It is a pleasure to have the opportunity to contribute to the issue of Journal of Autoimmunity honoring Dr. Noel Rose, a pioneer in autoimmunity. Doctor Rose laid some of the major fundamental groundwork in autoimmunity. His early studies on autoimmune thyroiditis are classics. I was fortunate to meet and get to know Noel while I was a junior scientist at the University of Michigan, Ann Arbor, when Noel became the chairman of Immunology and Microbiology at Wayne State University in Detroit. My mentor, Dr. Donald Shreffler, and Noel set up collaboration to study the genetics of autoimmune thyroiditis in inbred, congenic, and recombinant strains of mice. I became the primary contact person in our lab to work with Dr. Noel Rose and his associate, Dr. Yi-chi Kong. Eventually I became a co-investigator in Dr. Rose’s NIH grant, and we had several sessions with him to discuss data and to plan new experiments. I learned many of the fundamentals of autoimmunity from Noel. This interaction with Noel and his colleagues continued even after I moved to Washington University and then on to the Mayo Clinic. After moving to Johns Hopkins, Dr. Rose concentrated more on his pioneering studies on experimental autoimmune myocarditis. I continued to keep in touch with him. Doctor Rose is a gentleman and a scholar and I am happy he is my colleague and friend. I wish him many more years of productive research.
The main feature of the adaptive immunity is its diverse nature of immune response. The major player for adaptive immunity includes genes of Major Histocompatibility complex (MHC). It encodes for polymorphic HLA molecules that are critical for differentiating self from non-self. Mature T cells recognize foreign antigen when it is presented in context of self-MHC. The loss of self-tolerance of the immune system against the body’s own tissues/antigens leads to autoimmunity.
MHC and Autoimmunity
The hallmark of MHC molecules is its remarkable polymorphism, which dictates the immune response to specific antigens. Education of immune system in thymus teaches discrimination between self and non-self to ensure that an immune response is mounted against foreign antigen and not self. In the thymus, T cells are selected on the basis of their affinity and interaction with self-MHC molecules expressed in thymus. Thus HLA molecules play a critical role in shaping T cell repertoire in the thymus by presenting self-peptides. However, not all self-antigens are expressed in thymus thus T cells specific for some self antigens can escape negative selection in thymus. Familial clustering of autoimmune diseases and occurrence of autoimmunity in monozygotic twins suggest that predisposition to autoimmunity is under genetic control. Interestingly, the MHC gene complex is associated with most, if not all, of the common autoimmune conditions. Genes within the MHC region act as restricting elements for response to foreign antigens by T lymphocytes. Resistance to infection probably drives the polymorphism of this region.
Population studies have shown that predisposition to almost all human autoimmune diseases is linked to HLA genes, primarily the class II genes. Among these linkages, three MHC class II haplotypes stand out as the most autoimmune prone genes. Despite a number of studies demonstrating association of class II molecules with various autoimmune disease, the mechanisms to explain these associations remains obscure. In humans it is difficult to dissect the mechanism due to following reasons 1) there is a lack of knowledge of the autoantigens 2) time of sampling, 3) frequency of autoreactive cells and 4) huge genetic variation between individuals can make it difficult to interpret the results. The other problem has been the linkage disequilibrium of HLA class II alleles, DR and DQ, and heterozygosity which makes it difficult to interpret the association of a disease with a specific allele.
Myocarditis and cardiomyopathy
Myocarditis is an inflammatory heart disease defined pathologically as mononuclear or mixed cellular infiltration associated with myocyte necrosis and degeneration which can progress to dilated cardiomyopathy (DCM) (1). Heart disease is a major cause of mortality in young adults in advanced countries. It is difficult to diagnose due to varied clinical manifestations, thus the true incidence of disease are not known. In clinically diagnosed patients, a higher number of males over females have been reported (male: female ratio, 1.5:1) in some while other studies have observed similar incidence in both sexes. Evidence of myocarditis has been demonstrated in 20% of autopsy cases of presumed healthy individuals below 40 years of age. Several studies have indicated an aberrant immune response following viral-mediated damage to myocytes (2–5). A progression from viral myocarditis to dilated cardiomyopathy has been hypothesized even though the exact pathogenic mechanism remains uncertain. A wide variety of pathogens such as viruses, bacteria and protozoa have been associated with inflammatory heart disease. Among viruses, piconavirus coxsackie B3 (CVB3) is most commonly associated with myocarditis. It has been detected in heart of 40–50% of patients with dilated cardiomyopathy(6). Patients with myocarditis have been shown to have marked activation of TNF-α, IL-1 and IL-6(7). Persistent T cell activation by antigens intrinsic to myocardium that cross-react with viral peptides can lead to direct myocyte damage. In addition, evidence for a role of self-reactive T cells in myocarditis comes from myocardial antigen-specific T cells isolated from biopsy of patients with disease and induction of disease in SCID mice by transfer of T cells of patients with myocarditis(8).
There is a strong evidence of autoimmunity in myocarditis. Heart reactive antibodies are found in a large number of patients with myocarditis(9–12). Severity of cardiac impairment has been correlated with levels of autoantibodies in heart. Autoantigens for myocarditis include the β1 adrenoreceptor adenine nucleotide translocator (ANT), cardiac myosin, sarcolemmal and myolemmal proteins, extracellular matrix proteins and branched-chain keto acid hydrogenase (BCKD) (13). A high prevalence of autoantibodies has been described in unaffected members of an affected individual. These autoantibodies might be an early event in the sequence that leads to clinical disease, thus making these individuals at greater risk for development of cardiomyopathy. Using indirect immunofluorescence on human heart, organ-and disease-specific antibodies of IgG were observed in 33% of DCM patients(14) (15). All the antibodies were cardiac-specific. Western blotting identified one of them to be against myosin heavy alpha chain. Myosin comprises of an alpha and a beta chain. Myosin alpha chain is specific to cardiac myosin while beta chain is also expressed in skeletal myosin indicating organ-specific autoimmunity in myocarditis. Using ELISA, at least 20% of DCM patients had antibodies against α-myosin at the time of diagnosis. Among familial cases, increased frequency of patients (41%) and their relatives (20%) had these antibodies(14).
Recently several studies have shown an immunological basis of other systemic autoimmune disorder in patients with myocarditis ((16, 17). An increased prevalence of celiac disease (6%) and anti-tissue transglutaminase (tTg) has been described in patients with myocarditis and DCM. A gluten-free diet with immunosuppressive therapy is known to improve cardiac function in patients with celiac disease (CD) and autoimmune myocarditis. Increased frequency of DQ2 was observed in patients with cardiomyopathy and CD while myocarditis with increased tTg antibodies was associated with DQ8(18). Studies have suggested a potential role for tTg in human cardiomyocyte apoptosis (19{McDonough, 1999 #97)}. This demonstrates that autoimmune diseases might share some immunopathogenetic mechanisms.
Familial aggregation, presence of antibodies to cardiac antigens, association of HLA with DCM and antibodies, and abnormal expression of HLA class II on cardiac endothelium underscore the influence of genetic factors in development of autoimmune processes [(20–22)]. Individuals with HLA-DR4/DR1 and with HLA-DQ1, DQ5 have been shown to have increased susceptibility to myocarditis and dilated cardiomyopathy or both(23). Most of the studies have suggested a significant association of DCM with DR4. In addition positive association with DQB1*0601 and negative association with DR11 have been reported with DCM. Patients with serum antibodies against β1-adenoreceptor had decreased frequency of DR3 compared to patients negative for antibodies. HLA-DQB1*0602 has been associated with clinically overt disease and autoantibodies ((21). Theses studies point to an important role of MHC class II antigen in DCM. The discrepancy in MHC association with DCM could be due to sex and ethnic variability of the populations studied.
Experimental autoimmune myocarditis
Virus induced Myocarditis
As in humans, experimental autoimmune myocarditis (EAM) can be induced by infection with coxsackie B3 virus of susceptible mouse strains. The initial studies using Coxsackie virus infection for induction of myocarditis in mice came from Noel Rose’s group (reviewed in(24), (25). Mice injected with CVB intraperitoneally exhibit myocyte necrosis followed by inflammatory cell infiltrate and acute pathological damage in the myocardium after 7–10 days(26). A role of T cells was shown by marked reduction in myocardial injury during depletion study. DBA/2 mice with EMCV myocarditis have larger heart weight/body weight ratio than controls(27). The pathogenic mechanism involved in the transition from viral myocarditis to dilated cardiomyopathy remains to be elucidated. Persistent viral RNA in the heart tissue, aberrant immune system following viral infection and cell death have been put forth as some of the reasons for pathogenesis of dilated cardiomyopathy. However, development of CVB3-induced myocarditis is based on genetic differences among mice(28, 29). The induction of disease is affected by age, sex and strain of mouse and virus. A/J and BALB/c mice can develop acute myocarditis from day 7–14 post CVB and MCMV infection and chronic infection after day 28, which can persist for a long period. However, resistant C57BL/6 mice develop only mild acute myocarditis within 7–14 days of viral infection. Studies have confirmed that although in this model virus initiates the process, T cells play an instrumental role in disease pathogenesis.
In vivo studies using adoptive transfer of T cells and knockout mice provide further evidence that it is a T cell mediated disease. T cells from viral-infected heart can transfer disease when inoculated in to naïve mice (30). T cell depletion and athymic nude mice develop less severe disease to CVB(31). Mice lacking CD8 gene develop more severe disease than their heterozygote littermates, suggesting a role of CD8 cells in disease severity. On the other hand, CD4-deficient animals develop milder disease with an increased number of DN cells infiltrating heart. Using various knockout mice, role of p56lck, TNFRp55 ICOS and CD45 have been shown to be important in pathogenesis of autoimmune myocarditis ((32, 33). The pathogenesis of myocarditis is not fully understood, but there is sufficient evidence as described above that autoimmune response to cardiac myosin following infection in human and mouse model may contribute to the disease process.
Cardiac myosin induced Myocarditis
Experimental autoimmune myocarditis resembling human DCM can be induced in susceptible mice by immunization with purified foreign and murine cardiac specific α-myosin (34). The histopathology showed mononuclear infiltrates of CD4 T cells and antigen-presenting cells. Immunodominant peptides of cardiac α myosin have also been shown to induce myocarditis in susceptible strains of mice (35–37). Similar to viral-induced model, myosin-induced autoimmune myocarditis is also strain dependent suggesting MHC class II as an important genetic factor for susceptibility. Interestingly, the H2 a, s and f haplotypes are associated with increased morbidity in both viral and myosin induced models. Mice with H-2b haplotype are resistant to induced myocarditis. In both induced models, the prevalence and titer of cardiac specific antibodies are also MHC dependent. Transgenic expression of DQ6 and hCD4 rendered resistant H-2b mice susceptible to cardiac myosin induced myocarditis indicating a strong genetic influence (38). A strong T cell response accompanied by autoantibodies to cardiac α myosin heavy chain and strain-dependent response in experimental models of autoimmune myocarditis suggest MHC class II as an important genetic factor for susceptibility.
In addition to MHC, non-MHC loci are also involved in predisposition to autoimmune myocarditis (25, 39). Most mouse strains with congenic A background differing only at the MHC locus are susceptible to chronic autoimmune myocarditis while mouse strains with a B10 background are largely resistant to both viral and myosin-induced myocarditis model. Although both viral and myosin induced myocarditis show histological evidence of myocarditis and develop anti myosin antibodies, the myocarditis does not arise spontaneously thus potentially limiting the immune phenotype.
T cell mimicry in heart disease
Infectious pathogens, viruses, streptococci, Chlamydia and parasites have been suggested as etiologic factors of inflammatory heart disease (24). Molecular mimicry between pathogen and host antigen may break immune tolerance resulting in autoimmunity. During an infection, T cells that can recognize host and microbial epitopes may be important in the development of autoimmune responses. The inflammation in the heart may result from sequestered antigens like cardiac myosin during infection. For inflammatory heart disease, T cells reactive to cardiac myosin infiltrating the heart have been suggested to be important for pathogenesis of myocarditis and cardiomyopathy. Both humans and animal models generate cellular and humoral response to cardiac myosin. Structural and immunological mimicry between the streptococcal M protein and cardiac myosin in a rat model of myocarditis suggest T cell mimicry as a potential mechanism (40). Heart infiltrating T cells in myosin induced myocarditis proliferate to streptococcal M protein peptides. Also, focal myocarditis was observed in Balb/c mice immunized with peptides from streptococcal M5 protein homologous with cardiac myosin(41). The published data suggests mimicry between T cell epitopes in streptococcal peptide, Coxsackie virus and cardiac myosin. Streptococcal peptide homologous to cardiac myosin could prevent coxsackie induced myocarditis suggesting that T cell mimicry may be an important phenomenon for inflammatory heart disease.
Cytokines in Experimental Autoimmune Myocarditis
In humans and animal models of myocarditis, a prominent role of CD4 T cells and secreted cytokines has been demonstrated. In humans, myocarditis is heterogenous in nature with IFNγ driving a Th1-mediated disease in granulomatous myocarditis while macrophages and mast cells have been shown to secrete IL-4, TGFβ and IL-1β (33, 42, 43). In animals on the other hand, evidence regarding the role of cytokines is controversial. Several studies have reported an inflammatory role for Th1 cytokines and STAT4 in the induction of myocarditis, while its suppressive effects in myocarditis have been shown to occur via T-bet (44–46). Studies in IFNγ knockout mice are highly susceptible to EAM suggesting IFNγ is produced as a modulatory cytokine for protection purpose although at some point high levels of IFNγ and apoptosis may contribute to some pathology. Studies using in vivo IL-4 inhibition, IL-4 knockout and STAT6−/− mice have shown a controversial role of IL-4 in EAM. IL-4 has been shown to be important for pathogenesis in certain strains only (45, 47, 48).
Recent studies have shown a significant role of Th17 cells in autoimmunity. The IL-17 cytokine family consists of six members: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E/IL-25, and IL-17F. The best-characterized member of this family is IL-17A; its expression defines a newly discovered lineage of CD4+ effector T cells, the Th17 subset. Th17 cells can produce IL-17, IL-21, IL-22, IL-23 and IL-25. Recent data, however, suggest that EAM development critically depends on the IL-23-STAT4 axis (44). Using IL-12p35 and IL-12p40 knockout mice and neutralization studies, IL-23 was shown to be responsible for inflammatory heart disease although defective T cell response was only present in absence of both IL-12p40 and IL-12p35 (24, 49, 50). IL-23 has been suggested to promote a pathogenic IL-17-producing T cell population. Neutralization of IL-17 showed reduction in disease and heart autoantibody responses, suggesting that IL-17 is the critical effector cytokine responsible for EAM (49). However, IL-17A−/− mice are able to develop myocarditis with almost similar severity as wild type controls suggesting a role of other cytokines.
HLA class II transgenic mice as model of disease
Most of the work done in EAM, infectious as well as myosin induced, provided valuable information about the mechanism of autoimmunity. However, there are 2 confounding factors in the context of human disease, 1) the response to auto-antigen(s) is restricted by endogenous class II molecules and 2) no single virus has yet been defined as a cause for myocarditis in humans. In animal models, infection with various pathogens can lead to cardiomyopathy. Moreover, induction of disease can modulate the phenotype of disease.
Recent advent of HLA transgenic mice has helped in defining the role of genetic factors in human disease. Diane Mathis and Christopher Benoist (51) generated a mouse that lacked endogenous class II molecules (Aβo). In these mice, H2A β gene was disrupted so that no expression of H2A molecule was observed on the cell surface. These mice were utilized to generate transgenic mice that carried HLA alleles associated with various autoimmune diseases. Expression of only the human class II molecules in the thymus should lead to the development of DQ restricted CD4 T cells. With this hypothesis in mind, we introduced the human DQ8 gene into mouse class II deficient H2-Aβ° mice. Since these mice were of the H2b haplotype they had a natural nonfunctional Eα gene. These knockout mice do express the endogenous mouse class II Aα and Eβ gene. Mice expressing the HLA-DQ genes and lacking endogenous mouse class II expression were intercrossed to produce the DQ8.Aβ° mice (52). These mice expressed the DQ molecule in up to 25% of the peripheral blood lymphocytes. No endogenous mouse class II A or class II E molecules were detected. Further studies also showed that no hybrid HLA-DQβAα or HLA-DQαEβ molecules were generated in these mice.
The advent of mouse class II knock-out mice expressing human HLA-DR and HLA-DQ transgenes has significantly advanced the understanding of the role of individual HLA class II molecules (53). Aβo mice lack CD4 T cells. Introduction of HLA class II transgene in Aβo mice led to the expression of functional HLA class II molecule and reconstituted CD4 T cell compartment thus reconstituting CD4 restricted immune response to various peptides. The HLA transgene is considered self and mice are tolerant to the transgene.
Experimental data from various laboratories have shown that the HLA class II molecules in Aβo mice function similar to that in humans. The first evidence came from in vivo and in vitro studies done with super antigens in HLA transgenic mice. Bacterial super antigens (SAg) have lower affinity for mouse MHC class II than for human MHC class IImolecules. Because of this biologic characteristic, SAg-induced toxicity is much lower in mouse models than it is in humans. When immunized with SEB, HLA-DR3 transgenic mice respond to several log lower concentrations of super antigens and secrete higher levels of proinflammatory cytokines than wild-type mice (54). The response to super antigens in transgenic mice can result in toxic shock and is dependent on the polymorphism of class II alleles.
The second evidence comes from the peptide presentation by HLA transgenes in mice. The HLA transgenic mice respond to similar epitopes as observed in humans. In a comparison study DR3.Aβo mice recognized only one epitope comprising of aa 1–20 for heat shock protein (hsp) 65 of Mycobacterium tuberculosis as observed with human DR3-restricted T cells(55). The response was specific in DR3 mice as DQ8 mice did not respond to this peptide. These studies suggest that processing and presentation of the antigens in the context of class II molecules is similar in transgenic mice and humans. The third evidence that HLA transgenes in mice function similar to humans comes from studies with experimental autoimmune myasthenia gravis (EAMG) in DR3 transgenic mice (56). The wild type mice show a highly conserved TCRBV gene usage and CDR3 sequences in response to acetylcholine receptor (AChR), an autoantigen for myasthenia gravis patients. However, DR3-restricted murine hybridomas generated from DR3 mice immunized with AchR expressed a diverse set of T cell receptor B chains which is similar to that observed in humans. The TCRBV sequences from human MG patients were homologous to DR3 restricted murine clones, suggesting that human and mice can recognize similar epitopes and use similar CDR3 sequences for the recognition of the same peptide/MHC complex. Thus transgenic mice can provide an important insight into the peptide presentation by different classII alleles and the resulting pathogenesis.
Spontaneous model of Myocarditis and cardiomyopathy
Transgenic mice provide a unique tool to study the role of MHC molecules in disease association. MHC genes are the main restricting elements for specific response to foreign molecules by T lymphocytes and can provide important information concerning the immunopathogenesis and susceptibility to certain diseases. However, it is difficult to study the role of individual genes in humans. Humanized mice provide a valuable tool to study the unanswered questions. The background genes are controlled in the transgenic mice and all mice share a common environment. These conditions provide a means to study the role of MHC genes and non-MHC genes in disease pathogenesis.
Inbred non-obese diabetic (NOD) mice develop spontaneous autoimmune diabetes. Multiple genes contribute to the susceptibility; however the major susceptibility factor is the class II haplotype H-2Ag7. Ag7 has been shown to be structurally similar to HLA-DQ8 (57). We generated NOD.Aβo mice carrying HLA-DQ8 as a transgene in the absence of endogenous class II molecules. NOD.DQ8 mice were backcrossed to NOD.Aβo mice to generate congenic NOD background, N15, F15.Thus the only MHC molecule that can positively select T cells in thymus and present antigen in periphery are DQ8 molecules. We generated NOD.DQ8 mice with the hypothesis that DQ8 could replace Ag7 of NOD mice and develop spontaneous diabetes. NOD.DQ8 mice did not develop spontaneous diabetes as we had expected. However, a high mortality especially in female NOD/DQ8 mice was observed after 16 weeks of age (58). Morbidity was observed in 90% females with mean age of 18± 3 weeks while 20% male mice died around mean age of 25 weeks. To understand, the reason for mortality, we carried out histopathology of all organs (Figure 1). The histopathology showed that NOD.DQ8 mice develop myocarditis followed by dilated cardiomyopathy. Histologically, NOD.DQ8 hearts revealed a widespread, mixed inflammatory infiltrate consisting predominantly of lymphocytes, histiocytes and few neutrophils and eosinophils. The inflammatory lesions were associated with myocyte destruction without granuloma, giant cells or fibrosis suggesting development of spontaneous myocarditis in NOD.DQ8 mice. However, why NOD.DQ8 mice develop spontaneous autoimmunity is not clear. To understand if DQ8 is a requirement for expression of disease phenotype, we also examined another class II transgenic mouse with congenic NOD background, NOD.DR3.Aβo, for any signs of cardiac pathology. NOD mice do not develop spontaneous myocarditis. To understand if non-MHC genes may also contribute to pathogenesis, we studied DQ8 mice generated in congenic B10.Aβo. The control mice, B10.DQ8, NOD.DR3, transgene negative littermates and NOD mice survived with no signs of disease till about 50 weeks. Our studies supported the studies with induced models that polymorphism in class II is important in predisposition to myocarditis. Expression of DR3 transgene in NOD background does not lead to any spontaneous autoimmunity suggesting presence of DQ8 is critical for susceptibility to DCM in this model. NOD mice do not develop spontaneous myocarditis even though HLA-DQ8 is known to share structural homology with IAg7 suggesting that both can present different peptides. However, lack of disease in B10.DQ8 mice suggests contribution of non-MHC genes similar to that shown for induced models of myocarditis. The observations suggest that like other autoimmune disorders, disease expression in these mice requires both MHC and non-MHC genes.
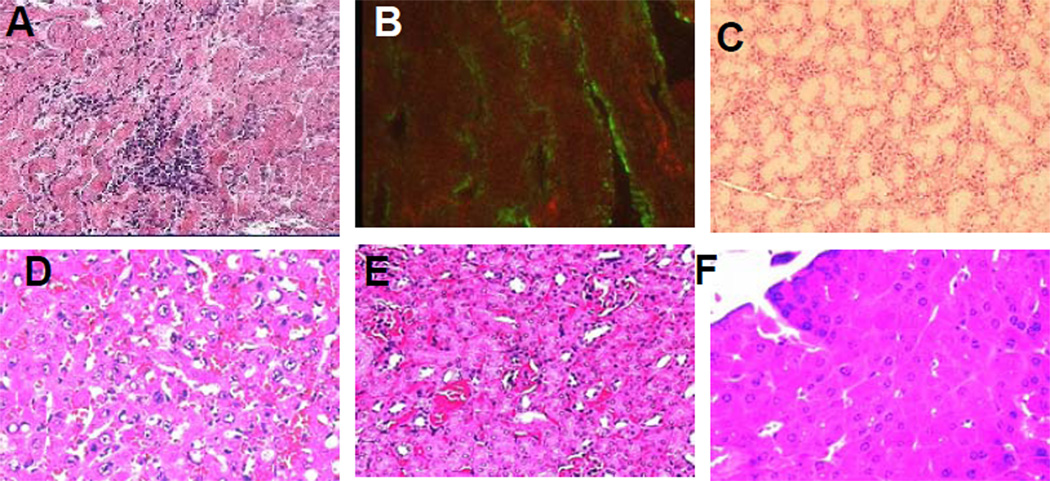
Figure 1.
NOD.DQ8.Abo mice develop spontaneous myocarditis leading to dilated cardiomyopathy. A) Histopathology of heart shows mononuclear infiltration along with myocyte destruction and B) IgG deposition in heart. Hematoxylin and Eosin stained section of C) thyroid gland D) Liver E) Kidney and F) Pancreas do not show any histopathology. Micrographs are at X100 magnification.
We showed for the first time that predominantly female NOD.DQ8 mice are susceptible to spontaneous myocarditis and cardiomyopathy. Autoimmunity is more common in women than men. 78% of autoimmune diseases occur in women (59). Interestingly, DR4/DQ8 is associated with another autoimmune disease, rheumatoid arthritis, which occurs more often in females. We believe that our model of spontaneous autoimmunity in females extends those observations. Extra-articular features like cardiovascular involvement in patients with severe RA is associated with the presence of DR4 (60). Since myocarditis is heterogenous in nature, it is possible that HLA-DQ8 may predispose females to autoimmune idiopathic myocarditis and DCM while males might develop DCM sequential to infectious etiology more commonly that is associated with other HLA molecules. Increased production of IFNγ and its contribution to disease in males that are CVB3 infected supports the notion (61).
Our data suggests that genetic factors in cardiomyopathy are not clearly defined in humans due to heterogenous nature of the disease and sex-specificity. It is possible that infectious and idiopathic cardiomyopathies are associated with different genetic factors. Studies in this model can greatly impact our understanding of the epistatic effect of HLA on autoimmunity in context of myocarditis and its relation to other autoimmune diseases.
Cardiomyopathy in NOD.DQ8 mice is similar to humans
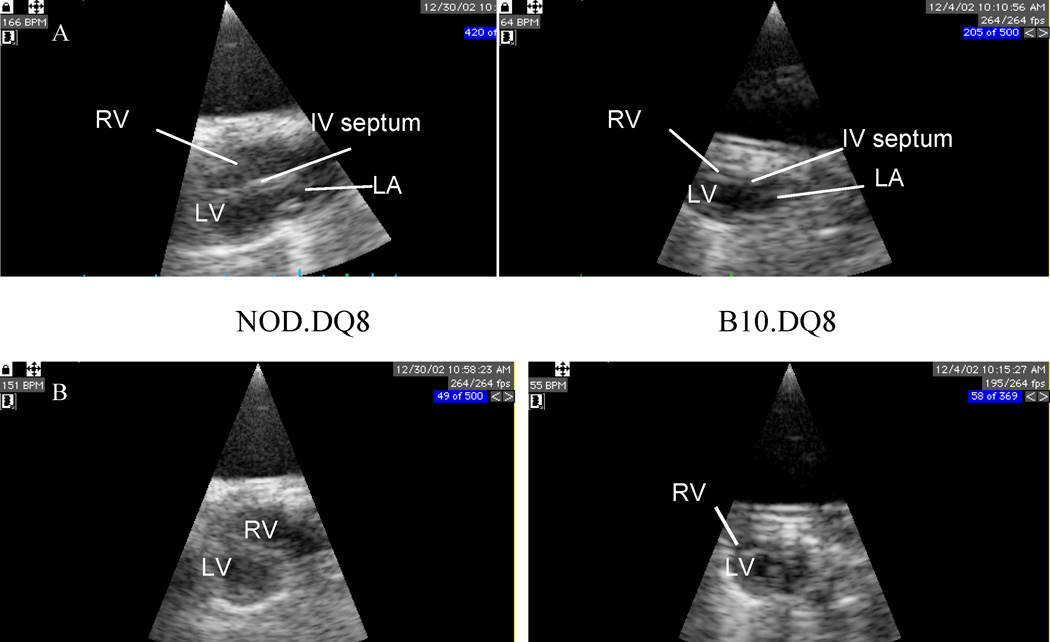
NOD.DQ8 affected mice showed cardiomegaly with a significantly higher heart/body weight ratio (0.01± 0.002) than control mice (.004±0.0006). Transthoracic echocardiography showed dilatation of both right ventricle (RV) and left ventricle (LV) with atrio-ventricular conduction block in NOD.DQ8 mice, in contrast to normal echo- and electro-cardiograms in B10.DQ8 mice The parasternal long axis (PSLA) and short axis (PSSA) in NOD.DQ8 mice (Figure 2) demonstrated systolic dysfunction as observed by fractional shortening and Circumferential shortening velocity similar to patients. To investigate if organs other than heart were also affected in NOD.DQ8 mice, histology of liver, lungs, pancreas, thyroid gland, salivary glands and kidney was done (Figure 1). Salivary glands showed scattered infiltration of cells, which is known to be a feature of sialadenitis in NOD mice. Pancreas, liver, kidney and thyroid gland showed no histopathology except few perivascular infiltrations of mononuclear cells was observed in lungs of NOD.DQ8 mice. B10.DQ8 mice, NOD.DR3 and negative littermates did not show any cardiomegaly or dysfunction of heart although salivary glands of NOD.DR3 mice did show scattered infiltration of cells.
Figure 2.
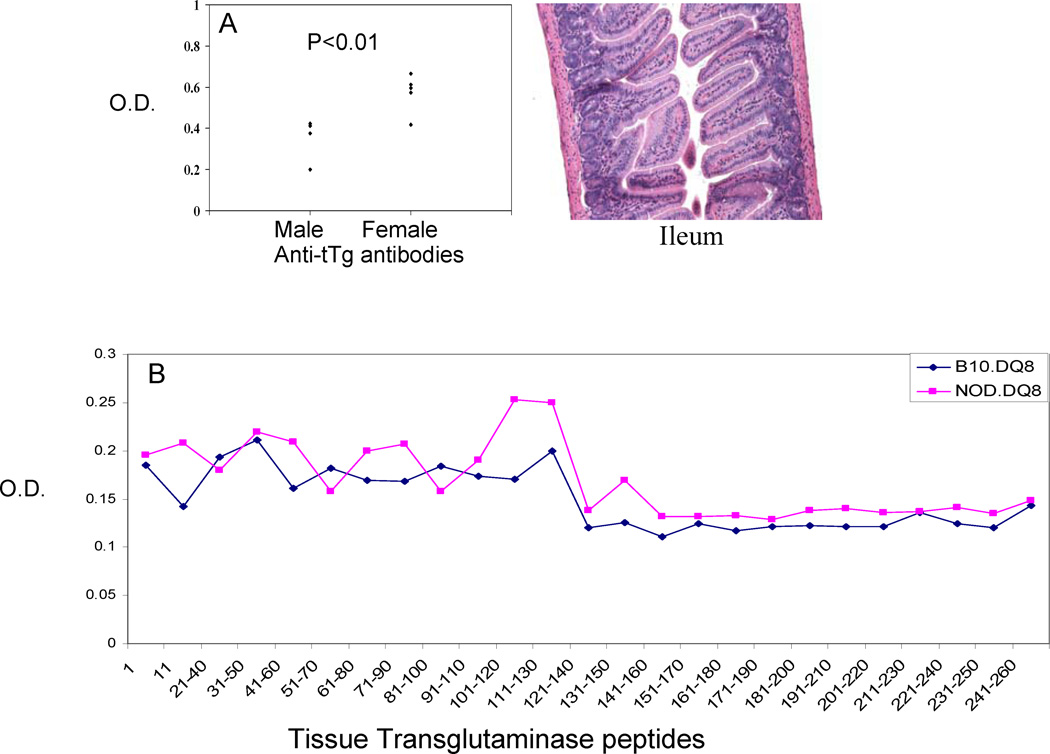
A) Anti-tag antibodies in male and female NOD.DQ8.Abo mice show higher levels in female mice. However, no enteropathy was observed in these mice as observed by H&E staining of sections of Ileum. B) Peptide ELISA using overlapping peptides of tissue transglutaminase shows a higher reactivity of NOD.DQ8 mice compared to B10.DQ8 mice. The region comprising aa 100–130 contained the most immunogenic epitopes.
Histopathology of hearts of NOD.DQ8 mice showed that by 8–10 weeks there was focal infiltration occupying around 20% of myocardium which by 15 weeks comprised of 70 % of myocardium. Both ventricles and the aorta were affected both with lesions distributed from endomyocardium to epicardium. Immunostaining of heart revealed an increased expression of DQ8 in NOD.DQ8 mice with myocarditis. In addition, mice produced cardiac myosin-specific IgG antibodies. Another similarity with human patients was an increased level of anti-tissue transglutaminase antibodies in mice with myocarditis. Affected female mice produce higher levels of autoantibodies compared to males (Figure 3A). Both B10.DQ8 and NOD.DQ8 mice produce anti-tTg antibodies, however antibodies in B10.DQ8 mice were very low. Peptide ELISA for antibodies to various peptides of tTg showed that region comprising of aa 100–130 was immunogenic in NOD.DQ8 mice (Figure 3B). Antibodies to tissue transglutaminase are a hallmark of celiac disease, however myocarditis mice did not show any enteropathy suggesting tissue transglutaminase and anti-Ttg antibodies complex may be involved locally in immune deposition and injury. Diseased mice showed IgG deposition in heart (Figure 1B).
Figure 3.
Echocardiographic assessment of heart of NOD.DQ8 and B10.DQ8 mice. A) Parasternal long axis view and B) Parasternal short axis view show dilation of both right and left inter-ventricle and left atrium with thickening of septum in hearts from NOD.DQ8 mice compared to B10.DQ8. RV-right ventricle, LV- left ventricle, LA-left atrium, and IV- intra ventricular.
Autoreactive response to cardiac myosin leads to spontaneous autoimmunity
Autoimmunity in the affected mice was evident by infiltrating mononuclear cells comprising of CD4 cells and macrophages in myocardium. NOD.DQ8 mice responded spontaneously to porcine cardiac myosin when challenged in vitro with it. B10.DQ8 and NOD mice generated a very low response suggesting NOD.DQ8 mice had cardiac myosin-reactive T cells that resulted in pathology. Delayed type response to cardiac myosin in these mice confirmed that NOD.DQ8 mice carry autoreactive T cells to heart myosin. In addition, NOD.DQ8 mice and not NOD.DR3 mice mount response to myosin derived peptide homologous between man and mouse. Since affected mice show higher expression of DQ8 in heart, presentation of peptides available from necrotic myocyte in heart to infiltrating autoreactive CD4 T cells may lead to amplification of inflammation. Heart infiltrating cells in mice have been shown to be generally restricted in Vb usage with most T cells being positive for Vβ8 (62, 63). We observed higher levels of antibodies to CDR1 of Vβ8.1 in NOD.DQ8 mice that developed disease compared to normal mice suggesting that the immune system in these mice is attempting to compensate immunological dysfunction by the production of immunomodulatory autoantibodies. Elevated antibodies to CDR1 of TCR Vβ8.1 have also been shown in sera of RA patients (64) suggesting autoimmunity may have common links. However, since TCR selection in thymus in B10.DQ8 and NOD.DQ8 mice should have been mediated by DQ8 in thymus, it suggests a mechanism in the periphery may lead to break in tolerance. The other autoimmune feature of NOD.DQ8 mice with myocarditis is the production of cardiac myosin specific antibodies of IgG1 and IgG2b subclass. Control mice, B10.DQ8 and NOD mice did not produce any autoantibodies.
Spontaneous cardiomyopathy is CD4 T cell dependent
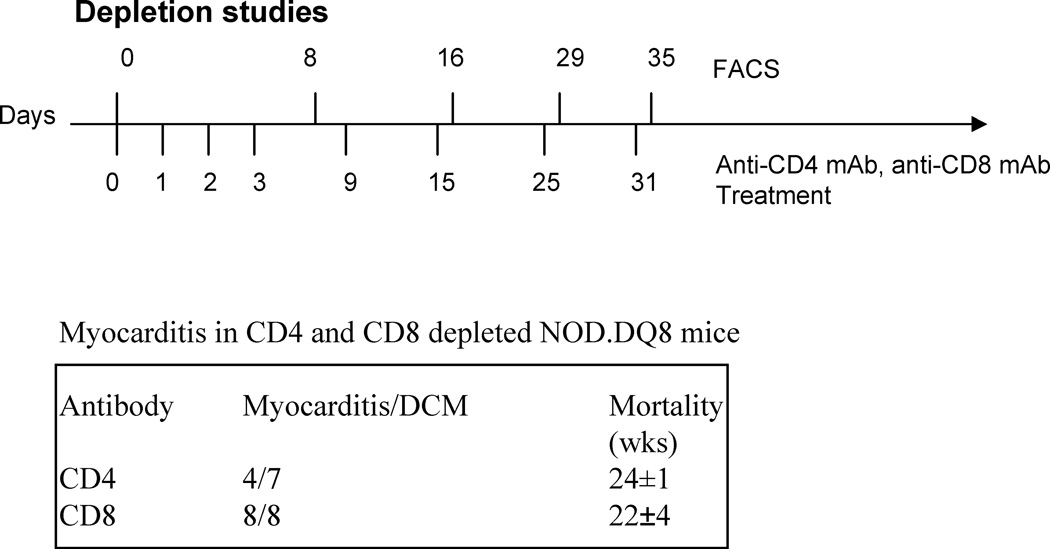
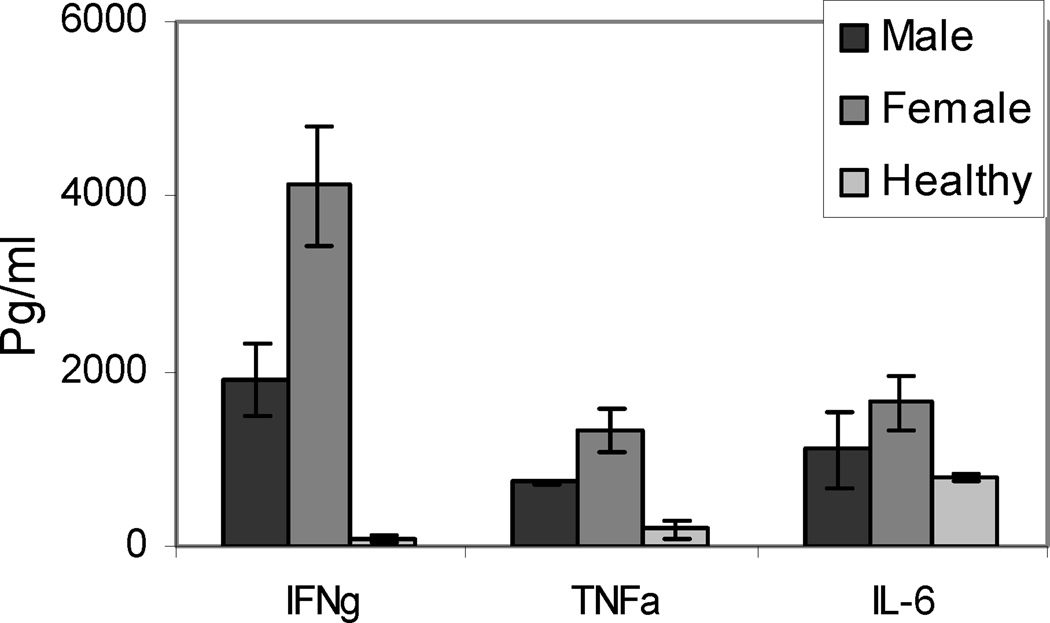
As CD4+ T cells are the predominant infiltrating cells in heart, we depleted CD4 T cells in vivo in NOD.DQ8 (4 females and 3 males) and B10.DQ8 (3M and 3F) mice using monoclonal antibody against mouse CD4 as shown in Figure 4. Depletion studies were started when mice were 6–8 weeks old, as that was the time when around 20% of myocardium was occupied by infiltrating cells. Almost all mice showed 99% depletion of CD4 cells as studied by FACS. PBS treated mice did not show any depletion. The CD4 depleted NOD.DQ8 mice still developed heart block and myocarditis but with delayed kinetics. Seventy five percent of CD4 depleted female mice were moribund around week 23 compared to 90% of PBS treated females that showed mortality around 18 weeks. However, B10.DQ8 mice did not show any evidence of disease. These studies suggest that CD4 T cells very likely play a role in the autoimmune myocarditis model. The autoreactive CD4 T cells infiltrate heart and secrete cytokines like IFNγ leading to recruitment and activation of macrophages. The infiltrating cells comprised of a huge number of macrophages. These activated macrophages can produce certain cytokines and chemokines starting a cascade of inflammation. Cytokine profile of myocarditis mice showed that mice with spontaneous myocarditis produced high amounts of IFNγ, IL6 and TNFα than healthy mice to cardiac myosin. Since predominantly females develop autoimmunity in our model, we analyzed sex specific difference in proinflammatory cytokines. There was no significant difference between male and female mice with myocarditis except IFNγ and TNFα were produced at higher levels in females (Figure 5). None of the mice produced IL-4 while both sexes produced IL-10. Production of prostaglandins, PGE2, mediated by PSG-2 has been shown to enhance IFN-γ production by Th1 T cells (65). Estrogen has been shown to significantly increase TNFα production. Thus an increased expression of prostaglandin (PGS2) under the influence of sex steroid may explain the gender differences in cytokine production and spontaneous myocarditis in NOD.DQ8 mice. As a significant role of TNFa is suggested in spontaneous and induced models, it is possible that anti-TNF therapy might work in patients with autoimmune myocarditis specially females similar to rheumatoid arthritis,
Figure 4.
Protocol for depleting CD4 and CD8 T cells in vivo. Mice were given intravenous injections of anti-CD4 (GK1.5) and anti-CD8 (lyt2) antibodies on indicated days. Presence of CD4 and CD8 cells in peripheral blood was analyzed by FACS after staining with specific conjugated antibodies on indicated days (top line). Most of the mice showed depletion of CD4 cells by 99% while CD8 T cells were depleted ranging between 85–95% in mice. Depleted mice were monitored for development of Myocarditis/dilated cardiomyopathy. CD4 depleted mice showed a later mortality as compared to nondepleted mice while CD8 depleted mice showed myocarditis in 100% of mice.
Figure 5.
Spontaneous cytokine production in response to in vitro challenge with porcine cardiac myosin in male and female mice with cardiomyopathy and healthy NOD.DQ8.Aβo mice.
CD8 T cells may act as regulatory T cells in spontaneous cardiomyopathy
Our studies with DQ8 mice using collagen-induced arthritis model have suggested a regulatory role of CD8 T cells in autoimmunity (66). Since we did not observe significant number of CD8 T cells in heart infiltrate, we set out to define function of CD8 T cells in pathogenesis of DCM. NOD.DQ8 mice were depleted of CD8 T cells in vivo with the hypothesis that depletion will lead to reduction in pathogenesis (Figure 4). However, depletion of CD8 T cells lead to DCM development in 100% of mice with mean mortality at around 23 weeks. This data suggested that CD8 T cells may be acting as regulatory T cells. CD8 deficient mice infected with CVB3 showed reduced disease confirming a role of CD8 as regulatory molecule in autoimmune myocarditis. This is also supported by studies showing that adoptive transfer of CD4+ T cells from NOD.DQ8 mice to Rag1−/− mice leads to much larger hearts compared to spontaneous DCM in NOD.DQ8 mice as enriched CD4 T cells in the absence of regulatory CD8 cells may lead to a severe outcome similar to that observed in arthritis model using DQ8.CD8−/− mice. NOD.DQ8.β2m° mice did not develop spontaneous myocarditis suggesting that class I bearing cells CD8, NKT and NK cells may be involved in autoimmune response (67). NOD and NOD.DQ8.Aβo mice differ in MHC class I molecules (Kd in NOD versus Kb in NOD.DQ8 mice). However, a role of MHC class I is ruled out as NOD.DR3.Abo and NOD.DQ8.Aβo mice share class I genes but the former mice do not develop spontaneous cardiomyopathy. NOD mice are deficient in NKT cells and it has been suggested that a further reduction in NKT cells leads to exacerbation of anti-islet autoimmunity suggesting NKT cells could also be involved in regulation. Recently, a role of CD8 Treg has been shown in GAD-IgG gene-transferred tolerance induction in NOD mice (68). These data are in contrast to popular belief that CD4 Treg can modulate adaptive T cell response while CD8 T cells are required for cytolytic activity. While the autoreactive CD8 T cells produce IFNg, regulatory CD8 T cells produce TGFβ and IL-10. Thus a defect in the T regulatory cells, CD4 or CD8, in combination with the cytokine milieu may decide the final outcome.
Concluding Remarks
MHC class II association with autoimmune disorders implies that autoantigen-specific T cells are important in the pathogenesis although the exact role of the MHC class II molecules in these disease processes is unknown. One way to study the role autoantigen/s in biologically relevant situation is to generate mice expressing human MHC alleles. While no single animal model perfectly mimics a human disease, experimental animal models afford opportunities to study the role of individual genes for investigating genetic, environmental and pathogenic aspects of an autoimmune disease.
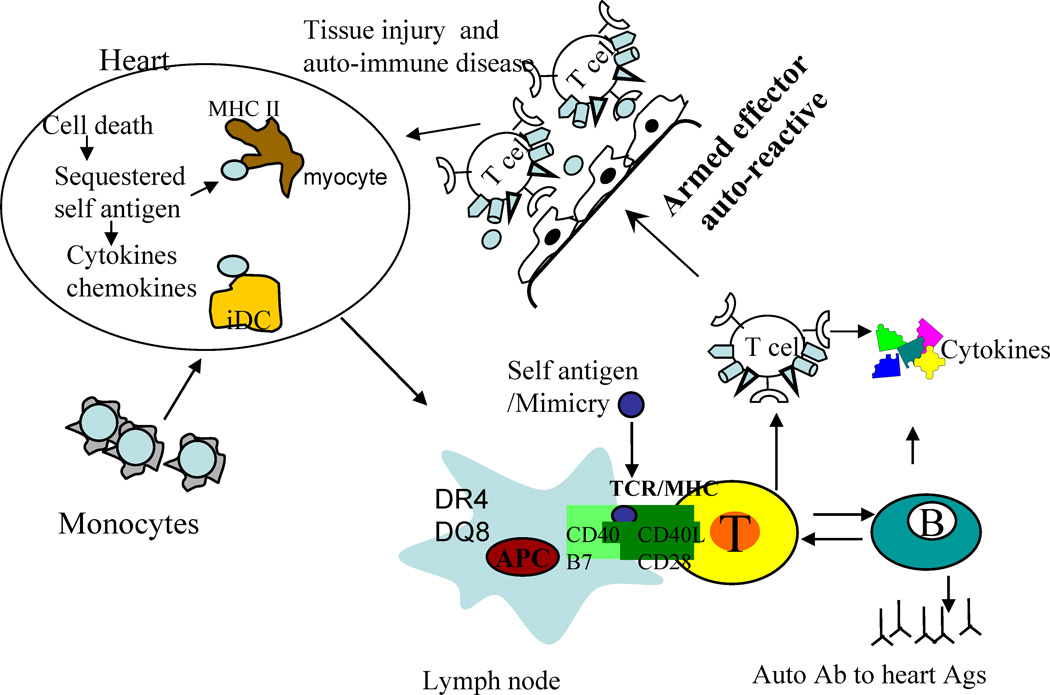
In humans, Myocarditis/DCM is heterogenous in nature and difficult to diagnose. It is essential to classify the disease in to categories based on the immunological profile. Based on the etiology of the disease, the treatment options for an individual may differ. The induced and spontaneous models of cardiomyopathy may represent different etiologies even though the affected organ is same. Defining mechanism of pathogenesis in both models will help define subclasses of disease. Ours and other findings bring forth following important observations 1) sex hormones likely contribute towards pathogenic response in this model, 2) Both MHC and non-MHC genes are required for pathogenesis 3) Disease is dependent on MHC class II polymorphism. In conclusion, this model bears similarities to human disease and spontaneous model should provide a valuable tool for exploring mechanisms involved in heart disease in women. In spontaneous model, DQ8 can positively select autoreactive T cells that can be presented in certain conditions in heart or in periphery and when these activated autoreactive T cells home to heart, an inflammatory scenario is produced by chemokines and cytokines thus amplifying the inflammatory loop. In induced model, a similar inflammation occurs in heart although it is due to insult by immunization or by virus (Figure 6).
Figure 6.
Schematic diagram of pathogenesis of myocarditis/dilated cardiomyopathy. Any damage to heart by viral infection can lead to cell death and availability of self antigen. Myocytes express class II molecules and in the event of inflammation, can present self antigen to infiltrating T cells. Alternatively, T cells and DCs in the periphery amplify the autoreactive response via antigen presentation, self or microbial epitopes that mimic heart antigen, and production of autoantibodies. These effector T cells can home to heart and lead to damage. Monocytes can carry antigens to heart and can activate DCs locally resulting in production of chemokines and inflammatory cytokines leading to infiltration of heart with T cells and mature DCs followed by destruction of myocytes and cardiomyopathy.
The HLA transgenic mice take us one step closer to understanding the role of HLA molecules, and accessory molecules involved in initiation and ensuing pathways after presentation of an autoantigen in inflammatory diseases. Finally, these mice can be utilized to try new therapeutic strategies like immunotherapies, gene therapy and vaccines and evaluate their usefulness in human population. It is fitting that we contribute this paper to the special issue dedicated to the enormous contributions of Dr. Noel Rose to autoimmunology. It is fitting because Noel Rose has contributed not only to autoimmune myocarditis, but also to the genetic contributions of autoimmunity. The field of autoimmunology is becoming increasingly recognized as a major discipline (69). We also acknowledge Dr. Rose’s long term commitment to autoimmunity (70–77), his development of the American Autoimmune Related Diseases Association (AARDA) and the workshops that result (78). Finally, we note that this issue is part of the Journal’s commitment in the recognition of outstanding figures in international autoimmunity (79–81).
Table.
Myocarditis and dilated cardiomyopathy in transgenic mice.
| NOD.DQ8 | B10. DQ8, NOD.DR3, NOD | |
|---|---|---|
| Mortality | ||
| Female | 89% | 0% |
| Male | 20% | |
| Onset | ||
| Female | 18±3wks | |
| Male | 25±3 | |
| Weight | ||
| Heart | .2–.25 gms | .12–.15 |
| Body | 22–25 gms | 25–30 |
| H/B ratio | .01–.05 | .004–.005 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim KS, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Reviews in medical virology. 2001;11:355–368. doi: 10.1002/rmv.326. [DOI] [PubMed] [Google Scholar]
- 2.Olinde KD, O'Connell JB. Inflammatory heart disease: pathogenesis, clinical manifestations, and treatment of myocarditis. Annual review of medicine. 1994;45:481–490. doi: 10.1146/annurev.med.45.1.481. [DOI] [PubMed] [Google Scholar]
- 3.Rose NR, Herskowitz A, Neumann DA, Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunology today. 1988;9:117–120. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- 4.Maisch B, Bauer E, Cirsi M, Kochsiek K. Cytolytic cross-reactive antibodies directed against the cardiac membrane and viral proteins in coxsackievirus B3 and B4 myocarditis. Characterization and pathogenetic relevance. Circulation. 1993;87:IV49–IV65. [PubMed] [Google Scholar]
- 5.Rose NR, Neumann DA, Herskowitz A, Traystman MD, Beisel KW. Genetics of susceptibility to viral myocarditis in mice. Pathology and immunopathology research. 1988;7:266–278. doi: 10.1159/000157122. [DOI] [PubMed] [Google Scholar]
- 6.Maier R, Krebs P, Ludewig B. Immunopathological basis of virus-induced myocarditis. Clinical & developmental immunology. 2004;11:1–5. doi: 10.1080/10446670410001670427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. British heart journal. 1994;72:561–566. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwimmbeck PL, Huber SA, Schultheiss HP. Roles of T cells in coxsackievirus B-induced disease. Current topics in microbiology and immunology. 1997;223:283–303. doi: 10.1007/978-3-642-60687-8_13. [DOI] [PubMed] [Google Scholar]
- 9.Caforio AL, Goldman JH, Haven AJ, Baig KM, McKenna WJ. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. International journal of cardiology. 1996;54:157–163. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- 10.Herskowitz A, Ahmed-Ansari A, Neumann DA, Beschorner WE, Rose NR, Soule LM, Burek CL, Sell KW, Baughman KL. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. Journal of the American College of Cardiology. 1990;15:624–632. doi: 10.1016/0735-1097(90)90637-5. [DOI] [PubMed] [Google Scholar]
- 11.Herskowitz A, Campbell S, Deckers J, Kasper EK, Boehmer J, Hadian D, Neumann DA, Baughman KL. Demographic features and prevalence of idiopathic myocarditis in patients undergoing endomyocardial biopsy. The American journal of cardiology. 1993;71:982–986. doi: 10.1016/0002-9149(93)90918-3. [DOI] [PubMed] [Google Scholar]
- 12.Neumann DA, Burek CL, Baughman KL, Rose NR, Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. Journal of the American College of Cardiology. 1990;16:839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- 13.Schultheiss HP. The significance of autoantibodies against the ADP/ATP carrier for the pathogenesis of myocarditis and dilated cardiomyopathy--clinical and experimental data. Springer seminars in immunopathology. 1989;11:15–30. doi: 10.1007/BF00197081. [DOI] [PubMed] [Google Scholar]
- 14.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 15.Bilinska ZT, Caforio AL, Grzybowski J, Michalak E, Kusmierczyk-Droszcz B, Goldman JH, Haven AJ, Rydlewska-Sadowska W, McKenna WJ, Ruzyllo W. Organ-specific cardiac autoantibodies in dilated cardiomyopathy. Frequency and clinical correlates in Polish patients. European heart journal. 1995;16:1907–1911. doi: 10.1093/oxfordjournals.eurheartj.a060846. [DOI] [PubMed] [Google Scholar]
- 16.Peracchi M, Trovato C, Longhi M, Gasparin M, Conte D, Tarantino C, Prati, and M. T. Bardella D. Tissue transglutaminase antibodies in patients with end-stage heart failure. The American journal of gastroenterology. 2002;97:2850–2854. doi: 10.1111/j.1572-0241.2002.07033.x. [DOI] [PubMed] [Google Scholar]
- 17.Curione M, Barbato M, De Biase L, Viola F, Lo Russo L, Cardi E. Prevalence of coeliac disease in idiopathic dilated cardiomyopathy. Lancet. 1999;354:222–223. doi: 10.1016/s0140-6736(99)01501-9. [DOI] [PubMed] [Google Scholar]
- 18.Frustaci A, Cuoco L, Chimenti C, Pieroni M, Fioravanti G, Gentiloni N, Maseri, and G. Gasbarrini A. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611–2618. doi: 10.1161/01.cir.0000017880.86166.87. [DOI] [PubMed] [Google Scholar]
- 19.Lotze U, Busch HJ, Aschoff A, Gluck B, Sigusch H, Jirikowski G, Stelzner, and H. R. Figull A. Damaged myocytes as detected by the colocalization of DNA fragmentation and tissue transglutaminase and their prognostic significance in enterovirus-associated dilated cardiomyopathy. European journal of clinical investigation. 2001;31:744–755. doi: 10.1046/j.1365-2362.2001.00878.x. [DOI] [PubMed] [Google Scholar]
- 20.Lozano MD, Rubocki RJ, Wilson JE, McManus BM, Wisecarver JL. Human leukocyte antigen class II associations in patients with idiopathic dilated cardiomyopathy. Myocarditis Treatment Trial Investigators. Journal of cardiac failure. 1997;3:97–103. doi: 10.1016/s1071-9164(97)90041-5. [DOI] [PubMed] [Google Scholar]
- 21.Limas C, Limas CJ, Boudoulas H, Bair R, Graber H, Sparks L, Wooley CF. Anti-beta-receptor antibodies in familial cardiomyopathy: correlation with HLADR and HLA-DQ gene polymorphisms. American heart journal. 1994;127:382–386. doi: 10.1016/0002-8703(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 22.Caforio AL, Iliceto S. Genetically determined myocarditis: clinical presentation and immunological characteristics. Current opinion in cardiology. 2008;23:219–226. doi: 10.1097/HCO.0b013e3282fbf572. [DOI] [PubMed] [Google Scholar]
- 23.Limas CJ. Autoimmunity in dilated cardiomyopathy and the major histocompatibility complex. International journal of cardiology. 1996;54:113–116. doi: 10.1016/0167-5273(96)02587-9. [DOI] [PubMed] [Google Scholar]
- 24.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Advances in immunology. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 25.Li HS, Ligons DL, Rose NR. Genetic complexity of autoimmune myocarditis. Autoimmunity reviews. 2008;7:168–173. doi: 10.1016/j.autrev.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herskowitz A, Beisel KW, Wolfgram LJ, Rose NR. Coxsackievirus B3 murine myocarditis: wide pathologic spectrum in genetically defined inbred strains. Human pathology. 1985;16:671–673. doi: 10.1016/s0046-8177(85)80149-0. [DOI] [PubMed] [Google Scholar]
- 27.Hirasawa M, Kitaura Y, Deguchi H, Ukimura A, Kawamura K. Spontaneous myocarditis in DBA/2 mice. Light microscopic study with transmission and X-ray analytical electron microscopic studies. Virchows Arch. 1998;432:461–468. doi: 10.1007/s004280050192. [DOI] [PubMed] [Google Scholar]
- 28.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. Journal of autoimmunity. 2001;16:175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 29.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- 30.Huber SA. Autoimmunity in myocarditis: relevance of animal models. Clinical immunology and immunopathology. 1997;83:93–102. doi: 10.1006/clin.1997.4342. [DOI] [PubMed] [Google Scholar]
- 31.Woodruff JF, Woodruff JJ. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]
- 32.Liu P, Aitken K, Kong YY, Opavsky MA, Martino T, Dawood F, Wen WH, Kozieradzki I, Bachmaier K, Straus D, Mak TW, Penninger JM. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nature medicine. 2000;6:429–434. doi: 10.1038/74689. [DOI] [PubMed] [Google Scholar]
- 33.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJ, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Afanasyeva M, Hill SL, Rose NR. Characterization of murine autoimmune myocarditis induced by self and foreign cardiac myosin. Autoimmunity. 1999;31:151–162. doi: 10.3109/08916939908994060. [DOI] [PubMed] [Google Scholar]
- 35.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 36.Donermeyer DL, Beisel KW, Allen PM, Smith SC. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. The Journal of experimental medicine. 1995;182:1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, Lenz DM, Zamborelli TJ, Penninger JM, Neu N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. The Journal of clinical investigation. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachmaier K, Neu N, Yeung RS, Mak TW, Liu P, Penninger JM. Generation of humanized mice susceptible to peptide-induced inflammatory heart disease. Circulation. 1999;99:1885–1891. doi: 10.1161/01.cir.99.14.1885. [DOI] [PubMed] [Google Scholar]
- 39.Ligons DL, Guler ML, Li HS, Rose NR. A locus on chromosome 1 promotes susceptibility of experimental autoimmune myocarditis and lymphocyte cell death. Clinical immunology (Orlando, Fla. 2009;130:74–82. doi: 10.1016/j.clim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham MW. T cell mimicry in inflammatory heart disease. Molecular immunology. 2004;40:1121–1127. doi: 10.1016/j.molimm.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Huber SA, Cunningham MW. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. J Immunol. 1996;156:3528–3534. [PubMed] [Google Scholar]
- 42.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. The American journal of pathology. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, Rose NR. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 44.Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha, and N. R. Rose AA. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway. Circulation. 2001;104:3145–3151. doi: 10.1161/hc5001.100629. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001;167:5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- 46.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. The Journal of experimental medicine. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afanasyeva M, Wang Y, Kaya Z, Park S, Zilliox MJ, Schofield BH, Hill, and N. R. Rose SL. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. The American journal of pathology. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonnella PA, Del Nido PJ, McGowan FX. Oral Tolerization with Cardiac Myosin Peptide (614-629) Ameliorates Experimental Autoimmune Myocarditis: Role of Stat 6 Genes in BALB/CJ Mice. Journal of clinical immunology. 2009 doi: 10.1007/s10875-009-9290-z. [DOI] [PubMed] [Google Scholar]
- 49.Sonderegger I, Rohn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. European journal of immunology. 2006;36:2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 50.Vogt L, Schmitz N, Kurrer MO, Bauer M, Hinton HI, Behnke S, Gatto D, Sebbel P, Beerli RR, Sonderegger I, Kopf M, Saudan P, Bachmann MF. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. The Journal of clinical investigation. 2006;116:2817–2826. doi: 10.1172/JCI25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 52.Nabozny GH, Baisch JM, Cheng S, Cosgrove D, Griffiths MM, Luthra, and C. S. David HS. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. The Journal of experimental medicine. 1996;183:27–37. doi: 10.1084/jem.183.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA class II transgenic mice mimic human inflammatory diseases. Advances in immunology. 2008;97:65–147. doi: 10.1016/S0065-2776(08)00002-3. [DOI] [PubMed] [Google Scholar]
- 54.DaSilva L, Welcher BC, Ulrich RG, Aman MJ, David CS, Bavari S. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. The Journal of infectious diseases. 2002;185:1754–1760. doi: 10.1086/340828. [DOI] [PubMed] [Google Scholar]
- 55.Geluk A, Taneja V, van Meijgaarden KE, de Vries RR, David CS, Ottenhoff TH. HLA-DR/DQ transgenic, class II deficient mice as a novel model to select for HSP T cell epitopes with immunotherapeutic or preventative vaccine potential. Biotherapy (Dordrecht, Netherlands) 1998;10:191–196. doi: 10.1007/BF02678296. [DOI] [PubMed] [Google Scholar]
- 56.Infante AJ, Baillargeon J, Kraig E, Lott L, Jackson C, Hammerling GJ, Raju, and C. David R. Evidence of a diverse T cell receptor repertoire for acetylcholine receptor, the autoantigen of myasthenia gravis. Journal of autoimmunity. 2003;21:167–174. doi: 10.1016/s0896-8411(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 57.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nature immunology. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 58.Taneja V, Behrens M, Cooper LT, Yamada S, Kita H, Redfield MM, Terzic, and C. David A. Spontaneous myocarditis mimicking human disease occurs in the presence of an appropriate MHC and non-MHC background in transgenic mice. Journal of molecular and cellular cardiology. 2007;42:1054–1064. doi: 10.1016/j.yjmcc.2007.03.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. The American journal of pathology. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez-Gay MA, Gonzalez-Juanatey C, Ollier WE. Endothelial dysfunction in rheumatoid arthritis: influence of HLA-DRB1 alleles. Autoimmunity reviews. 2004;3:301–304. doi: 10.1016/j.autrev.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, Fairweather D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain research. 2006;1126:139–147. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto Y, Jee Y, Sugisaki M. Successful TCR-based immunotherapy for autoimmune myocarditis with DNA vaccines after rapid identification of pathogenic TCR. J Immunol. 2000;164:2248–2254. doi: 10.4049/jimmunol.164.4.2248. [DOI] [PubMed] [Google Scholar]
- 63.Sepulveda RT, Marchalonis JJ, Watson RR. T-cell receptor vbeta8.1 peptide reduces coxsackievirus-induced cardiopathology in aged mice. Cardiovascular toxicology. 2005;5:21–28. doi: 10.1385/ct:5:1:021. [DOI] [PubMed] [Google Scholar]
- 64.Schluter SF, Adelman MK, Taneja V, David C, Yocum DE, Marchalonis JJ. Natural autoantibodies to TCR public idiotopes: potential roles in immunomodulation. Cellular and molecular biology (Noisy-le-Grand, France) 2003;49:193–207. [PubMed] [Google Scholar]
- 65.Bloom D, Jabrane-Ferrat N, Zeng L, Wu A, Li L, Lo D, Turck CW, An S, Goetzl EJ. Prostaglandin E2 enhancement of interferon-gamma production by antigen-stimulated type 1 helper T cells. Cellular immunology. 1999;194:21–27. doi: 10.1006/cimm.1999.1479. [DOI] [PubMed] [Google Scholar]
- 66.Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra H, David CS. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- 67.Hayward SL, Bautista-Lopez N, Suzuki K, Atrazhev A, Dickie P, Elliott JF. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J Immunol. 2006;176:7715–7725. doi: 10.4049/jimmunol.176.12.7715. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, Han G, Song L, Wang J, Chen G, Xu R, Yu M, Qian J, Shen B, Li Y. CD8+ regulatory T cells are responsible for GAD-IgG gene-transferred tolerance induction in NOD mice. Immunology. 2009;126:123–131. doi: 10.1111/j.1365-2567.2008.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmmun. 2008;31:325–330. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29:1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose NR, Witebsky E. Studies on organ specificity. II. Serological interrelationships among thyroid extracts of various species. J Immunol. 1955;75:282–290. [PubMed] [Google Scholar]
- 72.Rose NR, Brinckerhoff CE. Characteristics of some "natural" autoantibodies in rabbits. J Immunol. 1969;102:682–687. [PubMed] [Google Scholar]
- 73.Rose NR, Stylos WA. Splitting of human thyroglobulin. I. Reduction and alkylation. Clin Exp Immunol. 1969;5:129–140. [PMC free article] [PubMed] [Google Scholar]
- 74.Yativ N, Buskila D, Blank M, Burek CL, Rose NR, Shoenfeld Y. The detection of antithyroglobulin activity in human serum monoclonal immunoglobulins (monoclonal gammopathies) Immunol Res. 1993;12:330–337. doi: 10.1007/BF02935506. [DOI] [PubMed] [Google Scholar]
- 75.Caturegli P, Lupi I, Landek-Salgado M, Kimura H, Rose NR. Pituitary autoimmunity: 30 years later. Autoimmun Rev. 2008;7:631–637. doi: 10.1016/j.autrev.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rose NR. Genesis and evolution of diagnostic and clinical immunology. Clin Diagn Lab Immunol. 1999;6:289–290. doi: 10.1128/cdli.6.3.289-290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polley CR, Bacon LD, Rose NR. Spontaneous autoimmune thyroiditis in chickens. I. Effects of bursal reconstitution. J Immunol. 1981;127:1465–1468. [PubMed] [Google Scholar]
- 78.Mackay IR, Leskovsek NV, Rose NR. Cell damage and autoimmunity: a critical appraisal. J Autoimmun. 2008;30:5–11. doi: 10.1016/j.jaut.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whittingham S, Rowley MJ, Gershwin ME. A tribute to an outstanding immunologist - Ian Reay Mackay. J Autoimmun. 2008;31:197–200. doi: 10.1016/j.jaut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Gershwin ME. Bone marrow transplantation, refractory autoimmunity and the contributions of Susumu Ikehara. J Autoimmun. 2008;30:105–107. doi: 10.1016/j.jaut.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Blank M, Gershwin ME. Autoimmunity: from the mosaic to the kaleidoscope. J Autoimmun. 2008;30:1–4. doi: 10.1016/j.jaut.2007.11.015. [DOI] [PubMed] [Google Scholar]