Abstract
The global marine distributions of Cd and phosphate are closely correlated, which has led to Cd being considered as a marine micronutrient, despite its toxicity to life. The explanation for this nutrient-like behavior is unknown because there is only one identified biochemical function for Cd, an unusual Cd/Zn carbonic anhydrase. Recent developments in Cd isotope mass spectrometry have revealed that Cd uptake by phytoplankton causes isotopic fractionation in the open ocean and in culture. Here we investigate the physiochemical pathways that fractionate Cd isotopes by performing subcellular Cd isotope analysis on genetically modified microorganisms. We find that expression of the Cd/Zn carbonic anhydrase makes no difference to the Cd isotope composition of whole cells. Instead, a large proportion of the Cd is partitioned into cell membranes with a similar direction and magnitude of Cd isotopic fractionation to that seen in surface seawater. This observation is well explained if Cd is mistakenly imported with other divalent metals and subsequently managed by binding within the cell to avoid toxicity. This process may apply to other divalent metals, whereby nonspecific uptake and subsequent homeostasis may contribute to elemental and isotopic distributions in seawater, even for elements commonly considered as micronutrients.
Keywords: biological fractionation, isotope geochemistry, metal homeostasis, subcellular analysis, trace metal
In addition to the macronutrients nitrate, phosphate, and silicate, marine phytoplankton require many essential metals to function correctly (1). The uptake and utilization of these micronutrients results in large vertical isotopic and concentration gradients for many transition metals in seawater (2). In the open ocean, Cd concentrations are as low as a few picomoles per kilogram (2, 3), with associated highly fractionated Cd isotopic compositions of up to several permil (Fig. 1) (4). In culture, phytoplankton consume small quantities of Cd (5–7) and exhibit light Cd isotope compositions (8). Taken together, the available data suggest that the marine geochemistry of Cd is dominated by nutrient-like processes (i.e., required uptake). However, only one biochemical function for Cd is known: CdCA1, a Cd/Zn carbonic anhydrase from the marine diatom Thalassiosira weissflogii (9, 10). Although ubiquitous in natural waters (11), CdCA1 is absent in numerous phytoplankton including coccolithophores, cyanobacteria, archaea, and several species of diatom (11) (Table S1), and it is thus debateable whether the expression of this single enzyme can account for the nutrient-like distribution of Cd in the global ocean. Here we examine the isotopic fractionation of Cd associated with CdCA1 that has been expressed in vivo by the model microorganism, Escherichia coli. Because E. coli has no inherent use for Cd, our experimental design permits comparison of cultures both overexpressing and not expressing CdCA1 in otherwise identical experiments. Using a systematic experimental approach, we are able to identify the general physiochemical pathways that fractionate Cd isotopes.
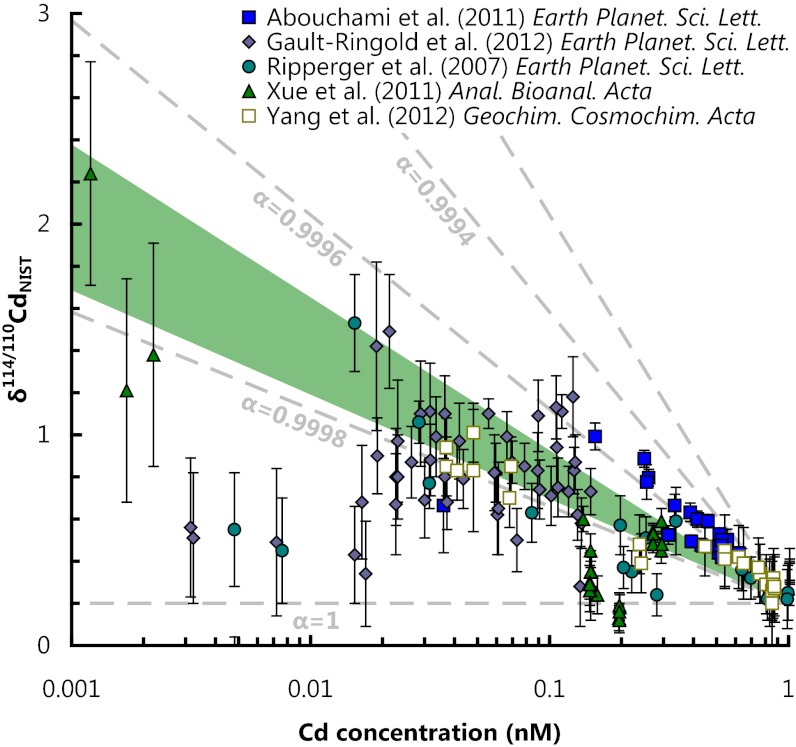
Fig. 1.
Comparison of dissolved Cd isotope compositions and Cd concentrations for all published analyses of seawater (4, 56–59). All data have been renormalized to NIST SRM 3108 (60). Dashed tie lines are shown for uptake that follows closed-system Rayleigh fractionation (as various α values) assuming an isotopically light removal phase, where αremoval-Cd(aq.) = (114Cd/110Cd)removal/(114Cd/110Cd)Cd(aq.). The fractionation factor observed for membrane storage (Cd homeostasis, αhomeo. = 0.99974 ± 0.00005, green shaded region) is consistent with the direction and magnitude of Cd isotope fractionation observed in modern seawater. The large scatter in the data is largely a consequence of water mass mixing of isotopically distinct water masses.
The role of CdCA1 expression in determining whole-cell isotopic compositions was investigated by introducing the CdCA1 coding sequence from T. weissflogii into the competent E. coli strain BL21(DE3). Seven replicate experiments were performed that differed only in the final cell washing step (SI Materials and Methods and Table S2). In each experiment, the culture suspension was divided into two parallel cultures that were grown with and without the addition of isopropyl-β-D-1-thiogalactopyranoside (IPTG; a chemical inducer) to promote overexpression of CdCA1 (Fig. 2). By adding IPTG to only 7 of the 14 cultures, we were able to independently study the role of CdCA1 expression in determining whole-cell Cd isotopic compositions without complications arising from differing physiologies. The 14 cultures were grown for up to 4 h and harvested during the exponential growth stage.
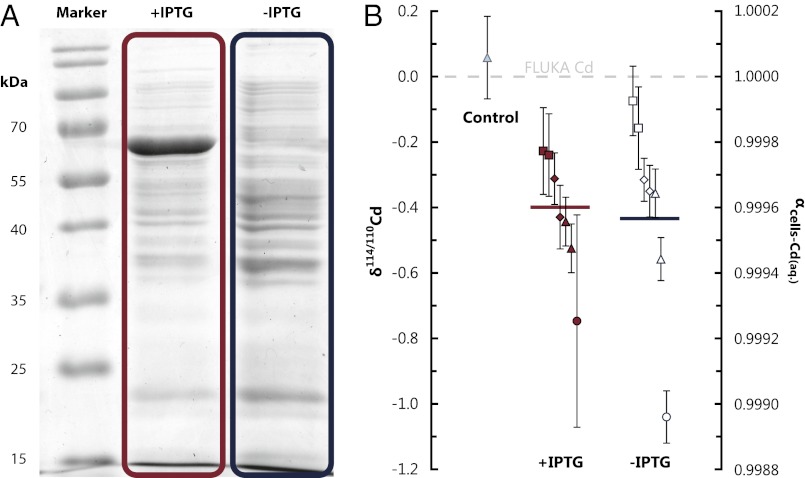
Fig. 2.
CdCA1 expression does not affect whole cell Cd isotopic compositions. (A) Image of an SDS/PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis) separation of cellular proteins (stained with Coomassie brilliant blue R-250). CdCA1 (with histidine tag) expression is seen in the induced cultures (+IPTG) at 67.5 kDa (10) but not in the uninduced cultures (no IPTG). (B) Whole cell isotopic compositions for the control, induced, and uninduced cultures (with the means of the induced and uninduced cultures, weighted by 1/uncertainty, shown as the red and blue horizontal bars, respectively). The mean values of the +IPTG and −IPTG populations are indistinguishable (P = 0.94, using an unpaired two-sample t test). Isotopic data are reported relative to the Cd in the growth solution and as fractionation factors: αcells-Cd(aq.). The control refers to cells that were harvested after ∼10-s exposure to Cd2+. The spread in Cd isotopic compositions relates primarily to the cell washing tests (SI Materials and Methods). When cells washed with the same treatment are compared (same symbols), +IPTG and –IPTG cultures are within analytical uncertainty.
Results
The whole-cell Cd isotope compositions in the 14 cultures from this study are lighter than the growth medium in experiments both with and without the expression of CdCA1. The weighted means of the two sets of cultures are αcells-Cd(aq.) = 0.99960 and αcells-Cd(aq.) = 0.99957 for induced and uninduced cultures, respectively (Fig. 2). The mean offset (αcells-Cd(aq.) ∼ 0.9996) is in agreement with the existing, albeit limited (and lower precision), Cd isotope dataset for cultured freshwater phytoplankton (αcells-Cd(aq.) = 0.9986 ± 0.0006; ref. 8) and the growing literature for other biologically active transition metals, whereby microorganisms exhibit a general preference for the light isotopes of a given element, e.g., Mo (12), Cu (13), Zn (14), Fe (15), and Ni (16). We rule out adsorption as the cause of the light isotopic fractionation observed for Cd in cultured cells, because a subset of cells plunged into the growth medium for a short time (∼10 s) resulted in no fractionation of Cd isotopes (Fig. 2, control; SI Materials and Methods).
To probe the processes responsible for Cd isotopic fractionation, we dissected the E. coli cells into key subcellular components. The cells were first mechanically separated by sonication and centrifugation into cell membranes and the bulk cytosol. The cytosolic components were further separated by chromatography into the nonspecifically bound Cd (termed Cd ligand: CdL), Cd bound in CdCA1, and denatured CdCA1 (Fig. 3).
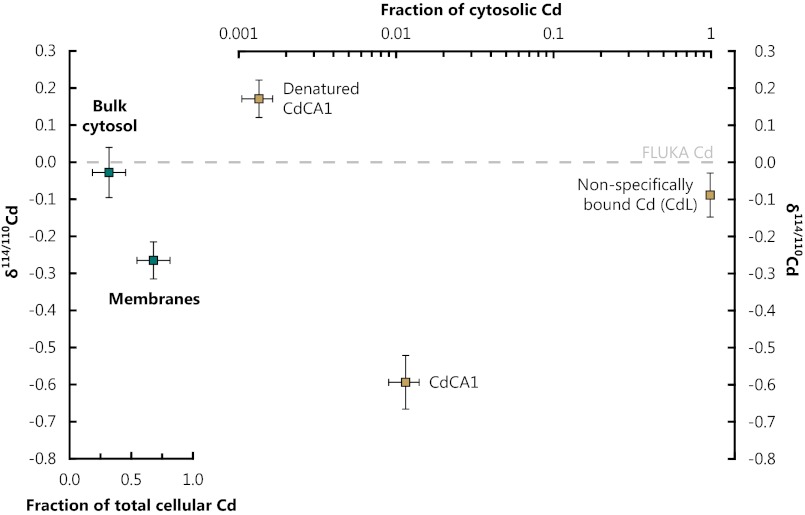
Fig. 3.
Subcellular mass balance for E. coli cells expressing CdCA1. (Left) Isotopic composition of the cytosol (pre-separation) and cell membranes, demonstrating that the membranes dominate whole-cell isotopic compositions. (Right) Isotopic composition of the cytosolic components (post chromatographic separation); note the log scale. Nonspecifically bound Cd within the cytosol is isotopically identical to the bulk Cd isotopic composition of the cytosol and accounts for nearly all cytosolic Cd (∼99%). CdCA1 is isotopically light by δ114/110Cd = −0.59 ‰, equivalent to αCdCA1-cytosol = 0.9994 ± 0.0001, whereas denatured CdCA1 exhibits the only positively fractionated Cd within the cell.
The subcellular separates exhibited a wide range of Cd isotope compositions (Table S3). The Cd bound in the cell membranes exhibited isotopic compositions negatively fractionated by δ114/110Cdmembranes = −0.26 ± 0.05 compared with the Luria-Bertani (LB) growth medium; δ114/110CdLB ≡ 0] and accounted for ∼68% of the total cellular burden. Cytosolic Cd (δ114/110Cdcytosol = −0.03 ± 0.07) is isotopically indistinguishable from the growth medium and contained the residual ∼32% of the cellular Cd (Fig. 3). The unfractionated Cd isotopic composition of the cytosol implies that metal acquisition by E. coli either does not fractionate Cd isotopes or that Cd isotopic equilibration across the membrane occurs quickly.
Further separation of the cytosolic components reveals similarly large δ114/110Cd variations within the cytosol (Figs. 3 and 4; Table S4). The largest pool of Cd in the cytosol was the nonspecifically bound Cd (CdL ∼ 99% of cytosolic Cd), with an isotope composition of δ114/110CdCdL = −0.09 ± 0.06 (i.e., within uncertainty of the bulk cytosol isotopic measurement). Insertion of Cd into CdCA1, which occurs in vivo within the cytosol, was observed to cause the largest isotopic fractionation within the cell (δ114/110CdCdCA1 = −0.59 ± 0.07 relative to the growth medium), but accounts for only ∼1% of the cytosolic Cd. In a full replicate culture, and subsequent multistep insertion and extraction of Cd into CdCA1, we obtained a statistically identical value of δ114/110Cd = −0.67 ± 0.07 (relative to the growth medium) for Cd bound in CdCA1. Denatured CdCA1 exhibited positive Cd isotope compositions (δ114/110Cddenat. CdCA1 = +0.17 ± 0.05) but is insignificant in terms of the cytosolic mass balance, accounting for ∼0.1% of cytosolic Cd. Although there is potential for Cd isotopic fractionation during protein extraction and purification chromatography, the subdivision of cells into membranes and bulk cytosol is a mechanical process; thus, the observation that the membranes dominate the whole-cell isotopic compositions is robust. This separation reveals that isotopic fractionation of Cd only occurs once Cd is inside the cell and is dominated by Cd translocation to the cell membranes (Fig. 4).
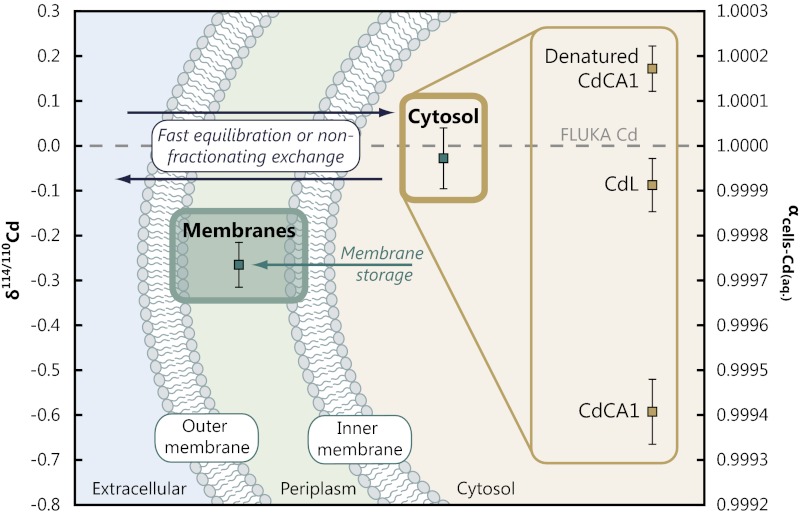
Fig. 4.
Proposed model for Cd isotopic fractionation in E. coli. Cd isotope compositions plotted on a schematic representation of the cell for each of the subcellular fractions, with interpretation. The measurements demonstrate that different components of E. coli cells exhibit multiple Cd isotope compositions. The bulk cytosolic measurement is isotopically indistinguishable from the growth solution, implying either rapid Cd isotopic equilibration across the membrane or that the uptake of Cd into the cytosol does not fractionate Cd isotopes.
Discussion
The macroscopic whole-cell experiments and subcellular mass balance both demonstrate that expression of the Cd enzyme, CdCA1, is not a significant contributor to the mass balance of Cd within the cell and hence whole-cell isotopic compositions (Fig. 3). Although Cd bound to CdCA1 has the most isotopically fractionated value observed, the small fraction of cellular Cd bound in this enzyme makes it insignificant to the overall cellular Cd isotope composition (in agreement with observations from the macroscopic experiments; Fig. 2). Instead, the Cd sequestered into the cell membrane drives the whole-cell isotopic composition. Importantly, this observation also explains why cells not expressing CdCA1 exhibit identically light Cd isotope compositions as those cells expressing it (Fig. 2). The size and direction of the membrane-associated fractionation is in agreement with Cd removal from seawater (Fig. 1).
Transport of Cd2+ into the cytosol likely occurs because of close geochemical similarities to Zn2+ (and/or other divalent metals, e.g., Co2+ or Mn2+), which plays an important role in many enzyme pathways (17, 18). Culturing experiments have demonstrated that the uptake of Cd into plankton—as with other divalent metals—occurs in direct proportion to dissolved [Cd2+], although this pattern can be modulated by competition with the availability of other metals (19, 20). For example, Zn limitation has been shown to increase Cd uptake in bacteria (21) and phytoplankton (22–24), with Fe restriction also promoting an increase in cellular Cd quotas in culture (24, 25). Cd is imported by a wide range of microorganisms as they accumulate geochemically similar trace elements for physiological use (26), thereby removing Cd from seawater.
The toxicity of Cd requires that it be managed if it enters a cell (27). One role of the membranes in E. coli, and many other microorganisms, is to ensure that metal homeostasis is maintained by sensing, transporting, and storing metals for subsequent use (17). E. coli cells have Cd management systems analogous to those of eukaryotic cells, including marine phytoplankton (28). There is genetic evidence in both pro- and eukaryotes for gene products that manage or confer tolerance to heavy-metal stress, including Cd (29, 30). Once internalized, Cd can interfere with essential cellular machinery, particularly through displacement of metals from their correct binding site (17). The cellular concentrations of Cd and other toxic metals must therefore be tightly regulated to prevent damage to cells (27). The mechanism of Cd management—binding with cysteine-rich peptides such as glutathione, phytochelatin, or metallothionein (31)—is a common, highly conserved feature of many organisms including bacteria, plants, fungi, and animals (28). The fate of these Cd complexes depends on the organism in question, although they are commonly exported to a vacuole (32, 33) or sometimes ejected from the cell (30, 34) where they cannot interfere with the critical cellular machinery in the cytosol. E. coli, which lack a vacuole, translocate Cd to their membranes (35) by binding Cd with glutathione, a precursor of (and functionally analogous to) phytochelatin (28). Cd is a particularly efficient metal for inducing production of metal-binding ligands in plankton (36), with analysis of field populations (37, 38) and laboratory cultures (39, 40) demonstrating that Cd-binding ligands are produced far in excess of that needed to sequester any intracellular Cd (and other metals, such as Cu and Zn; ref. 36). Our isotope data demonstrate that this process of homeostasis can lead to the retention of isotopically light inert Cd complexes by organisms (Fig. 4). This mechanism can account for the isotopic fractionation observed in experiments conducted both with and without expression of CdCA1 (Fig. 2). However, it remains to be tested whether there are different fractionation factors associated with various Cd-ligating peptides (e.g., phytochelatin vs. glutathione) and their ultimate fate within cells.
The implication of these experiments is that biologically mediated Cd isotope fractionation occurs from growth media as organisms inadvertently acquire Cd and sequester it into inert forms within the cell. Because all organisms share a common ability to manage Cd (28), but do not share CdCA1 (Table S1) (11), we contend that metal homeostasis, rather than physiological function, drives the vertical isotopic (4) and concentration gradients (2, 3) of Cd seen in the global ocean. The fractionation observed with nonspecific uptake and homeostasis (i.e., membrane storage) in E. coli is consistent with the direction and magnitude of Cd isotopic fractionation for published seawater data (Fig. 1). Together, previous culturing studies and the genetic data are consistent with the nonspecific uptake and homeostasis mechanism proposed here, thus providing an alternative explanation as to the cause of the Cd–P association seen in the global ocean (2, 3). In other words, all phytoplankton, regardless of their ability to use Cd, can contribute to the removal of Cd and fractionation of its isotopes in seawater (Fig. 1).
Nonspecific uptake and homeostasis may contribute to the distribution of other metals and their isotopic compositions in the oceans. Even metals with well-understood enzymatic roles (e.g., Fe) may exhibit a homeostasis control in regions of higher concentration (e.g., upwelling zones of the ocean). Despite the lack of similar subcellular analyses for other metal isotope systems, certain metal-specific storage proteins have been identified in phytoplankton [e.g., for Fe (41), Cu (42), Zn, or Mn (43)] that allow for the intracellular accumulation of metals during times of metal-replete conditions. This process of luxury uptake and storage has been observed in a wide range of phytoplankton lineages (44–46) and is one of a number of survival strategies plankton use during metal limitation (47). Subcellular isotopic analysis—as performed for Cd in this study—may enable testing of the relative importance of these mechanisms in setting oceanic distributions (and isotopic compositions) for other metals.
Materials and Methods
Preparation of Transgenic E. coli and Overexpression of CdCA1.
The pET15b-CdCA1 construct was obtained from Xu et al. (10). Briefly, the CdCA1 coding sequence for this construct was amplified from T. weissflogii cDNA. The PCR product was subsequently cloned into a pET15b expression vector (Novagen). This construct was transformed into a competent E. coli strain BL21(DE3). Positive transformants were selected on LB plates with ampicillin (100 μg/mL). The recombinants were also screened by PCR using the T7 promoter and T7 terminator primer. Positive clones were further sequenced with the same primer pair.
Cells were initially grown until the exponential stage of growth in Cd-free LB starter cultures at 37 °C to an optical density of 0.5–0.8 at 600 nm. Cd was subsequently added to a Cd2+ concentration of ∼60 μM (total, or analytical [Cd], was 0.5 mM, added as CdCl2), and the culture was split into two. The total Zn concentration of the growth medium, determined by inductively coupled plasma mass spectrometry (ICPMS) on a Cd-free starter culture, was ∼12 μM. The final Cd/Zn was ∼42 mol/mol, which is marginally higher than in previous studies (10), providing confidence that the Zn form of CdCA1 was not produced. The Cd content and isotopic composition of the growth medium did not change measurably during any experiment because of the high Cd concentrations used. CdCA1 overexpression was induced in 7 of the 14 cultures by addition of 1.0 mM IPTG to study the effect of CdCA1 expression on whole-cell isotopic compositions. Cells continued to grow in the exponential stage (determined by optical density) until they were harvested, typically after 4 h. Cells were harvested by centrifugation (5,000 × g for 15 min at 4 °C) and rinsed with 18.2 MΩ H2O, and the pellets were frozen at −80 °C until needed for further analysis.
CdCA1 Purification and Cleavage of the Histidine Tag.
Nickel columns were prepared by packing the His⋅Bind resin, Ni-charged, into chromatography columns (Novagen) according to the manufacturer’s protocol. Frozen bacterial pellets were defrosted and resuspended in 20 mL extraction buffer (20 mM Tris-Cl, pH 8.0, 100 mM Na2SO4), with one protease inhibitor tablet (Complete Protease Inhibitor Mixture Tablet, EDTA-free; Roche Diagnostics) and sonicated 4–6 times for 30-s bursts (on ice) with an interval of 1 min between sonications. The lysate was centrifuged, and the supernatant was filtered through a 0.45-μm filter. The resulting filtrate was loaded onto an equilibrated column at a flow rate of no faster than 1–2 mL/min; the pellet, composed mostly of cell membranes (and some other cellular debris), was stored for [Cd] and isotopic analysis. Nonspecifically bound proteins were removed and collected by washing with 40 mL wash buffer (20 mM Tris-Cl, pH 8.0, 100 mM Na2SO4) through the Ni columns. On-column cleavage of the target protein from the histidine tag was carried out by loading 20 mL elution buffer (20 mM Tris-Cl, pH 8.0, 100 mM Na2SO4, 20 mM imidazole) into the column. After the buffer had flowed through, the column was capped, and cleavage was allowed to occur overnight in the presence of 2 mL elution buffer with thrombin. The target protein was eluted the following day by adding 5 mL elution buffer and collecting the first 4 mL of the flow-through. Purified proteins were further concentrated by passing the extract through an Ultracel-30 membrane (Amicon Ultra-4 Centrifugal Filter Unit, 30-kDa cutoff; Millipore). Both the purified protein and the residual flow-through (denatured CdCA1) were stored for analysis.
SDS/PAGE and Protein Quantification.
Whole cell protein extracts were separated on 12% (vol/vol) SDS/PAGE using the Laemmli method (48, 49), and the gels were subsequently stained with Coomassie brilliant blue R-250. The protein concentration of each sample was determined using the Bradford method (50).
The distribution of CdCA1 homologues was investigated using BLAST (51), with the T. weissflogii CdCA1 protein sequence as query, on complete plankton genomes found in the Joint Genome Institute (JGI) and National Center for Biotechnology Information (NCBI) databases (Table S1).
Decomposition of Cells and Purification of Elemental Cd.
Whole cells and subcellular fractions were taken to dryness and weighed before acid digestion. Typically, 1 mL concentrated aqua regia was added per 15 mg (dry mass) of cells up to a maximum of 10 mL and refluxed at 80 °C for 24 h before evaporating to dryness at 120 °C and resampling in 2% HNO3 (vol/vol) for analysis by ICPMS. This process was repeated until no meniscus (caused by latent organic compounds) was observed in the suspension. To check for congruent dissolution, four bacterial isolates were subsampled and digested using a microwave treatment similar to the one described by Larner et al. (52), allowing direct comparison with the hotplate method. Cells were suspended in a mixture of 5 mL concentrated HNO3 and 3 mL 30% (weight percent) H2O2 and heated to 240 °C at 60 atm for 90 min. The measured Cd isotopic compositions from the two methods, following ion exchange chromatography, are within δ114/110Cd ≤ 0.03‰ of the alternative decomposition treatment (1:1 line R2 > 0.9; Table S5; Fig. S1), suggesting that the hotplate method was robust in ensuring congruent dissolution and liberation of Cd from cell organic matter.
Decomposed samples were analyzed for Cd concentrations on a Thermo ELEMENT 2 ICPMS. A suitable mass of Cd double spike (111Cd–113Cd) was then added, and the samples were purified using extraction chromatography (53, 54) in 200-μL shrink-fit polytetrafluoroethylene columns.
Isotopic Analysis of Cd.
Isotopic methods have been reported in detail previously (55). Briefly, samples were introduced into a Nu Instruments DSN-100 desolvation system (at 120 μL/min) with an Elemental Scientific PFA MicroFlow Nebulizer. The desolvated sample was introduced into a Nu Instruments Nu Plasma multi-collector-ICPMS. All ion currents from 110 AMU (110Cd, 110Pd) to 117 AMU (117Sn) were measured simultaneously in 40 × 10-s integrations, using a MATLAB-based script to iteratively deconvolve sample isotopic compositions from the spike-sample mixture and isobaric interferences (112Sn on 112Cd, 113In on 113Cd, and 114Sn on 114Cd). Final isotopic compositions are reported using δ-notation: δ114/110Cd = ([114Cd/110Cdsample/114Cd/110Cdstandard] – 1) × 1,000, relative to the starting Cd in the growth solution, FLUKA Cd (Sigma-Aldrich CdCl2, batch 20899). Because all Cd isotope compositions are reported relative to the Cd in the growth solution, the choice of reference standard was unimportant. The total analytical blank was negligible in all cases at 8 ± 6 pg Cd (1 SD, n = 7), equivalent to <0.1% of the Cd present on any single isotopic measurement.
Where possible, uncertainties are quoted as 2× SD of sample replicates (when n ≥ 5). When there was insufficient Cd to repeat the isotopic analyses at least five times, measurement uncertainties were derived from standard replicates, with similar Cd concentrations and spike/sample ratios that were run in the same analysis session, as this is generally a good approximation of the measurement uncertainty (53). (The uncertainty reported in the text is always the larger of the two.) The Cd isotopic composition of FLUKA Cd, relative to our in-house ICPMS standard OxCad (55), was determined as δ114/110Cd = +0.75 ± 0.10 (2 SD, n = 13).
Supplementary Material
Acknowledgments
We thank Y. Xu for the CdCA1 construct for use in these experiments; P. F. Holdship for help with the ICPMS; K. L. Karsh and F. M. M. Morel for helpful discussions; and two anonymous referees for particularly insightful comments. T.J.H. is supported by the Natural Environment Research Council (NE/G524060/1) and Nu Instruments. This work was funded by the Oxford-Princeton Research Partnership (R.E.M.R. and F. M. M. Morel), NERC, and the European Research Council (SP2-GA-2008-200915).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213857110/-/DCSupplemental.
References
- 1.Morel FMM, Price NM. The biogeochemical cycles of trace metals in the oceans. Science. 2003;300(5621):944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- 2.Bruland KW. Oceanographic distributions of cadmium, zinc, nickel, and copper in the North Pacific. Earth Planet Sci Lett. 1980;47(2):176–198. [Google Scholar]
- 3.Boyle EA, Sclater F, Edmond JM. On the marine geochemistry of cadmium. Nature. 1976;263(5572):42–44. [Google Scholar]
- 4.Ripperger S, Rehkämper M, Porcelli D, Halliday AN. Cadmium isotope fractionation in seawater–A signature of biological activity. Earth Planet Sci Lett. 2007;261(3-4):670–684. [Google Scholar]
- 5.Ho TY, et al. The elemental composition of some marine phytoplankton. J Phycol. 2003;39(6):1145–1159. [Google Scholar]
- 6.Price NM, Morel FMM. Cadmium and cobalt substitution for zinc in a marine diatom. Nature. 1990;344(6267):658–660. [Google Scholar]
- 7.Xu Y, Tang D, Shaked Y, Morel FMM. Zinc, cadmium, and cobalt interreplacement and relative use efficiencies in the coccolithophore Emiliania huxleyi. Limnol Oceanogr. 2007;52(5):2294–2305. [Google Scholar]
- 8.Lacan F, Francois R, Ji Y, Sherrell RM. Cadmium isotopic composition in the ocean. Geochim Cosmochim Acta. 2006;70(20):5104–5118. [Google Scholar]
- 9.Lane TW, et al. Biochemistry: A cadmium enzyme from a marine diatom. Nature. 2005;435(7038):42. doi: 10.1038/435042a. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Feng L, Jeffrey PD, Shi Y, Morel FMM. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature. 2008;452(7183):56–61. doi: 10.1038/nature06636. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Song B, Morel FMM. Diversity of the cadmium-containing carbonic anhydrase in marine diatoms and natural waters. Environ Microbiol. 2007;9(2):403–413. doi: 10.1111/j.1462-2920.2006.01151.x. [DOI] [PubMed] [Google Scholar]
- 12.Wasylenki LE, et al. Isotope fractionation during microbial metal uptake measured by MC-ICP-MS. J Anal at Spectrom. 2007;22(8):905–910. [Google Scholar]
- 13.Navarrete JU, Borrok DM, Viveros M, Ellzey JT. Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Geochim Cosmochim Acta. 2011;75(3):784–799. doi: 10.1016/j.gca.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John SG, Geis RW, Saito MA, Boyle EA. Zinc isotope fractionation during high-affinity and low-affinity zinc transport by the marine diatom Thalassiosira oceanica. Limnol Oceanogr. 2007;52(6):2710–2714. [Google Scholar]
- 15.Beard BL, et al. Iron isotope biosignatures. Science. 1999;285(5435):1889–1892. doi: 10.1126/science.285.5435.1889. [DOI] [PubMed] [Google Scholar]
- 16.Cameron V, Vance D, Archer C, House CH. A biomarker based on the stable isotopes of nickel. Proc Natl Acad Sci USA. 2009;106(27):10944–10948. doi: 10.1073/pnas.0900726106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7(1):25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 18.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460(7257):823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 19.Sunda WG. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front Microbiol. 2012;3:204. doi: 10.3389/fmicb.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twining BS, Baines SB. The trace metal composition of marine phytoplankton. Annu Rev Mar Sci. 2013;5:191–215. doi: 10.1146/annurev-marine-121211-172322. [DOI] [PubMed] [Google Scholar]
- 21.Laddaga RA, Silver S. Cadmium uptake in Escherichia coli K-12. J Bacteriol. 1985;162(3):1100–1105. doi: 10.1128/jb.162.3.1100-1105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen JT, Sherrell RM. Effects of dissolved carbon dioxide, zinc, and manganese on the cadmium to phosphorus ratio in natural phytoplankton assemblages. Limnol Oceanogr. 2005;50(4):1193–1204. [Google Scholar]
- 23.Sunda WG, Huntsman SA. Antagonisms between cadmium and zinc toxicity and manganese limitation in a coastal diatom. Limnol Oceanogr. 1996;41(3):373–387. [Google Scholar]
- 24.Sunda WG, Huntsman SA. Effect of Zn, Mn, and Fe on Cd accumulation in phytoplankton: Implications for oceanic Cd cycling. Limnol Oceanogr. 2000;45(7):1501–1516. [Google Scholar]
- 25.Lane ES, Jang K, Cullen JT, Maldonado MT. The interaction between inorganic iron and cadmium uptake in the marine diatom Thalassiosira oceanica. Limnol Oceanogr. 2008;53(5):1784–1789. [Google Scholar]
- 26.Nies DH. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid. 1992;27(1):17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 27.Finney LA, O’Halloran TV. Transition metal speciation in the cell: Insights from the chemistry of metal ion receptors. Science. 2003;300(5621):931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 28.Prévéral S, et al. A common highly conserved cadmium detoxification mechanism from bacteria to humans: heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. J Biol Chem. 2009;284(8):4936–4943. doi: 10.1074/jbc.M808130200. [DOI] [PubMed] [Google Scholar]
- 29.Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 30.Silver S, Phung T. A bacterial view of the periodic table: Genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol. 2005;32(11–12):587–605. doi: 10.1007/s10295-005-0019-6. [DOI] [PubMed] [Google Scholar]
- 31.Grill E, Winnacker EL, Zenk MH. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA. 1987;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza-Cózatl DG, et al. Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. J Biol Chem. 2010;285(52):40416–40426. doi: 10.1074/jbc.M110.155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortiz DF, Ruscitti T, McCue KF, Ow DW. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem. 1995;270(9):4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- 34.Lee JG, Ahner BA, Morel FMM. Export of cadmium and phytochelatin by the marine diatom Thalassiosira weissflogii. Environ Sci Technol. 1996;30(6):1814–1821. [Google Scholar]
- 35.Mitra RS, Gray RH, Chin B, Bernstein IA. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+ J Bacteriol. 1975;121(3):1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahner BA, Morel FMM. Phytochelatin production in marine algae. 2. Induction by various metals. Limnol Oceanogr. 1995;40(4):658–665. [Google Scholar]
- 37.Dupont CL, Moffett JW, Bidigare RR, Ahner BA. Distributions of dissolved and particulate biogenic thiols in the subartic pacific ocean. Deep Sea Res Part I Oceanogr Res Pap. 2006;53(12):1961–1974. [Google Scholar]
- 38.Ahner BA, Lee JG, Price NM, Morel FMM. Phytochelatin concentrations in the equatorial pacific. Deep Sea Res Part I Oceanogr Res Pap. 1998;45(11):1779–1796. [Google Scholar]
- 39.Ahner BA, Kong S, Morel FMM. Phytochelatin production in marine algae. 1. An interspecies comparison. Limnol Oceanogr. 1995;40(4):649–657. [Google Scholar]
- 40.Dupont CL, Ahner BA. Effects of copper, cadmium, and zinc on the production and exudation of thiols by Emiliania huxleyi. Limnol Oceanogr. 2005;50(2):508–515. [Google Scholar]
- 41.Marchetti A, et al. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature. 2009;457(7228):467–470. doi: 10.1038/nature07539. [DOI] [PubMed] [Google Scholar]
- 42.Dupont CL, Nelson RK, Bashir S, Moffett JW, Ahner BA. Novel copper-binding and nitrogen-rich thiols produced and exuded by Emiliania huxleyi. Limnol Oceanogr. 2004;49(5):1754–1762. [Google Scholar]
- 43.Tottey S, et al. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature. 2008;455(7216):1138–1142. doi: 10.1038/nature07340. [DOI] [PubMed] [Google Scholar]
- 44.Sunda WG, Huntsman SA. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar Chem. 1995;50(1-4):189–206. [Google Scholar]
- 45.Kustka A, Sañudo-Wilhelmy S, Carpenter EJ, Capone DG, Raven JA. A revised estimate of the iron use efficiency of nitrogen fixation, with special reference to the marine cyanobacterium Trichodesmium spp. (cyanophyta) J Phycol. 2003;39(1):12–25. [Google Scholar]
- 46.Kustka AB, Allen AE, Morel FMM. Sequence analysis and transcriptional regulation of iron acquisition genes in two marine diatoms. J Phycol. 2007;43(4):715–729. [Google Scholar]
- 47.Saito MA, et al. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc Natl Acad Sci USA. 2011;108(6):2184–2189. doi: 10.1073/pnas.1006943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory; 2001. p. 2344. [Google Scholar]
- 50.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 51.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 52.Larner F, et al. A new separation procedure for Cu prior to stable isotope analysis by MC-ICP-MS. J Anal at Spectrom. 2011;26(8):1627–1632. [Google Scholar]
- 53.Ripperger S, Rehkämper M. Precise determination of cadmium isotope fractionation in seawater by double spike MC-ICPMS. Geochim Cosmochim Acta. 2007;71(3):631–642. [Google Scholar]
- 54.Wombacher F, Rehkämper M, Mezger K, Münker C. Stable isotope compositions of cadmium in geological materials and meteorites determined by multiple-collector ICPMS. Geochim Cosmochim Acta. 2003;67(23):4639–4654. [Google Scholar]
- 55.Horner TJ, Rickaby REM, Henderson GM. Isotopic fractionation of cadmium into calcite. Earth Planet Sci Lett. 2011;312(1-2):243–253. [Google Scholar]
- 56.Abouchami W, et al. Modulation of the southern ocean cadmium isotope signature by ocean circulation and primary productivity. Earth Planet Sci Lett. 2011;305(1-2):83–91. [Google Scholar]
- 57.Xue Z, Rehkämper M, Schönbächler M, Statham PJ, Coles BJ. A new methodology for precise cadmium isotope analyses of seawater. Anal Bioanal Chem. 2012;402(2):883–893. doi: 10.1007/s00216-011-5487-0. [DOI] [PubMed] [Google Scholar]
- 58.Yang SC, Lee DC, Ho TY. The isotopic composition of cadmium in the water column of the South China Sea. Geochim Cosmochim Acta. 2012;98:66–77. [Google Scholar]
- 59.Gault-Ringold M, Adu T, Stirling CH, Frew RD, Hunter KA. Anomalous biogeochemical behavior of cadmium in subantarctic surface waters: Mechanistic constraints from cadmium isotopes. Earth Planet Sci Lett. 2012;341:94–103. [Google Scholar]
- 60.Abouchami W, et al. 2012. A common reference material for cadmium isotope studies—NIST SRM 3108 Cd. Geostand Geoanal Res, 10.1111/j.1751-908X.2012.00175.x.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.