Abstract
Gastroesophageal reflux disease (GORD) is highly prevalent in the general population. In the last decade, a potential relationship between Helicobacter pylori (H. pylori) eradication and GORD onset has been claimed. The main putative mechanism is the gastric acid hypersecretion that develops after bacterial cure in those patients with corpus-predominant gastritis. We performed a critical reappraisal of the intricate pathogenesis and clinical data available in this field. Oesophagitis onset after H. pylori eradication in duodenal ulcer patients has been ascribed to a gastric acid hypersecretion, which could develop following body gastritis healing. However, the absence of an acid hypersecretive status in these patients is documented by both pathophysiology and clinical studies. Indeed, duodenal ulcer recurrence is virtually abolished following H. pylori eradication. In addition, intra-oesophageal pH recording studies failed to demonstrated increased acid reflux following bacterial eradication. Moreover, oesophageal manometric studies suggest that H. pylori eradication would reduce - rather than favor - acid reflux into the oesophagus. Finally, data of clinical studies would suggest that H. pylori eradication is not significantly associated with either reflux symptoms or erosive oesophagitis onset, some data suggesting also an advantage in curing the infection when oesophagitis is already present. Therefore, the legend of “crazy acid” remains - as all the others - a fascinating, but imaginary tale.
Keywords: Helicobacter pylori, Oesophageal reflux, Oesophagitis, Eradication, Pathophysiology, Clinical studies
INTRODUCTION
Although Helicobacter pylori (H. pylori) infection prevalence is declining in developed countries, such infection remains a worldwide spread disease with a definite morbidity and mortality. Indeed, H. pylori may cause non-ulcer dyspepsia, peptic ulcer disease, and gastric tumors, including both low-grade mucosa-associated lymphoid tissue lymphoma and adenocarcinoma[1-3]. In addition, an interaction between H. pylori with non-steroidal, anti-inflammatory drugs in damaging the gastroduodenal mucosa has been also recognized[4]. Similarly, the role of H. pylori in the pathogenesis of different extra-digestive diseases has also been claimed. However, an association has been consistently proven only between H. pylori infection and both idiopathic thrombocytopenic purpura and idiopathic iron deficiency anemia[5,6], whilst conflicting data exist for other diseases[7-9].
Gastroesophageal reflux disease (GORD) is a highly prevalent condition in the general population[10]. It is characterized by the reflux of gastric contents into the oesophagus, leading to mucosal damage and⁄or typical and atypical symptoms[11]. Several factors may predispose patients to pathologic reflux, including hiatal hernia, lower esophageal sphincter (LES) hypotension, transient lower oesophageal sphincter relaxation, loss of esophageal peristaltic function, abdominal obesity, gastric hypersecretory states, and delayed gastric emptying[12].
In the last decade, a potential relationship between H. pylori infection and GORD has been claimed. In detail, it has been suggested that H. pylori eradication may cause both reflux symptoms and erosive oesophagitis. We performed a critical reappraisal of the intricate data available in such a topic.
DOES H. PYLORI ERADICATION CAUSE GORD?
The beginning
The first study suggesting a possible association between H. pylori and GORD was published by Labenz et al[13] on 1997. In this study, 25.8% of patients with duodenal ulcer (DU) cured for H. pylori infection developed erosive oesophagitis (Savary-Miller classification) at 3-year follow-up as compared to 12.9% of DU patients with ongoing infection (P < 0.001). Identified risk factors were male sex, severity of corpus gastritis, and weight gain. These results deserve some considerations. As shown in Figure 1, the incidence of erosive oesophagitis in H. pylori eradicated patients constantly increased at follow-up, so that at 3-year follow-up the curve did not still reach the plateau. This would probably suggest that an even higher oesophagitis incidence is expected at longer follow-up in these patients. However, this contrasts with data of a recent 7-year follow-up study which showed that reflux oesophagitis occurred in only 1.9% of DU patients following H. pylori eradication[14]. In addition, it should be noted that the curve of oesophagitis incidence in not eradicated DU patients showed a very similar slope as compared to that of eradicated patients (Figure 1). In detail, following a questionable delay of 1 year, reflux oesophagitis started to increase also in H. pylori infected patients, and the increase of incidence parallels with that of eradicated patients. A plausible explanation of such phenomenon could be that several not eradicated DU patients were taking anti-acid therapy, at least for the first year follow-up in this study. Indeed, this patient group included either control cases of H. pylori eradication trials (75 cases) or H. pylori eradication failure patients (141 cases) in whom DU symptoms most likely persisted[13]. Therefore, the observation of a distorted phenomenon could not be ruled out. On the other hand, it was observed that oesophagitis occurred more frequently in those H. pylori cured patients with weight gain > 2 kg [odds ratio (OR): 3.2; 95%CI: 1.2-9.4; P < 0.05] within 3-year follow-up. Unfortunately, both mean and range of weigh gain was not provided in this study. However, it is plausible that several patients changed their dietary and voluptuary habits following complete DU healing, with consequent body mass index (BMI) increase. The direct relationship between BMI and GORD is widely documented in literature, so that the “guilty” of erosive oesophagitis onset - at least in some patients - probably was the BMI increase rather than the H. pylori cure. Unfortunately, the lack of multivariate regression including H. pylori eradication as an independent risk factor for GORD does not allow to confirm the significant role observed solely at univariate analysis. Finally, the reason for which the risk of erosive oesophagitis incidence following H. pylori eradication was 3.6 (95%CI: 1.1-10.6) higher in males as compared to females remains unclear. This would contrast with the prevalence of GORD in the general population which equally occurs in both sexes[15].
Figure 1.
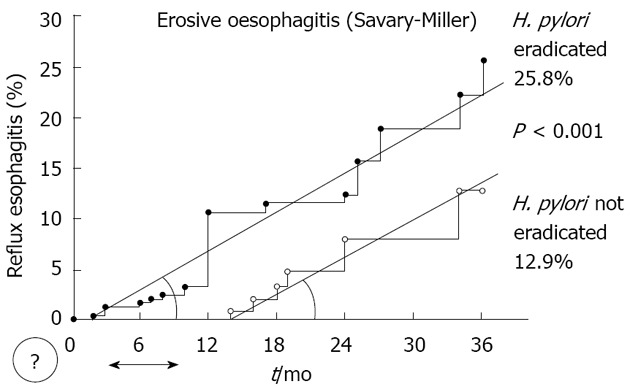
Incidence of erosive oesophagitis in Helicobacter pylori eradicated and not eradicated duodenal ulcer patients (Modified from reference 13). The comparable curve slopes would indicate a similar phenomenon. The final difference between patient groups depends on the 1-year delay between curve which remains unexplained. H. pylori: Helicobacter pylori.
The tale of a "crazy acid"
At least in theory, H. pylori eradication could cause GORD by: (1) recovering gastric acid secretion; (2) increasing the amount of reflux in the oesophagus; and (3) reducing the clearance of oesophageal acid exposure.
Regarding the first point, it has been observed that H. pylori-associated corpus-predominant gastritis is an independent risk factor (OR: 5.5; 95%CI: 2.8-13.6) for erosive oesophagitis development following H. pylori eradication in DU patients[13]. In this study, the body gastritis sum score before H. pylori eradication was 4.1 in those who developed oesophagitis as compared to 3.1 of those without oesophagitis onset. Therefore, it has been suggested that the higher degree of recovery leads into a higher acid secretion which, in turn, causes erosive oesophagitis[13]. However, by considering that the sum score (activity plus grade) of gastritis was ranging from 0 (absent) and 8 (severe), it is questionable that acid hypersecretion developed only in those patients in whom corpus gastritis score dropped from 4.1, but not from 3.1, to zero following bacterial cure. At this point, the crucial issue is to understand whether H. pylori eradication really causes gastric acid hypersecretion in DU patients. To our knowledge, neither pathophysiology nor clinical studies support such an event. It has been found that infected DU patients have a 3-fold increase of basal acid output (BAO) and a 6-fold increase of stimulated maximal acid output (MAO) as compared to uninfected controls[16]. Of note, both these alterations normalize 1-year following H. pylori eradication, suggesting that DU patients cured for the infection completely restore normal acid secretion[16]. The absence of an acid hypersecretive status in these patients is further documented by the clinical observation that DU recurrence is virtually abolished following H. pylori eradication[17]. Therefore, it is unclear the reason for which the hypothesized hypersecretion in DU patients after bacterial cure should cause erosive oesophagitis onset, but not DU recurrence. To search for a plausible explication, one could hypothesize the “tale of a crazy acid”. According to this theory, following the healing of corpus-predominant gastritis - a typical gastritis pattern of both gastric ulcer and cancer, but infrequent in DU[18] - acid hypersecretion occurs in some DU patients. In these patients the acid should invert its normal route - so failing to cause DU recurrence - and should turn into the oesophagus causing erosions (Figure 2). However, studies on 24-h intra-oesophageal pH recording before and after H. pylori eradication showed that the percentage time of pH < 4.0 did not significantly change following eradication[19,20]. Other putative mechanisms by which H. pylori eradication could favor GORD are alterations of both LES pressure and oesophageal peristalsis. However, two studies found no difference in basal LES pressure between H. pylori infected and matched controls[21,22], whilst another study showed an even lower basal LES pressure and higher rate of ineffective oesophageal motility in infected patients[23]. Based on these manometric observations, it could be expected that H. pylori eradication would reduce - rather than favor - acid reflux into the oesophagus.
Figure 2.
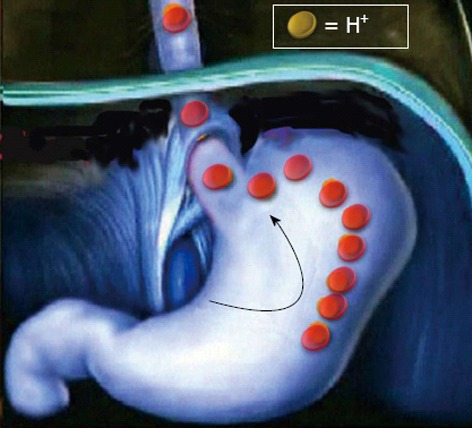
The “tale of a crazy acid”. Following Helicobacter pylori gastric in duodenal ulcer patients the hypothesized acid hypersecretion would U-turn into the oesophagus instead of in the duodenal bulb.
Clinical studies
Several clinical trials assessing the onset of both reflux symptoms and erosive oesophagitis have been performed. A recent meta-analysis evaluated data of 10 trials where data of patients treated for H. pylori infection were compared to those receiving placebo[24]. At 8-30 mo follow-up the incidence of reflux symptoms did not significantly differ between the two patient groups (17% vs 22.6%), with a trend even favoring bacterial eradication (OR: 0.81; 95%CI: 0.56-1.71). Likewise, erosive oesophagitis equally occurred in both groups (5% vs 5.1%; OR: 1.13; 95%CI: 0.72-1.78). Noteworthy, a study on 156 patients with both peptic ulcer and reflux oesophagitis found that the oesophageal lesions improved more frequently in H. pylori eradicated patients as compared to those with persistent infection[25]. Overall, these data would suggest that H. pylori eradication is not significantly associated with either reflux symptoms or erosive oesophagitis onset, some data suggesting also an advantage in curing the infection when oesophagitis is already present.
CONCLUSION
GORD is highly prevalent in the general population. Several altered mechanisms are involved in its pathogenesis, and different lifestyle and voluptuary habits have been identified as risk factors. In the last decade, H. pylori eradication has been charged to cause GORD. The main putative mechanism is that a gastric acid hypersecretion develops following bacterial cure in those patients with corpus-predominant gastritis. However, studies on acid secretion demonstrated that the hypersecretion status of both DU (BAO × 3; MAO × 6) and non-ulcer dyspeptic (MAO × 3) patients observed during H. pylori infection normalizes following bacterial eradication[16]. Indeed, DU does not recur following a successful H. pylori cure[17]. Therefore, the reported erosive oesophagitis onset after the infection cure in DU patients most likely depends on other factors rather gastric acid hypersecretion (Weight gain? Changes of voluptuary habits?). On the other hand, both oesophageal 24-h pH recording and manometry studies failed to demonstrate a significant role for H. pylori in GORD.
Gastric acid secretion may recover with H. pylori eradication in those patients with corpus-predominant (or atrophic) gastritis[26]. However, such a gastritis pattern is generally encountered in either gastric ulcer or cancer patients, and it is highly prevalent in Asian countries[18]. However, the reason for which such a hypersecretive status should cause GORD but not DU onset in these patients remains unclear.
The last but not the least, several, placebo-controlled, clinical trials have been recently summarized in a meta-analysis including near 4500 patients[25]. Data found that neither reflux symptoms nor erosive oesophagitis develop following H. pylori eradication. Therefore, the legend of “crazy acid” remains - as all the others - a fascinating, but imaginary tale!
Footnotes
P- Reviewer Shimatani T S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Alakkari A, Zullo A, O’Connor HJ. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2011;16 Suppl 1:33–37. doi: 10.1111/j.1523-5378.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 2.Zullo A, Hassan C, Cristofari F, Perri F, Morini S. Gastric low-grade mucosal-associated lymphoid tissue-lymphoma: Helicobacter pylori and beyond. World J Gastrointest Oncol. 2010;2:181–186. doi: 10.4251/wjgo.v2.i4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuccio L, Eusebi LH, Bazzoli F. Gastric cancer, Helicobacter pylori infection and other risk factors. World J Gastrointest Oncol. 2010;2:342–347. doi: 10.4251/wjgo.v2.i9.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zullo A, Hassan C, Campo SM, Morini S. Bleeding peptic ulcer in the elderly: risk factors and prevention strategies. Drugs Aging. 2007;24:815–828. doi: 10.2165/00002512-200724100-00003. [DOI] [PubMed] [Google Scholar]
- 5.Qu XH, Huang XL, Xiong P, Zhu CY, Huang YL, Lu LG, Sun X, Rong L, Zhong L, Sun DY, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16:886–896. doi: 10.3748/wjg.v16.i7.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D, Newland A, Amadori S, Bussel JB. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231–1240. doi: 10.1182/blood-2008-07-167155. [DOI] [PubMed] [Google Scholar]
- 7.Zullo A, Ridola L, Hassan C, Bruzzese V, Papini F, Vaira D. Glaucoma and Helicobacter pylori: eyes wide shut? Dig Liver Dis. 2012;44:627–628. doi: 10.1016/j.dld.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Zullo A, Hassan C, Morini S. Hepatic encephalopathy and Helicobacter pylori: a critical reappraisal. J Clin Gastroenterol. 2003;37:164–168. doi: 10.1097/00004836-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Sherman PM, Lin FY. Extradigestive manifestation of Helicobacter pylori infection in children and adolescents. Can J Gastroenterol. 2005;19:421–424. doi: 10.1155/2005/971974. [DOI] [PubMed] [Google Scholar]
- 10.Weber C, Davis CS, Fisichella PM. Current applications of evolving methodologies in gastroesophageal reflux disease testing. Dig Liver Dis. 2011;43:353–357. doi: 10.1016/j.dld.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920; quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Kwiatek MA, Kahrilas PJ. The pathophysiologic basis for epidemiologic trends in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2008;37:827–843, viii. doi: 10.1016/j.gtc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Labenz J, Blum AL, Bayerdörffer E, Meining A, Stolte M, Börsch G. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442–1447. doi: 10.1016/s0016-5085(97)70024-6. [DOI] [PubMed] [Google Scholar]
- 14.Maconi G, Sainaghi M, Molteni M, Bosani M, Gallus S, Ricci G, Alvisi V, Porro GB. Predictors of long-term outcome of functional dyspepsia and duodenal ulcer after successful Helicobacter pylori eradication--a 7-year follow-up study. Eur J Gastroenterol Hepatol. 2009;21:387–393. doi: 10.1097/MEG.0b013e3283069db0. [DOI] [PubMed] [Google Scholar]
- 15.Vakil N. Disease definition, clinical manifestations, epidemiology and natural history of GERD. Best Pract Res Clin Gastroenterol. 2010;24:759–764. doi: 10.1016/j.bpg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 16.el-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006;(2):CD003840. doi: 10.1002/14651858.CD003840.pub4. [DOI] [PubMed] [Google Scholar]
- 18.Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis. 2011;29:459–464. doi: 10.1159/000332213. [DOI] [PubMed] [Google Scholar]
- 19.Tefera S, Hatlebakk JG, Berstad A. The effect of Helicobacter pylori eradication on gastro-oesophageal reflux. Aliment Pharmacol Ther. 1999;13:915–920. doi: 10.1046/j.1365-2036.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu JC, Chan FK, Wong SK, Lee YT, Leung WK, Sung JJ. Effect of Helicobacter pylori eradication on oesophageal acid exposure in patients with reflux oesophagitis. Aliment Pharmacol Ther. 2002;16:545–552. doi: 10.1046/j.1365-2036.2002.01189.x. [DOI] [PubMed] [Google Scholar]
- 21.Shirota T, Kusano M, Kawamura O, Horikoshi T, Mori M, Sekiguchi T. Helicobacter pylori infection correlates with severity of reflux esophagitis: with manometry findings. J Gastroenterol. 1999;34:553–559. doi: 10.1007/s005350050372. [DOI] [PubMed] [Google Scholar]
- 22.Grande M, Cadeddu F, Villa M, Attinà GM, Muzi MG, Nigro C, Rulli F, Farinon AM. Helicobacter pylori and gastroesophageal reflux disease. World J Surg Oncol. 2008;6:74. doi: 10.1186/1477-7819-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JC, Lai AC, Wong SK, Chan FK, Leung WK, Sung JJ. Dysfunction of oesophageal motility in Helicobacter pylori-infected patients with reflux oesophagitis. Aliment Pharmacol Ther. 2001;15:1913–1919. doi: 10.1046/j.1365-2036.2001.01132.x. [DOI] [PubMed] [Google Scholar]
- 24.Saad AM, Choudhary A, Bechtold ML. Effect of Helicobacter pylori treatment on gastroesophageal reflux disease (GERD): meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2012;47:129–135. doi: 10.3109/00365521.2011.648955. [DOI] [PubMed] [Google Scholar]
- 25.Ishiki K, Mizuno M, Take S, Nagahara Y, Yoshida T, Yamamoto K, Okada H, Yokota K, Oguma K, Shiratori Y. Helicobacter pylori eradication improves pre-existing reflux esophagitis in patients with duodenal ulcer disease. Clin Gastroenterol Hepatol. 2004;2:474–479. doi: 10.1016/s1542-3565(04)00165-x. [DOI] [PubMed] [Google Scholar]
- 26.Haruma K, Mihara M, Okamoto E, Kusunoki H, Hananoki M, Tanaka S, Yoshihara M, Sumii K, Kajiyama G. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment Pharmacol Ther. 1999;13:155–162. doi: 10.1046/j.1365-2036.1999.00459.x. [DOI] [PubMed] [Google Scholar]


