release by cultured human smooth muscle cells. Relevance to asthma therapy. Am J Respir Cell Mol Biol. 2001;24:44–8. doi: 10.1165/ajrcmb.24.1.4027. [DOI] [PubMed] [Google Scholar]
- [117].Pang L, Knox AJ. Synergistic inhibition by beta(2)-agonists and corticosteroids on tumor necrosis factor-alpha-induced P from cultured airway E interleukin-8 release human smooth-muscle cells. Am J Respir Cell Mol Biol. 2000;23:79–85. doi: 10.1165/ajrcmb.23.1.3985. [DOI] [PubMed] [Google Scholar]
- [118].Halayko AJ, Tran T, Ji SY, Yamasaki A, Gosens R. Airway smooth muscle phenotype AC and function: interactions with current asthma therapies. Curr Drug Targets. 2006;7:525–40. doi: 10.2174/138945006776818728. [DOI] [PubMed] [Google Scholar]
- [119].Hirota JA, Nguyen TT, Schaafsma D, Sharma P, Tran T. Airway smooth muscle in asthma: phenotype plasticity and function. Pulm Pharmacol Ther. 2009;22:370–8. doi: 10.1016/j.pupt.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [120].Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol. 1996;270:L1040–51. doi: 10.1152/ajplung.1996.270.6.L1040. [DOI] [PubMed] [Google Scholar]
- [121].Liu HW, Halayko AJ, Fernandes DJ, Harmon GS, McCauley JA, Kocieniewski P, et al. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- [122].Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- [123].Blaker AL, Taylor JM, Mack CP. PKA-dependent phosphorylation of serum response factor inhibits smooth muscle-specific gene expression. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:2153–60. doi: 10.1161/ATVBAHA.109.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Davis A, Hogarth K, Fernandes D, Solway J, Niu J, Kolenko V, et al. Functional significance of protein kinase A activation by endothelin-1 and ATP: negative regulation of SRF-dependent gene expression by PKA. Cell Signal. 2003;15:597–604. doi: 10.1016/s0898-6568(02)00148-1. [DOI] [PubMed] [Google Scholar]
- [125].Roscioni SS, Dekkers BG, Prins AG, Menzen MH, Meurs H, Schmidt M, et al. cAMP inhibits modulation of airway smooth muscle phenotype via the exchange protein activated by cAMP (Epac) and protein kinase A. Br J Pharmacol. 2011;162:193–209. doi: 10.1111/j.1476-5381.2010.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–6. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- [127].Johnson M. The β-adrenoceptor. Am J Respir Crit Care Med. 1998;158:S146–53. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- [128].Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001;164:141–8. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
- [129].Nogami M, Romberger DJ, Rennard SI, Toews ML. TGF-beta 1 modulates beta-adrenergic receptor number and function in cultured human tracheal smooth muscle cells. Am J Physiol. 1994;266:L187–91. doi: 10.1152/ajplung.1994.266.2.L187. [DOI] [PubMed] [Google Scholar]
- [130].Hakonarson H, Herrick DJ, Serrano PG, Grunstein MM. Mechanism of cytokine-induced modulation of beta-adrenoceptor responsiveness in airway smooth muscle. J Clin Invest. 1996;97:2593–600. doi: 10.1172/JCI118708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, et al. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985;260:7094–101. [PubMed] [Google Scholar]
- [132].Belvisi MG, Saunders MA, Haddad el B, Hirst SJ, Yacoub MH, Barnes PJ, et al. Induction of cyclo-oxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of this cell type. Br J Pharmacol. 1997;120:910–6. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Penn RB, Panettieri RA, Jr., Benovic JL. Mechanisms of acute desensitization of the β2AR-adenylyl cyclase pathway in human airway smooth muscle. Am J Respir Cell Mol Biol. 1998;19:338–48. doi: 10.1165/ajrcmb.19.2.3025. [DOI] [PubMed] [Google Scholar]
- [134].Deshpande DA, Theriot BS, Penn RB, Walker JK. β-Arrestins specifically constrain β2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–41. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Penn RB, Pascual RM, Kim YM, Mundell SJ, Krymskaya VP, Panettieri RA, Jr., et al. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem. 2001;276:32648–56. doi: 10.1074/jbc.M104143200. [DOI] [PubMed] [Google Scholar]
- [136].Wang WC, Mihlbachler KA, Brunnett AC, Liggett SB. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway β2-adrenergic receptor physiologic signaling. Proc Natl Acad Sci U S A. 2009;106:15007–12. doi: 10.1073/pnas.0906034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, et al. β2-Adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem. 1997;272:23871–9. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- [138].January B, Seibold A, Allal C, Whaley BS, Knoll BJ, Moore RH, et al. Salmeterol-induced desensitization, internalization and phosphorylation of the human β2-adrenoceptor. Br J Pharmacol. 1998;123:701–11. doi: 10.1038/sj.bjp.0701658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, et al. β2-Adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci U S A. 2009;106:2435–40. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Ammar-Aouchiche Z, et al. Chronic exposure to β-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–62. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sears MR. Adverse effects of β-agonists. J Allergy Clin Immunol. 2002;110:S322–8. doi: 10.1067/mai.2002.129966. [DOI] [PubMed] [Google Scholar]